Abstract
Background
While diabetes has been linked to several cancers in the gastrointestinal (GI) tract, findings have been mixed for sites other than colorectal and liver cancer. We used the Women's Health Initiative (WHI) data and conducted a comprehensive assessment of associations between diabetes and GI malignancy (esophagus, stomach, liver, biliary, pancreas, colon and rectal).
Methods
145,765 postmenopausal women ages 50-79 enrolled in the WHI were followed for a mean 10.3 years. Cox proportional hazard regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between GI cancers and diagnosed diabetes, including its duration and treatment.
Results
Diabetes at enrollment was associated with increased risk for liver (HR = 3.00 95% CI 1.68-5.36), pancreatic (HR=1.64 95% CI: 1.16-2.33), colon (HR=1.37 95% CI: 1.13-1.65) and rectal (HR=1.90, 95%CI: 1.24-2.90) cancer. Diabetes severity, assessed by duration or need for pharmacotherapy, appeared to have stronger links to risk of liver, pancreatic and rectal cancer, but not colon cancer. There was no statistically significant association of diabetes with biliary, esophageal and stomach cancers.
Conclusion
Type 2 diabetes is associated with a significantly increased risk of cancers of the liver, pancreas, colon and rectum in postmenopausal women. Diabetes severity may further increase risk of pancreatic, liver and rectal cancer.
Impact
This study confirmed that diabetes increases risk of cancers of the liver, pancreas, colon and rectum. The suggestion that diabetes severity further increases these cancer risks requires future studies.
Background
Globally the prevalence of type 2 diabetes mellitus has been rapidly growing, and has become a major public health problem (1). Whereas cardiovascular complications are well-known in patients with diabetes, increasing evidence suggests that diabetic patients are also predisposed to developing many types of cancer (2, 3). Hyperinsulinemia acting through aberrations in the insulin-like growth factor pathways and steroid hormone metabolism, but also through independent mitogenic actions, is the main mechanism suggested to explain the association between diabetes and cancer (4). Several gastrointestinal tract (GI) cancers have been linked to diabetes, including colorectal cancer (5, 6), pancreatic cancer (7), and liver cancer (8, 9); however, a major question is whether diabetes independently increases risk for these cancers, or whether the diseases simply share common risk factors, as many studies have not thoroughly accounted for potential confounders (10). How risk of these cancer types is influenced by diabetes severity, diabetes treatment, or the duration of diabetes has not been thoroughly investigated.
The association of diabetes with other sites of cancer in the GI tract also is unclear. Studies on association of diabetes with biliary tract cancer have been mixed. Three have shown an excess risk for biliary cancer in patients with diabetes (11-13); one was a register-based study which was unable to adjust for most potential confounders (11), and two were composed mainly of men (12, 13). Another two studies examining diabetes did not observe an association with biliary cancer (14, 15). Similarly, the relationship between diabetes and esophageal or gastric cancer has not been established (13, 14).
In this large prospective study using data from the Women's Health Initiative (WHI) (16), we conducted a comprehensive assessment of the association between diabetes and risk of primary GI malignancies, including esophageal, stomach, liver, biliary tract, pancreas, colon, and rectal cancer. Our analyses included detailed information on potential confounders, treatment status, and duration of diabetes, and relied on central coding of all cases.
Materials and methods
Women's Health Initiative
The WHI was designed to address major causes of morbidity and mortality in postmenopausal women (16), and included both clinical trials (CT) and an observational study (OS). Details of the scientific rationale, eligibility requirements, and baseline characteristics of the participants in the WHI have been published elsewhere (17-21). Briefly, a total of 161,808 women aged 50 to 79 were recruited at 40 clinical centers throughout the United States between September 1, 1993 and December 31, 1998. The WHI CT includes four overlapping components: two Hormone Therapy Trials (27,347 women), a Dietary Modification Trial (48,835 women), and a Calcium/Vitamin D Supplementation Trial (36,282 women). Participants in the OS included 93,676 women who were screened for the CT but proved to be ineligible or unwilling to participate or were recruited through a direct invitation for the OS. The study was overseen by ethics committees at all 40 clinical centers and at the coordinating center, as well as by a data and safety monitoring board. All participants in the WHI gave informed consent and were followed prospectively.
