Summary
Purpose
It is generally accepted that blood–brain barrier (BBB) failure occurs as a result of CNS diseases, including epilepsy. However, evidences also suggest that BBB failure may be an etiological factor contributing to the development of seizures.
Methods
We monitored the onset of seizures in patients undergoing osmotic disruption of BBB (BBBD) followed by intraarterial chemotherapy (IAC) to treat primary brain lymphomas. Procedures were performed under barbiturate anesthesia. The effect of osmotic BBBD was also evaluated in naive pigs.
Results
Focal motor seizures occurred immediately after BBBD in 25% of procedures and originated contralateral to the hemisphere of BBBD. No seizures were observed when BBB was not breached and only IAC was administered. The only predictors of seizures were positive indices of BBBD, namely elevation of serum S100β levels and computed tomography (CT) scans. In a porcine model of BBBD, identical procedures generated an identical result, and sudden behavioral and electrographic (EEG) seizures correlated with successful BBB disruption. The contribution of tumor or chemotherapy to acute seizures was therefore excluded.
Conclusion
This is the first study to correlate extent of acute BBB openings and development of seizures in humans and in a large animal model of BBB opening. Acute vascular failure is sufficient to cause seizures in the absence of CNS pathologies or chemotherapy.
Keywords: Epileptogenesis, Endothelial cells, Cerebrovascular disease, Tight junctions
Seizures and epilepsy are commonly observed in conjunction with stroke, traumatic brain injury, and central nervous system infections, all conditions known to result in compromised BBB function. A point of debate is whether the compromised integrity of the BBB may be a prodromic component of the etiology of epilepsy or if BBB failure is simply a consequence of seizures. In support of the former is the fact that BBB disruption after acute head trauma is a well-known pathologic finding in both animal and humans studies (Schmidt and Grady, 1993; Grant and Janigro, 2004). This disruption may persist for weeks to years after the injury and may colocalize with abnormal EEG activity (Korn et al., 2005).
The increased interest in osmotic opening of the BBB as a viable mechanism of increased drug delivery to the brain provides an opportunity to explore the connection between BBB disruption and seizures in a more controlled, yet “human” environment. Osmotic opening of the blood–brain barrier by intravascular infusion of a hyperosmolar bolus of mannitol is mediated by vasodilatation and shrinkage of capillary endothelial cells. The cells shrink resulting in widening of the interendothelial tight junctions to an estimated radius of 200 Å (Kroll and Neuwelt, 1998). The marked increase in BBB permeability to intravascular substances (10 to 100-fold for small molecules) following the osmotic disruption procedure is due to both increased diffusion and bulk fluid flow across the tight junctions. The permeability effect is largely reversed within minutes (Armstrong et al., 1989; Greig et al., 1990). Loss of BBB integrity by intrarterial hyperosmotic mannitol has been shown, in rodents, to rapidly lead to EEG changes consistent with epileptic seizures (Fieschi et al., 1980); spike/wave complexes were interspersed with decreased EEG voltage and persisted for several hours after the BBB disruption event.
Given these findings, it is not surprising that seizures are a primary complication of osmotic BBB disruption; seizures occur in a relatively large number of patients (13–55%). This was initially attributed to the use of meglumine iothalamate, a known epileptogenic agent used as a contrast agent for computed tomography (CT). Seizures continued to occur when BBB disruption was monitored by radionuclide scanning rather than CT, albeit with decreased frequency (Neuwelt et al., 1983a, 1983b). However, the correlation between level of BBB disruption and probability of seizure events has not yet been studied, nor is it clear how BBB disruption and seizure events correlate temporally.
The main goal of our study was to investigate the temporal and quantitative correlation between intraarterial BBBD with mannitol and the development of seizures in humans and in a large animal model of osmotic BBB opening. In particular, we wished to test the hypothesis that increased levels of BBB disruption are more likely to result in seizures compared to attempts leading to modest opening of the BBB. The degree of opening was quantified radiologically by contrast CT scans and by serum analysis of S100β, a serum marker of blood–brain barrier integrity (Marchi et al., 2003a, 2003b, 2004; Fazio et al., 2004; Vogelbaum et al., 2005).
MATERIAL AND METHODS
BBB disruption in patients
All patients signed an informed consent according to institutional review protocols at The Cleveland Clinic Foundation and the Declaration of Helsinki. Eight patients (Table 1) with histologically proven nonacquired immunodeficiency syndrome Primary Central Nervous System Lymphoma (PCNSL) consented to participate in an institutional review board-approved protocol for the management of this disease at the Cleveland Clinic Foundation. This protocol involves concurrent intravenous chemotherapy and a treatment protocol including BBB disruption (Kroll and Neuwelt, 1998) followed by intraarterial instillation of chemotherapy (IAC). This subset of patients also agreed to additional blood draws for serum S100β sampling. The appropriate inclusion and exclusion of PCNSL patients on this protocol has been documented previously (Neuwelt et al., 1991). Treatment is approximately every 10 days for 3 months, then every 6 weeks for 1 year. The location and radiologic size of the tumors is presented in Table 2 and Fig. 3.
TABLE 1.
Patient characteristics and summary of results
| ID # | Age | Sex | IAC only | Seizures after IAC | IAC + BBBD | Seizures after IAC + BBBD (yes/no) | Previous AED |
|---|---|---|---|---|---|---|---|
| 1 | 57 | F | 0 | 0 | 17 | 2/15 | None |
| 2 | 38 | F | 0 | 0 | 14 | 7/7 | None |
| 3 | 72 | M | 3 | 0 | 11 | 7/4 | Phenytoin |
| 4 | 52 | F | 2 | 0 | 4 | 1/3 | Phenytoin |
| 5 | 33 | F | 2 | 0 | 7 | 4/3 | Phenytoin |
| 6 | 70 | F | 2 | 0 | 17 | 0/17 | Phenytoin |
| 7 | 20 | F | 2 | 0 | 20 | 3/17 | Phenytoin carbamazepine |
| 8 | 65 | F | 3 | 0 | 12 | 1/11 | None |
| Total | 14 | 0 | 102 | 25 | |||
| Mean | 50 | 0 | 3.1* | ||||
| SEM | ±18 | ±0 | ±0.9 |
BBBD, blood–brain barrier disruption; IAC, intraarterial chemotherapy.
SEM indicates the standard error of the mean. The asterisk indicates a significant (p < 0.02) difference between seizure occurrence in the IAC versus BBBD groups.
See text for details.
TABLE 2.
