Abstract
Scope
Birch pollen associated allergy to mung bean sprouts is caused by cross-reactivity between the birch pollen allergen Bet v 1 and the mung bean allergen Vig r 1. We aimed to determine the allergenicity of the cytokinin-specific binding protein from mung bean (Vig r 6), another allergen related to Bet v 1 with only 31% sequence identity.
Methods and results
Bet v 1, Gly m 4, Vig r 1, and Vig r 6 were produced in Escherichia coli. In an ELISA, 73 and 32% of Bet v 1-sensitized birch-allergic patients’ sera (n = 60) showed IgE binding to Vig r 1 and Vig r 6, respectively. Of 19 patients who reported allergic reactions or had positive prick-to-prick tests to mung bean sprouts, 79% showed IgE binding to Vig r 1 and 63% showed IgE binding to Vig r 6. Bet v 1 completely inhibited IgE binding to both mung bean allergens. Vig r 6 showed partial cross-reactivity with Vig r 1 and activated basophils sensitized with mung bean allergic patients’ sera.
Conclusion
We demonstrated IgE cross-reactivity despite low sequence identity between Vig r 6 and other Bet v 1-related allergens. Thus, IgE binding to Vig r 6 may contribute to birch pollinosis-associated mung bean sprout allergy.
Keywords: Bet v 1, CSBP, Food allergy, IgE cross-reactivity, Vig r 6
1 Introduction
Birch pollen is the most important cause of allergic rhinoconjunctivitis and bronchial asthma during spring in central and northern Europe [1]. Between 60 and 100% of birch pollen allergic patients have IgE specific for the major allergen, Bet v 1 [2]. Bet v 1 and related allergens belong to a large family of plant proteins named Bet v 1 family, which contains 11 subfamilies. The largest subfamily is the dicotyledonous PR-10 (where PR is pathogenesis-related) subfamily of PR proteins, which comprises all Bet v 1-related allergens and many nonallergenic proteins involved in plant defense. Other PR-10 proteins are known from monocotyledonous plants, conifers, and mosses [3,4].
About 70% of birch pollen allergic patients show IgE-mediated reactions to plant foods such as apple, hazelnut, carrot, soybean, and celery due to the cross-reactivity of Bet v 1 with related allergens present in these foods. Most patients develop only mild local reactions of the mucosa of the upper aerodigestive tract with itching and swelling, termed oral allergy syndrome (OAS) [5].
Vig r 1, a 16 kDa protein from mung bean (Vigna radiata) is an allergen from the PR-10 subfamily cross-reactive with Bet v 1 [6]. It shares 43% of its amino acid sequence with Bet v 1 and 75% with Gly m 4, the Bet v 1-related allergen from soybean [7]. Vig r 1 is mainly expressed in the sprouts of mung beans after a germination period of 4 days [6]. The seedlings are typical ingredients of raw Asian salads and may cause allergic reactions in some Bet v 1-sensitized birch pollen allergic patients. Similarly to birch pollen related soybean allergy, cases of severe reactions (throat tightness and nausea) were observed after consumption of mung bean sprouts [6]. Eight of ten birch pollen and mung bean allergic patients had specific IgE to recombinant Vig r 1 [6].
The cytokinin-specific binding protein (CSBP) from mung bean is a 17 kDa soluble cytosolic protein with high affinity to cytokinins [8], which are hormones involved in plant growth and differentiation [9]. Typical for hormone binding proteins, the physiological concentration of CSBP is very low, whereas PR-10 proteins are expressed in much higher amounts [10]. Purification of CSBP from 100 kg of mung bean sprouts yielded only 20 μg [11]. CSBP is a member of a small subfamily of the Bet v 1 family, but shows only distant sequence similarity with PR-10 proteins [3]. Sequences of homologous, uncharacterized proteins were found in other legumes such as lupine and soybean [3].
The purpose of our study was to explore the IgE-binding properties of mung bean CSBP and its cross-reactivity with Bet v 1 and Vig r 1. Despite its low sequence identity with PR-10 proteins, CSBP bound IgE from Bet v 1-sensitized patients sera, cross-reacted with Bet v 1 and Vig r 1, and was able to activate basophils sensitized with Bet v 1-specific IgE. Based on these data, mung bean CSBP was approved as an allergen by the International Union of Immunological Societies Allergen Nomenclature Sub-Committee (http://www.allergen.org/) and was designated Vig r 6.0101.
2 Materials and methods
2.1 Allergen surface comparison
Structures of Bet v 1, Gly m 4, and Vig r 6 were downloaded from the protein data bank (Table 1). A homology model of Vig r 1, based on the structure of PR10.2b from yellow lupine (protein data bank: 2qim; 67% sequence identity to Vig r 1) as the template, was downloaded from the Swiss-Model Repository [12].
Table 1.
Amino acid sequence and molecular surface comparison of the analyzed allergens
| PDB accession number | Uniprot accession number | Percent sequence identitya)/percent surface identityb) to |
||||
|---|---|---|---|---|---|---|
| Bet v 1.0101 | Gly m 4.0101 | Vig r 1.0101 | Vig r 6.0101 | |||
| Bet v 1.0101 | 1bv1 | P15494 | 100/100 | 48/52 | 43/47 | 31/29 |
| Gly m 4.0101 | 2k7h | P26987 | 100/100 | 75/71 | 25/25 | |
| Vig r 1.0101 | Homology model | Q2VU97 | 100/100 | 24/28 | ||
| Vig r 6.0101 | 2flh | Q9ZWP8 | 100/100 | |||
Sequence identity in a global alignment.
Percentage of the surface of the left allergen identical to corresponding patches on the surface of the top allergen.
Conservation of solvent accessible surface was computed using a modification of a previously published algorithm [13]. Residue contributions to the solvent accessible surface area were calculated with the GETAREA server (http://curie.utmb.edu/getarea.html; [14]) using a probe of 1.4 Å radius (corresponding to the size of a water molecule). We excluded the ligand binding cavity, which is inaccessible to antibody molecules, from the analysis by ignoring backbone and side chain contributions showing zero surface areas with a 3.5 Å probe. For identifying structurally equivalent residues, structures of Bet v 1-related allergens were aligned using the PscViewer extension [15] for UCSF chimera [16]. The conserved surface area of each protein was calculated by adding up backbone atom surface contributions of all residues structurally equivalent to a residue on the other protein and the side chain surface contributions of all residues identical to the corresponding ones in the other proteins.
