Abstract
Background: Heart failure (HF) is a major source of morbidity and mortality, particularly among the elderly. Magnesium, phosphorus, and calcium are micronutrients traditionally viewed in relation to bone health or chronic kidney disease. However, they also may be associated with risk of cardiovascular disease through a broad range of physiologic roles.
Objective: With the use of data from the Atherosclerosis Risk in Communities (ARIC) cohort, we tested the hypotheses that the incidence of HF is greater among individuals with low serum magnesium and those with high serum phosphorus and calcium.
Design: A total of 14,709 African Americans (27%) and whites from the ARIC cohort [aged 45–64 y at baseline (1987–1989)] were observed through 2009. Proportional hazards regression was used to explore associations between biomarkers and incident HF. Serum calcium was corrected for serum albumin. Models were adjusted for demographics, behaviors, and physiologic characteristics.
Results: A total of 2250 incident HF events accrued over a median follow-up of 20.6 y. Participants in the lowest (≤1.4 mEq/L) compared with the highest (≥1.8 mEq/L) category of magnesium were at greater HF risk (HR: 1.71; 95% CI: 1.46, 1.99). For phosphorus, there appeared to be a threshold whereby only those in the highest quintile were at greater HF risk [HR(Q5 vs Q1): 1.34; 95% CI: 1.16, 1.54]. Higher concentrations of calcium were also associated with greater risk of HF [HR(Q5 vs Q1): 1.24; 95% CI: 1.07, 1.43]. Results were not modified by race, sex, or kidney function and were similar when incident coronary heart disease was included as a time-varying covariate.
Conclusions: Low serum magnesium and high serum phosphorus and calcium were independently associated with greater risk of incident HF in this population-based cohort. Whether these biomarkers will be useful candidates for HF risk prediction or targets for prevention remains to be seen.
INTRODUCTION
Heart failure (HF)4 is a common cause of morbidity and mortality in the developed world. At 40 y of age, the lifetime risk for developing HF is 20% for both men and women (1). Although some risk factors for HF are firmly established [eg, increasing age, hypertension, diabetes, and antecedent myocardial infarction (MI)] (2), given the high societal burden of HF, there is interest in identifying new characteristics that may be associated with HF development.
Magnesium, phosphorus, and calcium are micronutrients traditionally viewed in relation to bone health or chronic kidney disease. However, they also may be associated with the risk of cardiovascular disease (CVD). Magnesium is believed to be linked to CVD risk through a broad range of physiologic roles; low serum concentrations have been associated with impaired glucose homeostasis and insulin action, elevated blood pressure, chronic inflammation, impaired vasomotor tone and peripheral blood flow, and electrocardiogram abnormalities (3). Elevated serum phosphorus is hypothesized to influence CVD risk through vascular calcification (4), myocardial fibrosis (5), and development of left ventricular hypertrophy (6). Higher serum calcium concentrations may promote CVD and atherogenesis through vascular calcification and increased coagulability (7, 8).
Previous studies have explored the relation of magnesium, phosphorus, and calcium to risk of CVD risk factors (4, 6, 9–13) and other CVD phenotypes (14–23), but relatively little is known about the association of these micronutrients to the risk of HF (13, 24). Using data from the Atherosclerosis Risk in Communities (ARIC) cohort, we tested the hypotheses that the incidence of HF is greater among individuals with low concentrations of serum magnesium and high concentrations of serum phosphorus and calcium.
MATERIALS AND METHODS
Study population
The ARIC Study (http://www2.cscc.unc.edu/aric/) is a multicenter population-based prospective cohort study that includes 15,792 predominantly white and black men and women who were aged 45–64 y in 1987–1989 (visit 1) and were recruited from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland (25). Four cohort reexaminations have taken place: 1990–1992, 1993–1995, 1996–1998, and 2011–2013. Local institutional review boards approved the ARIC protocol, and all participants gave informed consent.
For this analysis, we excluded participants who had prevalent HF at baseline (n = 752) or were missing information needed to ascertain HF prevalence (n = 287), as well as those who were neither African American nor white, and African Americans from the Minnesota and Maryland centers due to low numbers (n = 44). Our final analytic sample included 14,709 participants.
Exposure and covariate measurement
At baseline, ARIC participants underwent interviews, fasting venipuncture, and measurement of blood pressure and anthropometrics. Trained interviewers ascertained basic demographic data, medical history, and information about personal habits, such as diet, smoking status, physical activity, and medication use. Participants were asked to bring all medications, vitamins, and supplements taken in the 2 wk before the examination; all medication names were transcribed and coded. Height and weight were measured, with BMI calculated as weight (in kg) divided by the square of height (in m). Sitting blood pressure was measured in triplicate with a random-zero sphygmomanometer; the average of the latter 2 measurements were used in this analysis. Diabetes was defined by fasting blood glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, a self-report of physician diagnosis, or current medication use for diabetes.