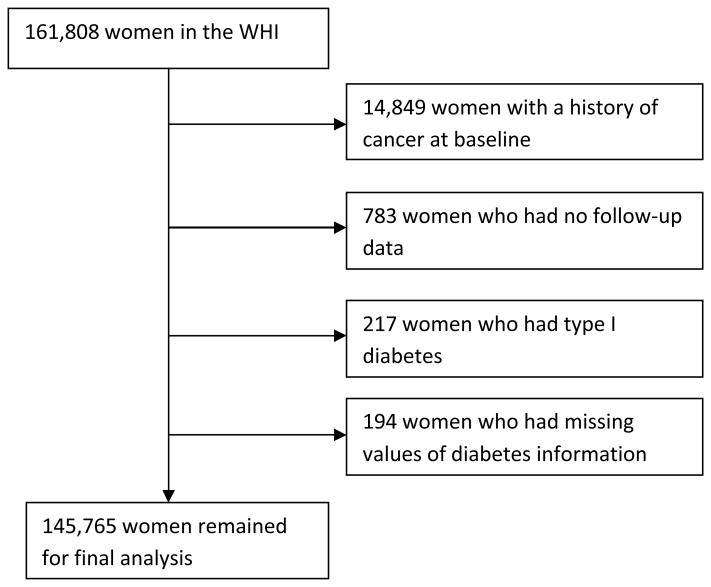
The following participants were excluded from the original cohort of 161,808 for this analysis: 14,849 women who had a history of cancer (except non-melanoma skin cancer) at baseline; 783 women who had no follow-up time; 217 women who were diagnosed with diabetes below age 20 or who were ever hospitalized for diabetic coma were considered to have type 1 diabetes; and 194 women who had missing values of the main exposures (including diabetes, age of diabetes onset and diabetes treatment). Our findings are based on the remaining 145,765 women who comprised our study cohort (Figure 1).
Figure 1. Summary of inclusion and exlusion criteria, and final cohort used for analysis.
Measurement of exposures, confounders and outcomes
Diabetes
Diabetes at enrollment (prevalent diabetes) was defined as a positive answer to a question “did a doctor ever say that you had sugar diabetes or high blood sugar when you were not pregnant”. Gestational diabetes was not included in our study. Treated diabetes (yes, no) at enrollment was defined as whether the participant reported ever being treated for diabetes with pills or insulin shots. Diabetes was further categorized as no diabetes, diabetes-not treated with medication, diabetes-treated with oral medications only, and diabetes-treated with insulin (either alone or in combination with oral medications). The duration of diabetes at enrollment was calculated as the difference between age when first diagnosed with diabetes and age at enrollment.
Incident diabetes was also determined during the study follow-up period based upon a positive response regarding newly prescribed treatment for diabetes with pills or insulin shots on either the semi-annual or annual WHI follow-up questionnaires. A validation study using medical record review has shown that 92% of prevalent diabetes and 82% of incident diabetes in WHI was confirmed, and that evidence of diabetes was found in only 5% of women who did not self-report it (Karen L. Margolis, unpublished data).
Confounders
The potential confounders considered in multivariable analyses included age at enrollment (<55, 55-59, 60-64, 65-69, 70-74, ≥75), ethnicity (American Indian or Alaska Native, Asian or Pacific Islander, Black or African-American, Hispanic/Latino, non-Hispanic white, and other), education (high school or less, some college/technical training, college or some post-college, and master's degree or higher), smoking status (never, former, current), body mass index (<18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, 35.0-39.9, >=40), waist-to-hip ratio (quintile), physical activity as (metabolic equivalent tasks [METs] per week: <5, 5-<10, 10-<20, 20-<30, 30 or more), alcohol intake (non-drinker, past drinker, <1drink per month, 1 drink/month-<1 drink/wk, 1-<7 drinks/wk, 7+drinks/wk), total daily energy intake (quintile), percent of daily dietary calories from fat (quintile), history of hormone therapy use (none, estrogen alone, estrogen and progestin, mixed) and non-steroidal anti-inflammatory drugs use (NSAID) (yes, no). In order to be included in the medication inventory at baseline, the NSAID had to be used at least once a week and used for at least the last 2 weeks at the time of data collection. Additional risk factors were adjusted for specific individual cancers, including whether women ever had liver disease for liver cancer, gallstone disease for biliary cancer; and stomach or duodenal ulcer disease as a Helicobacter pylori surrogate in the gastric cancer model.
Follow-up and ascertainment of cases
Initial cancer reports were ascertained by self-administered questionnaires (every 6 months in the CT through 2005, and annually in the CT after 2005 and in OS), with all cases subsequently confirmed by medical record review. All GI cancer cases were then coded centrally in accordance with the Surveillance Epidemiology and End Results (SEER) coding guidelines. For these analyses participants were followed to first cancer diagnosis of interest, date of death, loss to follow-up (including non-participation in the extension of WHI after 2005), or August 14, 2009, whichever occurred first. The completion rate of annual questionnaires was 93% - 96% through 2005. Among all living participants, the participant rate to the extension was about 73%.