Tumor burden and progression before and after BBBD
| ID # | Tumor location at admission/new enhancement during procedures | Tumor measurement at admission | Final response |
|---|---|---|---|
| 1 | Right Frontal intra-axial mass resected prior to BBBD treatment | No definite enhancement prior BBBD treatments | CR |
| 2 | No disease detected prior BBBD treatment | No definite enhancement prior BBBD treatments | CR |
| 3 | Right parietal mass resected prior to BBBD treatment. | Left parietal small enhancement | SD |
| Left small parietal mass | |||
| 4 | Bilateral low density lesion in temporal lobes | NA | PR |
| 5 | Multifocal disease mesial frontal | 2.5 cm × 1.7 cm × 1.8 cm = 7.65 cm3 | CR |
| Brain stem | 0.8 cm × 1.0 cm × 3.1 cm = 2.48 cm3 | CR | |
| 6 | Left basal ganglia | 4 cm × 3.4 cm × 3.8 cm = 51.68 cm3 | CR |
| Second left frontal lesion | 0.15 mm × 0.1 mm × 0.15 mm = 0.0023 mm3 | CR | |
| New right mesial cerebellum enhancement | 1.0 cm × 1.0 cm × 1.0 cm = 1.0 cm3 | PR | |
| 7 | Right frontal | 3.9 cm × 3.9 cm × 4.9 cm = 74.5 cm3 | CR |
| Left frontal | 2.7 cm × 2.0 cm × 3.2 cm = 17.28 cm3 | CR | |
| 8 | Left basal ganglia | 3.6 cm × 3.3 cm × 3.2 cm = 38.02 cm3 | CR |
CR, complete remission; PD, progressive disease; SD, stable disease; PR, partial response.
FIG. 3.
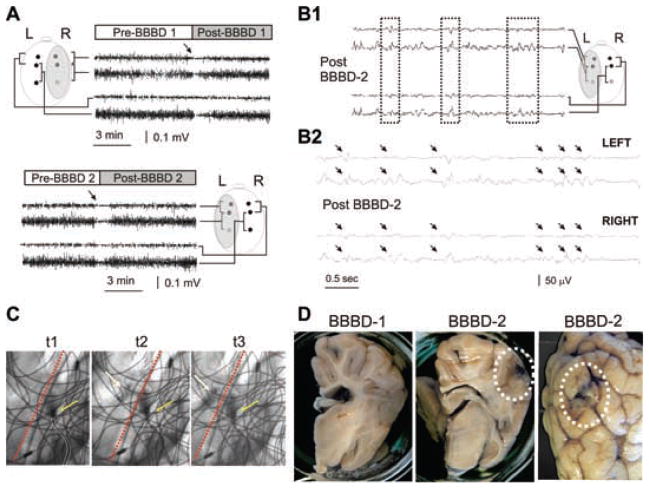
EEG correlates of acute blood–brain barrier disruption: a modest increase in BBB permeability does not lead to seizures. (A) Experimental setup for animal experiments. Electrodes were placed as indicated in the schematic diagram. The shaded area indicates the side of intraarterial mannitol injection. The tracings refer to frontal and parietal recordings as indicated. The upper panel refers to recordings obtained during the first BBBD attempt (BBBD1 throughout this figure), while the lower panel refers to BBBD2. Note the sudden decrease of EEG amplitude after either osmotic opening procedure. Also note the lack of obvious delayed EEG changes after BBBD1 or BBBD2. (B1) Spike-wave complexes observed after BBBD2. Note the signal reversal at different electrode locations (dashed boxes). Longer segment of recording (B2) to illustrate a cluster of spike-wave complexes seen after BBBD2 (arrows). Filters used for viewing during collection were 0.3 and 70 Hz (low pass and high pass, respectively); sampling rate was 200 Hz. (C) Indicates the site of catheterization (outlined in white) and its relationship to the midline (red dashed line). The yellow arrows indicate the extrusion of contrast agent at 1-s intervals (indicated by t 1–3). Note the slight contralateral diffusion of contrast. The wiring visible in the picture is the radiologic image of EEG electrodes and connectors. (D) Absence of Evans blue leakage after BBBD1 and minimal extravasation of the dye after BBB2. The dotted circles indicate spotty cortical leaks seen in the BBBD-2 but not BBBD-1 hemisphere. Serum S100β did not increase during these BBBD procedures (data not shown).
Specifically, these patients were treated with intraarterial injection of mannitol causing a temporary disruption of the BBB followed by a selective intracarotid chemotherapic injection. The procedure consists of the following steps: (1) Patient is taken to the operating room and general thiopental anesthesia is induced. (2) Catheterization of a selected intracranial artery (either an internal carotid or vertebral artery) is performed via a percutaneous transfemoral puncture on a given treatment day. (3) Mannitol (1.4 M) is administered intraarterially via the catheter at a predetermined rate of 3–12 cc/s for 30 s. (4) After the BBB is opened with mannitol, intraarterial methotrexate is infused. No seizures were associated with injection of contrast or chemotherapy in the absence of mannitol (see Fig. 1A). (5) Immediately following delivery of chemotherapy, nonionic contrast dye is given intravenously. (6) The patient is transported, still anesthetized, for a CT scan. This step is essential to determine and document the degree of BBB opening since better disruption portends better chemotherapy delivery across the barrier. Methods for grading the degree of BBBD and correlation of these grades with Hounsfield units were previously described (Roman-Goldstein et al., 1994a); degree of BBBD was graded by visual inspection as nil, fair, good, or excellent. MRI Gd++ enhancement is not compatible with the BBBD procedure due to toxicity (Roman-Goldstein et al., 1991, 1994b). (7) After the CT scan is completed the patient is awakened, extubated, and monitored in the hospital overnight. This is a 2-day procedure whereby two different intracranial vessels (typically an anterior circulation vessel, left or right internal carotid artery, on 1 day and the contralateral posterior circulation vessel right or left vertebral artery on the following day) are cannulated on consecutive treatment days for BBBD and instillation of chemotherapy. Blood samples were drawn 10 min prior to mannitol injection and 2–5 min after mannitol injection (Kapural et al., 2002; Marchi et al., 2003a). S100β was measured on all available blood samples by techniques described elsewhere (Marchi et al., 2003a, 2003b). A total of 102 BBB disruption procedures in eight patients were studied. Two opening procedures were excluded from further analysis because vascular spasms were observed. These two events belong to the no-seizure group. In addition, we analyzed data from 14 IAC procedures that were not preceded by BBBD.
FIG. 1.
