Abstract
Once considered primarily occupational, novel nanotechnology innovation and application has led to widespread domestic use and intentional biomedical exposures. With these exciting advances, the breadth and depth of toxicological considerations must also be expanded. The vascular system interacts with every tissue in the body, striving to homeostasis. Engineered nanomaterials (ENM) have been reported to distribute in many different organs and tissues. However, these observations have tended to use approaches requiring tissue homogenization and/or gross organ analyses. These techniques, while effective in establishing presence, preclude an exact determination of where ENM are deposited within a tissue. It is necessary to identify this exact distribution and deposition of ENM throughout the cardiovascular system, with respect to vascular hemodynamics and in vivo/ in vitro ENM modifications taken into account if nanotechnology is to achieve its full potential. Distinct levels of the vasculature will first be described as individual compartments. Then the vasculature will be considered as a whole. These unique compartments and biophysical conditions will be discussed in terms of their propensity to favor ENM deposition. Understanding levels of the vasculature will also be discussed. Ultimately, future studies must verify the mechanisms speculated on and presented herein.
Keywords: engineered nanomaterials, microcirculation, endothelial surface layer
Introduction
Advancements in nanotechnology have given rise to the potential for engineered nanomaterials (ENM) to broadly interact with every mammalian system. Therefore, human ENM exposures, once considered largely unintentional and/or occupational, are now also intentional. This is due to a variety of reasons associated with the wide availability of ENM and their desirability by the general population through the use of diverse commercial products such as surface coatings, filters, and cosmetics. Furthermore, future applicability of ENM in pharmaceuticals and biomedical devices will significantly advanced the rate which ENM are intentionally introduced to the body. The pulmonary system has long been the primary point of exposure in the field; however, the cardiovascular system has been more commonly observed to be at the greatest risk in terms of morbidity and mortality rates1.
The context of vascular ENM distribution is based upon the presumption that ENM will ultimately access to the vascular compartment to some point. Whether this access is intentional or unintentional, and how the internal milieu influences ENM properties, are outside the scope of this review (but should be addressed in future topics). While considerable evidence exists in the literature that ENM are capable of travelling in the plasma, interacting with endothelial cells and ultimately migrating from the vascular compartment to the extravascular compartment; relatively little attention has been focused on how the unique physical and biophysical in vivo conditions, innate to specific levels of the vasculature, influence ENM distribution, deposition, and action. This latter point is the purpose of this review.
Recently, ENM have been shown to distribute throughout the body2, 3; however, with the techniques currently available, it remains unclear as to exactly which anatomical/cellular region these particle(s) reside in at a given time of measurement (e.g. interstitium, intracellular compartmentalization, or trapped within the vasculature). To fully appreciate the complexity of vascular distribution, it is crucial to understand the compartments and specific levels of the cardiovascular system, where these nanomaterials may be interacting, unique considerations, and outcomes of these interactions.
Cardiovascular System
To more clearly discuss the vascular distribution of ENM, we will define the cardiovascular system in terms of compartments. In its basic form, the cardiovascular system functions as a central pump connected to a closed-loop arrangement of tubes, tasked with maintaining the delivery of nutrients and collection/removal of waste to a given tissue to preserve homeostasis. This homeostatic balance entails (but is not limited to) temperature, acid-base balance, blood pressure, blood flow, or nutrient-waste. The heart consists of two-pumps in series, identified as the left and right heart or the pulmonary-and-systemic systems, respectively. As the left ventricle contracts, freshly oxygenated blood is pumped into the aorta, then disseminated to the large and small arteries of the macrocirculation which progressively branch divergently toward the systemic tissues. The arterioles, capillaries, and venules within the tissue make up the microcirculation; upon exiting the tissues, the venules converge to veins, pool, and empty into the right atrium of the heart. The right heart pumps blood to the lungs (pulmonary circulation) under low pressure. Because the left heart pumps blood to all other tissues (systemic circulation), significantly higher pressures must be generated for proper blood flow distribution (as compared to the right heart). Moreover, because of these inherent differences between the compartments and the levels of the cardiovascular system, significant anatomical and physiological differences exist. These fundamental differences may be the foundation for many of the conditions and effects that ultimately influence ENM vascular distribution. We will try to highlight some of the most important mechanisms in these regards.
Macrocirculation
The macrocirculation consists of the major vessels which serve to deliver blood to and from the systemic organs. The compliant arteries act as a pressure reservoir, capable of transferring the pressures generated by left ventricular contraction (during systole) to a driving force that persists during diastole, allowing a constant blood flow to the tissues. This is also known as the Windkessel effect. The arterial compliance associated with the transfer of arterial pressures will act to create turbulent flow4, a hemodynamic outcome which may contribute to an increased ENM deposition in the vascular wall. In this case, turbulent flow verses laminar, may slow blood flow and promote settling of ENM out of centerline blood flow, or increase minor eddy currents that increase the likelihood of impaction and deposition into the vessel wall. This is especially prevalent at large artery bifurcations5 (this concept is now expanded on in the “Unique Considerations Influencing Vascular Distribution” section).
In addition to returning blood from the systemic organs to the heart, the veins function as a blood reservoir due to their capacitance or storage capacity. In this case, with respect to the distribution of ENM within the macrocirculation, the majority of ENM would reside within the venous circulation due to the high percentage of blood volume.
Microcirculation
The microcirculation is defined as all the vascular tissue contained within a given organ. The majority of the microvascular literature focuses on the arterioles, capillaries, and venules. However, the lymphatic system is, by this definition, also part of the microcirculation. Given their inherent and unique importance, the former and latter will be addressed in separate sections.
