Abstract
Recombinant adeno associated virus (rAAV) vectors are great tools for gene transfer due to their ability to mediate long-term gene expression. Recombinant AAVs have been used at various ages of development with no apparent toxicity. There are multiple ways of delivering AAV vectors to the CNS, depending on the stage of development of the mouse. In neonates, intravascular injections into the facial vein are often used. In adults, direct injections into target regions of the brain are achieved with great spatiotemporal control through stereotaxic surgeries. Recently, discoveries of new AAV vectors with the ability to cross the blood brain barrier have made it possible to also target the adult CNS by intravascular injections. rAAVs have been successfully used as gene transfer vehicles in multiple animal models of CNS disorders, and several clinical trials are currently underway.
Keywords: AAV, intracranial, intravascular, neonate, sterotaxic
INTRODUCTION
Adeno-associated virus (AAV) is a replication deficient DNA virus as a part of the Parvovirinae family in the Dependovirus genus. The single stranded 4.7kb DNA genome is composed of the rep and cap genes flanked by inverted terminal repeats (ITR). The AAV virion is a non-enveloped 20–25 nm icosahedral capsid composed of VP1, VP2, VP3 proteins encoded by the cap gene. AAV is highly prevalent in humans and other species, but to date there has been no disease associated with this virus (Afione et al., 1995). Recombinant AAV (rAAV) vectors for gene transfer are devoid of any coding sequences from the wild type virus and carry an ITR-flanked transcription cassette consisting of a promoter driving expression of a gene of interest and a polyadenylation signal. The ITR elements are necessary for vector genome replication and packaging during production in cell culture, and post-infection processing to generate stable transcriptionally active genomes in target cells. Recombinant AAV vectors have become one of the most widely used vehicles for CNS gene transfer because of their exceptional ability to mediate stable long-term gene expression in neurons with no apparent toxicity (Kaplitt et al., 1994). For these reasons these vectors have become part of a growing toolkit for studies of gene and cell function in the CNS, disease modeling and development of gene therapy approaches for neurodegenerative diseases. Importantly the CNS gene transfer properties of rAAV vectors appear relatively consistent across rodent species, and large animals such as cats, dogs, and monkeys (Asokan et al., 2012; Cotugno et al., 2011; Foust et al., 2010; Gagliardi and Bunnell, 2009; Gray et al., 2011). Clinical trials using rAAV are ongoing for different neurological diseases (Asokan et al., 2012), and the available data suggests that these vectors are capable of mediating long-term stable gene expression in the human brain as well (Hwu et al., 2012; Muramatsu et al., 2010).
The most commonly used technique to deliver rAAV vectors to the CNS is by direct infusion into the brain parenchyma using a stereotaxic instrument to guide needle placement. rAAV2 vectors were the first to be used for CNS gene transfer in animal models and human trials. Many subsequent studies have shown that rAAV vectors prepared with AAV1, AAV5, AAV8, AAV9, AAVrh10, and some other capsids are considerably more efficient than rAAV2 vectors for direct CNS gene transfer (Burger et al., 2004; Cearley et al., 2008; Cearley and Wolfe, 2006; Klein et al., 2006; Klein et al., 2008; Sondhi et al., 2007). Intraparenchymal infusion of rAAV vectors is the most effective approach to achieve structure specific gene transfer in the CNS, but is ineffective for widespread gene transfer as the vectors remain mostly localized to the injected structures. Recently rAAV9 and a few other vectors were shown to mediate widespread gene transfer to brain and spinal cord following systemic administration in neonatal and adult mice, cats and monkeys (Cotugno et al., 2011; Foust et al., 2009; Gray et al., 2011; Yang et al., 2012; Zhang et al., 2011b). Here we present protocols for stereotaxic intracranial injection of AAV vectors in adult mice, intravenous delivery of AAV vectors via the superficial temporal vein and tail vein in neonatal and adult mice, respectively.
BASIC PROTOCOL 1. Craniotomy and stereotaxic injection of AAV vectors into the brain of adult mice
The intracranial stereotaxic injection mode of delivery allows for targeting of specific structures in the adult mouse brain. Once mastered, it is a straightforward and safe way of delivering vector, with few if any side effects on the mouse. The limitation of stereotaxic injections is the requirement for specialized expensive equipment, time, and in some instances the need to validate the coordinates for each target structure in the mouse strain and age in the intended experiment as the available atlases were generated for adult C57BL/6 mouse brain (Paxinos & Franklin Mouse Brain Atlas C57Bl/6 adult male mouse, 26–30g (Paxinos and Franklin, 2001)). For this reason, it is recommended that a few pilot injections using a dye be done before embarking on large experiments, to make sure the intended structures are being correctly targeted. The titers for rAAVs injected intracranially are usually 1012–1013 vg/ml.
Materials
Xylazine (10 mg/kg)
Ketamine (100 mg/kg)
Sterile water
AAV vector to be injected (See Support Protocol 1)
Ophthalmic ointment (such as Puralube, Webster # 07-888-2572)
1ml syringes with permanent needle (Kendal Monojet #1188128012)
Small mouse shaver
Alcohol prep pads, 70% isopropyl alcohol
Povidone-Iodine prep pads
Sterile disposable scalpels (Dynarex # 4111)
Sterile cotton swabs (Puritan #25-806 1WC)
Hand held drill
Autoclaved drill bits
Autoclaved Hamilton gastight syringe, 10ul (Hamilton #7653-01)
Autoclaved Hamilton 33 gauge steel needles (Hamilton #7762-06)
Stereotaxic instrument (Stoelting 51730)
Injector (Ultra Micro Pump III; World Precision Instruments)
Syringe Pump controller (#SYS-MICRO4; World Precision Instruments)
Sterile wound reflex clip stapler (Kent Scientific #INS500345)
Sterile 9mm wound reflex clips clips (WPI 500346)
Wound reflex clip remover (Kent Scientific #INS500347)
Circulating water warming pad
Ketoprofen (100mg/ml)
Bottles of 10ml single dose 0.9% sodium chloride (Hospira #0409-4888-10)
Stereotaxic Atlas (Paxinos & Franklin, The Mouse Brain in Stereotaxic Coordinates (Paxinos and Franklin, 2001))
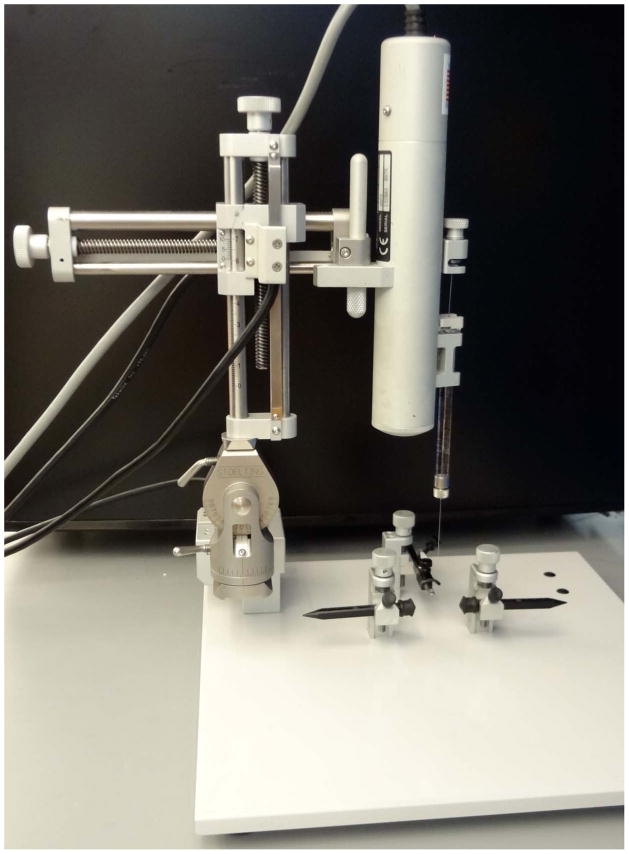
Before starting surgeries, set up the stereotaxic frame (FIG 1A) including loading the virus into the syringe, and attaching the syringe to the injector. The injection itself can also be done by hand by attaching the needle directly to the frame using a syringe holder. However, the manual approach is not compatible with a constant flow rate of delivery.