Statistical analysis
We evaluated baseline differences in sociodemographic and clinical characteristics according to diabetes status using a Chi-square test for categorical variables, and a t-test for continuous variables. Cox proportional hazards regression models were employed to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) in age-adjusted and multivariable-adjusted models. In the multivariable models, we adjusted for potential confounders as described above with and without adding obesity indices (BMI and waist to hip ratio). Different study cohorts (participation in OS or each CT, and different treatment assignments for each CT) were treated as strata in the model in order to take into account possible different baseline hazards in different sub-groups and any potential treatment effects.
The effect of exposure was examined employing different metrics. Our primary analysis was an evaluation of the effect of diabetes at time of study enrollment (prevalent diabetes), including its duration and treatment, on risk of individual GI cancers. In a secondary analysis, we considered all diabetes as an exposure, including incident diabetes newly occurring during WHI follow-up. In the analysis including both prevalent and incident diabetes, a time-dependent covariate in a Cox proportional hazards regression model was generated by taking account of changes in diabetes status during follow-up. The effect of treatment status was compared by entering multiplicative interaction terms in the models to compare untreated and treated diabetes, as well as treatment with oral agents only and treatment with insulin. Further, we compared diabetes treatment with metformin and non-metformin drugs. The effect of diabetes duration was compared by entering multiplicative interaction terms in the models to compare both categories (<10 years vs. >10 years) and continuous years of duration. The proportionality assumption was satisfied for all exposure variables of interest and potential confounding variables based on graphs of scaled Schoenfeld residuals (22), All statistical analyses were conducted using SAS (Version 9.2, SAS Institute, Cary, NC.)
Results
Baseline characteristics by diabetes status at enrollment are shown in Table 1. Compared to women with no diabetes, women with diabetes were significantly more likely to be older and have higher BMI, waist-to-hip ratio, physical inactivity, total daily energy intake, percent daily dietary calories from fat, and use of NSAIDS; and were significantly less likely to be white (non-Hispanic), have graduated from college, currently drink, ever have smoked, and have history of estrogen and progestin use. Women with diabetes were significantly less likely to report a family history of cancer and more likely to report a personal history of gallstones, liver disease, and stomach or duodenal ulcer. Among the 8,154 diabetic women, about 24.6% were receiving no diabetic pharmacologic treatment, 47.8% were using oral medications only and 27.6% were receiving insulin (Table 1). Overall, 12.7% were using metformin.
Table 1. Baseline characteristics of participants by diabetes status among 145,765 women at WHI enrollment.
| Variable | No Type 2-diabetes | Type 2 Diabetes | P value |
|---|---|---|---|
| Total number of women | 137,611 (94.4) | 8,154 (5.6) | |
| Age at baseline (mean, yrs) | 63.0 | 64.3 | <0.0001 |
| White, non-Hispanic-ethnicity (%) | 114,751 (83.4) | 5,318 (65.2) | <0.0001 |
| College graduate or above education (%) | 54,999 (40.0) | 2,219 (27.2) | <0.0001 |
| Body Mass Index (mean, kg/m2) | 27.7 | 32.1 | <0.0001 |
| Waist-to-hip ratio | 0.81 | 0.87 | <0.0001 |
| Physical activity (mean, METs/wk) | 12.6 | 9.2 | <0.0001 |
| Total energy intake (mean, Kcal/day) | 1627.5 | 1651.2 | 0.01 |
| Dietary percent calories from fat (%) | 32.6 | 35.0 | <0.0001 |
| Smoking status | <0.0001 | ||
| Never smokers | 69,652 (50.6) | 4,183 (51.3) | |
| Former smokers | 56,790 (41.3) | 3,287 (40.3) | |
| Current smokers | 9,433 (6.9) | 534 (6.6) | |
| Alcohol intake | <0.0001 | ||
| Non-drinker | 14,465 (10.5) | 1,477 (18.1) | |
| Past drinker | 23,551 (17.1) | 3,178 (39.0) | |
| Current drinker | 98,584 (71.6) | 3,403 (41.7) | |
| Non-steroidal anti-inflammatory drugs use (yes, %) | 25,918 (18.8) | 1,826 (22.4) | <0.0001 |