The arterioles, capillaries, and venules are imperative to maintenance of blood pressure and blood flow distribution. These vessels contribute significantly to blood pressure regulation and flow to enhance nutrient and waste exchange at the capillaries. The extent of this regulation varies by tissue type, is influenced centrally by the nervous system and locally in the tissue, and is manifest as vascular tone (or partial contraction). Vascular tone, while the product of many factors, is ultimately maintained through vascular smooth muscle control via neural or local signaling. Closely positioned to the vascular smooth muscle cells are the endothelial cells; coordinated interaction between these cells allows for vascular tone changes stemming from alterations within the local environment. Therefore any dysfunction associated with cellular sampling of the luminal environment, inappropriate signal transduction, or altered metabolic by-product production may lead to disturbances in vascular reactivity, blood distribution, or maintenance of tone. A reduction in reactivity could lead to local or systemic alterations in blood pressure and blood flow leading to devastating effects to downstream tissues. While described thoroughly below, microvascular ENM deposition would likely be highest in the arterioles, due to the combined influences of the endothelial surface layer, hydrodynamics, and bifurcations within this level.
Endothelium and the Endothelial Surface Layer
The intima is a dynamic system, comprised of a single layer of endothelial cells which serve to line the collective interior of the vasculature. This cellular lining is imperative for normal vascular function and homeostasis. It acts as a sensor, a physical barrier, a selectively permeable barrier, and a critical component of the inflammatory system by opposing leukocyte adherence until such trafficking is necessary6. Most importantly, the endothelium is capable of producing and secreting cyto- and chemokines. These include inflammatory markers, growth molecules, and modulators of vascular tone fundamental for maintenance of vascular tone (myogenic and sympathetic) and the appropriate distribution of blood; overall, acting as an autocrine, paracrine, and endocrine organ7.
As with many other cell types, endothelial cells are coated with a glycocalyx. The luminal glycocalyx of endothelial cells is particularly unique in that it physically and chemically interacts with the plasma; and as such, plays a vital role in processes such as endocrine/paracrine signaling, mechanotransduction, leukocyte trafficking, and permeability. The glycocalyx thickness has been highly studied and reported to be as thick as ~500 nm8. This measurement is highly variable and dependent on numerous factors including (but not limited to): vascular segment, luminal mechanics and measurement techniques. While considerable, this variation is not a primary concern as ENM (and their agglomerates in general) are well below 500 nm. As such, ENM should be physically quite able to interact with the glycocalyx from its outermost layer that interacts with the cell-free layer of plasma, to its innermost layer where proteoglycans, glycoproteins, and glycolipids project from the endothelial membrane into the membrane. Moreover, it is equally if not more important to recognize that a considerable body of evidence exists that a much thicker structure exists beyond the glycocalyx that is immediately adjacent to flowing blood and consists of adsorbed plasma constituents9. This layer, in conjunction with the glycocalyx, has been measured in the range of 500 nm to greater than 1 µm10, 11, and is collectively referred to as the endothelial surface layer (ESL).
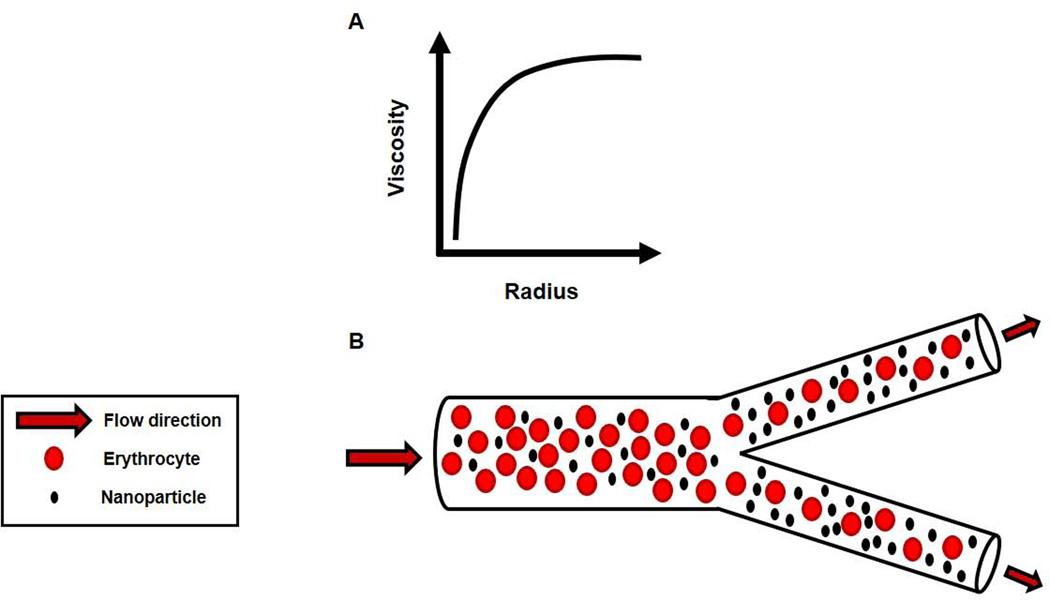
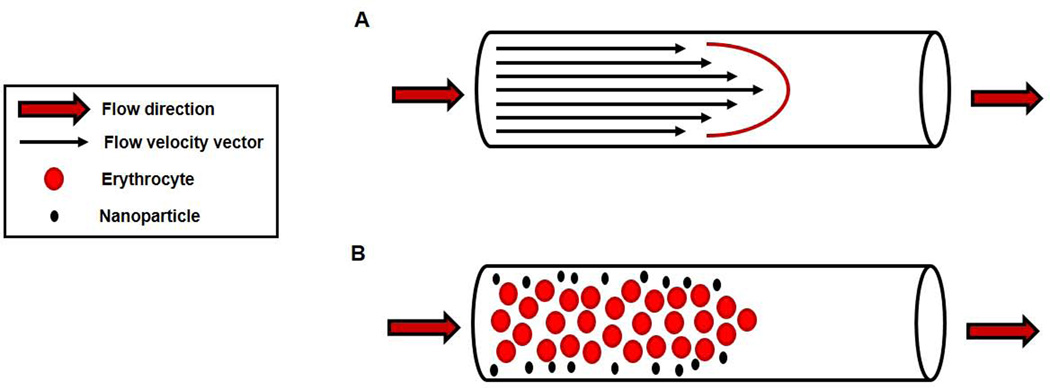
The ESL is an ideal environment for ENM deposition; however, neither the ESL nor the determinants of blood flow are homogeneous across the vascular network. This may be the very essence of ENM vascular distribution. At first consideration, it seems natural that ENM deposition should occur to a greater extent in the large arteries and veins, or the macrocirculation in general based on its tissue mass. However, blood flow has a greater tendency for turbulent or non-laminar flow in the macrocirculation. This would tend to keep ENM mixed well with erythrocytes, leukocytes, platelets and the various solutes typically found in the plasma. Thus, at the macrovascular level, ENM are suspended and contained within the plasma, but they have yet to be distributed and/or deposited. Vascular diameter decreases towards the capillaries; at a threshold of ~300 µm, a greater propensity for laminar flow exists in the microcirculation. This is an important property in terms of two physical effects: the Fahraeus effect12, and the Segre-Silberberg effect13. The Fahraeus effect describes the behavior of blood in decreasing microvessel diameter; wherein, hematocrit progressively deceases towards the capillaries. Considered on a per unit area perspective in these segments, plasma ENM concentration would increase as hematocrit decreases (FIGURE 1). In this case, ENM concentration would increase with decreasing vessel radius because the forces and anatomy, or the decreased blood velocity will not implicitly influence ENM suspended in the plasma. Alternatively, if ENM are bound to the cellular contents (RBC and other