Abstract
Retinoid X Receptor (RXR) regulates key cellular responses such as cell growth and development, and this regulation is frequently perturbed in various malignancies, including Hepatocellular Carcinoma (HCC). However, the molecule(s) that physically govern this deregulation are mostly unknown. Here, we identified RXR as an interacting partner of Astrocyte Elevated Gene-1 (AEG-1)/Metadherin (MTDH), an oncogene upregulated in all cancers. Upon interaction, AEG-1 profoundly inhibited RXR/Retinoic Acid Receptor (RAR)-mediated transcriptional activation. Consequently, AEG-1 markedly protected HCC and acute myeloid leukemia (AML) cells from retinoid- and rexinoid-induced cell death. In non-tumorigenic cells and primary hepatocytes, AEG-1/RXR co-localizes in the nucleus where AEG-1 interferes with recruitment of transcriptional co-activators to RXR preventing transcription of target genes. In tumor cells and AEG-1 transgenic hepatocytes, overexpressed AEG-1 entraps RXR in cytoplasm, precluding its nuclear translocation. Additionally, ERK, activated by AEG-1, phosphorylates RXR which leads to its functional inactivation and attenuation of ligand-dependent transactivation. In nude mice models, combination of all-trans retinoic acid (ATRA) and AEG-1 knockdown synergistically inhibited growth of human HCC xenografts. The present study establishes AEG-1 as a novel homeostatic regulator of RXR and RXR/RAR that might contribute to hepatocarcinogenesis. Targeting AEG-1 could sensitize HCC and AML patients to retinoid- and rexinoid-based therapeutics.
Keywords: Hepatocellular Carcinoma, Protein-protein interaction, Transcriptional regulation, Retinoic acid, Cancer therapeutics
INTRODUCTION
RXRs (RXR α,β,γ) are the master coordinators of cell growth, metabolism and development (1, 2). RXRs heterodimerize with one third of the 48 human nuclear receptor superfamily members, including RAR (3). RXR/RAR heterodimer mediates the retinoid-dependent transcription of genes involved in cell proliferation, differentiation and apoptosis. RXRs are frequently dysregulated due to altered expression and inactivation in various cancers including HCC, thyroid carcinoma and prostate cancer (4–6), which might lead to compromised RAR functions in these malignancies (7–9). However, molecular mechanisms or regulators that govern the differential expression and function of RXR still remain poorly understood.
AEG-1, also known as MTDH and LYRIC, is a multifunctional oncogene that is overexpressed in a wide variety of malignancies (10, 11). AEG-1 is significantly upregulated in >90% of human HCC patients, affecting diverse aspects of HCC pathogenesis, including proliferation, angiogenesis, invasion and metastasis (12, 13). Although numerous studies have established clinicopatholgical correlation of AEG-1 in cancer, the underlying molecular mechanism by which AEG-1 exerts its oncogenic functions requires further clarification. AEG-1 has been shown to interact with few proteins to drive tumorigenesis (10). In order to identify crucial AEG-1-interacting partners, we screened a human liver cDNA library by yeast two-hybrid (Y2H) assay (14), and identified RXR as a novel interacting partner of AEG-1.
Employing human HCC cell lines with endogenous low and high AEG-1 expression corresponding to low to high aggressive tumorigenic features, HCC cells with stable AEG-1 overexpression or knockdown and hepatocytes isolated from AEG-1 transgenic (Alb/AEG-1) (13) and knockout (AEG-1KO) mice, we have now deciphered how AEG-1 interaction with RXRα and RXRβ drives RXR-mediated functions in HCC. Our studies uncover the mechanism by which AEG-1 upregulation impacts RXR inactivation and retinoid-response contributing to carcinogenesis. Moreover, AEG-1 knockdown and chemical inhibition of AEG-1-mediated signaling enhances the efficacy of retinoids by sensitizing the cells leading to inhibited tumor growth in in vitro and xenograft models. AEG-1 inhibition might be an effective strategy to augment effects of retinoids in patients with diverse cancer indications.
MATERIAL AND METHODS
Generation of Alb/AEG-1 and AEG-1KO mice
Generation and characterization of a hepatocyte-specific AEG-1 transgenic mouse (Alb/AEG-1) in B6/CBA background have been described previously (13). AEG-1KO mouse was generated in C57B6/129Sv background and the procedure is described in detail in the supplemental information. All animal studies were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University, and were conducted in accordance with the Animal Welfare Act, the PHS Policy on Humane Care and Use of Laboratory Animals, and the U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training.
Tissue culture
HepG3, QGY-7703, THLE3, Hep3B, HuH7, and HEK-293 cells were cultured as reported earlier (12). Generation of Hep-PC-4 (control clone), Hep-AEG-1–14 (a C-terminal HA-tagged AEG-1 overexpressing clone), Hep-CTRLsi (control siRNA expressing clone) and Hep-AEG-1si (expressing AEG-1 shRNA) in HepG3 background has been described before (12, 14). A C-terminal HA-tagged AEG-1 construct mutated at LXXLL motif was stably expressed in HepG3 cells, Hep-AEG1-Lxxmut, and was generated following the same protocol as for Hep-AEG1–14 cells. The clones were selected and maintained in Hygromycin containing DMEM.
Primary cell culture and viability assay
Primary mouse hepatocytes were isolated from WT (B6/CBA), Alb/AEG-1, WT (C57B6/129Sv) and AEG-1KO mice in the Cell and Molecular Biology Core in VCU as described previously (13) and were plated on collagen-coated dishes (BD BioCoat collagen type I, BD Biosciences) and cultured in Williams E medium (SIGMA) containing NaHCO3, L-glutamine, insulin (1.5 µM) and dexamethasone (0.1 µM). For MTT assays, 1.0–1.5×104 mouse hepatocytes were plated in each well of a 96-well plate and treated with retinoids and rexinoids for respective time points as mentioned in the Figure legends. Cell viability was determined by standard MTT assay as described (12, 15, 16).
Transient transfection and luciferase reporter assays
Transfections and luciferase assays were done according to the manufacturer’s protocol for human HCC cells as described elsewhere (14, 17, 18) and primary hepatocytes (supplemental information). Each experiment was performed in triplicates and repeated three times to calculate means and standard error.
Total RNA extraction, cDNA preparation and Real time PCR
Total RNA was extracted from Human HepG3 cells, livers and hepatocytes of WT (B6/CBA and C57B6/129Sv), Alb/AEG-1, and AEG-1KO mouse using the QIAGEN miRNAeasy Mini Kit (QIAGEN, Hilden, Germany). cDNA preparation was done using ABI cDNA synthesis kit. Real-time polymerase chain reaction (RT-PCR) was performed using an ABI ViiA7 fast real-time PCR system and Taqman gene expression assays according to the manufacturer’s protocol (Applied Biosystems, Foster City, CA). The best available Taqman primers-probes spanning two exons for RARB, CYP26A1, NROB2, CRABP2, FOXA1, TLL1, RXRA and RXRB for human as well as mouse were purchased from ABI.
Chromatin Immunoprecipitation (ChIP) Assay
Sheared chromatin was prepared following the manufacturer’s instructions and was immunoprecipitated using RXRα (Santa Cruz Biotechnology), AHH3 and SRC-1 (Cell signaling) antibodies. The eluted DNA and inputs was subjected to PCR for RARB and HOXA1 genes. For RARB2 (Sense: 5’AGCTCTGTGAGAATCCTGGGAG3’, Antisense: 5’TAGACCCTCCT GCCTCTGAACA3’) and HOXA1 (Sense: 5’CTGGGG CAATCAGATTCAAACC3’, Antisense: 5’CTCAGATAAACTGCTGGGACTC3’) primers were used for PCR amplification using Taq PCRx polymerase kit (Invitrogen) following the manufacturer’s instructions. These PCRs were performed without enhancers and repeated at least three times.
Nude Mice Xenograft Studies
Subcutaneous xenografts were established in flanks of athymic nude mice using QGY-7703 cells (5 × 105). After 1 week, these mice were injected with ATRA (10 mg/kg) or DMSO i.p., a total of 7 injections, once every alternative day. One week after the final treatment, mice were sacrificed. Tumor volume was measured twice weekly with a caliper and calculated using the formula π/6 × larger diameter × (smaller diameter)2. Tumor samples were immunostained using antibodies against AEG-1, PCNA (Cell signaling), and Cleaved caspase 3 (Cell signaling). All experiments were performed with six-eight mice in each group and were repeated at least two times.
Statistical analysis
Data were represented as mean ± Standard Error of Mean (S.E.M) and analyzed for statistical significance using one-way analysis of variance (ANOVA) followed by Newman-Keuls test as a post hoc test. A p-value of <0.05 was considered as significant.