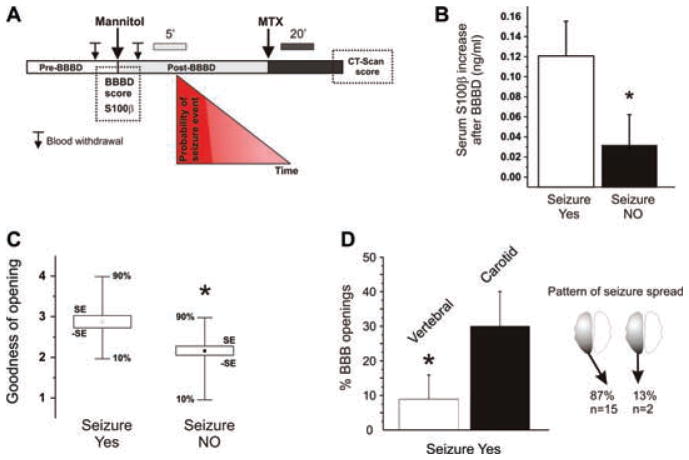
(A) Diagrammatic representation of the procedure, timing of serum S100β determination, CT scans, and probability of seizure development. Note the different time scale depicted above the post-BBBD and post-MTX intervals. Seizure probability was highest (red ) during the early post-BBBD period and before MTX. Serum S100β was measured before the onset of seizures. (B) Elevation of S100β serum levels immediately after mannitol infusion was larger in patients who eventually developed seizures after blood–brain barrier disruption. The values reported are the differences between serum S100β levels in blood drawn immediately before and immediately after (up to 5′) BBBD. (See references Kapural et al., 2002; Marchi et al., 2003a for details.) Baseline serum S100β was 0.04 ± 0.01 ng/ml. Note that the postmannitol S100β values were recorded prior to any seizure onset and are thus highly unlikely to reflect consequence of motor seizures. (C) BBBD efficiency correlates with occurrence of seizures: radiological indices of BBBD obtained by CT scans after BBBD. See text for details. The asterisks indicate p < 0.02. (D) Seizure events are more common after BBB disruption in the anterior circulation and seizures induced by intracarotid mannitol manifest usually contralaterally. The data show the percentage of seizures associated with vertebral or intracarotid mannitol injections. Note that BBB disruption of the anterior circulation was more likely to result in focal motor seizures, which most commonly occurred contralateral. The asterisks indicate p < 0.05.
Specific clinical conditions were evaluated in order to perform the IAC only or BBBD + IAC procedures. In general, only patients without significantly increased intracranial pressure, confirmed radiographically, were selected for BBB disruption. The following criteria were used: (1) no clinically significant dilation of the contralateral ventricular frontal horn, and (2) a patent quadrigeminal cistern. If these criteria were not met, patients would undergo intrarterial chemotherapy administration without prior BBBD. In this event, patients receive 1 day of in-patient intraarterial chemotherapy treatment under general anesthesia. The results in Fig. 1A refer to 14 procedures consisting of intrarterial chemotherapy not preceded by BBBD. Typically, when the tumor responded to the intraarterial treatment, the patients were then converted to treatment with BBBD followed by IA chemotherapy to enhance the delivery of chemotherapy to the brain.
Motor seizures consisting of rhythmic muscle contraction/relaxation (clonic activity) were documented for all procedures. They were considered focal if they affected one side of the body (leg, part of the face, or other isolated area). If focal, the side of onset was noted and correlated with the hemisphere undergoing BBBD.
Statistical analysis was performed by ANOVA with Origin 6.0 (Microcal Corporation, Northampton, MA, U.S.A).
BBB disruption in a porcine model
Two 35-kg Yorkshire pigs were placed under anesthesia using isofluorane (1–1.5%). Anesthesia was induced by intramuscular ketamine with the addition of xylazine (Rompun; 1–2 mg/kg) and atropine (0.02 mg/kg). In the supine position, the femoral artery was cannulated and under fluoroscopic guidance, the internal carotid artery catheterized. From a radiologic point of view, the procedure was essentially identical to that described above. The medical team responsible for the procedure was the same that was involved in human studies. We performed angiography with a right femoral artery cannulation and found that the swine cerebral circulatory system demonstrated a plexus of very small vessels, rete mirabile, in the base of the brain that was perfused by the ascending pharyngeal artery and reconstituted into the internal carotid artery downstream. Angiography was also performed to determine the rate and volume of an injection to opacify the ipsilateral carotid system; cross-filling of the contralateral (control side) was immediately evident in the porcine model. In this respect, the “hemispheric” nature of the BBB disruption was not immediately comparable to the human trial. However, the goodness of opening obtained was, as shown in human studies, variable. When measured by extravasation of the blood–brain barrier impermeant tracer Evans blue (Pekny et al., 1998), we observed one “nil” opening, a “fair” and a “good” opening of the blood–brain barrier. In this respect, the pig model faithfully reproduced the variable propensity of the human blood–brain barrier to osmotic opening.
The following equipment was utilized in collecting EEG data: Nihon Kohden EEG System (Nihon Kohden, Tokyo, Japan) Nihon Kohden Neurofax EEG 9000 Version 5–72 running on a Windows XP platform using DELL Optiplex GL280. A total of 13 channels, including ground, reference, EKG, and impedance electrodes were collected. A noncephalic reference was utilized for comparison. These tracings were collected on a Nihon Kohden JE910-A jackbox, using sterile stainless steel subdermal needle electrodes (13 × 0.40 mm with 0.5 × 27 Gauge) manufactured by Axon Systems, Hauppage, NY, U.S.A. The system was electrically isolated from the pig and the equipment used in performing angiographic procedures.
Serum S100β evaluation
Serum samples of S100β were obtained after induction of anesthesia, immediately prior and immediately after intraarterial mannitol infusion (Fig. 1A).
S100β ELISA analysis
At each time point, blood samples were collected and immediately centrifuged at 1,200 × g for 10 min, and the supernatant serum were stored at −80°C. The S100β concentration was measured in all samples by the Sangtec 100 ELISA method (Diasorin, Stillwater, MN, U.S.A.) using high- and low-level manufacturer provided controls to ensure proper assay performance.
S100β western blot analysis
Prior to electrophoresis, pig serum protein fractions were denatured by heating at 100°C for 5 min in a running buffer solution containing β-mercaptoethanol, and bromophenol blue tracking dye. An acrylamide gel (12%, precast gel; Bio-Rad Labs, Hercules, CA, U.S.A.) was run for approximately 3 h at constant voltage (80 V) until the bromophenol blue tracking dye migrated to the bottom edge of the gels. Proteins from the gel were transferred onto a blot of PVDF membrane using a 192-mM glycine, 25-mM Tris-base, 20% methanol buffer system at constant voltage (100 V) for 1 h. at 4°C. Following blocking for 2 h at room temperature with 5% milk protein in 10-mM Tris-HCl, 150-mM NaCl, pH 8.0, S100β protein was probed overnight at 4°C with primary S100β antibody (1:500; sheep antibovine S100β from QED Bioscience, Inc., San Diego, CA, U.S.A.). Blot was washed and treated with Rabbit Anti-Sheep IgG HRP conjugated secondary antibody (1:2000; Dako Corp., Carpinteria, CA, U.S.A.). Protein concentration was estimated according to the Bradford assay method. Relative expressions of proteins were determined by densitometric analysis using Phoretix 1D Advanced Software (Newcastle upon Tyne, United Kingdom).