flowing cells) in the plasma, it is entirely possible that ENM concentration may decrease as hematocrit decreases. The Segre-Silberberg effect, in conjunction with Poiseuille’s law describes the parabolic flow profile of suspensions following a laminar velocity profile. Here, erythrocytes moving at higher velocities display a tendency to remain in the flow centerline; whereas, ENM, smaller particles and/or non-cellular elements tend to migrate or be forced laterally towards the vessel wall (FIGURE 2). Given that these two effects occur almost exclusively at the microvascular level, it is highly likely that the majority of ENM distribution exists in the collective microvascular compartment of any given tissue/organ. After vessel wall deposition, it is improbable that the majority of ENM are subsequently translocated across the endothelial, basement membrane, smooth muscle, and adventitia layers of the vessel to the intercellular space between cells or within the intracellular compartment of a given tissue/organ (e.g. within hepatocytes, myofibrils, neurons).
FIGURE 1.
Fahraeus effect. A) Relationship between blood viscosity and microvessel diameter. B) As radius decreases, viscosity decreases because erythrocyte concentration, or hematocrit is decreasing per unit volume. Note that effect begins in microvessels ~<300 micrometers. Components are not drawn to scale, but for illustrative purposes.
FIGURE 2.
Segre-Silberberg effect, and Poiseuille flow. A) Red cell velocity under laminar flow conditions creates a parabolic flow profile. B) Flow profile favors larger plasma solutes concentrating around the centerline, and smaller plasma solutes tend to move towards the microvessel wall. Note that effect begins in microvessels ~<300 micrometers. Components are not drawn to scale, but for illustrative purposes.
While the above forces and structures seem paramount to describing ENM distribution, the bound state may be of equal or greater importance at any given time point after accessing the vascular compartment. In this regard, two states exist: unbound or bound. Acutely, in the unbound state ENM should tend to remain suspended in the plasma and therefore, largely within the microcirculation. However, with increasing time, the likelihood that unbound ENM extravasate to the extravascular space increases due to bulk flow principles. Conversely, in the bound state, ENM distribution would largely be dependent on the cellular fate. For example, ENM binding by and large would occur with RBC and could remain within the vascular compartment for the lifespan of the cell (up to 4 months).
The capacity for ENM to impact vascular tone and reactivity are relatively well known, and highly studied for a variety of reasons. It is outside the scope of this review to fully address all these topics. However, given the previous discussion that highlighted fundamental biophysical differences between macrovascular and microvascular segments, an opportunity exists to briefly address how such anatomical and biophysical differences may contribute to disparate or inconsistent reports in the literature. Depending on the ENM, and/or exposure route, alterations in endothelium-dependent and –independent vascular reactivity occur. The endothelium is a single layer of cells that line the entire cardiovascular system. An average endothelial cell is ~ 0.3 µm thick9, and this does not change throughout the various segments of the vascular tree. However, the medial smooth muscle layer of the aorta is approximately 1 mm; while the range of arteriolar smooth muscle layers typically range within tens of micrometers or less. Such a tremendous disparity of the endothelium-to-medial wall thickness ratios between macrovessels and microvessels is likely to be at the root of differential observations in the ENM literature.
While the literature regarding the ESL is limited, a measureable outcome of endothelial damage and/or dysfunction after ENM exposure has been established at multiple levels of the vasculature14. This can be considered the first step in cardiovascular disease, this is especially important as the endothelial dysfunction may be reversible at this point. ENM deposition within this layer would allow for direct cell-ENM interaction, possibly resulting in an increase in reactive oxygen species, localized oxidative stress, and oxidative scavenge15. A primary mediator is nitric oxide gas (NO), an endothelium-derived hyperpolarizing factor widely considered a primary regulator of vascular reactivity. NO molecules are produced within the endothelial cell, but act upon the vascular smooth muscle stimulating a cellular cascade leading to smooth muscle relaxation and vascular dilation16. NO production and bioavailability are crucial as a measure for endothelial function. Therefore, any reduction in NO bioavailability, limiting vascular reactivity, may be an indicator of endothelial dysfunction.
There are a number of theories regarding how ENM deposition may reduce local NO bioavailability. The majority revolves around direct interaction or redox-active radical scavenging ENM (e.g. cerium oxide)17,18. In this case, the redox potential of the ENM or established protein corona may act to scavenge NO, thereby reducing bioavailability and impairing vasodilation. It is further attractive to speculate that ENM trapped in the ESL would be strategically located to influence NO bioavailability.