| History of hormone therapy use | <0.0001 | ||
| none | 58,689 (42.7) | 4,486 (55.0) | |
| estrogen alone | 40,854 (29.7) | 2,414 (29.6) | |
| estrogen and progestin | 30,193 (21.9) | 1,007 (12.4) | |
| mixed | 7,875 (5.7) | 247 (3.0) | |
| Family history of cancer (%) | 64,071 (46.6) | 3,631 (44.5) | <0.0001 |
| Personal history of: | |||
| Liver disease (yes, %) | 3,127 (2.3) | 244 (3.0) | <0.0001 |
| Gallstone disease (yes, %) | 20,955 (15.2) | 2,292 (28.1) | <0.0001 |
| Stomach or duodenal ulcer (yes, %) | 8,440 (6.1) | 716 (8.8) | <0.0001 |
| Diabetes Treatment Status | |||
| Not treated | NA | 2,008 (24.6) | |
| Oral drugs use alone | NA | 3,898 (47.8) | |
| Insulin (alone and with oral drugs) | NA | 2,248 (27.6) | |
| Metformin (yes) | NA | 1036 (12.7) |
Women with a self-reported diabetes diagnosis at enrollment had significantly increased age-adjusted risk for liver, pancreatic, colon and rectal cancer, but risk for esophageal or stomach cancer was not significantly different from non-diabetic women. Although diabetes was also associated with excess biliary tract cancer risk, the HR did not reach significance. The magnitudes of risk in multivariable models were slightly attenuated from those in age-adjusted models, especially when models with further adjusted for BMI and waist to hip ratio. However, overall results for each type of GI cancer remained consistent (Table 2). Further, we assessed whether the effects of diabetes on risk of GI cancer differed by obesity status and did not detect significant interaction effect for any type of GI cancer (data not shown). When the analysis was limited to women with treated diabetes at baseline, the point estimates of risk were higher for liver, pancreas and rectal cancer. Similar results were obtained when considering incident treated diabetes diagnosed during follow-up (data not shown).
Table 2. Hazard ratios (HRs) and 95% confidence intervals (Cls) for gastrointestinal cancers incidence associated with diabetes status *.
| Type of cancer | No diabetes | Diabetes at enrollment | Treated Diabetes at enrollment | ||||
|---|---|---|---|---|---|---|---|
| Cases | Cases | Model 1 HR (95% CI) 1 |
Model 2 HR (95% CI) 2 |
Model 3 HR (95% CI) 3 |
Cases | Model 3 HR (95% CI) 3 |
|
| Esophageal | 50 | 3 | 1.10 (0.34 – 3.53) | 1.01 (0.31 – 3.32) | 0.80 (0.24 - 2.66) | 1 | 0.32 (0.04 - 2.40) |
| Stomach | 114 | 7 | 1.07 (0.50 – 2.29) | 0.80 (0.37 – 1.74) | 0.75 (0.34 - 1.65) | 5 | 0.70 (0.28 - 1.75) |
| Liver | 66 | 17 | 4.26 (2.49 – 7.29) | 3.67 (2.08 – 6.47) | 2.96 (1.66 - 5.31) | 15 | 3.47 (1.87 - 6.45) |
| Biliary | 114 | 11 | 1.68 (0.90 - 3.13) | 1.52 (0.80 – 2.87) | 1.25 (0.65 - 3.39) | 11 | 1.77 (0.92 - 3.41) |
| Pancreas | 378 | 39 | 1.79 (1.29 - 2.49) | 1.80 (1.28 - 2.54) | 1.63 (1.15 - 2.32) | 35 | 1.99 (1.38 - 2.88) |
| Colon | 1393 | 128 | 1.60 (1.33 - 1.91) | 1.51 (1.25 - 1.82) | 1.36 (1.12 - 1.64) | 95 | 1.31 (1.06 - 1.63) |
| Rectum | 238 | 27 | 1.95 (1.31 - 2.91) | 1.91 (1.26 - 2.88) | 1.88 (1.23 - 2.87) | 25 | 2.38 (1.53 - 3.71) |
In the models of this column (model 1), we only adjusted for age (<55, 55-59, 60-64, 65-69, 70-74, ≥75).
In the models of this column (model 2), we adjusted for age (<55, 55-59, 60-64, 65-69, 70-74, ≥75), ethnicity (American Indian or Alaska Native, Asian or Pacific Islander, Black or African-American, Hispanic/Latino, non-Hispanic white, and other), education (high school or less, some college/technical training, college or some post-college, and master's degree or higher), smoking status (never, former, current), physical activity (metabolic equivalent tasks [METs] per week: <5, 5-<10, 10-<20, 20-<30, 30 or more), alcohol intake (non-drinker, past drinker, <1drink per month, 1 drink/month-<1 drink/wk, 1-<7 drinks/wk, 7+drinks/wk), total daily energy intake (quintile), percent of daily dietary calories from fat (quintile), history of hormone therapy use (none, estrogen alone, estrogen and progestin, mixed) and non-steroidal anti-inflammatory drugs use (yes, no). In addition, we adjusted for an additional variable (whether women ever had stomach or duodenal ulcer disease) for stomach cancer; an additional variable (whether women ever had liver disease) for liver cancer; and gallstone disease for biliary cancer.