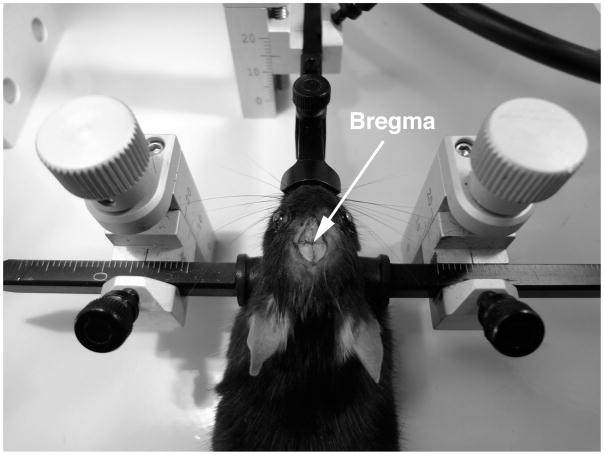
Figure 1.
Set up for stereotactic surgery. a) The stereotactic frame is assembled for surgery, and the injector and needle are attached attached. b) The animal is positioned in the stereotactic frame. The ear bars are positioned on either side of the head of the animal. The nose bar and ear bars are tightened securely. The head should be level and completely immobilized. The arrow indicates the bregma, the zero point for stereotactic coordinates.
The syringe and drill bits should be sterilized by autoclaving before use.
-
1
Anesthetize the mouse by intraperitoneal injection of the appropriate amount of ketamine and xylazine solution based on its body weight (see reagents and solutions section).
Ensure the animal is deeply anesthetized to prevent movement while in the frame. This is achieved when no movement is detected after a toe pinch. Ophthalmic ointment should be applied to the eyes at this point to prevent corneal drying. -
2
Shave the head of the mouse, removing as much hair as possible from between the ear and above the skull.
-
3
Place the mouse in the stereotaxic frame. First, scruff the animal, and hook the front teeth onto the hole in the bite grip of the stereotaxic frame. Next, gently pull back on the animal, position the ear bars in the ear canal*, and tighten, making sure that the skull is level. Lastly, gently bring the nose clamp down onto the nose. The animal’s head should now be firmly positioned in the frame. [FIG 1B]
To help with position the teeth onto the bite grip, it is sometimes helpful to use the stick end of the cotton swab to open the mouth of the animal. If hooked correctly, you should feel resistance while gently pulling back on the animal. Do not overtighten the nose grip. If the animal is properly positioned, gentle tapping on the head should not alter the positioning. -
3
Wipe the skin three times with povidone-iodine, followed by 70% alcohol.
-
4
Gently make an incision using a sterile scalpel. The incision should be large enough to give access to the area of the skull where burr holes will be made.
Use enough pressure to cut the skin, but not to cut into the skull. If you do get bleeding of the skull, dab with a cotton swab, and wait until bleeding stops. -
5
Using two sterile cotton swabs, gently separate the skin to expose the skull. Clean the exposed skull area with a sterile cotton swab.
Wait a few minutes for the skull to dry in order to better see the cranial sutures. [FIG 1C] Check again that the skull is level and properly aligned, and reposition if needed. -
6
Using the tip of the needle, measure the x and y coordinates of the bregma and use that as the zero point. Calculate the stereotaxic coordinates needed for the injection site. Move the needle over the injection site using the calculated coordinates.
The proper coordinates for the brain structures of interest can be determined using a stereotaxic atlas, but often need to be adjusted to take into account age and mouse strain. -
7
Use a small hand held drill to make a small burr hole (<1mm) in the skull over the injection site.
Apply gently downward pressure when drilling. Stop immediately once the drill bit has breached the skull, so as to not damage the dura or brain parenchyma. Some bleeding may occur if the dura or brain parenchyma is damaged. If that happens, use a cotton swab to dab the blood, and make sure the bleeding has stopped before injecting. -
8
Bring the needle back to the bregma and recalculate the coordinates needed for the injection. Bring the needle to the calculated x and y position, and lower into the burred hole, until the needle tip is just below the skull. This is your zero for the depth coordinates.
If the needle tip does not penetrate through the drilled hole, or if the hole does not correspond to the calculated coordinates, re-drill. -
9
Lower your needle the desired depth. Wait two minutes after the needle has been lowered, and then start the injection.
Infusion rates of 0.1–0.2 μl/min do not cause significant damage to the tissue. Higher infusion rates are often associated with reflux of the injectate along the needle track. -
10
After the injection has been completed, wait 2–5 minutes before raising the needle.
-
11
Remove the mouse from the frame, and close the incision by stapling the skin together with 9mm wound clips. Remove the wound clips 1 week later.
As an alternative, the incision can also be closed with sutures, such as Ethicon Monocryl 5–0 undyed 18″ sutures. -
12
Put the animal on a circulating water warming pad to keep it warm until it fully recovers from anesthesia and is able to regulate its own body temperature.
-
13
Once the animal has recovered, deliver ketoprofen subcutaneously (see reagents and solutions), and place the animal in a clean cage. Put wet food on the bottom of the cage. Monitor the animal for the next 48 hours. Deliver another dose of ketoprofen after 24 hours.