Lymphatics
Cardiac output in a healthy adult is approximately 7200 liters of blood per day. At the initial capillary level, ~20 liters of fluid per day is forced from the vasculature and enters the interstitial space as ultrafiltrate. By the end of the capillaries, ~17 liters of this ultrafiltrate has been reabsorbed back into the vascular compartment. The remaining ~3 liters of filtered fluid must constantly be collected, and ultimately returned to the vascular compartment proper or a catastrophic osmotic imbalance will result19. This return of excess filtered fluids is achieved by the lymphatic system. In addition to this primary function, the lymphatic system also plays a major role in host defense mechanisms, fat absorption and protein retention20. Despite the obvious importance of these critical functions in mammalian homeostasis, little to no information exists on ENM lymphatic distribution and influence.
The lymphatic system originates within tissues as bulbs or sacs formed by endothelial cells. This gives rise to tubes that are roughly larger than capillaries, and considered together, are referred to as the initial lymphatics. The lymphatic system as a whole operates under low pressure and flow (relative to the systemic circulation), which would favor ENM deposition/retention in it. ENM that have been filtered and not reabsorbed, or have accessed the extravascular compartment by other means would likely move via bulk flow into the initial lymphatics (as do other solutes in the fluids that regularly exit the intercellular space). The initial lymphatics converge towards the heart, and give rise to larger vessels referred to as the collecting lymphatics. These vessels are comprised of endothelial and smooth muscle cells. In conjunction with unidirectional valves, the vessels display phasic and tonic contractility that pumps lymph back into the blood via a connection just above the right atrium20. The collecting lymphatics and subsequent structures in the system respond to neural stimulation, paracrine stimuli, and hormonal/circulating factors. Because of its functional and structural similarities with other microvascular tissues, and common proximities, the lymphatics are not only subject, but may contribute, to inflammatory end-points associated with ENM exposure, but they may also contribute to a greater degree. We speculate that this may be the case as filtered ENM in this compartment would: a) present in higher concentrations than the systemic vasculature due to their pulsatile, low pressure, b) remain longer because of their low flow, c) have greater opportunity to interact with neutrophils, and d) contribute to the chemical broadcasting of inflammatory signals to the greater systemic circuit. At the time of preparing this review, no studies existed that directly explore these possibilities. However, computer modeling has recently been used to support the concept that inhaled ENM translocate to the systemic lymphatics21.
Hydrodynamics
Translocation
It is generally acknowledged that ENM translocation and biodistribution occur. This is the concept that ENM leave the system of original exposure or contact and migrate, through various mechanisms; that they are not absolutely contained within the primary exposure location (e.g., the pulmonary system after inhalation). Considerable evidence exists that suggests after initial exposure, nanomaterials have the propensity to biodistribute and accumulate throughout the system, presumably to the conditioning organs2, 3, 22, 23 possibly initiating a localized inflammation24. The notion that ENM preferentially deposit in conditioning organs, or organs responsible for treating the blood to maintain homeostasis (e.g. lungs, liver, kidney), is not based on their inherent importance or unique function; but rather the fact that these organs consistently see the greater portions of cardiac output. In other words, we speculate that ENM organ deposition is proportional to tissue blood volume flow. In the past, smaller non-fibrous nanomaterials were found in, but limited to the brain25, lung, liver, spleen, kidney, and heart26. More recently, with the advancements of fibrous nanomaterials, MWCNT translocations to major systemic organs (brain, heart, kidney, liver, diaphragm) have also been described2, 22. While the percentage of the lung burden that migrates is seemingly low, these migrations from the pulmonary system occur within 24-hours of exposure and have been confirmed nearly a year post-exposure2, 22, 27. It has been reported that regardless of the exposure route, cellular interactions and material functionalization may lead to translocation across phagocytotic and non-phagocytotic cellular membranes28. These findings support the hypothesis of direct particle interaction or localized inflammation due to particle injury could predispose a tissue to vascular dysfunction29.
Unique Considerations Influencing Vascular Distribution
Bifurcations
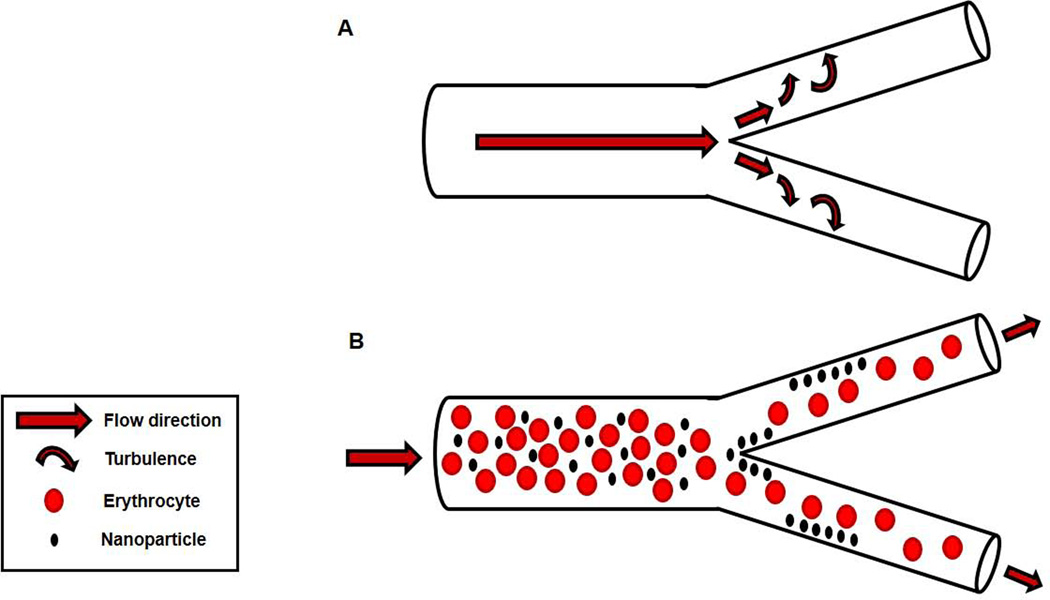
With respect to vascular nanomaterial distribution, the torrid conditions at large, conduit artery bifurcations create eddy currents (FIGURE 3). These eddies would favor ENM impaction in the vascular wall, depositing within the ESL, similar to that of cholesterol at the carotid bulb30.