RESULTS
The patient data relevant to this study are shown in Tables 1 and 2. Fig. 1A describes the timing of mannitol, methotrexate arterial infusion, blood sampling for S100β measurement, CT scan, and probability of seizure onset (red-to-white triangle). Note that S100β serum levels were always assessed immediately after BBBD with mannitol (5′, dotted box in Fig. 1A) and before the onset on focal motor seizures either in human and pig. We first evaluated the occurrence of behaviorally detectable motor seizures when patients received intraarterial chemotherapy without BBB disruption with mannitol. Previous studies (Kapural et al., 2002; Marchi et al., 2003a, 2003b) have shown that intraarterial chemotherapy does not per se cause any significant change in blood–brain barrier integrity. In the present study, patients undergoing intraarterial chemotherapy alone without BBB disruption never experienced motor seizures (n = 5 patients for a total of 14 IAC procedures). In contrast, 25% of 102 procedures in eight patients where intraarterial chemotherapy was administered after BBB disruption resulted in motor seizures. Note that five patients, who were initially treated with IAC without BBBD during 14 cycles, later received full BBB disruption treatments. Thus, IAC without BBBD did not cause seizures in the same patients in whom IAC with BBBD did.
When timing of seizure occurrence was analyzed, we found that seizures occurred exclusively within the time frame of the procedure, i.e., minutes and not hours after the administration of mannitol and disruption of the BBB. Occasionally, patients who had seized during the procedure continued to have additional periprocedural seizures (for up to 6 h postprocedure); however, seizures occurred most commonly prior to administration of either chemotherapic agents or contrast media (Fig. 1A). Only in two procedures seizures were detected during chemotherapy after BBB disruption. There was no association between occurrence of seizures and radiologic contrast infusion, since this was performed well after the occurrence of motor seizures (Fig. 1A). These results suggested a temporal and perhaps causal relationship between BBB disruption and onset of motor seizures. These data also excluded a significant contribution of chemotherapic agents or contrast media to the observed seizure behavior.
We examined the possibility that more widespread BBB opening were associated with occurrence of seizure. In particular, we assessed the degree of BBB disruption by CT scan and measured levels of S100β increase in serum. S100β has been widely used as a surrogate serum marker of BBB integrity (Mussack et al., 2002; Jaranyi et al., 2003; Sendrowski et al., 2004; Vogelbaum et al., 2005). S100β increases sharply in blood immediately after a successful BBBD procedure (Kapural et al., 2002; Marchi et al., 2003a) and is now a recognized alternative to contrast enhanced radiological exams for BBB integrity (Vogelbaum et al., 2005). The data in Fig. 1B show the results from eight patients where serum samples to measure the serum BBB indicator S100β were obtained immediately prior and immediately after BBBD (n = 97 procedures; serum S100β data from 5 BBBD were not available). The differential serum S100β post−BBBD-S100βpre−BBBD are indicators of blood–brain barrier leakage (Kapural et al., 2002; Marchi et al., 2003a, 2003b; Vogelbaum et al., 2005). Note that S100β was measured in blood before the onset of seizures, thus indicating goodness of the osmotic opening of the BBB and not BBB disruption induced by seizures.
In the same patients, BBBD was assessed by intraoperative CT scans (Roman-Goldstein et al., 1994a) taken approximately 30–120 min (Fig. 1A) after mannitol injection (Fig. 1C). The qualitative nil, fair, good, and excellent radiological scores were transformed into numeric values (1–4) for clarity and statistical analysis. Thus, we used a dual approach to uncover a possible link between the degree of BBBD and propensity toward the development of seizures. Note that regardless of the approach used (S100β or CT), seizures occurred preferentially during procedures associated with a successful blood–brain barrier disruption.
A direct cause–effect correlation between BBBD and motor seizures can be ascribed to disruption of brain homeostasis in proximity of the vessels where the BBB was breached. Thus, one expects that most seizures will originate contralateral to the site of BBBD, and that focal motor seizures would be more predominant during procedures affecting motor cortex (i.e., intracarotid application of mannitol) than in those where the vertebral circulation was used to deliver the osmotic agent. Our data show that this was indeed the case (Fig. 1D). BBB disruption by mannitol injection in the anterior circulation had a significantly higher probability of causing motor seizures. Furthermore, acute neurologic changes following intracarotid application almost invariably manifested as motor seizures occurring contralateral to the site of injection (Fig. 1D). It is possible that electrographic seizures arising from areas outside the motor cortex (e.g., occipital cortex) may have been occurring after disruption of the vertebral circulation.
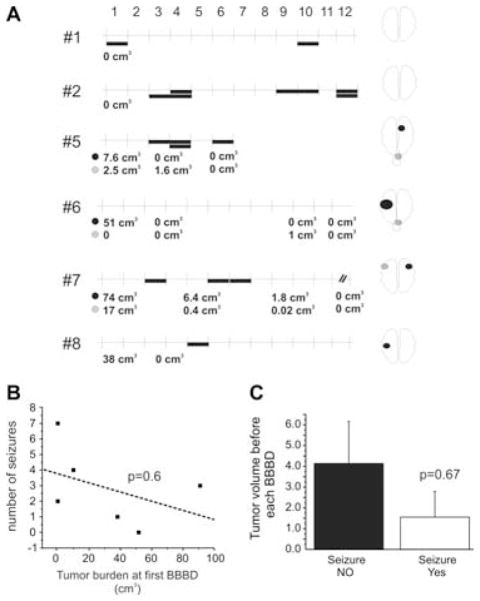
In one patient who underwent 11 BBBD, a seizure occurred only when the radiologic index was 2 or more, suggesting that even within the same course of treatment, seizures are promoted by successful BBB disruption. In the same patient, a BBBD with a good opening (radiological score of 3, and S100β increase immediately after BBBD of 0.507 ng/ml) was sufficient to cause a seizure, while the same procedure with marginal success in disruption of the BBB administered 24 h later did not (S100β increased by 0.014 ng/ml, which is an indication of no disruption (Marchi et al., 2004)). Since the tumor burden in this patient was obviously the same at 24-h interval, we concluded that size of the tumor was not a predictor or determinant of BBBD. This was also investigated in the whole cohort of patients. There was no association between tumor size or the number of procedures undergone since the beginning of treatment and development of seizures (Fig. 2), demonstrating a lack of correlation between tumor burden and effects of BBBD on brain excitability. Fig. 2B shows the correlation between volumetric tumor sizes determined by MRI 24 h before the first BBBD procedure and the total number of seizures that these patients experienced during the entire duration of the treatment. Fig. 2C summarizes the lack of correlation between volume of tumor(s) determined by MRI the day before a given BBBD procedure and the probability of developing seizures during that particular treatment session. Note the lack of positive correlation between tumor size and propensity toward the development of motor seizures.
FIG. 2.