RESULTS
Identification of RXRα and RXRβ as AEG-1-interacting partners
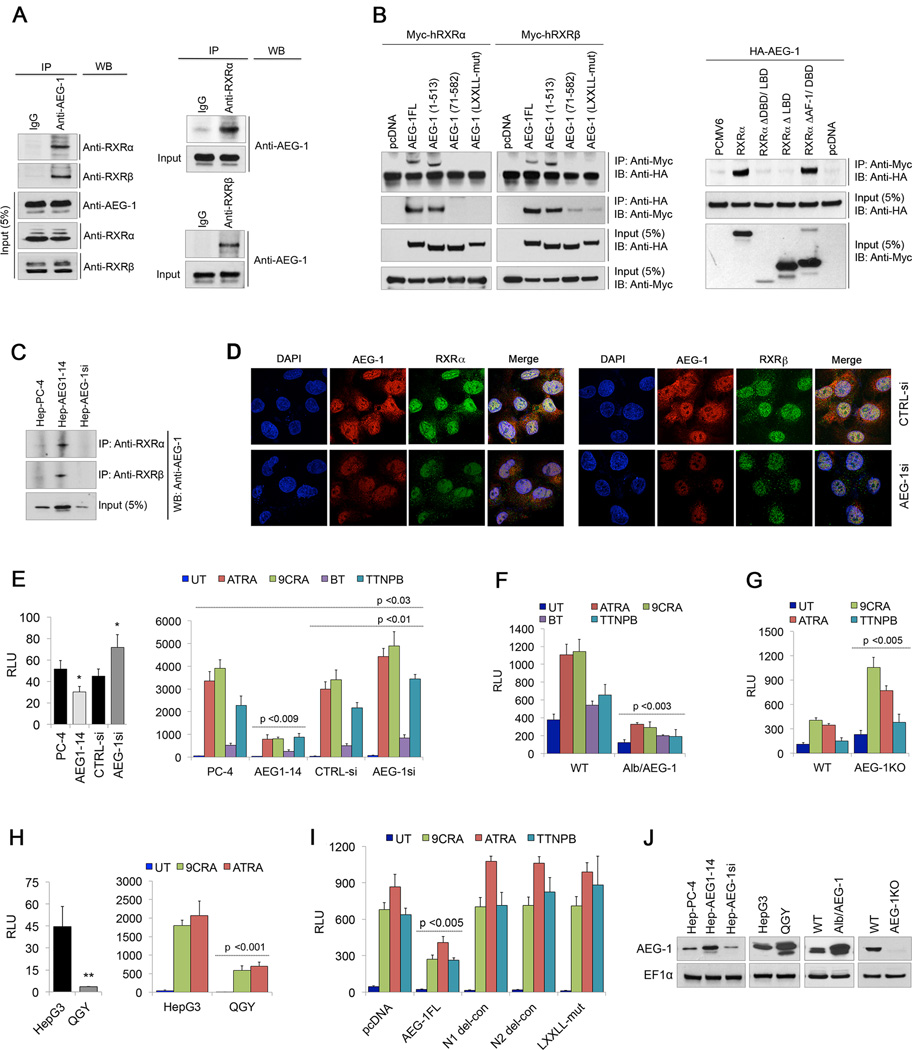
AEG-1 is a 582 a.a. protein with a transmembrane domain (51–72 a.a) and multiple nuclear localization signals. A number of AEG-1-interacting proteins have been identified that interact with its large C-terminal region (14, 17). However, a ‘LXXLL’ motif, by which co-activators and co-repressors interact with nuclear receptors (19), is located in the NH2-terminal region of AEG-1 suggesting that this region might be important for AEG-1 functions. Based upon this, a human liver cDNA library was screened by Y2H assay using the NH2-terminal 1–57 a.a. of AEG-1 as bait, which lead to identification of RXRβ as an AEG-1-interacting protein (Supplementary Table S1). Co-immunoprecipitation (IP) assays using lysates from human HCC cell line QGY-7703 demonstrated pull-down of RXRα and RXRβ by anti-AEG-1 antibody and vice versa confirming the interaction (Fig. 1A).
Fig. 1. AEG-1 interacts with RXRα and RXRβ abrogating RXR-dependent RARE promoter activity.
(A) Lysates from QGY-7703 cells were immunoprecipitated (IP) with anti-AEG-1 antibody and immunoblotted (IB or WB) with anti-RXRα and anti-RXRβ antibodies, and vice versa.
(B) HEK-293 cells were transiently transfected with Myc-tagged hRXRα or hRXRβ with control pcDNA3.1 vector, and HA-tagged full length AEG-1 (AEG-1FL, 1–582 a.a.), AEG-1 deletion constructs: C1 (1–513 a.a.) and N1 (71–582 a.a.), and AEG-1 LXXLL-mutant construct. After 48 hours, lysates were immunoprecipitated with anti-Myc antibody and IB conducted using anti-HA antibody, and vice versa. Cells were also transfected with HA-tagged AEG-1 with control PCMV6 vector, hRXRα (1–461 a.a.), RXRα deletion constructs: RXRαΔDBD/LBD (1–127 a.a.), RXRαΔLBD (1–208 a.a.) and RXRαΔAFD/DBD (226–461 a.a.). After 48 hours, lysates were immunoprecipitated using Myc and IB with HA antibodies, and vice versa. Long exposure of the blot showed non-specific bands in the lanes representing RXR deletion mutants which might be because of non-specific activity of the secondary antibody.
(C) Lysates from control HepG3 (Hep-PC-4), AEG-1-over-expressing (Hep-AEG-1–14) and AEG-1 knockdown (Hep-AEG-1si) clones were subjected to IP using anti-RXRα and anti-RXRβ antibodies, and IB with anti-AEG-1 antibody.
(D) Representative fluorescent confocal micrographs showing co-localization of AEG-1 and RXRα/ RXRβ in HepG3 cells transfected with control (CTRLsi) or AEG-1 (AEG-1si) siRNA.
(E–H) Cells were transfected with RARE luciferase reporter plasmid (RARE.Luc) and 48 hours post-transfection luciferase assay was performed. RARE activity was measured in untreated (UT) or upon treatment with 5µM ATRA, 2µM 9CRA, 400nm BT or 1µM TTNPB for 24 hours in PC-4, AEG1–14, CTRL-si and AEG-1si cells (E), WT and Alb/AEG-1 (F), WT vs AEG-1KO mice hepatocytes (G), and HepG3 vs. QGY7703 cells (H).
(I) RARE activity was measured 48 hours after co-transfection of pcDNA3.1, AEG-1FL, AEG-1 deletion mutants N1 and N2 (101–582), and AEG-1 LXXLL-mut constructs in HepG3 cells.
(J) Expression level of AEG-1 in different cells with AEG-1 over-expression or knockdown.
For A–C, five percent of cell lysates were used as inputs. For D–I, the luciferase data represents four different sets of mice respectively. All data represent mean ± SEM of three independent experiments with significant p-values denoted in the respective panels. *, p<0.05; **, p<0.001.
Deletion of a.a. 1–70 from N-terminus (N1) of AEG-1 and mutations in its LXXLL motif prevented the interaction of AEG-1 with RXRα and RXRβ (Fig. 1B). However, this interaction was maintained in full-length AEG-1 or upon deletion of C-terminal region (1–513) validating that RXRs indeed interacts with AEG-1 at its LXXLL motif. Further to check at which domain of RXR AEG-1 interacts with we made domain-specific deletion constructs of RXRα (Fig. S1). Deletion of the C-terminus Ligand-Binding AF-2 domain (LBD) of RXRα resulted in the loss of interaction, whereas deletion of its NH2-terminus AF-1 and DNA binding domain (DBD) did not affect the interaction of AEG-1 with RXRα (Fig. 1B). These findings demonstrated that AEG-1 binds RXRα at its LBD, the same domain where co-activators and co-repressors also bind.
Co-IP assays using stable clones of human HCC HepG3 cells overexpressing AEG-1 (Hep-AEG-1–14), AEG-1 knockdown (Hep-AEG-1si) and control clone Hep-PC-4 cells (12) confirmed that the interaction of AEG-1 with RXRα and RXRβ is indeed AEG-1-dependent (Fig. 1C). Double immunofluorescence analysis in HepG3 cells detected co-localization of AEG-1 with RXRα and RXRβ predominantly in the nuclear and peri-nuclear regions, which was not observed upon AEG-1 knockdown (Fig. 1D).
RAR/RXR-mediated promoter activities are inhibited by AEG-1
RAR/RXR binds to retinoic acid response elements (RARE) on the target promoters. Basal luciferase reporter activity of RARE-containing plasmid, pGL3-RARE-luc was decreased in Hep-AEG-1–14 clone and increased in Hep-AEG-1si cells compared to control Hep-PC-4 and Hep-CTRL-si clone, respectively (Fig. 1E). RARE activity remained suppressed even upon treatment with different retinoic acid/ retinoids (RA) and rexinoids (RXA) in Hep-AEG-1–14 cells, whereas it was significantly augmented in Hep-AEG-1si cells (Fig. 1E, Supplementary Fig. S2A). As a corollary, both basal and ligand-dependent RARE activity was significantly inhibited in Alb/AEG-1 hepatocytes (Fig. 1F) and amplified in AEG-1KO hepatocytes (Fig. 1G).
HepG3 cells express low levels of AEG-1 and are non-tumorigenic in nude mice while QGY-7703 cells express high levels of AEG-1 that generate very aggressive tumors (12). Consequently, both basal and ligand-dependent RARE activities were markedly less in QGY-7703 cells as compared to HepG3 cells (Fig. 1H). Transient overexpression of AEG-1 in HepG3 and HEK-293 cells inhibited while transient knockdown of AEG-1 by siRNA augmented ligand-dependent RARE activity (Supplementary Fig. S2B).