In the models of this column (model 3), we further adjusted for body mass index (<18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, 35.0-39.9, >=40), waist-to-hip ratio (quintile) besides all covariates in model 2.
When the analyses was stratified by type of diabetes treatment (Table 3), only colon cancer was significantly associated with both treated and untreated diabetes, while liver, pancreas and rectal cancer were significantly associated only with treated diabetes. There were neither strong nor significant differences in excess risk according to type of diabetes treatment (oral only vs. any insulin) observed for any type of GI cancer. However, when diabetes treatment was separated into metformin or non-metformin, only pancreatic cancer was significantly associated with metformin treatment, while all listed cancers were significantly associated with diabetes with non-metformin treatment. For colon cancer, relative risk was not significantly elevated for metformin treatment, but the point estimate was similar to that of non-metformin treatment (Table 3). Similarly, diabetes of longer duration also had somewhat stronger links to liver, pancreatic and rectal cancer (Table 4). This was statistically significant in the models in which duration of diabetes was included as a continuous variable for pancreatic cancer (HR 1.03, 95% CI 1.01-1.06, p=0.002) and rectal cancer (1.04, 95% CI 1.01-1.07, p=0.006) (data not shown). Unfortunately, given the very small number of esophageal and stomach cancer cases, we were unable to similarly evaluate the effects of diabetes treatment and duration on disease risk.
Table 3. Hazard ratios (HRs) and 95% confidence intervals (Cls) for gastrointestinal cancers incidence associated with treatment of diabetes at enrollment *.
| Type of cancer | diabetes-not on medication | diabetes –on oral medications only | diabetes – receiving insulin | Diabetes with Metformin treatment | Diabetes with Non-Metformin treatment | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | cases | HR (95% CI) | cases | HR (95% CI) | |
| Liver | 2 | 1.45 (0.35 – 5.97) | 10 | 3.74 (1.84 - 7.62) | 5 | 3.13 (1.19 - 8.25) | 1 | 1.49 (0.20 – 10.97) | 14 | 3.78 (2.00 – 7.15) |
| Biliary | 0 | - | 7 | 1.63 (0.74 - 3.60) | 4 | 1.88 (0.67 - 5.26) | 0 | - | 11 | 2.04 (1.06 – 3.92) |
| Pancreas | 4 | 0.68 (0.25 - 1.83) | 20 | 1.74 (1.09 - 2.77) | 15 | 2.42 (1.41 - 4.15) | 10 | 3.38 (1.78 - 6.44) | 25 | 1.16 (1.08 – 2.53) |
| Colon | 33 | 1.45 (1.02 - 2.05) | 59 | 1.28 (0.98 - 1.67) | 36 | 1.42 (1.01 – 1.99) | 18 | 1.53 (0.96 – 2.45) | 77 | 1.26 (1.00 – 1.60) |
| Rectum | 2 | 0.56 (0.14 - 2.26) | 15 | 2.16 (1.26 - 3.73) | 10 | 2.73 (1.41 - 5.29) | 2 | 1.17 (0.29 - 3.79) | 23 | 2.53 (1.60 - 4.00) |
In each multivariable model, we adjusted for age (<55, 55-59, 60-64, 65-69, 70-74, ≥75), ethnicity (American Indian or Alaska Native, Asian or Pacific Islander, Black or African-American, Hispanic/Latino, non-Hispanic white, and other), education (high school or less, some college/technical training, college or some post-college, and master's degree or higher), smoking status (never, former, current), body mass index (<18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, 35.0-39.9, >=40), waist-to-hip ratio (quintile), physical activity (metabolic equivalent tasks [METs] per week: <5, 5-<10, 10-<20, 20-<30, 30 or more), alcohol intake (non-drinker, past drinker, <1drink per month, 1 drink/month-<1 drink/wk, 1-<7 drinks/wk, 7+drinks/wk), total daily energy intake (quintile), percent of daily dietary calories from fat (quintile), history of hormone therapy use (none, estrogen alone, estrogen and progestin, mixed) and non-steroidal anti-inflammatory drugs use (yes, no). In addition, we adjusted for an additional variable (whether women ever had stomach or duodenal ulcer disease) for stomach cancer; an additional variable (whether women ever had liver disease) for liver cancer; and gallstone disease for biliary cancer.