Dilute 100 ul of ketoprofen (100g/ml) into 10ml of saline. Deliver 5ul/g of body weight. -
14
AAV-mediated transgene expression in the brain can be detected as soon as 3–7 days after direct intracranial injection.
Basic Protocol 2. Tail vein injection of AAV vectors in adult mice
Systemic delivery of AAV vectors in adult mice can be easily accomplished by tail vein injection. This delivery modality gives access to all organs in the body, and gene delivery to the brain is dependent on the transduction properties (e.g. ability to cross the BBB and cell tropism) of the AAV capsid of choice. rAAV9 vectors have been shown to mediate efficient widespread gene transfer to CNS after systemic delivery in neonatal and adult mice, cats and monkeys. Multiple organs and tissues are transduced at high efficiency by intravenous delivery of rAAV9 vectors. This method is fast and relatively easy to master. Since the volume used should not exceed 200ul, the rAAV vector titers used for this approach should be high (~1x1013 vg/ml) depending on the desired rAAV vector dose/mouse.
Materials
AAV vector to be injected diluted up to 200 ul in sterile 1xPBS (See Support Protocol 2)
27 3/8G 1ml allergy syringes (Becton, Dickinson and Company, #305541)
Infrared heat lamp
Mouse restrainer for tail vein injection (such as Kent Scientific #HLD-MS-T)
Alcohol prep pads, 70% isopropyl alcohol
Sterile gauze
Place the animal to be injected in a clean cage under a heat lamp for 5 minutes to allow vasodilation.
Load the 27 3/8G syringe with 200ul of the virus to be injected.
Place the animal in the mouse restrainer, and wipe the length of the tail with a 70% isopropyl alcohol pad.
-
Hold the tail taut and identify the tail veins running on each side of the tail [FIG 2]. Insert the needle tip into the vein, bevel up. The vein is close to the skin, so the needle should be inserted superficially. Inject the AAV vector.
If the injection is done correctly, the vein will blanche as soon as the injection starts. Also, if the needle is in the vein the injection will proceed easily with little resistance. If the needle has been inserted incorrectly, remove the needle and try again on another location in the tail vein closer to the body. -
Remove the needle and apply compression to the injection site with sterile gauze to ensure complete hemostasis.
If the injection was done correctly, a few drops of blood will flow from the injection site when the needle is removed. Return the animal to its home cage and observe for 15 minutes before returning to the holding room.
Expression of the AAV transgene may be seen as soon as two weeks.
Figure 2.
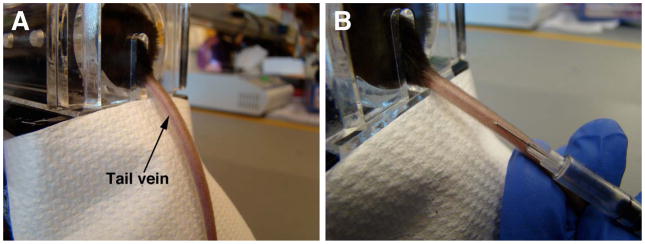
Intravenous delivery of rAAVs to adult mice. a) The animal is inserted into the mouse restrainer. The tail of the mouse is extended, and the tail vein is located. b) The needle is inserted bevel down, and parallel to the vein.
Basic Protocol 3. Intravenous delivery of rAAVs to neonatal mice
Intravenous injections are primarily used for peripheral tissue delivery but with the discovery of novel AAV capsids that can cross the blood brain barrier, intravenous injections can be used to deliver therapeutic rAAV vectors to the CNS of neonatal mice. The only vein that is accessible for neonatal intravenous injections is the superficial temporal/facial vein. This vein is visible just below the eye of the mouse as shown in Fig 3b. The method is inexpensive requiring very basic instruments although it will take considerable practice to master the technique. One major impediment is the final delivery volume cannot exceed 100ul, hence the rAAV vector titers used for this approach should be high (~1×1013 vg/ml).
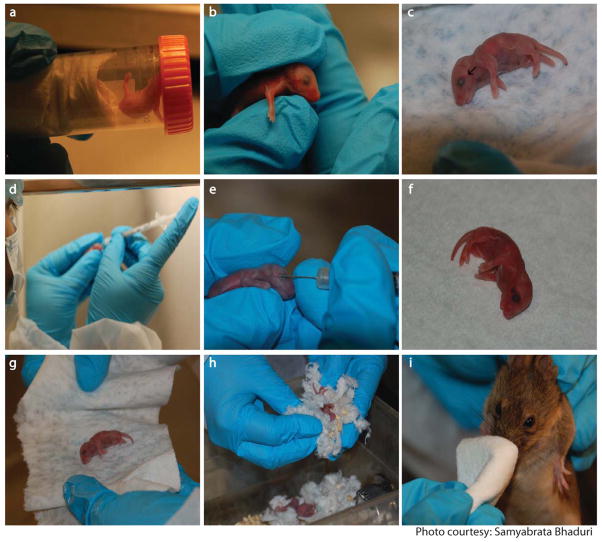
Figure 3.
Intravenous delivery of rAAVs to neonatal mice. Mouse pups are anesthetized in the makeshift anesthesia chamber containing isofluorane (a). Pups are positioned to expose the superficial temporal vein (b). Black arrow indicates the vein (c). Dosing syringe is positioned in dominant hand (d). Needle is slowly inserted into the vein and plunger is depressed (e). Blood flow is stemmed and appearance of a hematoma below the eye indicates successful injection (f). Pups are rolled in alcohol soaked tissue (g) followed by rubbing with dirty bedding (h) before putting them back in the cage. Dam is nose numbed with alcohol prior to introduction into the cage (i).
Materials
Isofluorane
AAV vector to be injected (see Support Protocol 2)
Anesthesia chamber for neonates made out of a 50ml Falcon tube stuffed with tissue paper
3/10 cc syringe with 31 gauge needle (8mm length needle) (such as: Becton, Dickinson and Company, Ultra-Fine II Short Needle Insulin Syringes)
-
Before anesthetizing pups, remove the dam from the cage and keep her out of sight of the procedure area. Add 1 ml of isofluorane to the tissue paper in the anesthesia chamber and tighten the cap to allow isofluorane to vaporize for 1 minute. Hold the chamber horizontally and place a pup inside and tighten the cap. Immediately remove the pup once it has completely stopped flailing its limbs.
Make sure that the pup is not left inside the chamber for too long because it might die. Be careful to avoid trapping the limbs of the pups in the lid. The pups usually remain anesthetized for 5 minutes. The pups lose color while anesthetized but become pink again as they regain consciousness. Before injection, check that the pup is alive by monitoring the rise and fall of rib cage. Hold the pup with less dominant hand, placing the thumb in the central axis of the pup’s body; position the index finger along the dorsal surface as shown in Fig 3b. The elbow should be resting on a surface. The vein to be injected is the superficial temporal vein (Figure 3c. black arrow).