2.2 Patients
In a retrospective study, residual serum samples of Bet v 1-sensitized birch pollen allergic patients admitted for routine diagnosis of pollen and food allergies to the allergy outpatient clinics Floridsdorfer Allergiezentrum and Ambulatorium für Allergie und klinische Immunologie, Vienna, Austria, were collected. The patients underwent no interventions related to this study. The use of anonymized serum samples and clinical records without obtaining written consent of the patients was approved by the ethics committee of the Medical University of Vienna (approval number 718/2010).
Birch pollen allergy was diagnosed based on case history, positive skin-prick tests to commercial birch pollen extracts (ALK-Abelló, Horsholm, Denmark), and positive Immuno-CAP to birch pollen extract and recombinant Bet v 1 (Thermo-Fisher, Uppsala, Sweden). Mung bean sprout sensitization was diagnosed based on positive prick-to-prick tests with fresh mung bean sprouts. The occurrence of food allergic reactions among sprout-sensitized patients was determined using a standardized questionnaire.
2.3 Recombinant allergens
Codon-optimized synthetic genes of Bet v 1.0101, Vig r 1.0101, Gly m 4.0101, and Vig r 6.0101 (see Table 1 for the protein sequence accession numbers) cloned into the expression vector pET-28a(+) (Merck Millipore, Darmstadt, Germany) were obtained from Eurofins MWG Operon (Ebersberg, Germany). The proteins were expressed in E. coli BL21[DE3] in LB medium at 37°C after induction with 1 mM isopropyl-β-D-thiogalactopyranoside. Purification of Vig r 1 and Vig 6, which contained a C-terminal 6× histidine tag, was achieved by metal chelate affinity and ion exchange chromatography. Bet v 1 and Gly m 4 were purified by a combination of hydrophobic interaction, ion exchange, and gel filtration chromatography.
SDS-PAGE, MALDI-TOF MS (Bruker Microflex, Bruker Daltonics, Billerica, MA, USA), and circular dichroism (CD) spectroscopy were used to verify protein purity and identity, mass, and secondary structure. CD spectra were measured at 0.1 mg/mL in 10 mM phosphate buffer, pH 7.4 in a J-810 spectropolarimeter (Jasco, Easten, MD, USA). Data from three measurements were accumulated. Thermal denaturation and renaturation were measured between 25 and 95°C in steps of 2°C with 5°C/min heating and cooling rates at a wavelength of 198 nm. The temperature at which the curve reached the average between the minimum and maximum ellipticities was defined as melting point.
2.4 IgE ELISA and ELISA inhibition assay
Allergens were adsorbed at 10 μg/mL to 96-well Maxisorp microtiter plates (Nunc, Roskilde, Denmark). Patients’ sera and five nonallergic individuals’ sera as negative controls, diluted 1:10–1:40, were incubated in duplicates over night. IgE detection was performed with alkaline phosphatase-labeled mouse anti-human IgE (BD Pharmingen, Heidelberg, Germany) and p-nitrophenyl phosphate (Sigma-Aldrich, St. Louis, MO, USA) and measured at 405 nm. All OD values were normalized to a substrate incubation period of 1 h. Results were considered positive if they exceeded the mean OD of the negative controls by more than three SDs.
For cross-inhibition experiments, sera were preincubated with tenfold serial dilutions (between 20 pg/ml and 200 μg/mL) of Bet v 1.0101, Vig r 1.0101, and Vig r 6.0101 before performing the immunoassay as described above. Percent inhibition was calculated using the OD values of sera without added allergens (0% inhibition) and the mean OD of the nonallergic control sera (100% inhibition).
2.5 Basophil activation assay
Degranulation assays with rat basophilic leukemia cells expressing the α-chain of the human FcεR1 receptor were performed as previously described [17]. Rat basophilic leukemia cells plated in sterile 96-well polystyrene cell culture plates (Corning, Inc., Corning, NY, USA) were passively sensitized with patients’ sera (diluted 1:10 or 1:20), which were depleted from IgG with Protein G HP Spin Trap columns (GE Healthcare), at 37°C over night. Tenfold serial dilutions of allergens (between 0.1 and 1000 ng/mL) were added for 1 h. The extent of degranulation was determined as β-hexosaminidase activity in the supernatants. Color development after adding p-nitrophenyl-N-acetyl-β-d-glucosaminide was measured at 405 nm. Percent activation was expressed relative to hexosaminidase activity of cells lysed with triton X-100 (100% activation) and the supernatant of untreated cells (0% activation).
2.6 Statistical analyses
Frequencies of IgE binding among unselected Bet v 1-sensitized patients and mung bean sprout sensitized patients were compared using the chi-squared test. The amounts of Bet v 1-specific IgE among Vig r 6-sensitized and Vig r 6-negative sera were compared using the Mann–Whitney test. The distributions of ELISA OD values of different allergens were compared using the Kruskal–Wallis test combined with Dunn’s multiple comparison test. Correlation of the amounts of IgE binding of the different allergens was calculated using Spearman’s rank correlation. p-values below 0.05 were considered significant. All analyses were performed and graphics generated in GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
3 Results
3.1 Structural comparison
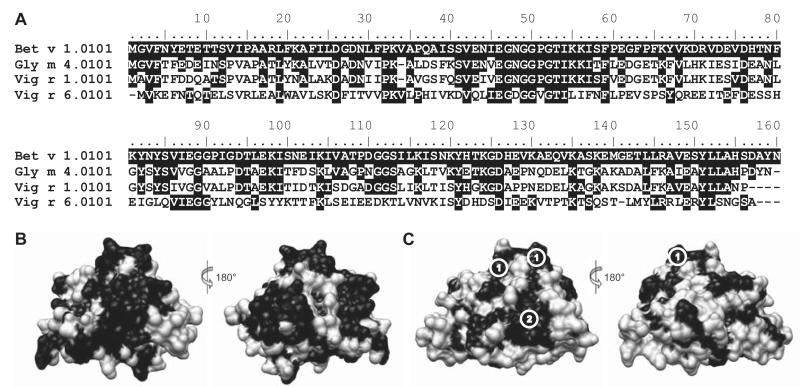
The results of a structural comparison of the molecular surfaces of Bet v 1, Gly m 4, Vig r 1, and Vig r 6 are shown in Table 1 and Fig. 1. Bet v 1 showed 47% surface identity to Vig r 1, with conserved patches distributed over most parts of the surface (Fig. 1B). Only 29% of the surface area of Bet v 1 was identical to Vig r 6 (Fig. 1C). Nevertheless, the surface of Vig r 6 contained two contiguous conserved patches large enough for representing potential cross-reactive epitopes. One area (42-QLIEGDGGVGTI-53; corresponding sequence in Bet v 1: 42-ENIEGNGGPGTI-53; conserved residues in bold) had an accessible surface area of 411 Å2. A neighboring but nonoverlapping patch (28- TVVPKVLPHIKVDV-41, Y149; corresponding sequence in Bet v 1: 28-NLFPKVAPQAISSV-41, Y150; 352 Å2) was centered on residue K32.