Lack of correlation between seizure occurrence (indicated by filled bars in A), tumor size or site and treatment cycle. (A) Seizure occurrence in six patients where volumetric tumor analysis was performed at each treatment episode. Each treatment refers to two subsequent BBBD at 24-h interval (see Methods). Thus, two seizure episodes may have occurred during the same treatment. The vertical lines refer to sequential treatments indicated by the numbers (1–12). The numbers at the left are patient ID’s as per Tables 1 and 2. The numbers below each graph represent the MRI volume of the tumor at the time indicated. Tumor location is schematically shown in the drawing to the right. When more than one tumor site was present, two color-coded symbols are used to match their location shown to the right with their size. (B) Lack of correlation between tumor size at beginning of first treatment and total seizure numbers during the whole treatment period. (C) Cumulative tumor burden measured in patients at time of BBBD leading to seizures or not. Tumor size does not correlate with occurrence of seizures (p = 0.6). In fact, on average, smaller tumor size was present at the time of seizure occurrence further ruling out a contribution of the tumor to epileptogenicity.
As shown in Table 1, three out of eight patients (numbers 1, 2, and 8) were not prophylactically treated with antiepileptic drugs while the other five (numbers 3–7) received daily phenytoin or carbamazepine to reduce the risk of tumor-related seizures. To our knowledge, none of these patients ever experienced seizures before enrollment in the BBBD program. Interestingly, BBBD-triggered seizures occurred with equal probability in both groups, suggesting no significant influence of previous AED regimen.
While the data so far presented were all pointing to acute blood–brain barrier failure as a trigger of motor seizures, our intraoperative experimental design prevented us from directly measuring electrographic seizures in these patients. This was primarily due to logistic issues, such as removal of EEG wires during CT scanning, etc. Furthermore, while the results in Fig. 2 clearly show no correlation between tumor presence or size and seizures, we could not exclude some effect of preexisting lymphoma or ongoing peritumoral infiltration processes in determining seizures. Finally, one may object that while seizures were more often seen before MTX injection, a persistent long-term effect of chemotherapy could not be ruled out. In other words, it is unlikely but possible that previous chemotherapic exposures were responsible for lingering effects leading to seizures. To address all these issues, we used an animal model of acute BBB disruption based on an identical procedure performed by the same medical team on naive adult pigs. In these experiments, we were able to isolate BBBD from other variables (tumor, chemotherapy) and also perform EEG analysis during and after the BBBD procedure. The results of these experiments are shown in Figs. 3–5.
FIG. 5.
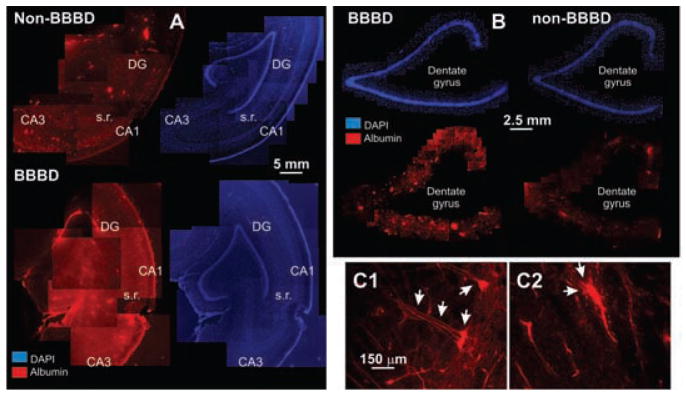
Histological analysis Evans blue extravasation. (A) Low-power micrographs showing the widespread leakage of the albumin-Evans blue complex (red signal) in the disrupted (BBBD) hemisphere compared to non-BBBD. The corresponding image was obtained by nuclear staining with DAPI to illustrate the relationship between serum leakage and anatomical structures. DG-dentate gyrus; s.r., stratum radiatum. (B) Higher-power images demonstrating selective leakage of the dentate gyrus in neighboring sections. (C1–C2) In the regions of Evans blue extravasation, neuronal uptake (C1) was frequently observed. Note that the “filling” of the cell extends to both basal and apical dendrites of these CA1 pyramidal cells. C2 shows vascular profiles and surrounding leakage.
A total of three blood–brain barrier disruptions were performed on two animals. Identical procedures and personnel were used. EEG electrodes were positioned on the animal’s skull before BBBD as indicated in the insets. Fig. 3 shows the result of an experiment where the success of the BBBD was evaluated by measurements of S100β immediatelly before and after mannitol infusion and also by extravasation of Evans blue. The first procedure was performed on the right hemisphere, while the second was performed on the left. There was no Evans blue extravasation following the first procedure as judged by postmortem inspection of the hemisphere. Consistent with this finding was the fact that S100β levels did not change after mannitol injection (data not shown). Based on these considerations, the “goodness of opening” was deemed to be nil, according to the scale used for patient evaluation. During and after this first procedure, there were no significant EEG changes suggestive of cortical synchronization or seizure-like activity. However, there were significant EEG changes seconds after mannitol injection regardless of the site of injection (see arrows in EEG tracing in Fig. 3A). The first change was a bilateral “flattening” of EEG signals. This was not due to a filtering artifact, since reduction of EEG signal was similarly observed on unfiltered traces (see, e.g., Fig. 4). The mechanism of this phenomenon is unknown.
FIG. 4.
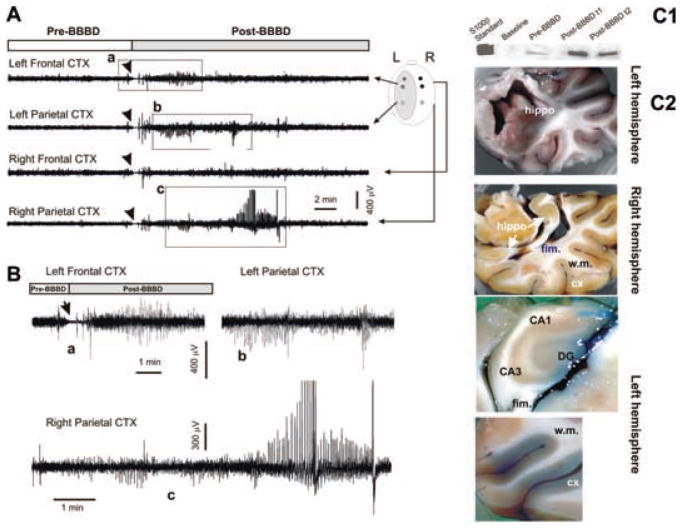
EEG correlates of acute blood–brain barrier disruption: a widespread increase in BBB permeability leads to seizures. (A) EEG recordings revealed a sharp increase in activity after BBBD in this animal. The BBBD was performed on the left hemisphere where activity was predominant. Motor seizures predominated on the right side. The traces in (B) are magnified segments as indicated by the dashed boxes in A. The arrows point to the EEG slowing that followed mannitol infusion (see also Fig. 4). Filters used for viewing during collection were 0.08 and 300 Hz (low pass and high pass, respectively); sampling rate was 1000 Hz. (C1–C2) Serum S100β increased occurred immediately post-BBBD, as assessed by western blot. Morphological demonstration of successful hemispheric blood–brain barrier disruption by Evans blue staining. Note the ubiquitous leakage in the left hemisphere and the absence of extravasation in the right. w.m: white matter; DG: dentate gyrus; fim: fimbria.