Hold the needle (bevel facing upwards) with thumb and middle finger of dominant hand (resting the elbows on a surface) with the index finger poised over the plunger. Position the needle tip over the superficial vein at an angle of 10–20° before the vein bifurcates. Slowly insert the tip of the needle and depress the plunger with the index finger.
-
Slowly withdraw needle and immediately press the point of injection with absorbent gauze.
Successful injection is marked by blanching of the vein during injection followed by pulsatile flow of the injectate through the local vascular network and the appearance of a round hematoma (Fig 3f.) -
Once bleeding stops, rub the pup with a tissue lightly sprayed with 70% alcohol and then with the dirty bedding to reestablish their natural scent and put it back into the cage. Before re-introducing the dam into the cage, numb its nose with a tissue soaked in alcohol.
Nose-numbing prevents the dam from smelling foreign smells on the pups (blood, gloves etc) which eventually leads to rejection of the pups.
Support Protocol 1: Preparing rAAV Vector for Intracranial delivery
The following steps outline how to prepare a rAAV vector for delivery into a mouse, using a sterile Hamilton gastight syringe fitter with a 33G needle.
Materials
Sterile filtered 1x phosphate buffered saline
Autoclaved sterile gas-tight Hamilton syringe fitted with 33G needle
Sterile pipette tips
Sterile pipette
Sterile tubes
Surgical tools autoclaving pouch
Assemble the syringe and needle. Pull up 0.5M sterile NaOH into the syringe and let sit for ten minutes. Repeat 2 more times. Make sure there are no air bubbles in the syringe.
-
Flush the syringe with sterile water 25 times.
NaOH is used to destroy any vector left over from previous injections. Thus, it is very important that all NaOH is removed from the syringe, as any left over solution will destroy the vector to be used in the injection. Alternatively, designated syringes can be used for each virus, eliminating the need for a NaOH wash. Sterilize the syringe by autoclaving in a surgical tool autoclave pouch. Wrap the needle in aluminum foil to prevent it from puncturing the pouch.
Thaw rAAV vector on ice.
-
Calculate the total amount of vector to be used the day of the surgery. If needed, dilute the vector to the proper concentration using sterile PBS. Use sterile tubes, tips and pipettes. Work in a biosafety cabinet to maintain sterility of the AAV vector solution.
Vectors stocks should be stored at −80°C. Do not refreeze a vial once it has been thawed. Prepare fresh dilutions of virus each day of surgery. Suggested titers to be used are mentioned at the beginning of each Protocol. -
Remove the autoclaved syringe from the pouch and flush with sterile water. Pull up the prepared vector into the syringe. Make sure that there are no air bubbles in the syringe.
If bubbles are being pulled up, it is sometimes helpful to first spin down the tube containing the prepared vector.
Support Protocol 2: Preparing rAAV Vector for IV Delivery
The following steps outline how to prepare a rAAV vector for delivery into a mouse, via tail vein or facial vein injection.
Materials
Sterile filtered 1x phosphate buffered saline
Sterile pipette tips
Sterile pipette
Sterile tubes
Thaw rAAV vector on ice.
-
Calculate the total amount of vector to be used the day of the surgery. If needed, dilute the vector to the proper concentration in 100 or 200 μl total volume (if injecting neonates or adults, respectively) using sterile filtered PBS. Use sterile tubes, tips and pipettes.
The same volume of vector should be injected across all experimental groups. Hence the most dilute vector will determine the volume and maximum dose possible.Vectors stocks should be stored at −80°C, and should be used immediately. Do not refreeze a vial once it has been thawed. Prepare fresh dilutions of virus each day of injection.
Support Protocol 3: Collection of brain for histological analysis
The following steps outline how to perform a basic animal perfusion, and collection of the brain. Perfusions with paraformaldehyde (PFA) are useful in preserving mouse tissue for an extended period of time, while maintaining tissue integrity and structure. Collection of the intact brain is important for assessing parameters such as distribution of transduced cells, tissue inflammation, and neuropathology. However, it is important to know the downstream studies to be carried out in the brain, as PFA perfusion may interfere with some assays. In such cases, the animal can be perfused only with PBS, or the brain can be removed from a freshly sacrificed animal, and immediately frozen.
Materials
1x PBS
Freshly prepared 4% paraformaldehyde fixative solution [See Reagents and Solutions]
Anesthesia (Ketamine/Xylazine)
Surgical scissors
Flat tweezers
Curved hemostatic forceps
Scalp vein set (butterfly needle) 25¾G (Exel #26708)
Peristalic pump (Fisher #13-876)
Three way stopcock with attached tubing
15ml plastic conical tube
Note: Perform all steps in a fume hood!
-
1
Connect the peristalic pump to the cannulated needles, and set the ends of the valved tubing in ice cold PFA or PBS. [FIG 4]
-
2
Anesthetize the mouse by intraperitoneal injection of twice the amount of ketamine and xylazine anesthesia solution normally used for survival surgeries.
The perfusion must be started quickly after the animal is deeply anesthetized, as it must be done while the heart is still beating. -
3
Open the abdomen using surgical scissors, and cut up the sides until the diaphragm is visible.
-
4
Open the thoracic cavity by cutting through the diaphragm and then through the ribcage on each side. Be careful not to nick any organs, especially the heart. Use the retractor to flap back the ribcage and expose the heart.
-
5
Insert the cannula/needle into the left ventricle of the heart by entering at the apex and going straight up. Make a small incision into the right atrium.
-
6
Perfuse the mouse using the peristalic pump set at a constant slow flow rate (7ml/min) with ice-cold PBS for 3 minutes followed by ice-cold 4% paraformaldehyde fixative solution for 7 minutes.
If the perfusion is performed correctly, the liver will go from dark red to a beige coloring within the first minute of PBS flowing through the animal. It is normal to observe a tail twitch during the perfusion. If the perfusion was successful, the animal should be completely stiff at the end of the seven minutes. -
7
Separate the head of the animal from the rest of the body at the top of the spine.
-
8
Peel back the skin of the head until the whole skull is exposed. The peeled back skin can be used to get a good grip on the head.
-
9
Using small scissors, cut any excess tissue at the bottom of the skull. Then, using the flat scissors, gently peel away the top of the skull from the brain.
The skull should easily come off in small pieces. Position the tweezers up against the skull at the edges and push up. Be careful to not push the tweezers into the brain as it is soft and can be easily damaged. -
11
Once the top of the skull has been removed, carefully extract the brain, and store in a 15ml conical tube in 4% paraformaldehyde fixative solution overnight at 4°C.