Transient expression of full length AEG-1 which interacts with RXRs decreased RARE activity, while AEG-1 deletion constructs N1 and N2, (lacking NH2-terminal 70 and 100 a.a., respectively) and LXXLL mutant expression construct failed to inhibit RARE activity in HepG3 (Fig. 1I) or HEK-293 cells (Supplementary Fig. S2C). AEG-1 expression level in the cells used in this study is shown in Fig. 1J. Taken together, AEG-1-RXR interaction affected RXRs negatively and AEG-1 upregulation led to a decrease in RXR-dependent RARE reporter activity. These activities were increased upon inhibiting this interaction, suggesting that AEG-1 provides a homeostatic balance to RXR functions.
AEG-1 provides protection from retinoids and rexinoid-induced inhibition of cell growth
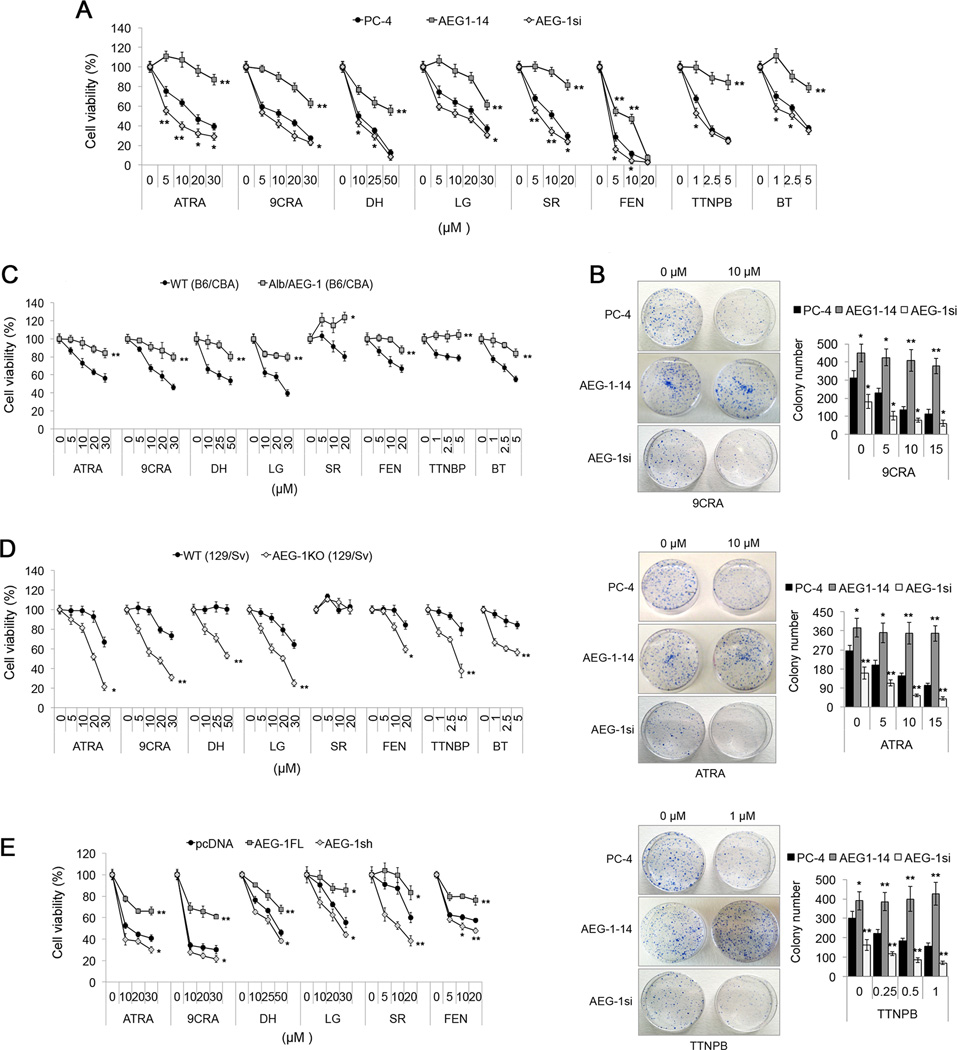
Since RAR/RXR pathway mediates anti-proliferative effects of retinoids and rexinoids, we determined the extent to which AEG-1 might provide protection. Using eight different RA and RXA and their synthetic analogues at multiple doses (Supplementary Table S2), we documented that Hep-AEG1–14 cells displayed 2–3-fold resistance and Hep-AEG-1si cells showed enhanced sensitivity to different RA/RXA treatment at different doses up to 96 h, when compared to control Hep-PC-4 cells (Fig. 2A; Supplementary Fig. S3A–H). Colony formation assays also confirmed marked protection from RA- and RXA-mediated inhibition of cell growth in Hep-AEG-1–14 cells and potentiation of cell growth inhibition in Hep-AEG-1si cells (Fig. 2B). Similarly, QGY-7703 cells with basal high AEG-1 expression displayed resistance towards RA/RXA as compared to HepG3 (Supplementary Fig. S3Q).
Fig. 2. AEG-1 protects cells from retinoid and rexinoid-induced apoptosis.
(A–D) Cell viability of PC-4, AEG1–14 and AEG-1si cells was determined upon treatment with the indicated RA or RXA for 48 hours by MTT assay (A), and, by colony formation assay after treatment with 9CRA, ATRA or TTNPB at the indicated concentrations (B). Colonies were scored after 12 days. Cell viability in WT vs Alb/AEG-1 at 96 hours (C), and in WT vs AEG1-KO mice hepatocytes at 48 hours (D) with indicated RA/RXA.
(E) HL-60 cells were transiently transfected with pcDNA, AEG1-FL or siAEG-1. Cell viability was detected upon treatment with the indicated drugs for 48 hours post transfections.
All data represent mean ± SEM of three independent experiments. *, p< 0.02; **, p<0.001.
WT hepatocytes showed <25% cell growth inhibition at 48 h while only <5% in Alb/AEG-1 hepatocytes, even at high doses of different RA/RXA (data not shown). At 96 h, WT hepatocytes showed variable cell viability with different doses of retinoids and rexinoids. However, Alb/AEG-1 hepatocytes were noticeably protected from inhibition of cell growth with >80% viability (Fig. 2C). Conversely, AEG-1KO hepatocytes demonstrated marked decrease in cell growth with retinoids and rexinoids at 48 to 96 h in a dose-dependent manner (Fig. 2D; Supplementary Fig. S3I–P). We next tested whether AEG-1 had any effect on retinoid-mediated killing in other cancer cells such as human AML HL-60 cells. Notably, profound resistance was obtained with AEG-1 transient expression, and decreased cell viability was observed with AEG-1 knockdown upon treatment of different RA/RXA (Fig. 2E).
AEG-1 inhibits expression of retinoids-target genes
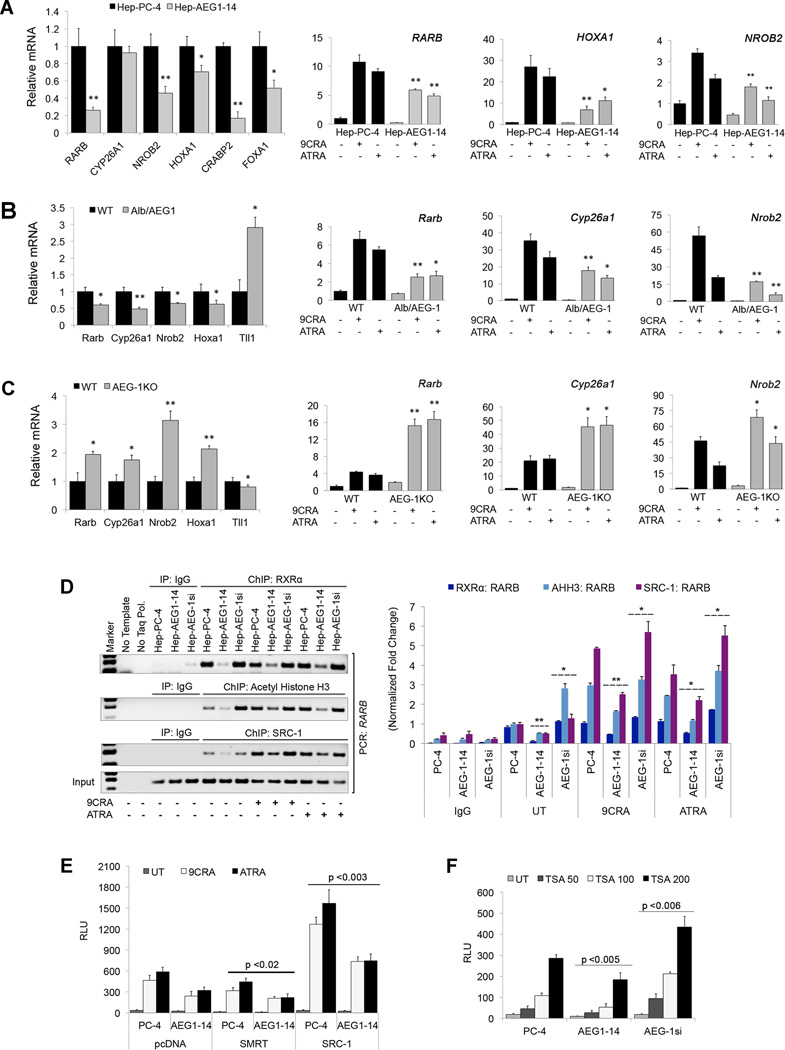
Relative mRNA levels were measured for representative RAR/RXR target genes, Retinoid Acid Receptor beta (RARB), Cytochrome P450 26A1 (CYP26A1), Nuclear receptor subfamily 0, group B, member 2 (NR0B2), Homeobox A1 (HOXA1), Cellular retinoic acid-binding protein 2 (CRABP2) and Forkhead box protein A1 (FOXA1). Hep-AEG-1–14 cells showed significant downregulation of all these genes at basal level and post 9CRA or ATRA treatment, when compared to control Hep-PC-4 (Fig. 3A; Supplementary Fig. S4A). Similar decrease and corresponding increase in the above-mentioned genes were also observed in Alb/AEG-1 and AEG-1KO hepatocytes, respectively (Fig. 3B–C; Supplementary Fig. S4B–C). The RA-negatively regulated gene, Tll1 showed significant upregulation in Alb/AEG-1 hepatocytes with or without ligand, and a reduction in AEG-1KO hepatocytes at basal level (Supplementary Fig. S4B–C). Downregulation of RA-responsive gene, RARB, which determines cell growth and apoptosis, was confirmed in additional Alb/AEG-1 mice (Supplementary Fig. S4D).