FIGURE 3.
Bifurcation-dependent generation of turbulent flow. A) Blood flow into a bifurcation divides blood flow into two daughter arteries; this division also generates turbulent blood flow, or eddy currents that impacts on the arterial wall. B) Blood flow into a bifurcation and resultant turbulent blood flow impaction deposits ENM on the arterial wall.
In contrast, the local vascular characteristics specific to the microcirculation would directly lead to the widespread distribution of nanomaterials throughout a tissue of interest (rather than impacted in a few turbulent prone zones). The slow blood flow and low blood pressure within the capillaries favor nanomaterials to settle. Recent work has detailed the specific requirements for renal excretion31, including assessments of size and particle coatings. Overall, to prevent toxic accumulation, ENM must be less than 5.5 nm to allow for efficient renal filtration and excretion31. Due to particle agglomeration and protein interaction, it is plausible that the majority of ENM within the microcirculation will be larger than this criterion. Therefore, the majority of ENM would reside (and be trapped) within the microcirculation and/or impair glomerular filtration.
Aspect Ratio
As with the toxicities associated with pulmonary exposures, the aspect ratio of the nanomaterial needs to be taken into consideration in the context of nanomaterials and laminar flow. Concerns revolving around smaller ENM (TiO2, C60) involve ENM agglomeration leads to organ accumulation that initially and eventually affects vascular hemodynamics. Alternatively, these ENM may initiate local cellular dysfunction associated with direct interaction stemming from redox potentials. Whereas, an ENM with a high-aspect-ratio (CNT, nanobelts, nanowires) are more likely to interrupt blood flow, disturb signaling patterns, and initiate endothelial cell dysfunction. Locally, a single high-aspect-ratio fiber possesses the potential to pierce the cell membrane under conditions of turbulent flow or bifurcation and deposit in the vessel wall. Dependent on length, this fiber may also disrupt laminar flow at the site of impact and, therefore to downstream tissues; while a more gross consideration would be agglomeration of these foreign materials within the vessel, or an ENM-induced thrombosis leading to reduced blood flow, ENM thrombus formation, vascular occlusion, and ischemia of downstream tissues.
Protein Corona
An additional consideration for the distribution of nanomaterials to the vascular environment is a “protein corona” or protein covering over the surface of the nanomaterial. The protein corona can differ based on the ENM size, shape, composition, and functionalization state, thereby changing the ENM interaction, size, and toxicity32, 33. Additionally, these protein interactions can be described as “hard” or direct interaction with the ENM or “soft”, a secondary protein layer. With respect to the ENM and biological implications, these coatings may inhibit intended action through the prevention of targeted binding or an activated immune response32, 34. These varying protein corona may have vascular consequences as well, lead to a disruption of signaling patterns (specifically those associated with inflammation, leukocyte adhesion and rolling, and sensing of shear stress), endothelial dysfunction, and increase in thrombosis due to ENM aggregation. The hard protein corona may have a strong effect on ENM distribution and deposition, as the soft corona seems more transient at this time. The bound hard corona may prevent bound ENM from migrating further due to size or hydrophobic/hydrophobic state, the ENM may be effectively stuck either within the microvasculature (due to size) or interstitial compartment (due to size or functionalization). How the protein corona influences ENM interactions and deposition within the ESL remains to be determined, but it would appear likely that interactions between the soft corona and outer ESL are most likely.
ENM Functionalization
Up to this point we have discussed ENM vascular distribution and deposition as a point of consideration and awareness; however, functionalization, future programmability, and intentional intravenous exposure of ENM highlight the potential for biomedical use. As opposed to the in vivo modifications associated with the formation of a protein corona, functionalization of an ENM can be achieved by adding a functional group to the surface of the ENM (e.g. carboxyl, iron, amines, polyethylene glycol (PEG), etc) thereby altering the physiochemical parameters associated with the ENM (e.g. charge, shape, pH, solubility, etc). Currently, the greatest biomedical promise is perhaps the systemic use of ENM as imaging and contrasting agents; however, use as drug-delivery vehicles, targeting or directing release is also on the imminent horizon. In this application, consideration and manipulation of the endothelial glycocalyx and ESL may play a role in pharmaceutical ENM deposition. For example, magnetofluorescent nanomaterials have been shown to preferentially deposit at sites of atherosclerosis due to macrophage interactions35. While this outcome demonstrated an enhancement of plaque imaging via florescence microscopy and magnetic resonance imaging, pharmaceutical impregnation of the ENM could also have therapeutic results35.
In Vitro
Many in vitro and computational models have been designed to evaluate the ENM toxicokinetics within the vascular circulations, mimicking vascular bifurcations36, vascular adhesion and impaction5, and cellular interactions33. Overall the transition between in vitro and in vivo studies, specifically with respect to experimental conditions, remains to be properly addressed3. While these studies continue to provide the necessary framework and substantiation of evidence, we must continue to address the additive effect of dynamic systems found within the unique environment of the microcirculation.
Considerations and Alternate Models
With the advancements in biomedical nanomaterial engineering, a shift in perception has occurred regarding routes of possible exposure and susceptible populations. The perceived “norm” of healthy male workers exposed via pulmonary exposures during an occupational exposure is no longer universally applicable. Therefore, alternative routes of ENM exposure and models must be considered with respect to cardiovascular distribution and toxicity37.
Alternate Routes
Injections
Within the realm of biomedical applications, ENM injections of ENM are intuitively a systemic exposure. Some of these interactions may be more acutely contained or “localized” (subcutaneous/ intraperitoneal/ intramuscular), however over time all will lead to systemic interactions via clearance through the lymph and/or blood.