The second procedure was more successful and small, patchy leaky spots of Evans blue were observed in gray and white matter regions of the interested hemisphere (BBBD2 in Fig. 3D). After BBBD2, the only changes observed consisted of spike-wave complexes that were seen in both left (disrupted) and right (undisrupted) hemispheres (arrows in Fig. 3B). These changes were not accompanied by any significant behavioral seizure. Fig. 3C depicts the radiological appearance of the cerebral vasculature and the positioning of electrodes.
In a subsequent trial on a different animal, an excellent hemispheric (left) blood–brain barrier disruption was obtained after a single mannitol infusion (Fig. 4). This was evident by inspection of postmortem gross brain anatomy (Fig. 4C1) and by comparing serum S100β changes after the BBBD procedure (Fig. 4C2). The hippocampus and cortex of the disrupted hemisphere were stained with Evans blue, while the white matter appeared largely unstained. Electrophysiologically, the early EEG flattening was identical to the changes seen during other, less successful procedures (e.g., Fig. 3). Immediately after EEG suppression, high frequency and high amplitude signals appeared ispilateral to the disrupted hemisphere (Fig. 4A–B). These rapidly spread to the contralateral hemisphere. During this time, the animal experienced obvious motor seizures characterized by head elevation and body/limb extension.
As a complement to the S100β analysis as means of measurement of the level of BBBD, vascular extravasation of Evans blue was evaluated by fluorescent microscopy. The red signal shown in Fig. 5 depicts the vascular and parenchymal staining of autofluorescent Evans blue bound to albumin. Note that albumin extravasation in the left (BBBD) hemisphere was much more prominent compared to the right. Interestingly, albumin cellular uptake was seen in neurons.
DISCUSSION
The main finding of our work is that failure of the endothelial protection of the CNS can lead to acute seizures, occurring contralateral to the hemisphere where the blood–brain barrier is disrupted. This implies a spatial relationship between the area of BBBD and the focal generation of epileptogenicity. The novel aspect of our research is the fact that we were able for the first time to correlate the extent and location of BBB damage to the probability of abnormal brain responses. Furthermore, we are the first to demonstrate that iatrogenic seizures occur due to BBBD and not underlying medical conditions such as tumor or chemotherapy. Finally, this is the first study to compare in a controlled study specifically designed for this purpose, human data with data obtained in a large animal model.
Human versus animal studies
In patients, seizures occurred immediately after blood–brain barrier disruption in approximately one quarter of the procedures. This happened in spite of heavy premedication with anesthetic dosages of the antiepileptic drug thiopental. There was no significant correlation of seizure occurrence with age, gender, tumor size or site, AED regimen, or number of prior BBBD treatments. The only predictors of motor seizures were positive indices of blood–brain barrier disruption, namely serum S100β levels and radiological evaluation by contrast-enhanced CT scans. The results from human data were confirmed in a porcine model of osmotic BBB disruption, and therefore in the absence of potential confounders such as chemotherapy and presence of lymphoma. The most parsimonious explanation of our findings is that acute blood–brain barrier openings may lead to electrographic and behavioral seizures.
In humans, seizures were detected only by direct behavioral examination, since EEG recordings were very difficult to perform on patients undergoing CT angiographic procedures followed by contrast CT scans. We were, however, able to successfully record the correlates of these behavioral seizures in pigs, where electrographic seizures were recorded concomitant to behavioral changes. In addition, previous animal studies by others (Fieschi et al., 1980) have shown that intracarotid mannitol provokes electrographic seizures characterized by spike/wave complexes and synchronization of neuronal activity as detected by high-voltage low-frequency spiking. Taken together, these findings support the hypothesis that electrographic and behavioral seizures can be induced by disruption of the blood–brain barrier independent from other CNS pathologies (tumor) or chemotherapy.
There are at least two factors other than blood–brain barrier failure that may have accounted for the observed results. First, mannitol may be per se epileptogenic, or neuronal excitability could be affected by increased brain osmolarity. This seems highly unlikely since increased osmolarity reduces neuronal excitability (Schwartzkroin et al., 1998). Mannitol has been recently shown to exert antiepileptic actions (Haglund and Hochman, 2005) and has been used for many years, albeit at lower concentrations, to prevent complications after a variety of brain insults. In addition, it is possible that methotrexate may have caused direct brain toxicity leading to seizures. While this appears to be highly unlikely given the fact that chemotherapy was administered in human after seizures developed and was not employed in the porcine model of BBBD, it must nevertheless be taken into account given a recent report of status epilepticus following intraventricular injection of the drug (Naing et al., 2005). Interestingly, the authors of this study suggested that the effect of methotrexate may be due to its deleterious effects on blood–brain barrier permeability (Phillips et al., 1987) and not via a direct effect on neurons, further supporting our hypothesis linking BBB failure to proepileptic changes in neuronal excitability.
BBB and seizures
Although the blood–brain barrier prevents the penetration of many blood constituents into the brain extracellular space, the effects of blood–brain barrier failure in the pathogenesis of cortical diseases are unknown (for a review, see Grant and Janigro (2004)). One of the main confounding factors has always been the difficulty of finding an exact cause-relationship framework encompassing BBB opening and onset of disease. This is particularly obvious when dealing with seizure disorders, since it is known that seizures cause breakdown of the BBB (Janigro, 1999). That human blood–brain barrier disruption causes behavioral and electrographic seizures has not been directly demonstrated before this study, but there are several reports that support an etiological link between blood–brain barrier failure and proepileptogenic CNS changes; some of these have been reviewed elsewhere (Janigro, 1999). Seiffert and colleagues (Seiffert et al., 2004) have shown that chronic, focal opening of the BBB causes reactive changes in the brain that lead to abnormal excitability. Furthermore, Korn et al., have recently shown that focal cortical dysfunction occurs in conjunction with BBB disruption and other vascular changes in postconcussion syndrome, a condition that is known to be epileptogenic (Korn et al., 2005). There is at least one epileptic pathology, Alexander’s disease, characterized clinically by development of megalencephaly in infancy accompanied by progressive spasticity, seizures, and dementia (Alexander, 1949). The molecular mechanism involved is a gain of function mutation of the gene that encodes GFAP (Mignot et al., 2004; Li et al., 2005). Interestingly, altered GFAP expression leads to delayed formation of the BBB (Pekny et al., 1998), a finding confirmed radiologically in these patients (Shiihara et al., 2002). These findings suggest that disruption of the BBB may be a predisposing or etiologically relevant player in epileptogenesis. An alternative hypothesis is that widespread BBB opening could facilitate not only acute seizure but also an enduring neuronal hyperexcitability.