Table 4. Hazard ratios (HRs) and 95% confidence intervals (Cls) for gastrointestinal cancers incidence associated with duration of diabetes at enrollment *.
| Duration of diabetes at enrollment | ||||
|---|---|---|---|---|
| Type of cancer | <10 years | 10 or more years | ||
| Cases | HR (95% CI) | Cases | HR (95% CI) | |
| Liver | 9 | 2.49 (1.19 - 5.21) | 8 | 3.77 (1.74 - 8.17) |
| Biliary | 6 | 1.05 (0.45 - 2.44) | 5 | 1.62 (0.65 - 4.05) |
| Pancreas | 20 | 1.32 (0.83 - 2.10) | 19 | 2.17 (1.35 - 3.50) |
| Colon | 83 | 1.39 (1.10 - 1.75) | 45 | 1.31 (0.97 - 1.77) |
| Rectum | 16 | 1.72 (1.02 - 2.91) | 11 | 2.17 (1.16 - 4.04) |
In each multivariable model, we adjusted for age (<55, 55-59, 60-64, 65-69, 70-74, ≥75), ethnicity (American Indian or Alaska Native, Asian or Pacific Islander, Black or African-American, Hispanic/Latino, non-Hispanic white, and other), education (high school or less, some college/technical training, college or some post-college, and master's degree or higher), smoking status (never, former, current), body mass index (<18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, 35.0-39.9, >=40), waist-to-hip ratio (quintile), physical activity (metabolic equivalent tasks [METs] per week: <5, 5-<10, 10-<20, 20-<30, 30 or more), alcohol intake (non-drinker, past drinker, <1drink per month, 1 drink/month-<1 drink/wk, 1-<7 drinks/wk, 7+drinks/wk), total daily energy intake (quintile), percent of daily dietary calories from fat (quintile), history of hormone therapy use (none, estrogen alone, estrogen and progestin, mixed) and non-steroidal anti-inflammatory drugs use (yes, no). In addition, we adjusted for an additional variable (whether women ever had stomach or duodenal ulcer disease) for stomach cancer; an additional variable (whether women ever had liver disease) for liver cancer; and gallstone disease for biliary cancer.
Discussion
In this large prospective study of postmenopausal women followed for ∼10 years, diabetes mellitus was significantly associated with an increased risk for liver, pancreatic, colon and rectal cancers. In particular, women with treated diabetes or longer duration of diabetes appeared to have greater risk of liver, pancreatic and rectal cancer. Although relatively few women were treated with metformin, it was significantly associated with increased pancreatic risk. However, we cannot evaluate the associations for other GI cancer sites (i.e. biliary, esophagus and stomach) due to lack of statistical power.
Accumulating of experimental and epidemiological evidence suggests that hyperinsulinemia may be the underlying mechanism to explain the association between diabetes and cancer. Insulin could be directly related to risk by promoting tumor proliferation; or it could affect risk by modulating circulating levels of growth factors and their binding proteins or by competing with their specific receptors in target tissues (23). Insulin activates IGF-I receptor, known to have growth-promoting effects (4). Thus, reduced insulin sensitivity with compensatory hyperinsulinemia leads to an elevated level of IGF-I and stimulation of cell proliferation. Excess insulin could also reduce the level of IGF-BP1 with resultant increases in the levels of circulating free, bioactive IGF-I. The IGF-I could act as a growth stimulus in preneoplastic and neoplastic cells (4).
Previous observational studies have also reported that diabetes is associated with an increased risk for liver, pancreatic and colorectal cancer (2, 5-8), though questions remained about the validity of these observed relationships. One concern is that the association between diabetes and cancer risk may simply reflect common underlying risk factors (e.g. excess body weight, diet, and/or lack of physical activity). Moreover, few studies were able to adjust for important confounding factors such as waist circumference or waist-hip ratio, a better measure of visceral fat than BMI.(10) In our study, adjustment for BMI or adiposity did not materially alter the diabetes-cancer relations observed indicating that confounding by adiposity is unlikely to account for these associations. However, our study was unable to adjust for Hepatitis B virus infection (HBV) and Hepatitis C virus infection (HCV), two important risks for liver cancer, which may have confounded the observed diabetes-liver relationship. However, according to Walker AM's finding (24), the degree of confounding is very low even though both exposure-confounder odds-ratio (ECOR) and confounder -disease relationship (RRC) are very stronger (for example, 1.4 apparent relative risk caused by confounding requires both ECOR and RRC are larger than 3). Thus, our observed association between diabetes and liver cancer is unlikely explained by confounding of HBV and HCV.