Fig. 3. AEG-1 suppresses RAR/RXR target genes by interfering with RXR-binding and co-activators recruitment to the gene promoters.
(A–C) Relative mRNA levels of representative RAR/RXR target genes in PC-4 and AEG1–14 UT cells (left panel), and that of RARB, HOXA1 and NROB2 (A), in WT and Alb/AEG-1 (B), and WT and AEG-1KO mice hepatocytes (C) in untreated (left panels) or treated with 2µM 9CRA or 5µM ATRA (right panels).
(D) ChIP assays were performed using anti-RXRα, anti-Acetyl Histone H3 and anti-SRC-1 antibodies in PC-4, AEG1–14, AEG-1si cells with or without 9CRA and ATRA, and PCR primers amplifying the promoter regions of RARB. The graphical representation for densitometry quantification of the immunoprecipitated RARB promoter has been given in right panel.
(E–F) RARE activity in PC-4, AEG1–14 and AEG-1si cells co-transfected with pcDNA3.1, SMRT or SRC-1 expression plasmids with or without ligands (E), or treated with indicated doses (in nM) of Trichostatin A (TSA) (F).
Data represents mean ± SEM of three independent experiments with significant p-values indicated in respective panels or *, p<0.05; **, p<0.001.
AEG-1 impairs the RXR binding and co-activator recruitment to RXR/RAR target genes
Chromatin Immunoprecipitation (ChIP) assays were performed using the RA-target genes RARB and HOXA1. In the absence of ligand, significantly decreased and increased recruitment of RXRα was observed in both target gene promoters in Hep-AEG-1–14 and Hep-AEG-1si cells, respectively as compared to Hep-PC-4 cells (Fig. 3D, Supplementary Fig. S4E). Similar effects were also observed for Acetyl Histone H3 (AHH3) and Steroid Receptor Coactivator-1 (SRC-1). Upon treatment with 9CRA or ATRA, RXRα recruitment did not change substantially, while both AHH3 and SRC-1 recruitment was significantly augmented in all three cell lines. However, ligand-induced recruitment of AHH3 and SRC-1 was notably less in Hep-AEG-1–14 cells and more in Hep-AEG-1si cells. (Fig. 3D, Supplementary Fig. S4E). To check whether AEG-1 interferes with DNA-binding property of RXR, EMSA was performed in a cell-free system using in-vitro translated RARβ, RXRα and AEG-1 using a DR5 RARE response element as probe. RARβ/RXR heterodimer efficiently bound to DNA in the absence or presence of ATRA, which was not affected by addition of AEG-1 (data not shown). These findings suggest that AEG-1 does not interfere with DNA binding or heterodimerization of RXR with its partners rather it might modulate co-activator/co-repressor recruitment. The decreased recruitment of RXRα to RARB or HOXA1 promoter in Hep-AEG-1–14 cells might be due to reduced levels of nuclear RXRs in these cells, a hypothesis we will address in subsequent sections.
Next, we measured RARE reporter activity in control and AEG-1 overexpressing cells with co-expression of a co-repressor Silencing mediator for retinoid/thyroid-hormone receptors (SMRT) or a co-activator, SRC-1. SMRT overexpression led to a minimal but significant reduction in RARE activity (Fig. 3E). Interestingly, SRC-1 overexpression profoundly elevated RARE activity in Hep-AEG-1–14 cells similar to Hep-PC-4 cells without SRC-1 overexpression (Fig. 3E). Histone deacetylase inhibitor, Trichostatin A (TSA), markedly induced basal RARE activity in a dose-dependent manner (Fig. 3F). Collectively, these findings suggest that overexpressed AEG-1 competes with the co-activators to bind RXR by interacting through the LXXLL motif maintaining histones in a deacetylated state.
AEG-1 upregulation leads to cytoplasmic translocation of RXRs
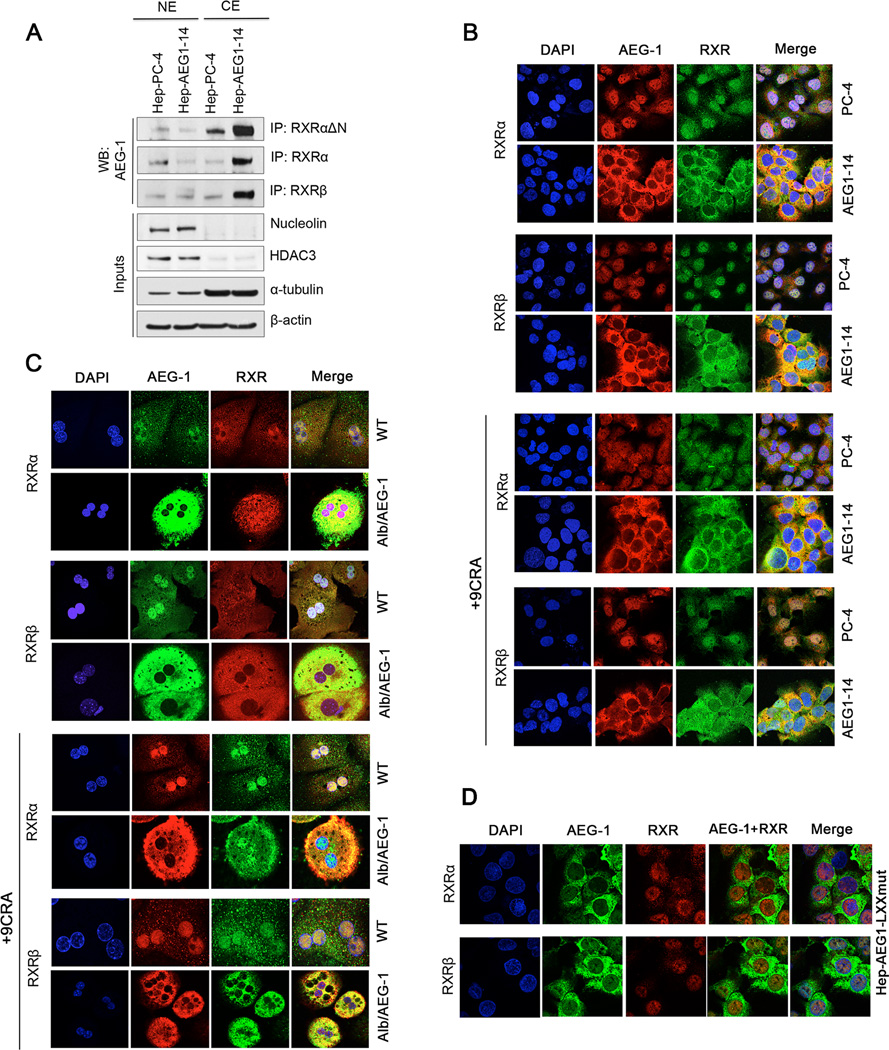
Co-IP analysis using nuclear and cytoplasmic fractions demonstrated that AEG-1 interaction with RXRs mainly occurs in the cytoplasm of Hep-AEG-1–14 cells and in the nucleus in control cells (Fig. 4A). Immunofluorescence studies revealed that AEG-1 resides mainly in the nucleus of control Hep-PC-4 cells and WT hepatocytes, which show low AEG-1 expression (Fig. 4B–C, Supplementary Fig. S5). However, in Hep-AEG-1–14 cells and Alb/AEG-1 hepatocytes, AEG-1 displays predominantly cytoplasmic localization (Fig. 4B–C, Supplementary Fig. S5), which has been attributed to the enhanced monoubiquitination and stabilization (13, 20). Interestingly, AEG-1 overexpression and cytoplasmic translocation also resulted in the coordinated translocation of RXRα and RXRβ from nucleus to the cytoplasm in Hep-AEG-1–14 cells. While in Hep-PC-4 cells, AEG-1/RXR interaction took place predominantly in the nucleus (Fig. 4B, Supplementary Fig. S5). As nuclear receptors shuttle between the cytoplasm and the nucleus, we determined whether cytoplasmic retention of RXRs might depend on ligand. Treatment with 9CRA or ATRA at various doses up to 24 hours, failed to induce nuclear translocation of RXRα and RXRβ in AEG-1 overexpressing cells suggesting that AEG-1 entraps RXRs into cytoplasm obstructing their nuclear import (Fig. 4B, Supplementary Fig. S5).