Figure 1.
Sequence and structural comparison of the allergens examined in this study. (A) Sequence alignment of Bet v 1 with Bet v 1-related allergens from soybean and mung bean. (B, C) Structural comparison of Vig r 1 (B) and Vig r 6 (C), respectively, with Bet v 1. Black: residues or surface patches identical to Bet v 1; Encircled numbers: Conserved potential cross-reactive epitopes of Vig r 6; 1: p-loop; 2: surface patch centered on residue K32. The sequence alignment was generated using BioEdit 7.1.3.0 [36]. Structures were aligned and visualized using UCSF Chimera 1.6.2 [16].
3.2 Validation of the recombinant allergens
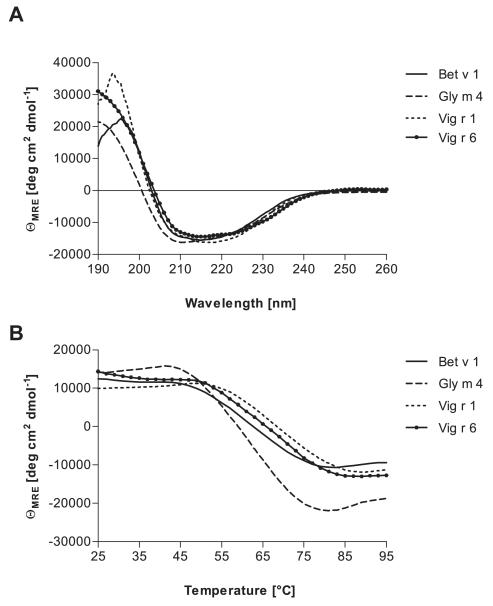
CD spectra revealed that the secondary structure contents of the purified recombinant Bet v 1.0101, Vig r 1.0101, Vig r 6.0101, and Gly m 4.0101 were nearly identical, showing the characteristics of folded proteins with mixed α-helical and β-strand structures (Fig. 2A). Thermal denaturation of these allergens revealed a similarly low extent of heat resistance with melting points of 61°C for Gly m 4, 62°C for Bet v 1, 66°C for Vig r 6, and 69°C for Vig r 1 (Fig. 2B). With all four proteins, complete renaturation was observed after cooling the samples back to 25°C (data not shown).
Figure 2.
CD spectra of purified allergens. (A) Secondary structure contents of Bet v 1.0101, Gly m 4.0101, Vig r 1.0101, and Vig r 6.0101 measured at room temperature and pH 7.4. (B) Thermal denaturation determined at 198 nm between 25 and 95°C.
Molecular masses of the recombinant proteins measured by MALDI-TOF were equivalent to those calculated from the sequences after removal of the N-terminal Met (shown in brackets). Bet v 1 with 17 439 Da (17 440 Da), Gly m 4 with 16 641 Da (16 641 Da), Vig r 1 with 17 121 Da (17 123 Da including the 6xHis-tag), and Vig r 6 with 18 666 Da (18 660 Da including the 6xHis-tag and the N-terminal Met).
3.3 Patients’ characteristics
Two panels of sera were used for the immunological assays (Table 2). Panel A consisted of 60 sera from unselected Bet v 1-sensitized patients with and without associated food allergies. This panel served as a large control population to asses the extent of IgE corecognition of mung bean allergens among Bet v 1-sensitized patients. Panel B comprised 19 sera of Bet v 1-sensitized patients with positive prick-to-prick tests to fresh mung bean sprouts. Five of these reported OAS after consumption of raw but not of cooked sprouts, three patients reported no symptoms after eating sprouts, all other patients had not knowingly consumed raw sprouts before (Table 2 and Supporting Information Table 1).
Table 2.
Clinical and serological data of the patients included in this study
| Panel Aa) | Panel Bb) | |
|---|---|---|
| Number (male/female) | 60 (29/31) | 19 (4/15) |
| Mean age (range) | 33 (7–79) | 42 (18–68) |
| Reported plant food allergy | ||
| Any plant food | 27 (45%) | 19 (100%) |
| Soy milk (yes/no/not consumed) |
Not known | 13/2/4 |
| Raw mung bean sprouts (yes/no/not consumed) |
Not known | 5/3/11 |
| Positive prick-to-prick test to fresh mung beans |
Not done | 19 (100%) |
| IgE ELISA | ||
| Bet v 1.0101 | 60 (100%) | 19 (100%) |
| Gly m 4.0101 | 52 (87%) | 17 (89%) |
| Vig r 1.0101 | 44 (73%) | 15 (79%) |
| Vig r 6.0101 | 19 (32%) | 12 (63%) |
Sera from unselected Bet v 1-sensitized patients with and without associated food allergies.
Sera from Bet v 1-sensitized patients with positive prick-to-prick tests to fresh mung bean sprouts.