Another concern is that some of the observed associations may be due to early chronic disease that may influence glucose homeostasis. For example, the diabetes-liver cancer association may be due to reverse causality. End stage liver disease itself can cause glucose intolerance and overt diabetes (25) and diabetes has been associated with a spectrum of liver disease (26, 27), ranging from nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH) and cirrhosis, which enhance susceptibility to liver cancer (28-30). Although it is difficult to establish clearly that diabetes indeed preceded any underlying chronic liver disease in our cohort the findings of the diabetes-liver cancer relationship were not materially altered when the analysis was further adjusted for self-reported liver disease at baseline. In addition, another risk factor for liver cancer, HCV, may itself cause diabetes. However, HCV is linked to a lower proportion of liver cancer than diabetes (30). Similarly, abnormal glucose metabolism due to pancreatic cancer may also explain the apparent diabetes-pancreatic cancer association observed by us and others. However, a positive association between diabetes and pancreatic cancer risk has been found when restricted to diabetes that precedes the diagnosis of pancreatic cancer by at least 5 years (31). Our finding of an increased risk associated with diabetes 10 years or more years suggests that reverse causation is unlikely to explain the association.
Whether diabetes is associated with biliary tract cancer is controversial. An elevated risk of biliary tract cancer has been suggested in some (11-13, 32), but not all studies (14, 15, 33). Our study noted a significant increase in biliary tract cancer risk only among diabetic women who were treated with non-metformin drug therapy. The relationship between diabetes and esophageal or gastric cancer is also uncertain (13, 14). There was a significantly decreased risk for esophageal cancer among men reported in one study (13) and a significantly increased risk for gastric cancer among women reported in another study (14). However, as in our study, the majority of studies did not find an association for either cancer, perhaps due to the small number of cases (32, 33).
Our data suggest that diabetes requiring pharmacologic treatment had stronger links to liver, pancreatic and rectal cancer, but not to colon cancer. Diabetes requiring pharmacologic treatment may be a proxy for more severe or longer duration diabetes, which may further increase cancer risk. Our data did not show the risk of any GI cancer to differ between type 2 diabetes patients receiving insulin and oral medication only; but suggested that longer duration of diabetes may increase the risk of liver, pancreatic and rectal cancer. Since longer duration of diabetes is correlated with the need for pharmacologic treatment, the independent role of these is not clear. Type 2 diabetes patients begin to require insulin therapy when there is a significant decline in endogenous insulin production. Similarly, insulin is more commonly prescribed in patients with a longer duration of type 2 diabetes and is used more often in those with one or more comorbid conditions that preclude the use of oral medications (2). In addition, once type 2 diabetes mellitus patients become insulin dependent, patient are treated with different analogues of insulin that may have different pharmacokinetic and pharmocodynamic profiles (34). For example, studies observed that patients with type 2 diabetes exposed to sulfonylureas and exogenous insulin had a significantly increased risk of cancer-related mortality compared with patients exposed to metformin (35, 36). This was suggested by our analyses stratified treatment into metformin and non-metformin. An increased risk of GI cancer was noted in patients with non-metformin treatment but not in patients with metformin treatment with an exception for pancreatic cancer. However, due to small women in metformin drug use group, power limits our ability to draw firm conclusions.
Strengths of our study include the prospective design, detailed information on exposure, central coding of cancer diagnoses, and information on potential confounders, including data on waist circumference and waist-to-hip ratio. However, there are some study limitations as well. Our results pertain to post-menopausal women who were generally well-educated, although the cohort is relatively diverse both geographically and racially. Diabetes status relied on self-report and no medical records were achieved. Thus some degree of under reported diabetes may be in our non-diabetes group, which may make our estimates more conservative. Further, it is possible that a few participants might have had diabetes type 1 rather than type 2. However, a validation study in the WHI has shown a high concordance of self-report with a gold standard based on medical record review and with medication inventories (37). In addition, our measure of diabetes duration is likely an underestimate, since women could have had undiagnosed diabetes for an unknown length of time. Further, the power for examining the association of diabetes with several rare cancers is low, as is power for examining the treatment and duration of diabetes for all cancers other than colon, and for addressing association of specific type of anti-diabetes drugs with specific cancer sites. In addition, the study was conducted in postmenopausal women, so our findings may not be generalized to other populations.