-
12
The following day transfer the brain to 30% sucrose in 1X PBS, and store at 4°C until it has sunk to the bottom.
Depending on the application and purpose, the brain can be fixed using many different solutions, or simply fresh frozen. Thin sections of the brain (4–6 μm) are the preferred method for detailed neuropathological analysis of the brain, and require the tissue to be embedded in paraffin (not descried here). Cryostat sections of frozen brain tissue (fixed in paraformaldehyde or non-fixed fresh) are also commonly used in many applications.
Figure 4.
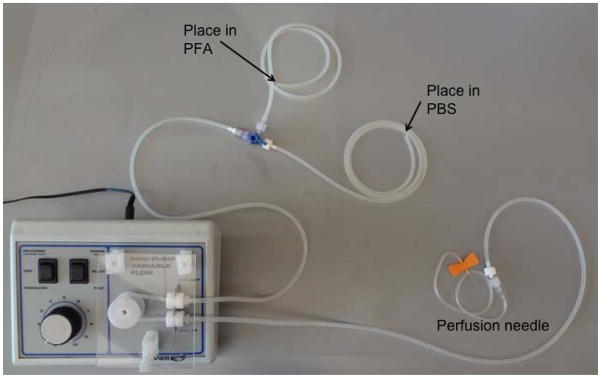
Set up of peristaltic pump for transcardiac perfusion. The tubing is wound around the peristaltic pump. The single end is connected to a cannulated needle. The two ends with the stopcock valve are placed in the PFA or PBS.
Support Protocol 4: Embedding brain for cryostat sectioning
The following steps outline how to embed a brain for cryostat sectioning. Embedding the brain is a good way to preserve it long term, while maintaining tissue integrity. It is also versatile as it allows for many different histological stains to be used post sectioning. However, the media used to embed should be chosen based on the post sectioning technique that will be used, as different section sizes require different embedding procedures.
Materials
1x PBS
Fume Hood
Paper towels
Optimal Cutting Temperature (OCT) media (such as Thermo Scientific’s NEG50)
Plastic disposable embedding molds (Electron Microscopy Sciences #70182)
2-O-Methyl butane
Dry ice, two buckets
Transfer pipettes
-
Set up a 2 O-Methylbutane/dry ice bath in a fume hood.
Put a layer of dry ice in the bottom of a bucket, and cover with 2 O-Methylbutane. The liquid should be almost covering all of the dry ice.
-
Fill a brain mold with OCT. Make sure there are no bubbles.
If bubbles form, use a transfer pipette to remove them. Take brain out of 30% sucrose solution after it has sunk, and rinse with 1xPBS. Dry completely by blotting on paper towels.
Put the brain in the OCT filled mold, with the olfactory bulb pointing up. Make sure the brain is perfectly straight. Remove any bubbles near the brain using a transfer pipette.
Place the mold containing the brain in the 2 O-Methylbutane/dry ice bath. The mold should sit level on the dry ice, and should be covered by liquid on all sides. Do not let any liquid go over the top of the mold.
Once all of the OCT has solidified, move the mold to the bucket with just dry ice, and let it dry.
Store the embedded brains at −80°C until they are ready to be cryosectioned.
REAGENTS AND SOLUTIONS
Ketamine/Xylazine anesthesia solution
| Xylazine (20mg/ml stock concentration) | 0.05ml (1mg/ml final concentration, 10mg/kg dose to mouse) |
| Ketamine (100mg/ml stock concentration) | 0.1ml (10mg/ml final concentration, 100mg/kg dose to mouse) |
| Sterile Isotonic Saline | 0.85ml |
| Total cocktail: | 1ml |
Dose of cocktail to mouse: 10 ul/g of body weight
| Weight (g) | Dose (ml) |
|---|---|
| 10 | 0.1 |
| 12 | 0.12 |
| 15 | 0.15 |
| 18 | 0.18 |
| 20 | 0.2 |
| 25 | 0.25 |
| 30 | 0.3 |
Use a xylazine stock of 20mg/ml and a ketamine stock of 100 mg/ml. Using strictly aseptic technique, take 0.1 ml ketamine + 0.05 ml xylazine + 0.85 ml of sterile saline. Place this mixture in a sterile, stoppered, drug-dosing bottle. Make fresh anesthesia each day of surgery.
Weigh the animal and calculate the dose of anesthetic to be administered. Use an insulin syringe to deliver the anesthetic by intraperitoneal injection. Check status of the animal ten minutes after injection, by toe pinch. If the animal is still responsive, administer another 10ul of anesthesia. Wait another ten minutes and check responsiveness again. If the animal is still responsive, deliver another 5ul of anesthesia.
Ketoprofen analgesia
| Ketoprofen (100mg/ml stock concentration) | 0.1ml (5mg/kg dose to mouse) |
| Sterile Isotonic Saline | 9ml |
| Total cocktail: | 1ml |
Dose per animal: 5ul/g of body weight.
Use a ketoprofen stock of 100mg/ml. Using strictly aseptic technique, remove 0.1ml from a bottle of sterile isotonic saline, so it now contains 0.9ml. Next, take 0.1 ml of ketoprofen and inject it into the 0.9ml bottle of sterile isotonic saline. Weigh the animal. Use an insulin syringe to deliver the anesthetic by subcutaneous injection.
Other acceptable analgesia: Acetaminophen, Acetylsalicylic Acid; Butorphanol; Codeine; Ibuprofen; Meperidine; Methadone; Morphine; Nalbuphine; Oxymorphone; Pentazocine.
4% Paraformaldahyde Fixative Solution
NOTE: All steps involving paraformaldehyde powder must be done in a fume hood.
| DI water (mL) | 750 | 375 | 188 |
| 10M NaOH (mL) | 0.600 | 0.300 | 0.150 |
| Paraformaldehyde powder (g) | 40 | 20 | 10 |
| 10X PBS (mL) | 100 | 50 | 25 |
| 12M HCl (mL) | Adjust to pH 7.2–7.4 | ||
| Final Volume (mL) | 1000 | 500 | 250 |
Place the DI water in a beaker and stir on a hot plate to approximately 100°C.
Add the 10M NaOH, and the paraformaldahyde powder.
Once all the powder has dissolved cool the solution to room temperature.
Add the 10X PBS.
Adjust the pH to 7.2–7.4 using 12M HCl.
Bring to final volume with DI water.
Store at 4°C wrapped in foil to protect from light.