3.4 Patterns of IgE binding to mung bean sprout allergens
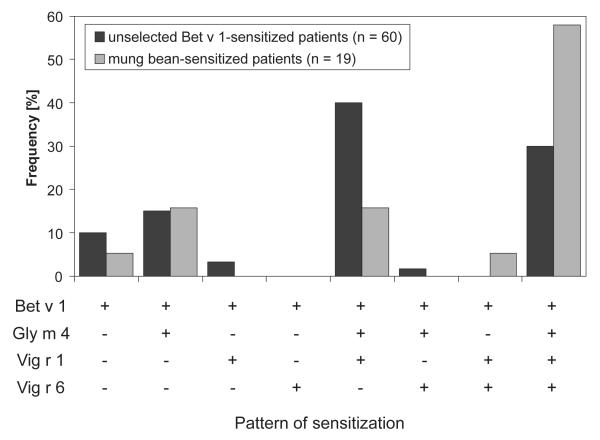
An overview of the frequencies of IgE binding to Bet v 1, Gly m 4, and mung bean sprout allergens is shown in Table 2. ELISA OD values of all individual patients of panel B are listed in the Supporting Information Table 1. All sera showed IgE binding to Bet v 1. The percentages of IgE binding to Gly m 4 and Vig r 1 were similar in both the unselected Bet v 1-sensitized and the mung bean sprout sensitized groups. In contrast, the frequency of IgE binding to Vig r 6 was significantly (p = 0.01) higher among mung bean sprout sensitized (12 of 19; 63%) than among unselected Bet v 1-sensitized patients (19 of 60; 32%).
Considering only the OD values of sera containing allergen-specific IgE, the amounts of IgE specific for Gly m 4 (median OD per hour: 0.34) and Vig r 1 (median OD per h: 0.16), assessed based on ELISA OD values and serum dilutions, were significantly (p < 0.001) lower than for Bet v 1 (median OD per h: 2.57). Furthermore, the amounts of Vig r 6-specific IgE (median OD per h: 0.02) were significantly (p < 0.05) lower than the Vig r 1-specific IgE values.
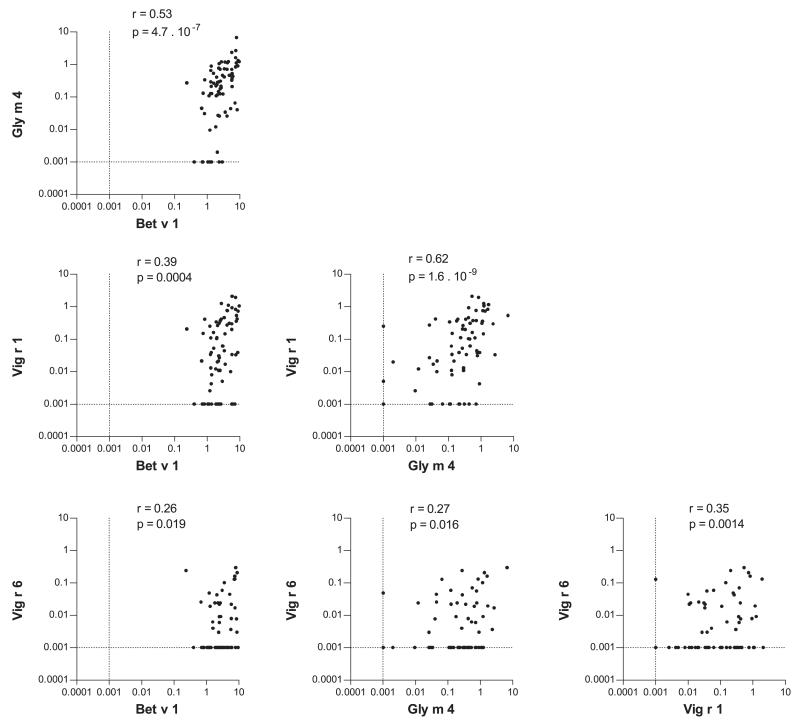
IgE of most sera from both groups either recognized all tested allergens (18 of 60 in panel A; 11 of 19 in panel B) or Bet v 1, Gly m 4, and Vig r 1 (24 of 60 in panel A; 3 of 19 in panel B; Fig. 3). Of the 31 sera from both groups with IgE binding to Vig r 6, 29 also recognized both Gly m 4 and Vig r 1, one serum each bound to one of those allergens. Combining the ELISA data of both patient groups, the amounts of IgE binding to different allergens were moderately correlated (Fig. 4). The tightest correlation was observed between IgE binding to Gly m 4 and Vig r 1 (r = 0.62; p = 1.6 × 10−9). The lowest extent of correlation was found between IgE binding to Vig r 6 and to the other allergens. Separate analyses for both serum panels yielded similar results (data not shown).
Figure 3.
Sensitization profiles of unselected Bet v 1-sensitized patients (Panel A) and mung bean sensitized patients (Panel B) determined by IgE ELISA.
Figure 4.
Correlation of the amounts of allergen-specific IgE. IgE ELISA OD values for the different allergens were normalized to a substrate incubation period of 1 h. Negative values were set to 0.001. Spearman’s rank correlation coefficients (r values) and p values were calculated using GraphPad Prism.