Intravenous injections of MWCNT are designed to mimic those associated with biomedical applications of the material (contrasting agents, drug delivery vehicles, etc26). This type of contact also leads to systemic exposure to and organ accumulation of the injected material via vascular distribution. It was also noted that the tissues mount a tissue specific “nanoparticle-induced inflammatory response” in reaction to this direct interaction and deposition38; this response is more detrimental with materials of a higher aspect ratio38. Evaluation of alveolar regions of the lung after pulmonary exposures document MWCNT deposition and penetrations39; it is reasonable to suspect that the endothelial vascular lining would suffer the same fate after intravenous exposure, ENM deposition, and speculated penetration. These types of intravenous exposures have led to additional pertinent questions: (1) if the inflammation remains locally around the deposition site or if it can spillover to a systemic reaction, due to the systemic exposure, (2) what is the timeline of the inflammatory response, and (3) is there an eventual clearance of the injected particles?
Implantable Devices
Nanomaterials have also been considered within the biomedical community for advancements of implantable devices, such as stents, pumps, pacemakers40, 41. These coatings could lead to an additional avenue for exposure. The material is essentially deposited upon implantation; therefore, any degradation of the coating would either enter the blood (luminal side of a stent) or the lymph (implantable pump). However, prior to degradation, it is crucial to consider the vascular implications of the microenvironment surrounding the implanted devices. If coated entirely by ENM, the implant could be entirely surrounded by a protein corona. It plausible that the corona may initiate a systemic inflammatory cascade (or host response), similar to that of tissue rejection, as the proteins interacts with the implanted (and possibly functionalized) ENM coating. These corona or inflammatory cascade could initiate platelet activation, increasing thrombogenic activity, in an already compromised setting42. While the possibilities are exciting and novel, the long term implications of the interactions must first be determined.
Alternate Models
Females and Pregnancy
The role of gender in virtually all biomedical research is commonly avoided due to complexities associated with reproductive physiology. This has created a gender disparity in our overall understanding of numerous female outcomes, and toxicology is no exception. Exposure during pregnancy and use of a female model has led to distinct areas of research associated with ENM distribution: (1) uterine development during pregnancy and (2) placental development and function.
Pregnancy and successful gestation demands rapid uterine vascular remodeling, growth, and functional changes specific to the macro- and microvascular compartments to support fetal development43. Uterine dysfunction at any level is capable of influencing fetal development and/or health. Secondly, the development of the placenta, a transient fetaomaternal vascular organ, is crucial during successful gestation. Placental blood flow, uncharacteristically turbulent in a microvascular environment, would seem to predispose for ENM impaction and deposition/retention.
Recently, groups have recently demonstrated translocation of PEG-SWCNTs to the tissues supporting fetal development (placenta and yolk sac) after IV injection and zinc oxide nanomaterials were distributed to the liver and kidney within the fetal tissues after maternal ingestion44, 45. These materials have also been measured in the mammary tissue or milk of lactating dams45, 46. Some groups have also initiated investigations of female fertility after MWCNT exposure45, 47 however these studies are in their infancy. We have reported that, ENM exposures during gestation can lead to significant microvascular dysfunction in maternal and fetal vessels48. It is unclear at this time if this dysfunction persists into adulthood and/or creates a foundation for the development of adult disease. Given the far reaching potential of this possibility, future studies and resources must be expanded and escalated in this area.
Comorbidities
While the literature is sparse, considerations and future studies should also better explore if any pre-existing cardiovascular compromise or confounding variable, must also be taken into account. These include models of pre-existing disease (e.g. hypertension, diabetes, obesity, and atherosclerosis) and age. Hypertension, or an increase in blood pressure, would directly influence the laminar flow, alter the glycocalyx and endothelial surface layer, and ENM impaction at bifurcation sites49. The progression and end stages of diabetes could reduce systemic ENM distribution, as vasculopathy progresses. Obesity is a unique condition, with respect to ENM deposition. Adipose tissue has only recently been recognized as an endocrine organ, with the release of adipokines50. The signaling patterns associated with obesity, satiety, and consumption are not well defined; ENM may influence these signaling molecules.
The progression of each of the above disease models includes the development of microvascular rarefaction, a reduction of vascular density, which only serves to exacerbate pathology through reduced perfusion51–53. To compensate for this reduction in blood flow, blood pressures are increased to drive more blood to ischemic area. With respect to nanomaterial distribution, this reduction of vascular density would concentrate the ENM within a tissue of interest; in combination with an increased pressure, turbulent flow would persist, increasing ENM deposition and impaction in the remaining microvessels.
With respect to atherosclerosis, magnetofluorescent nanomaterials injected into a mouse model of atherosclerosis demonstrated an enhancement of plaque via magnetic resonance imaging and florescence microscopy35. While valuable for biomedical imaging, this preferential deposition in combination with turbulent flow and ENM impaction may potentially accelerate the rupture of a vulnerable plaque. In general ENM and comorbidity studies are limited at this point, but seemingly aim toward acceleration of a disease state or exacerbation of a pre-existing condition.
SUMMARY
Numerous studies exist that indicate ENM translocate to systemic organs. It remains to be shown exactly where within the vasculature and a related organ that ENM distribute. We have provided fundamental biophysical properties that are observed primarily at the microvascular level that would favor ENM deposition in microvascular tissue, rather than the intracellular space of organs. This possibility must be fully studied to determine exactly where ENM are depositing in a specific organ (e.g. are the ENM simply trapped in the microcirculation of an organ of interest because of biophysical predisposition, or have they truly migrated into the organ because of its unique function?).
Given the potential for ENM to gain access to the vascular compartments and beyond, there are clear understudied areas within the literature. These topics include: (1) the ESL, glycocalyx, and protein corona interactions which may lead to ENM physical or chemical compartmentalization leading to direct endothelial contact, (2) the lymphatic compartment and how ENM confinement may affect systemic inflammatory outcomes, (3) the hormonal variations found within females may have protective effects as found with other models of exposure54, and (4) the additive effect of co-morbidities and ENM exposure which may exacerbate a delicate physiological balance. While research focusing on advancement of nanotechnology is vital, studies directly addressing these physiological possibilities should be considered, at the very least, an equal priority.