Fig. 4. AEG-1 regulates intracellular localization of RXRs.
(A) Nuclear and cytoplasmic extracts prepared from PC-4 and AEG1–14 cells were subjected to IP using anti-RXRΔN (that recognizes all RXR isotypes), anti-RXRα and anti-RXRβ antibodies and WB with anti-AEG-1 antibody.
(B–D) Representative confocal photomicrographs to analyze co-localization of AEG-1 and RXRs using anti-AEG-1, anti-RXRα and anti-RXRβ antibodies in PC-4/AEG1–14 cells (B) and WT/Alb/AEG-1 mice hepatocytes (C) with no or 9CRA treatment for 2 hours; and in HepG3 cells stably overexpressing LXXLL-mutated AEG-1 (D).
For 4C, Multinucleation is a common feature of primary mouse hepatocytes which mostly have two nuclei and frequently one-four nuclei.
In WT hepatocytes, RXRα is distributed throughout the cell but more in the nucleus, and RXRβ showed universal staining. 9CRA treatment induced the import of RXRα and RXRβ into the nucleus (Fig. 4C). In contrast, both RXRα and RXRβ were mostly cytoplasmic in Alb/AEG-1 hepatocytes and 9CRA treatment did not alter this profile significantly (Fig. 4C). Dominant to complete cytoplasmic localization of AEG-1 and RXRs was dependent upon AEG-1 expression level (Supplementary Fig. S6A–E). Cytoplasmic translocation of AEG-1 and RXR was not affected upon culturing them on collagen-fibronectin matrix and synchronization (Supplementary Fig. S6F–G)
Double IF of AEG-1 with RXRα and RXRβ was performed using HepG3 stable clone overexpressing AEG-1 mutated at LXXLL motif (Hep-AEG1-LXXmut). Interestingly, both RXRα and RXRβ remains nuclear and do not translocate to cytoplasm in these cells (Fig. 4D) confirming that AEG-1 indeed brings RXRs to the cytoplasm which is prevented upon losing the interaction.
AEG-1 induces phosphorylation and inactivation of RXRs by activating ERK and p38MAPK signaling
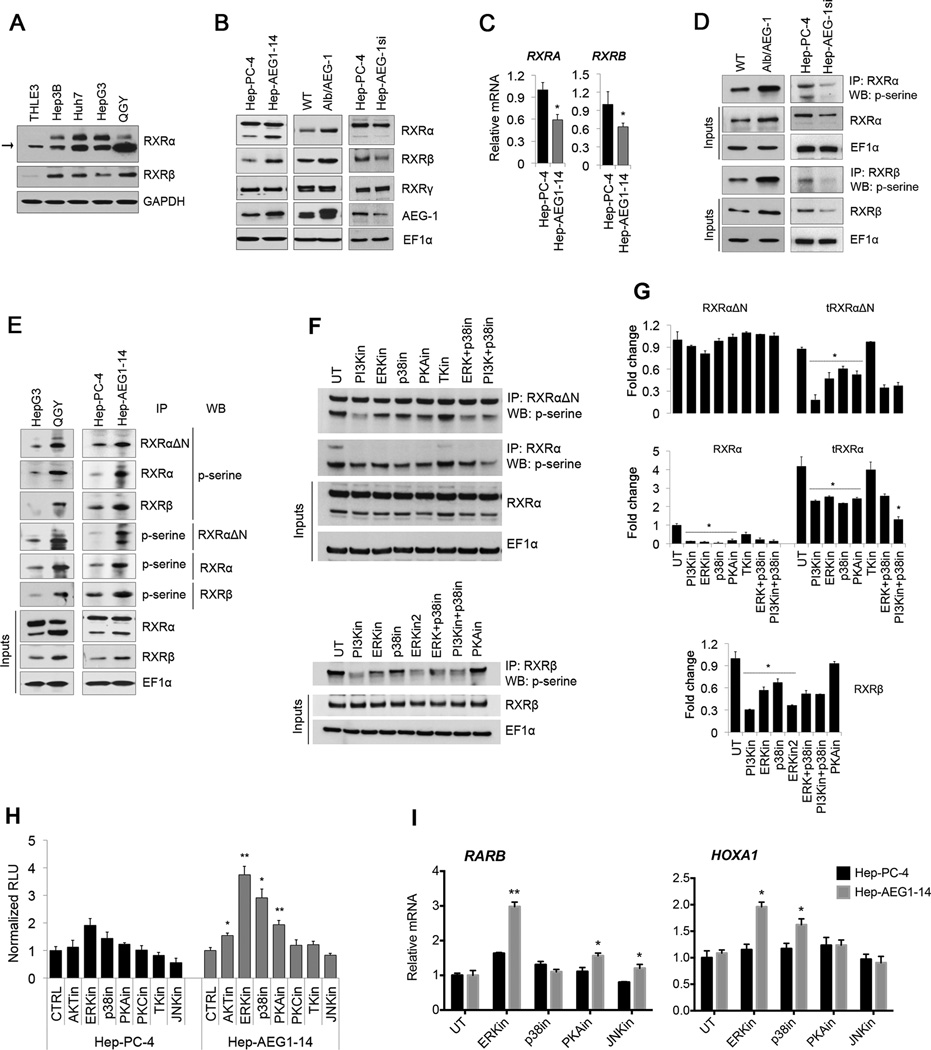
Both RXRα and RXRβ levels were significantly higher in HCC cells compared to normal immortal human hepatocytes (THLE-3) cells (Fig. 5A). Moreover, truncated form of RXRα (tRXRα, arrow in Fig. 5A) accumulated more in different HCC cell lines. RXRα and RXRβ were also upregulated in AEG-1 overexpressing systems: Hep-AEG-1–14 cells and Alb/AEG-1 hepatocytes and downregulated in AEG-1 knockdown cells (HepAEG-1si) (Fig. 5B; Supplementary Fig. S7A). However, their mRNA levels were reduced in Hep-AEG-1–14 cells and Alb/AEG-1 hepatocytes (Fig. 5C; Supplementary Fig. S7B–C). These findings suggested potential post-translational modification of RXRs by AEG-1. Interestingly, phosphorylation of RXRα and RXRβ at serine residues was conspicuously induced in different AEG-1 overexpression systems: Alb/AEG-1 hepatocytes, QGY-7703 and Hep-AEG-1–14 cells (Fig. 5D–E). Phosphorylation was inhibited in Hep-AEG-1si cells in comparison to control cells which might reflect the reduction of total RXR (Fig. 5D). More phosphorylated RXRα was detected in both the nucleus and cytoplasm of Hep-AEG-1–14 cells although phospho-forms of tRXRα and RXRβ remained prevalent in the nucleus (Supplementary Fig. S7D). Inhibition of nuclear phosphorylated RXRα and RXRβ in Hep-AEG-1si cells might explain increased RARE reporter activity, expression of RAR/RXR target genes and apoptosis (Fig. 1 and 2).
Fig. 5. AEG-1 induces RXR phosphorylation by activating ERK, p38MAPK and PKA signaling.
(A) Expression analysis of RXRα and RXRβ in different HCC cell lines and human immortalized hepatocytes THLE3 cells.
(B–C) Expression of RXRα, RXRβ, RXRγ and AEG-1 in human Hep-PC-4 vs. Hep-AEG1–14 cells and WT vs. Alb/AEG-1 mice hepatocytes at protein level detected with western blot (B) and at mRNA levels by qPCR (C).
(D–E) Phosphorylation levels of RXRα and RXRβ were determined by immunoprecipitating cell lysates with anti-RXRΔN, anti-RXRα and anti-RXRβ antibodies followed by WB with anti-phospho-serine (p-serine) antibody and vice-versa, for WT vs. Alb/AEG-1 mice hepatocytes, Hep-PC4 vs. Hep-AEG-1si (D), HepG3 vs. QGY-7703, and PC-4 vs. AEG1–14 cells (E).
(F–G) Decreased levels of phosphorylated RXRα and RXRβ in Hep-AEG1–14 cells after treatment with inhibitors of the indicated kinases. IP was performed with anti-RXRΔN and anti-RXRα and anti-RXRβ antibodies and WB was performed for p-serine. Graphical representations of densitometry quantification are shown in G. Cells were treated with different inhibitors in DMEM containing 1% charcoal stripped FBS for 24 hours. For concentrations used and detailed information, please see Table S2.
(H–I) RARE activity (H), expression of representative RXR/RAR target genes, RARB and HOXA1 (I) in Hep-PC-4 and Hep-AEG1–14 cells after inhibition of different kinases.