3.5 Cross-reactivity between Bet v 1 and mung bean allergens
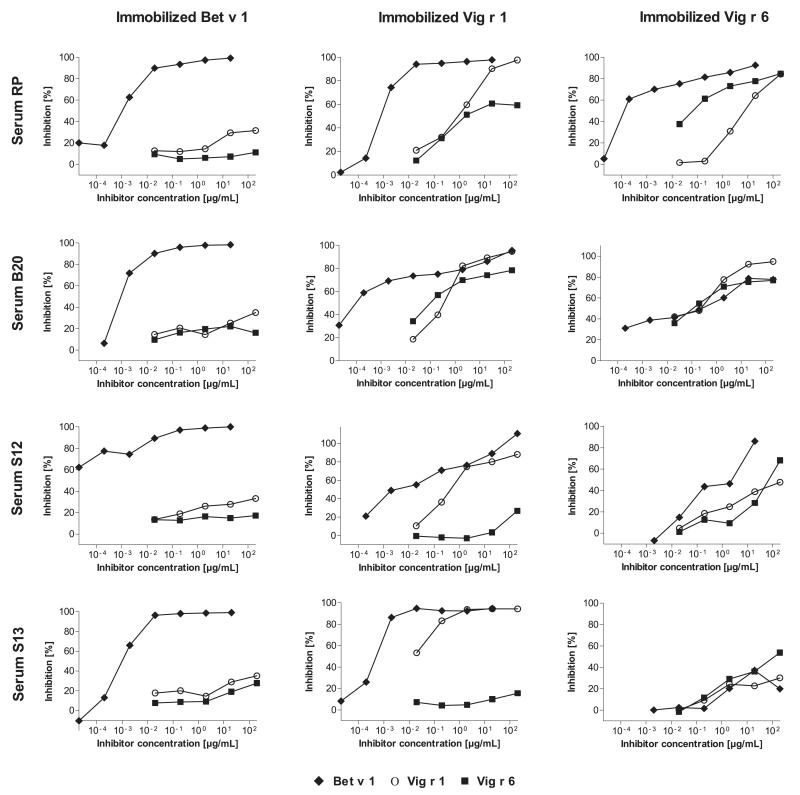
ELISA cross-inhibition experiments with four representative sera are shown in Fig. 5. Preincubation with Bet v 1 completely inhibited IgE binding to Vig r 1 with all and to Vig r 6 with two tested sera. With the other two sera, IgE binding to Vig r 6 was inhibited by Bet v 1 by 80 and 40%. In contrast, both mung bean allergens reduced IgE binding to Bet v 1 by only 10–35% (Fig. 5, left column). The extent of cross-reactivity between Vig r 1 and Vig r 6 depended on the tested serum. While Vig r 1 inhibited IgE binding to Vig r 6 by 30–95% (Fig. 5, right column), inhibition in the opposite direction resulted in reduction of IgE binding to Vig r 1 between 15 and 80% (Fig. 5, middle column).
Figure 5.
IgE ELISA inhibition. One serum from panel A (RP) and three sera from panel B (B20, S12, and S13) were selected. Bet v 1, Vig r 1, and Vig r 6 were immobilized to microtiter plates at 10 μg/mL. Sera were preincubated with these allergens at concentrations between 20 pg/ml and 200 μg/mL and residual IgE binding to the immobilized allergens measured.
A comparison of the relative affinities of patients’ IgE to the tested allergens, as assessed by the inhibitor concentrations required to reach half-maximum inhibition (IC50), revealed large differences. With immobilized Vig r 1, inhibition with Bet v 1 reached half-maximum values between 1 and 10 ng/mL. In contrast, the IC50 for Vig r 1 as inhibitor was 100–1000 times higher. The IC50 of Vig r 6 was similar to that of Vig r 1 with two sera, while with the other two sera, inhibition with Vig r 6 did not reach plateau values at 200 μg/mL (Fig. 5, middle column).With immobilized Vig r 6, the inhibition curves of all three allergens were similar for two sera, whereas IC50 values increased in the order Bet v 1 < Vig r 1 < Vig r 6 in one serum and Bet v 1 < Vig r 6 < Vig r 1 in the remaining serum (Fig. 5, right column).
3.6 Basophil activation assay
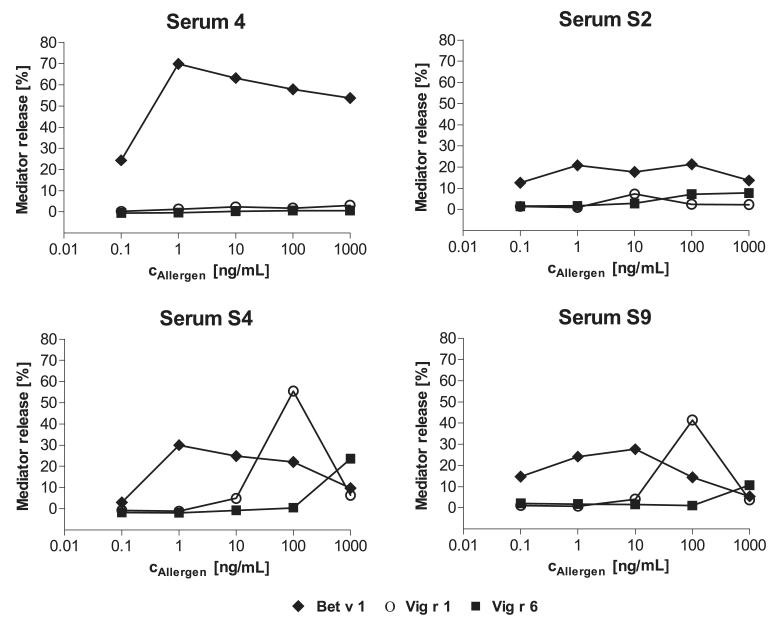
One serum from the unselected Bet v 1-sensitized group as control and three sera of Bet v 1-sensitized patients with positive prick-to-prick tests to mung bean sprouts were tested in a basophil activation assay (Fig. 6). While cells sensitized with the control serum were activated only after incubation with Bet v 1, the other three sera showed maximum hexosaminidase release between 5 and 60% with all three allergens. In accordance with the affinity differences observed in the inhibition ELISA experiments, the allergen concentration eliciting maximum mediator release differed. Bet v 1 induced maximum release at concentrations between 1 and 10 ng/mL, whereas maximum release after incubation with Vig r 1 was reached between 10 and 100 ng/mL. Mediator release after treatment with Vig r 6 was only observed at 100 ng/mL with one serum and 1000 ng/mL, the highest tested concentration, with the other two sera. The degranulation activities of Bet v 1 and Vig r 1 decreased at high allergen concentrations, most likely due to saturation of cell-bound IgE with allergen.
Figure 6.
Basophil activation assay. Rat basophilic leukemia cells were sensitized with one serum from the Bet v 1-sensitized group (serum 4) and with three sera from the panel of mung bean sprout sensitized patients (S2, S4, and S9), and then incubated with Bet v 1, Vig r 1, and Vig r 6. Activation was determined by measuring β-hexosaminidase activity in the supernatants.