Reference List
- 1.Schwartz J, Morris R. Air pollution and hospital admissions for cardiovascular disease in Detroit, Michigan. Am J Epidemiol. 1995;142:23–35. doi: 10.1093/oxfordjournals.aje.a117541. [DOI] [PubMed] [Google Scholar]
- 2.Mercer RR, Scabilloni JF, Hubbs AF, Wang L, Battelli LA, McKinney W, Castranova V, Porter DW. Extrapulmonary transport of MWCNT following inhalation exposure. Part Fibre Toxicol. 2013;10:38. doi: 10.1186/1743-8977-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev. 2011;40:1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 4.Freis ED. Studies in hemodynamics and hypertension. Hypertension. 2001;38:1–5. doi: 10.1161/01.hyp.38.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Hossain SS, Zhang Y, Liang X, Hussain F, Ferrari M, Hughes TJ, Decuzzi P. In silico vascular modeling for personalized nanoparticle delivery. Nanomedicine (Lond) 2013;8:343–357. doi: 10.2217/nnm.12.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ley K, Reutershan J. Leucocyte-endothelial interactions in health and disease. Handb Exp Pharmacol. 2006:97–133. doi: 10.1007/3-540-36028-x_4. [DOI] [PubMed] [Google Scholar]
- 7.Inagami T, Naruse M, Hoover R. Endothelium as an endocrine organ. Annu Rev Physiol. 1995;57:171–189. doi: 10.1146/annurev.ph.57.030195.001131. [DOI] [PubMed] [Google Scholar]
- 8.Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann Biomed Eng. 2012;40:828–839. doi: 10.1007/s10439-011-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 10.van Haaren PM, VanBavel E, Vink H, Spaan JA. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physiol. 2003;285:H2848–H2856. doi: 10.1152/ajpheart.00117.2003. [DOI] [PubMed] [Google Scholar]
- 11.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- 12.Fahraeus R. The suspension stability of the blood. Physiological Reviews. 1929;9:241–274. [Google Scholar]
- 13.Segre G, Silberberg A. Radial particle displacements in poiseuille flow of suspensions. Nature. 1961;189:209–210. [Google Scholar]
- 14.Meng J, Yang XD, Jia L, Liang XJ, Wang C. Impacts of nanoparticles on cardiovascular diseases: modulating metabolism and function of endothelial cells. Curr Drug Metab. 2012;13:1123–1129. doi: 10.2174/138920012802850056. [DOI] [PubMed] [Google Scholar]
- 15.Koren E, Torchilin VP. Drug carriers for vascular drug delivery. IUBMB Life. 2011;63:586–595. doi: 10.1002/iub.496. [DOI] [PubMed] [Google Scholar]
- 16.Rapoport RM, Murad F. Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J Cyclic Nucleotide Protein Phosphor Res. 1983;9:281–296. [PubMed] [Google Scholar]
- 17.Karakoti A, Singh S, Dowding JM, Seal S, Self WT. Redox-active radical scavenging nanomaterials. Chem Soc Rev. 2010;39:4422–4432. doi: 10.1039/b919677n. [DOI] [PubMed] [Google Scholar]
- 18.Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int. 2013;2013:942916. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zawieja D, Weld P, Gashev A. Microlymphatic Biology. In: Tuma RF, Duran WN, Ley K, editors. Handbook of Physiology: Microcirculation. San Diego: Elsevier; 2008. pp. 125–158. [Google Scholar]
- 20.Kesler CT, Liao S, Munn LL, Padera TP. Lymphatic vessels in health and disease. Wiley Interdiscip Rev Syst Biol Med. 2013;5:111–124. doi: 10.1002/wsbm.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolanjiyil AV, Kleinstreuer C. Nanoparticle mass transfer from lung airways to systemic regions--Part II: Multi-compartmental modeling. J Biomech Eng. 2013;135:121004. doi: 10.1115/1.4025333. [DOI] [PubMed] [Google Scholar]
- 22.Stapleton PA, Minarchick VC, Cumpston AM, McKinney W, Chen BT, Sager TM, Frazer DG, Mercer RR, Scabilloni J, Andrew ME, Castranova V, Nurkiewicz TR. Impairment of coronary arteriolar endothelium-dependent dilation after multi-walled carbon nanotube inhalation: a time-course study. Int J Mol Sci. 2012;13:13781–13803. doi: 10.3390/ijms131113781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokel RA, Tseng MT, Dan M, Unrine JM, Graham UM, Wu P, Grulke EA. Biodistribution and biopersistence of ceria engineered nanomaterials: size dependence. Nanomedicine. 2013;9:398–407. doi: 10.1016/j.nano.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Reddy AR, Krishna DR, Reddy YN, Himabindu V. Translocation and extra pulmonary toxicities of multi wall carbon nanotubes in rats. Toxicol Mech Methods. 2010;20:267–272. doi: 10.3109/15376516.2010.484077. [DOI] [PubMed] [Google Scholar]
- 25.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- 26.Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews IP, Gregory CJ, Aljayyoussi G, Morris CJ, McDonald I, Hoogendoorn B, Gumbleton M. Maximal extent of translocation of single-walled carbon nanotubes from lung airways of the rat. Environ Toxicol Pharmacol. 2013;35:461–464. doi: 10.1016/j.etap.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Lacerda L, Russier J, Pastorin G, Herrero MA, Venturelli E, Dumortier H, Al-Jamal KT, Prato M, Kostarelos K, Bianco A. Translocation mechanisms of chemically functionalised carbon nanotubes across plasma membranes. Biomaterials. 2012;33:3334–3343. doi: 10.1016/j.biomaterials.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Stapleton PA, Goodwill AG, James ME, Brock RW, Frisbee JC. Hypercholesterolemia and microvascular dysfunction: interventional strategies. J Inflamm (Lond) 2010;7:54. doi: 10.1186/1476-9255-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liepsch D, Pflugbeil G, Matsuo T, Lesniak B. Flow visualization and 1- and 3-D laser-Doppler-anemometer measurements in models of human carotid arteries. Clin Hemorheol Microcirc. 1998;18:1–30. [PubMed] [Google Scholar]