BBB, seizures, and brain tumors
An obvious caveat of the human study presented here is the fact that all patients were affected by PCNSL and were thus at risk for pre- or proepileptogenic changes regardless of their BBB status. In fact, most of the patients in the study received antiepileptic drugs to prevent tumor-induced seizures. Based on the animal study performed concomitantly to the human investigation, we are confident that presence of tumors was not an essential component of the acute seizure. In addition, we are convinced that tumorigenesis did not affect the interpretation of our results inasmuch as: (1) There was no correlation between tumor size and BBBD-induced seizures; (2) There was no correlation between stages of treatment (e.g., large tumor or recurrence, or no radiologically visible tumor) and BBBD-associated seizures; (3) There was no correlation between tumor location and propensity toward seizures; and (4) Patients receiving prophylactic AEDs did not have a significantly higher risk than those who did not (data not shown). Finally, in humans, seizures developed with one exception, contralateral to the site of BBBD and independent of tumor location. Seizures were seen predominantly when the anterior circulation was affected, supporting a direct link between the site of blood–brain barrier failure and abnormal activation of the motor cortex.
Sudden onset of unprovoked seizures is not necessarily related to preexisting central nervous system pathologies, as assessed by CT scan and MRI, but are a consequence of trauma, high temperature, or iatrogenic interventions (Newburger et al., 1993; Frey, 2003; Gaynor et al., 2005; Clancy et al., 2005). Thus, seizures represent a “nonspecific” pathological response of neurons to a broad variety of prodromic stimuli. Occurrence of a seizure in the early postoperative period after surgical procedures is a marker for a central nervous system injury and has been associated with adverse neurodevelopmental sequelae (Newburger et al., 1993; Clancy et al., 2005; Gaynor et al., 2005). Our hypothesis is that failure of the blood–brain barrier represents a crucial event leading to entry into the brain of systemic factors normally excluded from the brain, thus contributing to the onset of CNS pathologies such as seizures. The present data, together with previous independent reports (Fieschi et al., 1980; Seiffert et al., 2004), show that BBB damage facilitates the onset of seizures. Damage of the BBB occurs in response to head injury, stroke, or acute systemic inflammation. Under these circumstances, it is plausible that bloodborne substances, including normal blood constituents (e.g., glutamate, potassium), disease-related products (e.g., cytokines), xenobiotics, or heavy metals, can alter brain homeostasis and neuronal activity.
In conclusion, we have shown that acute, transient openings of the blood–brain barrier lead to behavioral and electrographic seizures that correlate with the degree of BBB disruption. We suggest that cerebrovascular changes may be an important etiologic cofactor in a variety of seizure disorders.
Acknowledgments
This work was supported in part by the National Institutes of Health (NIH-NS43284, NIH-HL51614, NIH-NS46513, NIH-NS049514, and NIH-NS38195) to DJ. We would also like to acknowledge Drs. Marco De Curtis and Giorgio Battaglia for insightful discussion on the role of the blood–brain barrier in epileptogenesis. We would like to thank Dr. Marianna Bugiani for helpful comments on the manuscript.
References
- Alexander WS. Progressive fibrinoid degeneration of fibrillary astrocytes associated with mental retardation in a hydrocephalic infant. Brain. 1949;72:373–381. doi: 10.1093/brain/72.3.373. [DOI] [PubMed] [Google Scholar]
- Armstrong BK, Smith QR, Rapoport SI, Strohalm J, Kopacek J, Duncan R. Osmotic opening of the blood-brain barrier permeability to N-(2-hydroxypropyl) methacrylamide copolymers. Effects of polymer m.w., charge and hydrophobicity. Journal of Controlled Release. 1989;10:27–35. [Google Scholar]
- Clancy RR, Sharif U, Ichord R, Spray TL, Nicolson S, Tabbutt S, Wernovsky G, Gaynor JW. Electrographic neonatal seizures after infant heart surgery. Epilepsia. 2005;46:84–90. doi: 10.1111/j.0013-9580.2005.22504.x. [DOI] [PubMed] [Google Scholar]
- Fazio V, Bhudia SK, Marchi N, Aumayr B, Janigro D. Peripheral detection of S100beta during cardiothoracic surgery: what are we really measuring? The Annals of Thoracic Surgery. 2004;78:46–52. doi: 10.1016/j.athoracsur.2003.11.042. [DOI] [PubMed] [Google Scholar]
- Fieschi C, Lenzi GL, Zanette E, Orzi F, Passero S. Effects on EEG of the osmotic opening of the blood-brain barrier in rats. Life Sciences. 1980;27:239–243. doi: 10.1016/0024-3205(80)90143-5. [DOI] [PubMed] [Google Scholar]
- Frey LC. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44(suppl 10):11–17. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- Gaynor JW, Nicolson SC, Jarvik GP, Wernovsky G, Montenegro LM, Burnham NB, Hartman DM, Louie A, Spray TL, Clancy RR. Increasing duration of deep hypothermic circulatory arrest is associated with an increased incidence of postoperative electroencephalographic seizures. The Journal of Thoracic and Cardiovascular Surgery. 2005;130:1278–1286. doi: 10.1016/j.jtcvs.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant GA, Janigro D. The blood-brain barrier. In: Winn HR, editor. Youmans neurological surgery. Saunders; Philadelphia, PA: 2004. pp. 153–174. [Google Scholar]
- Greig NH, Daly EM, Sweeney DJ, Rapoport SI. Pharmacokinetics of chlorambuciltertiary butyl ester, a lipophilic chlorambucil derivative that achieves and maintains high concentrations in brain. Cancer Chemotherapy and Pharmacology. 1990;25:320–325. doi: 10.1007/BF00686230. [DOI] [PubMed] [Google Scholar]
- Haglund MM, Hochman DW. Furosemide and mannitol suppression of epileptic activity in the human brain. Journal of Neurophysiology. 2005;94:907–918. doi: 10.1152/jn.00944.2004. [DOI] [PubMed] [Google Scholar]
- Janigro D. Blood-brain barrier, ion homeostasis and epilepsy: possible implications towards the understanding of ketogenic diet mechanisms. Epilepsy Research. 1999;37:223–232. doi: 10.1016/s0920-1211(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Jaranyi Z, Szekely M, Bobek I, Galfy I, Geller L, Selmeci L. Impairment of blood-brain barrier integrity during carotid surgery as assessed by serum S-100B protein concentrations. Clinical Chemistry and Laboratory Medicine. 2003;41:1320–1322. doi: 10.1515/CCLM.2003.201. [DOI] [PubMed] [Google Scholar]