4 Discussion
Mung bean sprouts are often falsely labeled “soy sprouts”. While mung bean is closely related to soybean, soybean sprouts are considerably less frequently consumed. Both bean species are typical ingredients of the Asian cuisine. With the growing popularity of Asian food in western countries, the risk of soy and mung bean allergy has increased [18]. Gly m 4 from soybean as well as Vig r 1 from mung bean have been reported as Bet v 1-related allergens from the PR-10 subfamily, posing an additional risk for birch pollen allergic patients who are prone to associated food allergies. Both have shown cross-reactivity with Bet v 1 and with each other [6,7].
In our study, we focused on the new allergen Vig r 6, a Bet v 1-related protein not belonging to the PR-10 subfamily, whose occurrence in mung bean seedlings has previously been shown [8, 11]. Despite its relatedness with Bet v 1, no data have been published on its binding to Bet v 1-sensitized patients’ IgE or cross-reactivity with Bet v 1. Furthermore, until now only allergens from the PR-10 subfamily have been described to cross-react with Bet v 1 [3]. The sole exception has been Act d 11 from kiwi fruit, which belongs to the major latex proteins/ripening-related proteins subfamily and shows only around 20% sequence identity to PR-10 subfamily members [19]. Similarly to mung bean sprouts, kiwi fruit contains two Bet v 1-related allergens, Act d 8, a member of the PR-10 subfamily [20], and Act d 11.
Vig r 6 has only 31% sequence identity with Bet v 1 (Table 1). We have previously proposed that homologous allergens with identities less than 50% rarely show IgE cross-reactivity [21]. However, most IgE antibodies bind to surface-exposed patches formed by residues discontinuously distributed over the protein sequence [22]. Hence, we compared the antibody-accessible molecular surfaces of Bet v 1 and Vig r 6 to identify potential cross-reactive conformational epitopes. Despite a low overall surface identity of 29%, we found two adjacent conserved patches. One contained a glycine-rich loop named the p-loop, which is the region with the highest extent of sequence conservation among Bet v 1-related proteins [23]. This region was previously identified as an immunodominant IgE epitope based on the crystal structure of Bet v 1 in complex with an IgE-inhibiting Fab’ fragment [24,25] and decreased IgE-binding capacities after mutation of the central residue E45 in Bet v 1 and Pru av 1, the Bet v 1 homologue from cherry [25,26]. Since Vig r 6 contains a glutamate residue at position 45, a substantial fraction of IgE specific for this allergen most likely binds to the p-loop epitope.
In connection with allergy risk assessment of genetically modified foods, potential transgenes have to be compared with a database of known allergens. Potential cross-reactivity is assumed if a sequence identity greater than 35% to a known allergen is found, using a sliding window of 80 residues [27]. A comparison of Vig r 6 with the AllergenOnline database (www.allergenonline.org) yielded 42% identity to several Bet v 1 isoallergens despite a much lower identity over the complete sequence (data not shown). Hence, this example justifies the very conservative choice of the 35% sequence identity threshold.
We compared the frequencies of IgE binding to Vig r 6 among sera of unselected Bet v 1-sensitized patients with and without food allergy with the binding frequency of sera from mung bean sensitized patients. The frequency of IgE binding to Vig r 6 was almost twice as high in the mung bean sensitized group (63% of 19) than in the unselected group (32% of 60). Nevertheless, all Vig r 6 sensitized patients’ sera also recognized Vig r 1 and/or Gly m 4. Thus, the inclusion of Vig r 6 in a diagnostic test will not increase the frequency of detecting mung bean sensitization.
We showed IgE cross-reactivity between Bet v 1, Vig r 1, and Vig r 6. Among Bet v 1-related allergens, Bet v 1 is the primary sensitizing agent with the highest affinity to birch pollen allergic patients’ IgE [28]. The results of our inhibition assays were consistent with those previous observations. IgE binding to Vig r 1 and Vig r 6 was completely inhibited by Bet v 1 but not vice versa (Fig. 5). The results of the IgE cross-inhibition between Vig r 1 and Vig r 6 were highly patient dependent. These differences and the low extent of correlation of the amounts of IgE binding to Vig r 1 and Vig r 6 (Fig. 4) indicate that cross-sensitizations between Bet v 1 and Vig r 1 on the one hand and Bet v 1 and Vig r 6 on the other hand occur independently of each other.
Comparison of the allergen concentrations required for half-maximum inhibition revealed that Vig r 1 and Vig r 6 had a 100- to 1000-fold lower affinity to allergen-specific IgE than Bet v 1 (Fig. 5). In line with the ELISA inhibition data, a measurable mediator release in the basophil activation assays was obtained for Vig r 6 only at tenfold higher allergen concentrations than for Vig r 1 and at 100- or 1000-fold higher concentration than for Bet v 1 (Fig. 6). Similarly, Api g 1, the clinically well-established elicitor of birch pollen associated celery allergy, induced basophil activation only at 100–1000 times higher concentrations than Bet v 1 [29]. While high-affinity allergen-specific IgE is generally considered a prerequisite for activating mast cells and basophils, experiments with monoclonal recombinant IgE antibodies specific for the house dust mite allergen Der p 2 previously showed that activation of basophils may occur already after cross-linking of a high-affinity with a low-affinity IgE antibody [30]. Consequently, the presence of low-affinity allergen-specific IgE in patients’ sera might contribute to allergic reactions.
Food processing can have great impact on the elicitation of allergic reactions. Despite being structurally highly similar, considerable differences in the thermal stabilities of Bet v 1-related allergens were shown. Thermal stability of Mal d 1 was lower than of Api g 1, Dau c 1, and Gly m 4 [31–34]. After heating at neutral pH, Bet v 1, Gly m 4, Vig r 1, and Vig r 6 started unfolding above 60°C and were fully denatured at 95°C (Fig. 2). Nevertheless, they regained their native structures after cooling down to room temperature, similarly to other Bet v 1-related allergens [32]. All patients of our study population with OAS after consumption of raw mung bean sprouts tolerated cooked ones. Notwithstanding, if cooked mung bean sprouts are consumed as a cold dish such as a salad, the allergens might be renatured and pose a risk for some patients with birch pollen fruit syndrome. However, the influence of the food matrix on the stability of allergens should not be neglected. It has previously been shown that the major cherry allergen, Pru av 1, despite refolding after heating in neutral buffer solution, did not regain its IgE binding ability after heat treatment in the presence of carbohydrates [35].