For F–I, selective inhibitors for PI3K, ERK, JNK, TK were used at final 15µM concentration and PKA, PKC and p38 MAPK at 2 µM. Data represents mean ± SEM of three independent experiments. *: p<0.02 and **: p<0.001.
Different kinases have been reported to phosphorylate RXRα in HCC (21–23). We tested which kinase might be responsible for AEG-1-dependent RXR phosphorylation in HCC cells by using specific inhibitors (Table S2). Inhibition of PKA, PKC, JNK, ERK and p38MAPK led to downregulation of pRXRα and p-tRXRα in AEG-1-specific manner (Fig. 5F–G; Supplementary Fig. S7E–G). pRXRβ was also reduced to a greater extent in Hep-AEG1–14 cells upon inhibiting PI3K, JNK, ERK, p38MAPK and TK (Fig. 5F–G; Supplementary Fig. S7E–G). RXRΔN antibody detecting all isotypes of RXR showed none to minimal effect of the inhibitors on the full-length RXR. However, RXRα specific antibody detected inhibition of both pRXRα and p-tRXRα (5F–G). Moreover, combined inhibition of PI3K and p38MAPK synergistically decreased phosphorylation of tRXRα.
RARE activity was significantly augmented in AEG-1 stable and transient overexpressing cells upon inhibition of ERK, PKA and p38MAPK (Fig. 5H; Supplementary Fig. S8A–B). ERK has been shown to phosphorylate RXRα at the serine 260 residue in HCC, and inhibition of ERK-mediated RXR phosphorylation led to most increase in RARE activity in a dose- and AEG-1-dependent manner (Supplementary Fig. S8C). We also checked the expression of representative RXR/RAR target genes; RARB, HOXA1, CYP26A1 and NR0B2 upon inhibition of various kinases (Fig. 5I; Supplementary Fig. S8D). ERK inhibition retrieved the expression of all the genes at various extents in AEG-1 over-expressing cells. PKA and p38MAPK inhibitors specifically increased the expression of RARB and HOXA1/FOXA1, respectively. Notably, siRNA-mediated knockdown of AEG-1 in HepG3 (~1.3 folds) and QGY-7703 cells (~2 folds) and RXRα overexpression in only QGY-7703 cells augmented ligand-dependent RARE activity (Supplementary Fig. S8E) confirming the AEG-1 caused inactivation of RXR in tumorigenic HCC cells, which was significantly retrieved by AEG-1 knockdown or RXRα overexpression.
Synergistic anti-tumor effect of AEG-1 inhibition and ATRA administration in HCC xenografts in nude mice
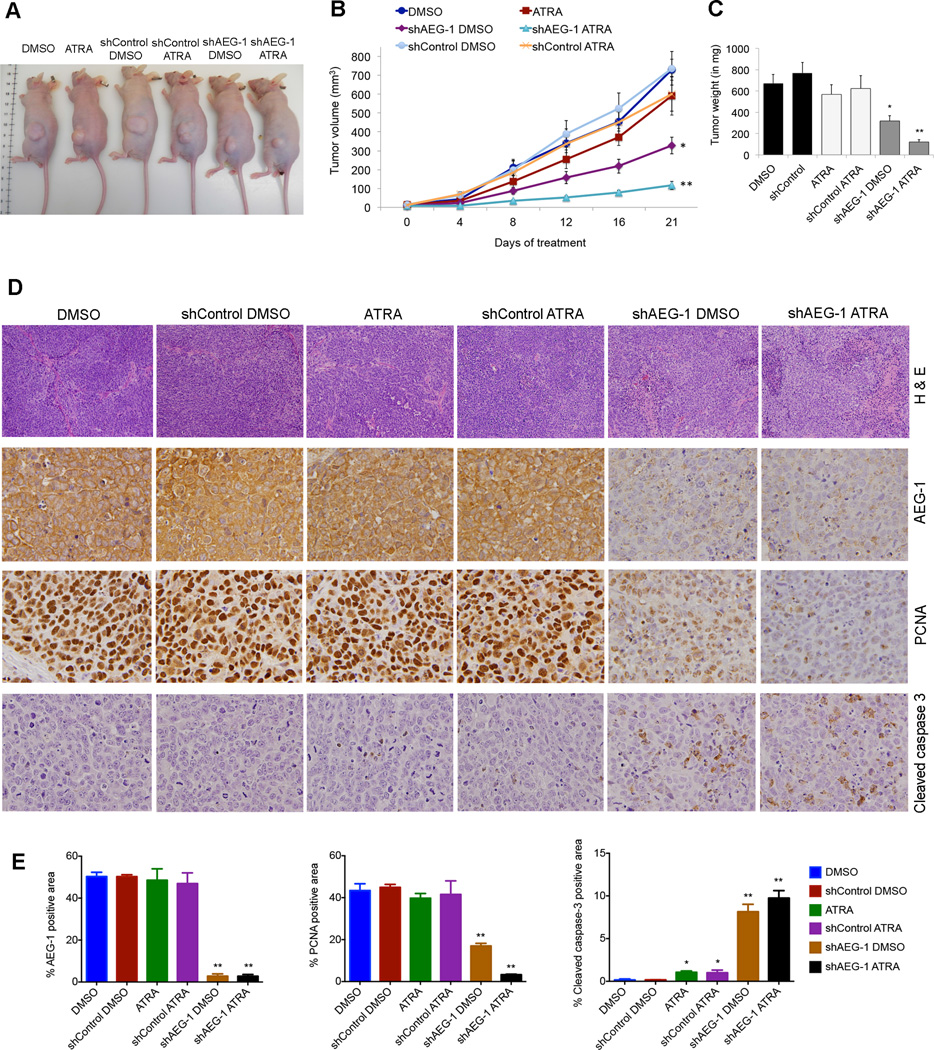
The in vitro growth-suppressing function of retinoids upon AEG-1 knockdown was extended by in vivo assays. Since, QGY-7703 cells develop aggressive tumors in nude mice, we transduced them with none, control lentivirus expressing scrambled shRNA (shControl) and AEG-1 knockdown lentivirus (shAEG-1) (24). These cells were then subcutaneously xenografted into the flanks of male athymic nude mice. One week after implantation, when tumor is well established, the mice were treated with i.p. injections of DMSO (vehicle) or ATRA (10 mg/kg body weight). In DMSO-treated only and DMSO-treated shControl groups, tumor gradually increased in size as reflected by tumor volume measured twice a week, and tumor weight at the final day of experiment (Fig. 6A–C). In ATRA treated groups; only two of the eight mice showed reduction in tumor growth which was not significant. In the shAEG-1 DMSO-treated animals, the tumor growth was significantly suppressed as compared to controls and ATRA-treated group. Interestingly, the shAEG-1 ATRA-treated animals grew none to minimal tumors in size and weight (Fig. 6A–C). Three out of eight shAEG-1 ATRA-treated mice showed no tumor or smaller tumor than 0 day of treatment. Their combination effect was calculated as 0.78 (<1= synergistic) indicating synergism. Histological analysis of the tumors revealed significantly increased necrosis/ apoptosis in shAEG-1 ATRA-treated group compared to others (Fig. 6D top panel). Immunohistochemical studies confirmed knockdown of AEG-1 in shAEG-1 groups (Fig. 6D–E). In addition, there was marked downregulation of the cell proliferating marker PCNA and upregulation of apoptosis marker Cleaved caspase-3 in tumors derived from shAEG-1 ATRA, shAEG-1 and ATRA, respectively as compared with the DMSO control and shControl DMSO groups (Fig. 6D–E).
Fig. 6. Combination of AEG-1 knockdown and ATRA treatment synergistically inhibits tumor growth in nude mice.
Subcutaneous xenografts were established in athymic nude mice, using QGY-7703 cells, either untreated or transduced with lenti.shControl or lenti.shAEG-1.
(A) Representative photograph of tumor-bearing mice at the end of the study.
(B–C) Measurement of tumor volume at the indicated time points (B), and tumor weight at the end of study (C).
(D) Tumor sections were stained for Hematoxylene and Eosine (H&E) and immunostained for AEG-1, PCNA and Cleaved caspase-3.
(E) Graphical representation for quantification of immunostaining of four representative images for each group. Data represent mean ± SEM. *, p< 0.05; **, p<0.001.
DISCUSSION
Molecular mechanism(s) by which RXR controls diverse cellular functions including cell proliferation and metabolism has been thoroughly investigated. However only a few reports have addressed the molecular regulators of RXR itself, which might be indispensable in evaluating and improving retinoid- and rexinoid-based chemotherapy. Our current observations suggest that AEG-1 negatively regulates functions of RXRs by interacting through the LXXLL motif at the AF-2 ligand-binding domain of RXR thereby interfering with co-activator recruitment and subsequent histone acetylation. AEG-1 is a highly evolutionary conserved gene present only in vertebrates. In rodents, ‘LXXLL’ motif is present as ‘LRELL’ while in primates as ‘LREML’. This change from leucine to methionine in primates might affect binding affinity between AEG-1 and RXRs and determine the strength, degree and duration of inhibition.