- Kapural M, Krizanac-Bengez L, Barnett G, Perl J, Masaryk T, Apollo D, Rasmussen P, Mayberg MR, Janigro D. Serum S-100beta as a possible marker of blood-brain barrier disruption. Brain Research. 2002;940:102–104. doi: 10.1016/s0006-8993(02)02586-6. [DOI] [PubMed] [Google Scholar]
- Korn A, Golan H, Melamed I, Pascual-Marqui R, Friedman A. Focal cortical dysfunction and blood-brain barrier disruption in patients with Postconcussion syndrome. Journal of Clinical Neurophysiology. 2005;22:1–9. doi: 10.1097/01.wnp.0000150973.24324.a7. [DOI] [PubMed] [Google Scholar]
- Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42:1083–1099. doi: 10.1097/00006123-199805000-00082. [DOI] [PubMed] [Google Scholar]
- Li R, Johnson AB, Salomons G, Goldman JE, Naidu S, Quinlan R, Cree B, Ruyle SZ, Banwell B, D’Hooghe M, Siebert JR, Rolf CM, Cox H, Reddy A, Gutierrez-Solana LG, Collins A, Weller RO, Messing A, van der Knaap MS, Brenner M. Glial fibrillary acidic protein mutations in infantile, juvenile, and adult forms of Alexander disease. Annals of Neurology. 2005;57:310–326. doi: 10.1002/ana.20406. [DOI] [PubMed] [Google Scholar]
- Marchi N, Cavaglia M, Bhudia S, Hallene K, Janigro D. Peripheral markers of blood-brain barrier damage. Clinica Chimica Acta. 2004;342:1–12. doi: 10.1016/j.cccn.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Marchi N, Fazio V, Cucullo L, Kight K, Masary KT, Barnett G, Vogelbaum M, Kinter M, Rasmussen P, Mayberg MR, Janigro D. Serum transthyretin as a possible marker of blood-to-CSF barrier disruption. Journal of Neuroscience. 2003a;23:1949–1955. doi: 10.1523/JNEUROSCI.23-05-01949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Rasmussen PA, Kapural M, Fazio V, Cavaglia M, Janigro D. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restorative Neurology and Neuroscience. 2003b;21:109–121. [PMC free article] [PubMed] [Google Scholar]
- Mignot C, Boespflug-Tanguy O, Gelot A, Dautigny A, Pham-Dinh D, Rodriguez D. Alexander disease: putative mechanisms of an astrocytic encephalopathy. Cellular and Molecular Life Sciences. 2004;61:369–385. doi: 10.1007/s00018-003-3143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussack T, Biberthaler P, Geisenberger T, Gippner-Steppert C, Steckmeier B, Mutschler W, Jochum M. Assessment of early brain damage in carotid endarterectomy: evaluation of S-100B serum levels and somatosensory evoked potentials in a pilot study. World Journal of Surgery. 2002;26:1251–1255. doi: 10.1007/s00268-002-6547-6. [DOI] [PubMed] [Google Scholar]
- Naing A, Luong D, Extermann M. Methotrexate-induced status epilepticus. American Journal of Hematology. 2005;80:35–37. doi: 10.1002/ajh.20365. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Glasberg M, Frenkel E, Barnett P. Neurotoxicity of chemotherapeutic agents after blood-brain barrier modification: neuropathological studies. Annals of Neurology. 1983a;14:316–324. doi: 10.1002/ana.410140310. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Goldman DL, Dahlborg SA, Crossen J, Ramsey F, Roman-Goldstein S, Braziel R, Dana B. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: prolonged survival and preservation of cognitive function. Journal of Clinical Oncology. 1991;9:1580–1590. doi: 10.1200/JCO.1991.9.9.1580. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Specht HD, Howieson J, Haines JE, Bennett MJ, Hill SA, Frenkel EP. Osmotic blood-brain barrier modification: clinical documentation by enhanced CT scanning and/or radionuclide brain scanning. AJR American Journal of Roentgenology. 1983b;141:829–835. doi: 10.2214/ajr.141.4.829. [DOI] [PubMed] [Google Scholar]
- Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Kuban KC, Farrell DM, Holmes GL, Helmers SL, Constantinou J. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. The New England Journal of Medicine. 1993;329:1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- Pekny M, Stanness KA, Eliasson C, Betsholtz C, Janigro D. Impaired induction of blood-brain barrier properties in aortic endothelial cells by astrocytes from GFAP-deficient mice. Glia. 1998;22:390–400. [PubMed] [Google Scholar]
- Phillips PC, Dhawan V, Strother SC, Sidtis JJ, Evans AC, Allen JC, Rottenberg DA. Reduced cerebral glucose metabolism and increased brain capillary permeability following high-dose methotrexate chemotherapy: a positron emission tomographic study. Annals of Neurology. 1987;21:59–63. doi: 10.1002/ana.410210111. [DOI] [PubMed] [Google Scholar]
- Roman-Goldstein S, Clunie DA, Stevens J, Hogan R, Monard J, Ramsey F, Neuwelt EA. Osmotic blood-brain barrier disruption: CT and radionuclide imaging. AJNR American Journal of Neuroradiology. 1994a;15:581–590. [PMC free article] [PubMed] [Google Scholar]
- Roman-Goldstein SM, Barnett PA, McCormick CI, Ball MJ, Ramsey F, Neuwelt EA. Effects of gadopentetate dimeglumine administration after osmotic blood- brain barrier disruption: toxicity and MR imaging findings. AJNR American Journal of Neuroradiology. 1991;12:885–890. [PMC free article] [PubMed] [Google Scholar]
- Roman-Goldstein SM, Barnett PA, McCormick CI, Szumowski J, Shannon EM, Ramsey FL, Mass M, Neuwelt EA. Effects of Gd-DTPA after osmotic BBB disruption in a rodent model: toxicity and MR findings. Journal of Computer Assisted Tomography. 1994b;18:731–736. doi: 10.1097/00004728-199409000-00010. [DOI] [PubMed] [Google Scholar]
- Schmidt RH, Grady MS. Regional patterns of blood-brain barrier breakdown following central and lateral fluid percussion injury in rodents. Journal of Neurotrauma. 1993;10:415–430. doi: 10.1089/neu.1993.10.415. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Baraban SC, Hochman DW. Osmolarity, ionic flux, and changes in brain excitability. Epilepsy Research. 1998;32:275–285. doi: 10.1016/s0920-1211(98)00058-8. [DOI] [PubMed] [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. Journal of Neuroscience. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendrowski K, Sobaniec W, Sobaniec-Lotowska ME, Lewczuk P. S-100 protein as marker of the blood-brain barrier disruption in children with internal hydrocephalus and epilepsy–a preliminary study. Roczniki Akademii Medycznej w Bialymstoku. 2004;49(suppl 1):236–238. [PubMed] [Google Scholar]
- Shiihara T, Kato M, Honma T, Ohtaki S, Sawaishi Y, Hayasaka K. Fluctuation of computed tomographic findings in white matter in Alexander’s disease. Journal of Child Neurology. 2002;17:227–230. doi: 10.1177/088307380201700316. [DOI] [PubMed] [Google Scholar]
- Vogelbaum MA, Masaryk T, Mazzone P, Mekhail T, Fazio V, McCartney S, Marchi N, Kanner A, Janigro D. S100beta as a predictor of brain metastases: brain versus cerebrovascular damage. Cancer. 2005;104:817–824. doi: 10.1002/cncr.21220. [DOI] [PubMed] [Google Scholar]