In conclusion, we showed unexpected IgE cross-reactivity between Bet v 1 and its distant homologue Vig r 6 despite their low sequence identity. A potential clinical relevance of this allergen for mung bean sprout allergy is supported by the significantly increased frequency of IgE binding to Vig r 6 among mung bean sprout sensitized compared to other birch pollen allergic patients and the capability of Vig r 6 to activate sensitized basophils. On the other hand, the low affinity of Vig r 6-specific IgE and the low concentration of this allergen in mung bean sprouts might limit the clinical significance of Vig r 6.
Supplementary Material
Acknowledgments
The study was supported by grants P-22559-B11 (to C.R.) and SFB-F4608 (to H.B.) from the Austrian Science Fund.
Abbreviations
- CD
circular dichroism
- CSBP
cytokinin-specific binding protein
- OAS
oral allergy syndrome
- PR
pathogenesis-related
Footnotes
Potential conflict of interest statement: Stefan Vieths has received speaker’s honoraria from Phadia, Germany and Fresenius Akademie, Germany. He served as a paid consultant as expert reviewer for the FARRP AllergenOnline database. S.V. has also received research funding from Monsanto Company and Pioneer Hi-Bred International, Inc. All other authors had no conflicts of interest to declare.
Additional supporting information may be found in the online version of this article at the publisher’s web-site
5 References
- [1].D’Amato G, Spieksma FT, Liccardi G, Jager S, et al. Pollen-related allergy in Europe. Allergy. 1998;53:567–578. doi: 10.1111/j.1398-9995.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- [2].Moverare R, Westritschnig K, Svensson M, Hayek B, et al. Different IgE reactivity profiles in birch pollen-sensitive patients from six European populations revealed by recombinant allergens: an imprint of local sensitization. Int. Arch. Allergy Immunol. 2002;128:325–335. doi: 10.1159/000063855. [DOI] [PubMed] [Google Scholar]
- [3].Radauer C, Breiteneder H. Evolutionary biology of plant food allergens. J. Allergy Clin. Immunol. 2007;120:518–525. doi: 10.1016/j.jaci.2007.07.024. [DOI] [PubMed] [Google Scholar]
- [4].Radauer C, Lackner P, Breiteneder H. The Bet v 1 fold: an ancient, versatile scaffold for binding of large, hydrophobic ligands. BMC Evol. Biol. 2008;8:286. doi: 10.1186/1471-2148-8-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vieths S, Scheurer S, Ballmer-Weber B. Current understanding of cross-reactivity of food allergens and pollen. Ann. NY Acad. Sci. 2002;964:47–68. doi: 10.1111/j.1749-6632.2002.tb04132.x. [DOI] [PubMed] [Google Scholar]
- [6].Mittag D, Vieths S, Vogel L, Wagner-Loew D, et al. Birch pollen-related food allergy to legumes: identification and characterization of the Bet v 1 homologue in mungbean (Vigna radiata), Vig r 1. Clin. Exp. Allergy. 2005;35:1049–1055. doi: 10.1111/j.1365-2222.2005.02309.x. [DOI] [PubMed] [Google Scholar]
- [7].Kleine-Tebbe J, Vogel L, Crowell DN, Haustein UF, et al. Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v 1- related PR-10 protein in soybean, SAM22. J. Allergy Clin. Immunol. 2002;110:797–804. doi: 10.1067/mai.2002.128946. [DOI] [PubMed] [Google Scholar]
- [8].Nagata R, Kawachi E, Hashimoto Y, Shudo K. Cytokininspecific binding protein in etiolated mung bean seedlings. Biochem. Biophys. Res. Commun. 1993;191:543–549. doi: 10.1006/bbrc.1993.1252. [DOI] [PubMed] [Google Scholar]
- [9].Werner T, Schmulling T. Cytokinin action in plant development. Curr. Opin. Plant Biol. 2009;12:527–538. doi: 10.1016/j.pbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- [10].Pasternak O, Bujacz GD, Fujimoto Y, Hashimoto Y, et al. Crystal structure of Vigna radiata cytokinin-specific binding protein in complex with zeatin. Plant Cell. 2006;18:2622–2634. doi: 10.1105/tpc.105.037119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fujimoto Y, Nagata R, Fukasawa H, Yano K, et al. Purification and cDNA cloning of cytokinin-specific binding protein from mung bean (Vigna radiata) Eur. J. Biochem. 1998;258:794–802. doi: 10.1046/j.1432-1327.1998.2580794.x. [DOI] [PubMed] [Google Scholar]
- [12].Kiefer F, Arnold K, Kunzli M, Bordoli L, et al. The SWISS-MODEL repository and associated resources. Nucl. Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jenkins JA, Griffiths-Jones S, Shewry PR, Breiteneder H, et al. Structural relatedness of plant food allergens with specific reference to cross-reactive allergens: an in silico analysis. J. Allergy Clin. Immunol. 2005;115:163–170. doi: 10.1016/j.jaci.2004.10.026. [DOI] [PubMed] [Google Scholar]
- [14].Fraczkiewicz R, Braun W. Exact and efficient analytical calculation of the accessible surface areas and their gradients for macromolecules. J. Comp. Chem. 1998;19:319–333. [Google Scholar]
- [15].Berbalk C, Schwaiger CS, Lackner P. Accuracy analysis of multiple structure alignments. Protein Sci. 2009;18:2027–2035. doi: 10.1002/pro.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pettersen EF, Goddard TD, Huang CC, Couch GS, et al. UCSF chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- [17].Wagner S, Radauer C, Bublin M, Hoffmann-Sommergruber K, et al. Naturally occurring hypoallergenic Bet v 1 isoforms fail to induce IgE responses in individuals with birch pollen allergy. J. Allergy Clin. Immunol. 2008;121:246–252. doi: 10.1016/j.jaci.2007.08.006. [DOI] [PubMed] [Google Scholar]