- 31.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty IB, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundqvist M. Nanoparticles: Tracking protein corona over time. Nat Nanotechnol. 2013;8:701–702. doi: 10.1038/nnano.2013.196. [DOI] [PubMed] [Google Scholar]
- 33.Shannahan JH, Brown JM, Chen R, Ke PC, Lai X, Mitra S, Witzmann FA. Comparison of nanotube-protein corona composition in cell culture media. Small. 2013;9:2171–2181. doi: 10.1002/smll.201202243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shannahan JH, Lai X, Ke PC, Podila R, Brown JM, Witzmann FA. Silver nanoparticle protein corona composition in cell culture media. PLoS One. 2013;8:e74001. doi: 10.1371/journal.pone.0074001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaffer FA, Nahrendorf M, Sosnovik D, Kelly KA, Aikawa E, Weissleder R. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging. 2006;5:85–92. [PubMed] [Google Scholar]
- 36.Tan J, Shah S, Thomas A, Ou-Yang HD, Liu Y. The influence of size, shape and vessel geometry on nanoparticle distribution. Microfluid Nanofluidics. 2013;14:77–87. doi: 10.1007/s10404-012-1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stapleton PA, Minarchick VC, McCawley M, Knuckles TL, Nurkiewicz TR. Xenobiotic particle exposure and microvascular endpoints: a call to arms. Microcirculation. 2012;19:126–142. doi: 10.1111/j.1549-8719.2011.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Podila R, Shannahan JH, Rao AM, Brown JM. Intravenously delivered graphene nanosheets and multiwalled carbon nanotubes induce site-specific Th2 inflammatory responses via the IL-33/ST2 axis. Int J Nanomedicine. 2013;8:1733–1748. doi: 10.2147/IJN.S44211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Schwegler-Berry D, Castranova V, Porter DW. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part Fibre Toxicol. 2010;7:28. doi: 10.1186/1743-8977-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mooney E, Mackle JN, Blond DJ, O'Cearbhaill E, Shaw G, Blau WJ, Barry FP, Barron V, Murphy JM. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials. 2012;33:6132–6139. doi: 10.1016/j.biomaterials.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 41.Karagkiozaki V, Vavoulidis E, Karagiannidis PG, Gioti M, Fatouros DG, Vizirianakis IS, Logothetidis S. Development of a nanoporous and multilayer drug-delivery platform for medical implants. Int J Nanomedicine. 2012;7:5327–5338. doi: 10.2147/IJN.S31185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karagkiozaki V, Karagiannidis PG, Kalfagiannis N, Kavatzikidou P, Patsalas P, Georgiou D, Logothetidis S. Novel nanostructured biomaterials: implications for coronary stent thrombosis. Int J Nanomedicine. 2012;7:6063–6076. doi: 10.2147/IJN.S34320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osol G, Moore LG. Maternal uterine vascular remodeling during pregnancy. Microcirculation. 2013 doi: 10.1111/micc.12080. [DOI] [PubMed] [Google Scholar]
- 44.Campagnolo L, Massimiani M, Palmieri G, Bernardini R, Sacchetti C, Bergamaschi A, Vecchione L, Magrini A, Bottini M, Pietroiusti A. Biodistribution and toxicity of pegylated single wall carbon nanotubes in pregnant mice. Part Fibre Toxicol. 2013;10:21. doi: 10.1186/1743-8977-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jo E, Seo G, Kwon JT, Lee M, Lee B, Eom I, Kim P, Choi K. Exposure to zinc oxide nanoparticles affects reproductive development and biodistribution in offspring rats. J Toxicol Sci. 2013;38:525–530. doi: 10.2131/jts.38.525. [DOI] [PubMed] [Google Scholar]
- 46.Sumner SC, Fennell TR, Snyder RW, Taylor GF, Lewin AH. Distribution of carbon-14 labeled C60 ([14C]C60) in the pregnant and in the lactating dam and the effect of C60 exposure on the biochemical profile of urine. J Appl Toxicol. 2010;30:354–360. doi: 10.1002/jat.1503. [DOI] [PubMed] [Google Scholar]
- 47.Hougaard KS, Jackson P, Jensen KA, Sloth JJ, Loschner K, Larsen EH, Birkedal RK, Vibenholt A, Boisen AM, Wallin H, Vogel U. Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in mice. Part Fibre Toxicol. 2010;7:16. doi: 10.1186/1743-8977-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stapleton PA, Minarchick VC, Yi J, Engels K, McBride CR, Nurkiewicz TR. Maternal engineered nanomaterial exposure and fetal microvascular function: does the Barker hypothesis apply? Am J Obstet Gynecol. 2013;209:227–211. doi: 10.1016/j.ajog.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hennerici M, Burrig KF, Daffertshofer M. Flow pattern and structural changes at carotid bifurcation in hypertensive cynomolgus monkeys. Hypertension. 1989;13:315–321. doi: 10.1161/01.hyp.13.4.315. [DOI] [PubMed] [Google Scholar]
- 50.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145–1158. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- 51.Henrich WL. Approach to volume control, cardiac preservation, and blood pressure control in the pre-end-stage renal disease patient. J Am Soc Nephrol. 1998;9:S63–S65. [PubMed] [Google Scholar]
- 52.Gilbert RE. Endothelial loss and repair in the vascular complications of diabetes: pathogenetic mechanisms and therapeutic implications. Circ J. 2013;77:849–856. doi: 10.1253/circj.cj-13-0236. [DOI] [PubMed] [Google Scholar]
- 53.Frisbee JC, Delp MD. Vascular function in the metabolic syndrome and the effects on skeletal muscle perfusion: lessons from the obese Zucker rat. Essays Biochem. 2006;42:145–161. doi: 10.1042/bse0420145. [DOI] [PubMed] [Google Scholar]
- 54.Prisby RD, Muller-Delp J, Delp MD, Nurkiewicz TR. Age, gender, and hormonal status modulate the vascular toxicity of the diesel exhaust extract phenanthraquinone. J Toxicol Environ Health A. 2008;71:464–470. doi: 10.1080/15287390701839349. [DOI] [PubMed] [Google Scholar]