In conclusion, in postmenopausal women type 2 diabetes is associated with a significantly increased risk of cancers of the liver and pancreas, as well as colon and rectal cancer. The suggestion that diabetes severity, assessed by need for pharmacologic therapy and longer duration of diagnosis, further increase risk of pancreatic, liver and rectal cancer requires further study.
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. A short list of WHI investigators is given in an appendix.
Appendix
Short List of WHI Investigators
Program Office
(National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center
(Fred Hutchinson Cancer Research Center, Seattle, WA)
Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles Kooperberg; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers
(Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Haleh Sangi-Haghpeykar; (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence S. Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Erin LeBlanc; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O'Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael S. Simon.
Women's Health Initiative Memory Study
(Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
References
- 1.Stovring H, Andersen M, Beck-Nielsen H, Green A, Vach W. Rising prevalence of diabetes: evidence from a Danish pharmaco-epidemiological database. Lancet. 2003;362:537–8. doi: 10.1016/S0140-6736(03)14116-5. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2011;33:1674–85. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 364:829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 5.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 6.Berster JM, Goke B. Type 2 diabetes mellitus as risk factor for colorectal cancer. Arch Physiol Biochem. 2008;114:84–98. doi: 10.1080/13813450802008455. [DOI] [PubMed] [Google Scholar]
- 7.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–80. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 116:1938–46. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grote VA, Becker S, Kaaks R. Diabetes mellitus type 2 - an independent risk factor for cancer? Exp Clin Endocrinol Diabetes. 2011;118:4–8. doi: 10.1055/s-0029-1243193. [DOI] [PubMed] [Google Scholar]
- 11.Adami HO, Chow WH, Nyren O, Berne C, Linet MS, Ekbom A, et al. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472–7. doi: 10.1093/jnci/88.20.1472. [DOI] [PubMed] [Google Scholar]
- 12.Jamal MM, Yoon EJ, Vega KJ, Hashemzadeh M, Chang KJ. Diabetes mellitus as a risk factor for gastrointestinal cancer among American veterans. World J Gastroenterol. 2009;15:5274–8. doi: 10.3748/wjg.15.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011 doi: 10.1002/ijc.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166:1871–7. doi: 10.1001/archinte.166.17.1871. [DOI] [PubMed] [Google Scholar]
- 15.La Vecchia C, Negri E, Decarli A, Franceschi S. Diabetes mellitus and the risk of primary liver cancer. Int J Cancer. 1997;73:204–7. doi: 10.1002/(sici)1097-0215(19971009)73:2<204::aid-ijc7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 17.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 18.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S98–106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 19.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 20.Ritenbaugh C, Patterson RE, Chlebowski RT, Caan B, Fels-Tinker L, Howard B, et al. The Women's Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S87–97. doi: 10.1016/s1047-2797(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 21.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women's Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S78–86. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 22.Hess KR. Graphical Methods for Assessing Violations of the Proportional Hazards Assumption in Cox Regression. Statistics in Medicine. 1995;14:1707–23. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 23.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem. 2008;114:63–70. doi: 10.1080/13813450801954451. [DOI] [PubMed] [Google Scholar]
- 24.Walker A. An introduction to the methods of epidemiology. USA: Epidemiology Resources Inc.; 1991. Observation and Inference. [Google Scholar]
- 25.Petrides AS, Vogt C, Schulze-Berge D, Matthews D, Strohmeyer G. Pathogenesis of glucose intolerance and diabetes mellitus in cirrhosis. Hepatology. 1994;19:616–27. doi: 10.1002/hep.1840190312. [DOI] [PubMed] [Google Scholar]
- 26.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–62. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 27.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–10. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 28.Zen Y, Katayanagi K, Tsuneyama K, Harada K, Araki I, Nakanuma Y. Hepatocellular carcinoma arising in non-alcoholic steatohepatitis. Pathol Int. 2001;51:127–31. doi: 10.1046/j.1440-1827.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 29.Davila JA. Diabetes and Hepatocellular Carcinoma: What Role Does Diabetes Have in the Presence of Other Known Risk Factors? American Journal of Gastroenterology. 2010;105:632–4. doi: 10.1038/ajg.2009.715. [DOI] [PubMed] [Google Scholar]
- 30.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 26:2183–91. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 31.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. Jama-Journal of the American Medical Association. 2000;283:2552–8. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 32.Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360–5. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 33.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. American Journal of Epidemiology. 2004;159:1160–7. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 34.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 35.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–5. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulforrylureas or insulin. Diabetes Care. 2006;29:254–8. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 37.Margolis KL, Lihong Q, Brzyski R, Bonds DE, Howard BV, Kempainen S, et al. Validity of diabetes self-reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5:240–7. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]