- [18].Ballmer-Weber BK, Vieths S. Soy allergy in perspective. Curr. Opin. Allergy Clin. Immunol. 2008;8:270–275. doi: 10.1097/ACI.0b013e3282ffb157. [DOI] [PubMed] [Google Scholar]
- [19].D’Avino R, Bernardi ML, Wallner M, Palazzo P, et al. Kiwifruit Act d 11 is the first member of the ripening-related protein family identified as an allergen. Allergy. 2011;66:870–877. doi: 10.1111/j.1398-9995.2011.02555.x. [DOI] [PubMed] [Google Scholar]
- [20].Oberhuber C, Bulley SM, Ballmer-Weber BK, Bublin M, et al. Characterization of Bet v 1-related allergens from kiwifruit relevant for patients with combined kiwifruit and birch pollen allergy. Mol. Nutr. Food Res. 2008;52:S230–S240. doi: 10.1002/mnfr.200800146. [DOI] [PubMed] [Google Scholar]
- [21].Radauer C, Breiteneder H. Pollen allergens are restricted to few protein families and show distinct patterns of species distribution. J. Allergy Clin. Immunol. 2006;117:141–147. doi: 10.1016/j.jaci.2005.09.010. [DOI] [PubMed] [Google Scholar]
- [22].Aalberse RC, Crameri R. IgE-binding epitopes: a reappraisal. Allergy. 2011;66:1261–1274. doi: 10.1111/j.1398-9995.2011.02656.x. [DOI] [PubMed] [Google Scholar]
- [23].Liu JJ, Ekramoddoullah AKM. The family 10 of plant pathogenesis-related proteins: their structure, regulation, and function in response to biotic and abiotic stresses. Physiol. Mol. Plant Pathol. 2006;68:3–13. [Google Scholar]
- [24].Mirza O, Henriksen A, Ipsen H, Larsen JN, et al. Dominant epitopes and allergic cross-reactivity: complex formation between a Fab fragment of a monoclonal murine IgG antibody and the major allergen from birch pollen Bet v 1. J. Immunol. 2000;165:331–338. doi: 10.4049/jimmunol.165.1.331. [DOI] [PubMed] [Google Scholar]
- [25].Spangfort MD, Mirza O, Ipsen H, Van Neerven RJ, et al. Dominating IgE-binding epitope of Bet v 1, the major allergen of birch pollen, characterized by X-ray crystallogra phy and site-directed mutagenesis. J. Immunol. 2003;171:3084–3090. doi: 10.4049/jimmunol.171.6.3084. [DOI] [PubMed] [Google Scholar]
- [26].Neudecker P, Lehmann K, Nerkamp J, Haase T, et al. Mutational epitope analysis of Pru av 1 and Api g 1, the major allergens of cherry (Prunus avium) and celery (Apium graveolens): correlating IgE reactivity with three-dimensional structure. Biochem. J. 2003;376:97–107. doi: 10.1042/BJ20031057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Goodman RE, Vieths S, Sampson HA, Hill D, et al. Allergenicity assessment of genetically modified crops – what makes sense? Nat. Biotechnol. 2008;26:73–81. doi: 10.1038/nbt1343. [DOI] [PubMed] [Google Scholar]
- [28].Kazemi-Shirazi L, Pauli G, Purohit A, Spitzauer S, et al. Quantitative IgE inhibition experiments with purified recombinant allergens indicate pollen-derived allergens as the sensitizing agents responsible for many forms of plant food allergy. J. Allergy Clin. Immunol. 2000;105:116–125. doi: 10.1016/s0091-6749(00)90186-6. [DOI] [PubMed] [Google Scholar]
- [29].Schimek EM, Zwolfer B, Briza P, Jahn-Schmid B, et al. Gastrointestinal digestion of Bet v 1-homologous food allergens destroys their mediator-releasing, but not T cell-activating, capacity. J. Allergy Clin. Immunol. 2005;116:1327–1333. doi: 10.1016/j.jaci.2005.09.007. [DOI] [PubMed] [Google Scholar]
- [30].Christensen LH, Holm J, Lund G, Riise E, et al. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J. Allergy Clin. Immunol. 2008;122:298–304. doi: 10.1016/j.jaci.2008.05.026. [DOI] [PubMed] [Google Scholar]
- [31].Ballmer-Weber BK, Hoffmann A, Wuthrich B, Luttkopf D, et al. Influence of food processing on the allergenicity of celery: DBPCFC with celery spice and cooked celery in patients with celery allergy. Allergy. 2002;57:228–235. doi: 10.1034/j.1398-9995.2002.1o3319.x. [DOI] [PubMed] [Google Scholar]
- [32].Bollen MA, Wichers HJ, Helsper JPFG, Savelkoul HFJ, et al. Thermodynamic characterization of the PR-10 allergens Bet v 1, Api g 1 and Dau c 1 and pH-dependence of nApi g 1 and nDau c 1. Food Chem. 2010;119:241–248. [Google Scholar]
- [33].Mills EN, Sancho AI, Rigby NM, Jenkins JA, et al. Impact of food processing on the structural and allergenic properties of food allergens. Mol. Nutr. Food Res. 2009;53:963–969. doi: 10.1002/mnfr.200800236. [DOI] [PubMed] [Google Scholar]
- [34].Mittag D, Vieths S, Vogel L, Becker WM, et al. Soybean allergy in patients allergic to birch pollen: clinical investigation and molecular characterization of allergens. J. Allergy Clin. Immunol. 2004;113:148–154. doi: 10.1016/j.jaci.2003.09.030. [DOI] [PubMed] [Google Scholar]
- [35].Gruber P, Vieths S, Wangorsch A, Nerkamp J, et al. Mail-lard reaction and enzymatic browning affect the allergenicity of Pru av 1, the major allergen from cherry (Prunus avium) J. Agric. Food Chem. 2004;52:4002–4007. doi: 10.1021/jf035458+. [DOI] [PubMed] [Google Scholar]
- [36].Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999;41:95–98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.