COMMENTARY
Background Information
Recombinant AAV serotype 2 vectors were the first to be used for CNS gene transfer in animal models (Kaplitt et al., 1994) and human clinical trials for neurodegenerative diseases (Eberling et al., 2008; Janson et al., 2002; Kaplitt et al., 2007; Worgall et al., 2008). Until recently the only viable approach for AAV-mediated gene delivery to the CNS was based on direct intraparenchymal infusion as systemic infusion of AAV2 vectors proved to be ineffective at transducing cells in the CNS, presumably due to the blood brain barrier. Direct infusion of rAAV vectors into specific brain regions has proven extraordinarily powerful to probe gene and cell function in the CNS, and development of new therapies for neurodegenerative diseases. The distribution of rAAV vectors in the brain can be improved by co-infusion with substances that change the capsid interaction with cell surface receptors (Mastakov et al., 2001), and convection enhanced delivery (CED) (Bankiewicz et al., 2000; Cunningham et al., 2000; Cunningham et al., 2008; Hadaczek et al., 2006; Nguyen et al., 2001). CED is more commonly implemented in large animals and in humans to achieve better distribution of rAAV vectors over much larger structures from a small number of injections. The most important advancement impacting the efficiency and distribution of AAV-mediated gene transfer to the CNS has come from the cloning and identification of new AAV capsid genes (Gao et al., 2005). To date more than 120 different AAV capsids have been identified and many have been engineered for production of new rAAV vectors. Numerous studies have shown that rAAV1, rAAV5, rAAV8, rAAV9, rAAVrh10 and some other vectors are considerably more efficient than rAAV2 vectors for direct CNS gene transfer (Burger et al., 2004; Cearley et al., 2008; Cearley and Wolfe, 2006; Klein et al., 2006; Klein et al., 2008; Sondhi et al., 2007). In addition to the distribution imparted by the infusion pressure in the targeted structure, it appears that rAAV vectors can travel long distances in the CNS via axonal transport (Cearley et al., 2008; Cearley and Wolfe, 2006; Cearley and Wolfe, 2007; Hadaczek et al., 2004; Kells et al., 2009; Salegio et al.). The direction of transport, anterograde or retrograde, appears to be capsid specific (Salegio et al.). Most rAAV vectors appear to transduce mostly neurons upon direct infusion into the brain parenchym. Although the tropism of rAAV vectors is thought to be largely dependent on the capsid, there is increasing recognition that it can also be influenced by the rAAV purification method (Klein et al., 2008), and the type of promoter used to drive gene expression (Snyder et al., 2011). New AAV capsid and advanced infusion techniques have contributed greatly to improve the distribution of rAAV vectors in the CNS delivered by direct intraparenchymal infusion. Nonetheless this approach remains best suited for localized gene transfer applications.
The recent discovery that systemic infusion of rAAV9 and several other vectors in neonatal and adult mice led to widespread gene transfer to the CNS (Foust et al., 2009; Yang et al., 2012; Zhang et al., 2011b) marked the beginning of a new era in gene therapy for neurodegenerative diseases. This approach would be beneficial for genetic diseases affecting widely dispersed cell populations in the CNS, or diseases where both the CNS and peripheral organs are involved as is the case for many lysosomal storage diseases. The degree of neuronal gene transfer and therapeutic benefit in a mouse model of spinalmuscular atrophy (SMA) appear to be age dependent (Foust et al., 2010). In adult animals rAAV9 vectors appear to transduce mostly astrocytes and brain endothelial cells (Foust et al., 2009; Yang et al., 2012), while rAAVrh10 vectos appear to transduce additional cell types of the CNS, including neurons(Yang et al., 2012). Studies in primates infused with rAAV9 vector at different post-natal ages showed efficient transduction of spinal cord motor neurons in animals ranging from 1 day to 3 years old (oldest animal tested). In the cerebrum most transduced cells appeared to be astrocytes (Bevan et al., 2011). rAAV9 vectors appear to be particularly efficient for gene transfer to spinal cord and Purkinje cells in the cerebellum, making them excellent candidates for therapeutic development for diseases such as amyotrophic lateral sclerosis (ALS), SMA, and spinocerebellar ataxias where these structures and cell types are particularly affected. The mechanism used by rAAV9 vectors to cross the blood brain barrier, ie receptor mediated transcytosis or otherwise, is unknown at the moment. It appears that rAAV9 vectors use galactose (Shen et al., 2011) as its receptor, and the galactose-binding domain on the capsid has been recently identified (Bell et al., 2012). Apparently the BBB-crossing properties of rAAV9 after systemic delivery in mice is shared by a number of other novel rAAV vectors including rAAVrh.10, rAAVrh.39 and rAAVrh.43 (Yang et al., 2012; Zhang et al., 2011a). With the discovery of a wide variety of vectors that can cross the BBB, intravenous delivery of therapeutic rAAVs to alleviate CNS disorders is becoming an increasingly viable option.
The presence of neutralizing antibodies in animals (Rapti et al., 2012) and patients could limit the effectiveness of systemic delivery of BBB-crossing rAAV vectors as a means to achieve global gene delivery to the CNS (Samaranch et al., 2012). However epidemiological studies suggest that the percentage of the population sero-positive for neutralizing antibodies against newer AAV serotypes is lower than AAV2 ((Boutin et al., 2010) (Calcedo et al., 2009; van der Marel et al., 2011). Even in sero-positive patients, neutralizing antibodies may be reduced or eliminated by means of apheresis to allow gene targeting (Monteilhet et al., 2011). Another aspect to consider with systemic delivery of these novel rAAV vectors is the high levels of transduction of peripheral organs such as skeletal muscle, liver and heart (Bevan et al., 2011; Xie et al., 2011), which in many instances may be advantageous, such as in lysosomal storage diseases with CNS and peripheral pathology, but could also be detrimental depending on the transgene being expressed. Strategies to de-target transgene expression from peripheral organs may be accomplished using cell specific promoters, or miRNA-regulation (Xie et al., 2011).
Critical Parameters and Troubleshooting
Craniotomy and stereotaxic injection of AAV vectors into the brain of adult mice
The main difficulty encountered with the stereotaxic injection delivery method is the variability across mouse strains and age. The injection depends on coordinates for brain structures determined from a particular mouse strain, and calculated in relationship to the bregma. Thus, the injection may miss the target structure due to skull irregularities. Furthermore, if the mouse to be injected is particularly small, the head itself may be smaller than the reference mouse brain, and thus the coordinates would not match. One way to prevent this problem is to first inject an animal using a vector encoding a reporter gene, and examine the brain of that animal after a few weeks. For a faster, but less accurate answer, dye can be injected, the animal immediately sacrificed, and the brain dissected to determine the site of injection.
Tail vein injection of AAV vectors into adult mice
The biggest challenge in performing this technique is correctly inserting the needle into the vein. However, practice will easily solve this problem. The most important thing to remember is to keep the needle shaft at a small angle to the tail surface, insert it superficially with bevel up. Using the heat lamp before injection helps to make the tail vein more visible due to vasodilation.