We document that AEG-1 not only inhibits RXR-mediated transcriptional regulation, but also induces RXR phosphorylation via ERK and p38MAPK pathways, which are known to be activated by AEG-1 (12). Phosphorylated RXRs are resistant to ubiquitin-proteasome mediated degradation and act dominant negatively for normal RXR functions by abrogating heterodimerization and coactivator(s) recruitment (25, 26), which is indispensable to drive the oncogenic functions. Therefore, inhibition of these molecules suppressed AEG-1-mediated phosphorylation of RXR and rescued transcriptional activation of genes regulating cell proliferation in HCC. RXRα is proteolytically degraded generating N-terminally truncated 44-kDa receptor (tRXRα) that interacts with the p85α subunit of PI3K and activate pro-tumorigenic PI3K/AKT signaling (27). Activation of PI3K/Akt pathway regulates AEG-1-mediated resistance to apoptosis (28), and AEG-1-induced tRXRα generation might lead to PI3K/Akt activation.
Our findings reveal that overexpressed cytoplasmic AEG-1 in tumorigenic cells leads to dominant to exclusive cytoplasmic translocation of RXRα and RXRβ. RXRs shuttle between the cytoplasmic and nuclear compartments during certain stages of development, in response to ligand, differentiation, apoptosis, and inflammation (29–32). Further, tRXRα has been shown to be exclusively cytoplasmic (27). Overall, RXRs might be regulated by AEG-1 through histone (de)acetylation in normal cells and via cytoplasmic entrapping and phosphorylation in human cancer cells. It should be noted that treatment with ERK inhibitor increased RA-dependent gene expression but did not increase nuclear translocation of RXR (data not shown). We observed phosphorylation of both nuclear and cytoplasmic RXR in AEG-1-overexpressing cells (Fig. S7D). ERK inhibition might dephosphorylate nuclear RXR leading to its increased activity without resulting in nuclear translocation of cytoplasmic RXR which remains entrapped in the cytoplasm because of physical interaction with AEG-1.
One important consequence of RXRs inhibition by AEG-1 is abrogation of RA-induced gene transcription. Inactivation or downregulation of RARB and other RAR/RXR downstream genes have been reported in various malignancies with increased cell survival and augmentation of disease (8). A transgenic mouse expressing dominant negative RARα develops spontaneous HCC (9). By negatively regulating the retinoid-inducible genes, such as RARB and CRABP2, important regulators of normal cell growth, AEG-1 provided significant protection from cell growth inhibition by different retinoids/rexinoids while AEG-1 knockdown potentiated these effects. Similar level of AEG-1-mediated resistance in HL-60 cells provided the importance of AEG-1 in determining response to retinoid, a frontline therapy for leukemia.
Retinoids and rexinoids have been evaluated as candidates for cancer chemoprevention from past two decades (33, 34), however there have been multiple drawbacks and limitations. One important factor is that retinoid signaling is often lost or compromised in carcinogenesis leading to retinoid-ineffectiveness and resistant (35–37). An important reason is the inactivation/inhibition of RXR and/or RAR pathways in various cancers including breast cancer, HCC and AML (36–38). Combination of AEG-1 inhibition with ATRA treatment resulted in tremendous synergistic inhibition of tumor growth in HCC xenograft assays, and demonstrated reduced proliferation and increased apoptosis. Targeting AEG-1 could enhance the efficacy of retinoids- and rexinoids-based therapeutics overcoming drawbacks in HCC and other malignancies including leukemia. This hypothesis needs to be confirmed using orthotopic xenograft models in nude mice and endogenous tumor models.
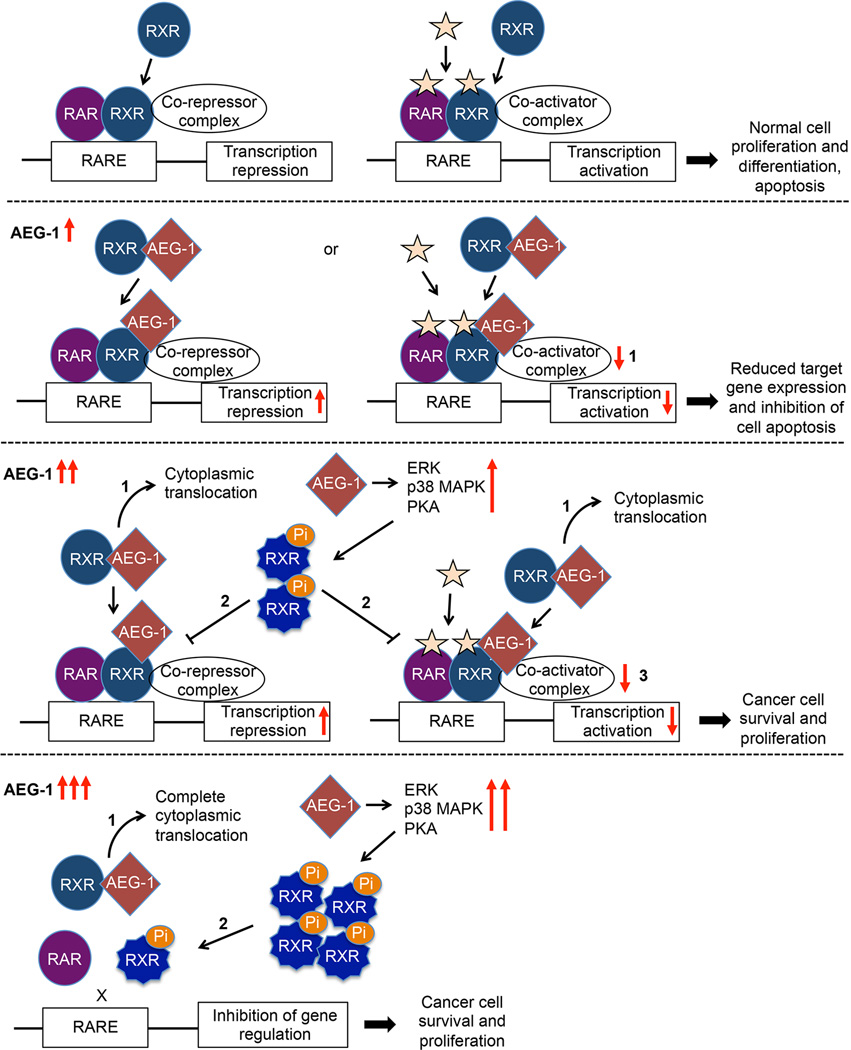
In summary, a schematic representation is presented to describe AEG-1 driven regulation of RXRα and β (Fig. 7). Generally, RXR heterodimerizes with RAR and binds to target gene promoters recruiting co-repressor complex in absence of ligand, and co-activators in presence of ligand, thus regulating transcription of target genes for normal cell proliferation and apoptosis. AEG-1 in the nucleus of normal non-tumorigenic cell balances this phenomenon by interfering with co-activator recruitment. When AEG-1 increases and accumulates in the cytoplasm of tumorigenic cells, this equilibrium is perturbed. First, AEG-1 translocates and entraps RXRα and β into cytoplasm obstructing RXR binding and transcriptional activation of target genes. Second, AEG-1 induces phosphorylation of RXRs that act dominant negatively on normal RXR. Lastly, RXRα truncation is also elevated by AEG-1. All together, AEG-1 upregulation causes inactivation of RXRs thereby negatively impacting downstream signaling, thus favoring unrestrained cancer cell proliferation leading to HCC and other cancers.
Fig. 7. Schematic model illustrating the molecular mechanism by which AEG-1 regulates RXR signaling.
In the second panel, 1: In normal cells, AEG-1 is adequately expressed and resides in the nucleus influencing histone (de)acetylation. As AEG-1 expression level increases, it potentially inhibits RXRs in three different manners (third and fourth panels). 1: AEG-1 preferentially entraps RXR into cytoplasm. 2: AEG-1 induces phosphorylation of RXRs. 3: Also, remaining AEG-1 in nucleus modulates the binding of co-activators or co-repressors.
Supplementary Material
ACKNOWLEDGEMENTS
Present study was supported in part by grants from The James S. McDonnell Foundation and National Cancer Institute Grant R01 CA138540 (DS) and National Institutes of Health Grants R01 CA134721 (PBF). DS is the Harrison Endowed Scholar in Cancer Research and Blick scholar. PBF holds the Thelma Newmeyer Corman Chair in Cancer Research.
Abbreviations
- HCC
Hepatocellular Carcinoma
- 9CRA
9-cis Retinoic Acid
- ATRA
all-trans Retinoic Acid
- BT
Bexarotene
- RLU
relative luciferase unit
- DH
Docosahexaenoic Acid
- LG
- SR
SR11237
- FEN
Fenretinide
- ERK
Extracellular signal-regulated kinases
- MAPK
Mitogen-activated protein kinases
- PKA
Protein kinase A
- PKC
Protein kinase C
- JNK
c-Jun N-terminal kinases
- PI3K
Phosphatidylinositide 3-kinases
- TK
Tyrosine Kinase
Footnotes
Disclosure:
All authors have no potential conflicts.