Facial vein injection of AAV vectors into neonates
The major challenge in performing this technique is correctly inserting the needle into the vein. The vein is small and fragile and steady hands are necessary, as well as a quick injection to minimize the time the needle spends in the vein. It is easy to move the needle tip, and end up with a subcutaneous injection instead. This will be obvious by the formation of a subcutaneous bulge that will form near the injection site. The best way to overcome this challenge is to first practice with a colored dye, which makes it obvious that the injection is done correctly. Furthermore, it is recommended that the injector’s arms be firmly planted on a steady surface, to minimize any unwanted movement.
Collection of brain tissue to be used for histology
The challenge in collecting perfused brain tissue is keeping the tissue intact and free of nicks while removing the skull. Careful use of the tweezers against the skull and small movements will lead to an intact brain. It is recommended that several tweezers be tried to determine which is best for the user.
It is also important that the 4% paraformaldehyde fixative solution be made fresh and used within a week. It should always be kept cold, and shielded from light. All work done with paraformaldehyde should be done in a fume hood.
Most problems encountered during perfusion are due to incorrect placement of the cannula/needle in the left ventricle of the heart. Often the needle is placed by mistake in the right ventricle leading to incorrect or lack of systemic perfusion with fixative. If the liver does not change color from deep red to a beige color stop the perfusion and readjust the needle. Once the perfusion has started the needle should not be moved anymore, so as to not disturb its placement. Furthermore, if an organ is nicked during the procedure it can also cause a leakage point, and impair the procedure. In the case that the perfusion is not fully successful, the animal will be limp and the organs soft. If this occurs, remove the organs and place in 4% paraformaldehyde fixative solution overnight at 4° C. This is usually sufficient to obtain good fixation of the tissues, but blood may still be present in the organs.
Embedding brain for cryostat sectioning
The challenge in embedding a brain for cryostat sectioning is making sure the brain is frozen orthogonal to the bottom surface of the embedding mold to facilitate subsequent orientation of the block in the cryostat. If the brain is at an angle it will be more challenging to cut sections with a desired orientation such as coronal, saggital, or longitudinal. Thus, when putting the brain in the OCT filled mold, forceps can be used to make sure it is in the correct position. Then, when the mold is moved to the 2 O-Methylbutane/dry ice bath, it should be placed on a flat surface so the brain doesn’t shift in the OCT. It may be helpful to devise a device to float the mold on, in a similar way that floating devices are used to incubate small 1.5 mL tubes in water baths.
Anticipated Results
Craniotomy and stereotaxic injection of AAV vectors into the brain of adult mice
Correct delivery of the vector to the brain structure of interest should lead to distribution of AAV-transduced cells throughout the injected structure, and in some instances also in synaptically connected strcutures. Expression of the transgene should be detectable as early as one week post injection, with peak expression after 3–4 weeks. Cellular localization and distribution of the protein encoded by the transgene will depend on the endogenous properties of the protein, as well as the serotype and promoter used in the vector.
Tail vein injection of AAV vectors into adult mice
Correct delivery of the vector should lead to distribution of rAAV vector throughout the animal. rAAV mediated gene expression in different tissues is determined by the AAV capsid and promoter used for the particular experiment. Expression of the transgene should be detectable as early as one week post injection, with peak expression after 2–6 weeks, depending on the AAV capsid. The kinetics of gene expression after intravenous delivery varies considerably between rAAV2 vectors and others such as rAAV8 or rAAV9.
Facial vein injection of AAV vectors into neonates
The transduction profile after systemic intravenous delivery in neonatal mice will be dependent on the AAV capsid and eukaryotic promoter used in the recombinant vector. Expression of the transgene should be seen as early as 1 week post injection, with peak expression after ~ 3 weeks. It is important to consider that for the most part rAAVs vectors do not integrate into the host cell genome and as such the vector genomes will tend to be lost due to cell division as the organs grow.
Time Considerations
Craniotomy and stereotaxic injection of AAV vectors into the brain of adult mice
Once mastered, the technique should take less than an hour, depending on the rate of injection, total volume injected, and number of injection sites. The mice will typically become responsive within two hours after injection of anesthesia.
Tail vein injection of AAV vectors into adult and neonatal mice
Once mastered, the technique should take less than 5 minutes per animal. Many animals can be done during one session, the limiting step being the large amount of vector required for each animal.
Acknowledgments
This work was supported by Public Health Service grants from the National Institutes of Health UL1RR031982, P01 HL059407, P01AI100263-01, R01NS076991-01 (to GG), and U01NS064096, R01NS066310 and R01HD060576 (to MSE) as well as in part by a UMSS internal grant (to GG).
Footnotes
Figure 1B shows an alternative method of securing the skull, using ear bar adaptors.
Literature Cited
- Afione SA, Conrad CK, Flotte TR. Gene therapy vectors as drug delivery systems. Clin Pharmacokinet. 1995;28:181–189. doi: 10.2165/00003088-199528030-00001. [DOI] [PubMed] [Google Scholar]
- Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, Cunningham J, Budinger TF, Harvey-White J. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- Bell CL, Gurda BL, Van Vliet K, Agbandje-McKenna M, Wilson JM. Identification of the galactose binding domain of the adeno-associated virus serotype 9 capsid. J Virol. 2012;86:7326–7333. doi: 10.1128/JVI.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L, Chan CM, McCrate M, Chicoine LG, Coley BD, Porensky PN, Kolb SJ, Mendell JR, Burghes AH, Kaspar BK. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther. 2011;19:1971–1980. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Vandenberghe LH, Parente MK, Carnish ER, Wilson JM, Wolfe JH. Expanded Repertoire of AAV Vector Serotypes Mediate Unique Patterns of Transduction in Mouse Brain. Mol Ther. 2008 doi: 10.1038/mt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27:9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotugno G, Annunziata P, Tessitore A, O’Malley T, Capalbo A, Faella A, Bartolomeo R, O’Donnell P, Wang P, Russo F, Sleeper MM, Knox VW, Fernandez S, Levanduski L, Hopwood J, De Leonibus E, Haskins M, Auricchio A. Long-term amelioration of feline Mucopolysaccharidosis VI after AAV-mediated liver gene transfer. Mol Ther. 2011;19:461–469. doi: 10.1038/mt.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J, Oiwa Y, Nagy D, Podsakoff G, Colosi P, Bankiewicz KS. Distribution of AAV-TK following intracranial convection-enhanced delivery into rats. Cell Transplant. 2000;9:585–594. doi: 10.1177/096368970000900504. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Pivirotto P, Bringas J, Suzuki B, Vijay S, Sanftner L, Kitamura M, Chan C, Bankiewicz KS. Biodistribution of Adeno-associated Virus Type-2 in Nonhuman Primates after Convection-enhanced Delivery to Brain. Mol Ther. 2008 doi: 10.1038/mt.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, Aminoff MJ. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, Morales PR, Rich MM, Burghes AH, Kaspar BK. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28:271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gagliardi C, Bunnell BA. Large animal models of neurological disorders for gene therapy. ILAR J. 2009;50:128–143. doi: 10.1093/ilar.50.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadaczek P, Kohutnicka M, Krauze MT, Bringas J, Pivirotto P, Cunningham J, Bankiewicz K. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17:291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- Hadaczek P, Mirek H, Bringas J, Cunningham J, Bankiewicz K. Basic fibroblast growth factor enhances transduction, distribution, and axonal transport of adeno-associated virus type 2 vector in rat brain. Hum Gene Ther. 2004;15:469–479. doi: 10.1089/10430340460745793. [DOI] [PubMed] [Google Scholar]
- Hwu WL, Muramatsu S, Tseng SH, Tzen KY, Lee NC, Chien YH, Snyder RO, Byrne BJ, Tai CH, Wu RM. Gene therapy for aromatic L-amino acid decarboxylase deficiency. Sci Transl Med. 2012;4:134ra161. doi: 10.1126/scitranslmed.3003640. [DOI] [PubMed] [Google Scholar]
- Janson C, McPhee S, Bilaniuk L, Haselgrove J, Testaiuti M, Freese A, Wang DJ, Shera D, Hurh P, Rupin J, Saslow E, Goldfarb O, Goldberg M, Larijani G, Sharrar W, Liouterman L, Camp A, Kolodny E, Samulski J, Leone P. Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther. 2002;13:1391–1412. doi: 10.1089/104303402760128612. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O’Malley KL, During MJ. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- Kells AP, Hadaczek P, Yin D, Bringas J, Varenika V, Forsayeth J, Bankiewicz KS. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proc Natl Acad Sci U S A. 2009;106:2407–2411. doi: 10.1073/pnas.0810682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol Ther. 2006;13:517–527. doi: 10.1016/j.ymthe.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP. AAV8, 9, Rh10, Rh43 Vector Gene Transfer in the Rat Brain: Effects of Serotype, Promoter and Purification Method. Mol Ther. 2008;16:89–96. doi: 10.1038/sj.mt.6300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastakov MY, Baer K, Xu R, Fitzsimons H, During MJ. Combined injection of rAAV with mannitol enhances gene expression in the rat brain. Mol Ther. 2001;3:225–232. doi: 10.1006/mthe.2001.0246. [DOI] [PubMed] [Google Scholar]
- Monteilhet V, Saheb S, Boutin S, Leborgne C, Veron P, Montus MF, Moullier P, Benveniste O, Masurier C. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther. 2011;19:2084–2091. doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K, Kawakami T, Urabe M, Kume A, Sato T, Watanabe E, Ozawa K, Nakano I. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol Ther. 2010;18:1731–1735. doi: 10.1038/mt.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JB, Sanchez-Pernaute R, Cunningham J, Bankiewicz KS. Convection-enhanced delivery of AAV-2 combined with heparin increases TK gene transfer in the rat brain. Neuroreport. 2001;12:1961–1964. doi: 10.1097/00001756-200107030-00037. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. Academic Press; San Diego: 2001. [Google Scholar]
- Rapti K, Louis-Jeune V, Kohlbrenner E, Ishikawa K, Ladage D, Zolotukhin S, Hajjar RJ, Weber T. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol Ther. 2012;20:73–83. doi: 10.1038/mt.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salegio EA, Samaranch L, Kells AP, Mittermeyer G, San Sebastian W, Zhou S, Beyer J, Forsayeth J, Bankiewicz KS. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther. doi: 10.1038/gt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranch L, Salegio EA, San Sebastian W, Kells AP, Foust KD, Bringas JR, Lamarre C, Forsayeth J, Kaspar BK, Bankiewicz KS. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther. 2012;23:382–389. doi: 10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Bryant KD, Brown SM, Randell SH, Asokan A. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J Biol Chem. 2011;286:13532–13540. doi: 10.1074/jbc.M110.210922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder BR, Gray SJ, Quach ET, Huang JW, Leung CH, Samulski RJ, Boulis NM, Federici T. Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery. Hum Gene Ther. 2011;22:1129–1135. doi: 10.1089/hum.2011.008. [DOI] [PubMed] [Google Scholar]
- Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM, Wilson JM, Crystal RG. Enhanced Survival of the LINCL Mouse Following CLN2 Gene Transfer Using the rh.10 Rhesus Macaque-derived Adeno-associated Virus Vector. Mol Ther. 2007;15:481–491. doi: 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- van der Marel S, Comijn EM, Verspaget HW, van Deventer S, van den Brink GR, Petry H, Hommes DW, Ferreira V. Neutralizing antibodies against adeno-associated viruses in inflammatory bowel disease patients: implications for gene therapy. Inflamm Bowel Dis. 2011;17:2436–2442. doi: 10.1002/ibd.21673. [DOI] [PubMed] [Google Scholar]
- Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N, Dyke JP, Ballon D, Heier L, Greenwald BM, Christos P, Mazumdar M, Souweidane MM, Kaplitt MG, Crystal RG. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther. 2008;19:463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
- Xie J, Xie Q, Zhang H, Ameres SL, Hung JH, Su Q, He R, Mu X, Seher Ahmed S, Park S, Kato H, Li C, Mueller C, Mello CC, Weng Z, Flotte TR, Zamore PD, Gao G. MicroRNA-regulated, systemically delivered rAAV9: a step closer to CNS-restricted transgene expression. Mol Ther. 2011;19:526–535. doi: 10.1038/mt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Cao C, Zhang H, Su Q, Ahmed SS, Zhong L, He R, Sena-Esteves M, Flotte TR, Brown R, Xu Z, Guangping G. Intravasuclar Delivery of rAAVrh.8 Generates Widespreading Transduction of Neuronal and Glial Cell Types in the Adult Mouse Central Nervous System. American Society of Gene & Cell Therapy 15th Annual Meeting; May 16–19, 2012; Philadelphia, Pennsylvania, USA. 2012. May, [Google Scholar]
- Zhang H, Yang B, Mu X, Ahmed SS, Su Q, He R, Wang H, Mueller C, Sena-Esteves M, Brown R, Xu Z, Gao G. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol Ther. 2011a;8:1440–1448. doi: 10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang B, Mu X, Ahmed SS, Su Q, He R, Wang H, Mueller C, Sena-Esteves M, Brown R, Xu Z, Gao G. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol Ther. 2011b;19:1440–1448. doi: 10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]