REFERENCES
- 1.Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990;345:224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- 2.De Luca LM. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991;5:2924–2933. [PubMed] [Google Scholar]
- 3.Lefebvre P, Benomar Y, Staels B. Retinoid X receptors: common heterodimerization partners with distinct functions. Trends Endocrinol Metab. 2010;21:676–683. doi: 10.1016/j.tem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Matsushima-Nishiwaki R, Shidoji Y, Nishiwaki S, Yamada T, Moriwaki H, Muto Y. Aberrant metabolism of retinoid X receptor proteins in human hepatocellular carcinoma. Mol Cell Endocrinol. 1996;121:179–190. doi: 10.1016/0303-7207(96)03863-4. [DOI] [PubMed] [Google Scholar]
- 5.Takiyama Y, Miyokawa N, Sugawara A, Kato S, Ito K, Sato K, Oikawa K, et al. Decreased expression of retinoid X receptor isoforms in human thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:5851–5861. doi: 10.1210/jc.2003-032036. [DOI] [PubMed] [Google Scholar]
- 6.Zhong C, Yang S, Huang J, Cohen MB, Roy-Burman P. Aberration in the expression of the retinoid receptor, RXRalpha, in prostate cancer. Cancer Biol Ther. 2003;2:179–184. doi: 10.4161/cbt.2.2.281. [DOI] [PubMed] [Google Scholar]
- 7.Picard E, Seguin C, Monhoven N, Rochette-Egly C, Siat J, Borrelly J, Martinet Y, et al. Expression of retinoid receptor genes and proteins in non-small-cell lung cancer. J Natl Cancer Inst. 1999;91:1059–1066. doi: 10.1093/jnci/91.12.1059. [DOI] [PubMed] [Google Scholar]
- 8.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nature Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 9.Yanagitani A, Yamada S, Yasui S, Shimomura T, Murai R, Murawaki Y, Hashiguchi K, et al. Retinoic acid receptor alpha dominant negative form causes steatohepatitis and liver tumors in transgenic mice. Hepatology. 2004;40:366–375. doi: 10.1002/hep.20335. [DOI] [PubMed] [Google Scholar]
- 10.Yoo BK, Emdad L, Lee SG, Su ZZ, Santhekadur P, Chen D, Gredler R, et al. Astrocyte elevated gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology. Pharmacol Ther. 2011;130:1–8. doi: 10.1016/j.pharmthera.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu G, Wei Y, Kang Y. The multifaceted role of MTDH/AEG-1 in cancer progression. Clin Cancer Res. 2009;15:5615–5620. doi: 10.1158/1078-0432.CCR-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, Mills AS, et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Invest. 2009;119:465–477. doi: 10.1172/JCI36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava J, Siddiq A, Emdad L, Santhekadur PK, Chen D, Gredler R, Shen XN, et al. Astrocyte elevated gene-1 promotes hepatocarcinogenesis: novel insights from a mouse model. Hepatology. 2012;56:1782–1791. doi: 10.1002/hep.25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo BK, Santhekadur PK, Gredler R, Chen D, Emdad L, Bhutia S, Pannell L, et al. Increased RNA-induced silencing complex (RISC) activity contributes to hepatocellular carcinoma. Hepatology. 2011;53:1538–1548. doi: 10.1002/hep.24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo BK, Chen D, Su ZZ, Gredler R, Yoo J, Shah K, Fisher PB, et al. Molecular mechanism of chemoresistance by astrocyte elevated gene-1. Cancer Res. 2010;70:3249–3258. doi: 10.1158/0008-5472.CAN-09-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkar D, Lebedeva IV, Su ZZ, Park ES, Chatman L, Vozhilla N, Dent P, et al. Eradication of therapy-resistant human prostate tumors using a cancer terminator virus. Cancer Res. 2007;67:5434–5442. doi: 10.1158/0008-5472.CAN-07-0195. [DOI] [PubMed] [Google Scholar]
- 17.Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB. Molecular basis of nuclear factor-kappaB activation by astrocyte elevated gene-1. Cancer Res. 2008;68:1478–1484. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
- 18.Santhekadur PK, Das SK, Gredler R, Chen D, Srivastava J, Robertson C, Baldwin AS, Jr, et al. Multifunction protein staphylococcal nuclease domain containing 1 (SND1) promotes tumor angiogenesis in human hepatocellular carcinoma through novel pathway that involves nuclear factor kappaB and miR-221. J Biol Chem. 2012;287:13952–13958. doi: 10.1074/jbc.M111.321646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 20.Thirkettle HJ, Girling J, Warren AY, Mills IG, Sahadevan K, Leung H, Hamdy F, et al. LYRIC/AEG-1 is targeted to different subcellular compartments by ubiquitinylation and intrinsic nuclear localization signals. Clin Cancer Res. 2009;15:3003–3013. doi: 10.1158/1078-0432.CCR-08-2046. [DOI] [PubMed] [Google Scholar]
- 21.Mascanfroni ID, Montesinos Mdel M, Alamino VA, Susperreguy S, Nicola JP, Ilarregui JM, Masini-Repiso AM, et al. Nuclear factor (NF)-kappaB-dependent thyroid hormone receptor beta1 expression controls dendritic cell function via Akt signaling. J Biol Chem. 2010;285:9569–9582. doi: 10.1074/jbc.M109.071241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harish S, Ashok MS, Khanam T, Rangarajan PN. Serine 27, a human retinoid X receptor alpha residue, phosphorylated by protein kinase A is essential for cyclic AMP-mediated downregulation of RXRalpha function. Biochem Biophys Res Comm. 2000;279:853–857. doi: 10.1006/bbrc.2000.4043. [DOI] [PubMed] [Google Scholar]
- 23.Adam-Stitah S, Penna L, Chambon P, Rochette-Egly C. Hyperphosphorylation of the retinoid X receptor alpha by activated c-Jun NH2-terminal kinases. J Biol Chem. 1999;274:18932–18941. doi: 10.1074/jbc.274.27.18932. [DOI] [PubMed] [Google Scholar]
- 24.Yoo BK, Gredler R, Vozhilla N, Su ZZ, Chen D, Forcier T, Shah K, et al. Identification of genes conferring resistance to 5-fluorouracil. Proc Natl Acad Sci USA. 2009;106:12938–12943. doi: 10.1073/pnas.0901451106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macoritto M, Nguyen-Yamamoto L, Huang DC, Samuel S, Yang XF, Wang TT, White JH, et al. Phosphorylation of the human retinoid X receptor alpha at serine 260 impairs coactivator(s) recruitment and induces hormone resistance to multiple ligands. J Biol Chem. 2008;283:4943–4956. doi: 10.1074/jbc.M707517200. [DOI] [PubMed] [Google Scholar]
- 26.Matsushima-Nishiwaki R, Okuno M, Adachi S, Sano T, Akita K, Moriwaki H, Friedman SL, et al. Phosphorylation of retinoid X receptor alpha at serine 260 impairs its metabolism and function in human hepatocellular carcinoma. Cancer Res. 2001;61:7675–7682. [PubMed] [Google Scholar]
- 27.Zhou H, Liu W, Su Y, Wei Z, Liu J, Kolluri SK, Wu H, et al. NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling. Cancer Cell. 2010;17:560–573. doi: 10.1016/j.ccr.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 29.Fukunaka K, Saito T, Wataba K, Ashihara K, Ito E, Kudo R. Changes in expression and subcellular localization of nuclear retinoic acid receptors in human endometrial epithelium during the menstrual cycle. Mol Hum Reprod. 2001;7:437–446. doi: 10.1093/molehr/7.5.437. [DOI] [PubMed] [Google Scholar]
- 30.Ghose R, Zimmerman TL, Thevananther S, Karpen SJ. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: A novel mechanism for reduced hepatic gene expression in inflammation. Nuclear Recep. 2004;2:4. doi: 10.1186/1478-1336-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka T, Dancheck BL, Trifiletti LC, Birnkrant RE, Taylor BJ, Garfield SH, Thorgeirsson U, et al. Altered localization of retinoid X receptor alpha coincides with loss of retinoid responsiveness in human breast cancer MDA-MB-231 cells. Mol Cell Biol. 2004;24:3972–3982. doi: 10.1128/MCB.24.9.3972-3982.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao X, Liu W, Lin F, Li H, Kolluri SK, Lin B, Han YH, et al. Retinoid X receptor regulates Nur77/TR3-dependent apoptosis [corrected] by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol. 2004;24:9705–9725. doi: 10.1128/MCB.24.22.9705-9725.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nature Rev Cancer. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka T, De Luca LM. Therapeutic potential of "rexinoids" in cancer prevention and treatment. Cancer Res. 2009;69:4945–4947. doi: 10.1158/0008-5472.CAN-08-4407. [DOI] [PubMed] [Google Scholar]
- 35.Xu XC. Tumor-suppressive activity of retinoic acid receptor-beta in cancer. Cancer Lett. 2007;253:14–24. doi: 10.1016/j.canlet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farias EF, Arapshian A, Bleiweiss IJ, Waxman S, Zelent A, Mira YLR. Retinoic acid receptor alpha2 is a growth suppressor epigenetically silenced in MCF-7 human breast cancer cells. Cell Growth Diff. 2002;13:335–341. [PubMed] [Google Scholar]
- 37.Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Ann Rev Pathol. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 38.Johansson HJ, Sanchez BC, Mundt F, Forshed J, Kovacs A, Panizza E, Hultin-Rosenberg L, et al. Retinoic acid receptor alpha is associated with tamoxifen resistance in breast cancer. Nature Comm. 2013;4:2175. doi: 10.1038/ncomms3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.