Fasting (12-h) blood samples were drawn, and plasma and serum were frozen at −70°C until analyzed (26). Serum magnesium was measured by the Gindler and Heth procedure by using the metallochromic dye calmagite [1-(1-hydroxy-4-methyl-2-phenylazo)-2-napthol-4sulfonic acid]. Using split samples sent 1 wk apart, the within-participant laboratory CV was 3.6%. Serum phosphorus was quantified by using methods based on ammonium molybdate (CV: 7.6%), serum calcium with an approach based on o-cresolphthalein complexone (CV: 1.1%), serum albumin with a bromocresol green colorimetric assay (CV: 2.8%), and serum creatinine by using a modified kinetic Jaffe–picric acid (CV: 3.7%). As is typically done in clinical settings, serum calcium was adjusted for serum albumin by using the following equation: corrected calcium = measured total calcium (mg/dL) + 0.8 [4.0 − serum albumin (g/dL)]. Throughout the article, all results reported for calcium are based on the serum albumin–corrected variable. Measurement of serum magnesium, phosphorus, and calcium was repeated at visit 2 by using similar methods. Estimated glomerular filtration rate was calculated by using serum creatinine (27) then categorized according to established clinical cutoffs: ≥90, 60–89, and 15–59 mL · min–1 · 1.73 m–2.
HF and coronary heart disease ascertainment
Prevalent HF at baseline was defined as the following: 1) an affirmative response to “Were any of the medications you took during the last 2 weeks for heart failure?” or 2) stage 3 or “manifest heart failure” by Gothenburg criteria (28, 29). Prevalent coronary heart disease (CHD) was defined by self-reported previous physician diagnosis of MI or coronary revascularization, or prevalent MI by 12-lead electrocardiogram.
Incident cardiovascular events through 31 December 31 2009 were identified through annual telephone calls to ARIC cohort participants to identify all hospitalizations, review of local hospital discharge indexes, and retrieval of death certificates. HF incidence was defined as the first occurrence of either a hospitalization that included an International Classification of Diseases, 9th Revision (ICD-9) discharge code of 428 (428.0–428.9) among the primary or secondary diagnoses or else a death certificate with an ICD-9 code of 428 or an International Classification of Diseases, 10th Revision code of I50 among any of the listed diagnoses or underlying causes of death (29). ARIC has shown the validity of ICD-9 code 428 to be moderately high compared with medical record review, with a sensitivity of 93% for identifying acute decompensated heart failure (30). Incident CHD was defined by a definite or probable MI, coronary angioplasty, coronary artery bypass surgery, or CHD death, according to previously described procedures (31).
Data analysis
Baseline characteristics of participants are described by using means and proportions stratified by levels of the exposures. Cox proportional hazards regression was used to explore associations between serum magnesium, phosphorus, calcium, and risk of incident HF. Restricted cubic splines were used to visually depict the associations. In the spline models, biomarker values were truncated at the 1st and 99th percentiles to minimize the influence of extreme values at either tail of the distributions. Serum magnesium values were reported to only one decimal place; therefore, we were not able to categorize serum magnesium as equal quintiles. As such, a priori, we categorized serum magnesium in a manner that most closely approximated quintiles. The highest category was used as the reference. Serum phosphorus and calcium were modeled as quintiles, with the lowest group being the reference. P values are reported for the linear trends across the quintiles.
Our first model adjusted for age, sex, race, and ARIC field center. Model 2 also adjusted for baseline behavioral characteristics (education, physical activity, and smoking status) and BMI. Model 3 further adjusted for prevalent diabetes and CHD, as well as baseline estimated glomerular filtration rate categories, systolic blood pressure, any hypertension medication use in the past 2 wk, LDL cholesterol, HDL cholesterol, triglycerides, and antihyperlipidemic medication use in the past 2 wk. In model 4, incident CHD was included as a time-dependent covariate.
Interaction terms were used to evaluate whether race, sex, hypertension, diabetes, kidney function, or prevalent MI modified the associations of serum magnesium, phosphorus, and calcium to the risk of incident HF. The proportional hazards assumption was evaluated quantitatively by testing the interaction between the biomarkers and ln(time) and qualitatively by inspection of ln(−ln) survival curves for biomarker categories. For serum magnesium, sensitivity analyses were conducted adjusting for alcohol intake (g/d) as a covariate, because heavy alcohol intake is associated with low magnesium concentrations. For both magnesium and calcium, sensitivity analyses excluded participants taking diuretics (n = 2114), because diuretics have been shown to lower serum magnesium and calcium concentrations.
RESULTS
Our final analytic sample included 14,709 ARIC participants who at baseline had a mean age of 54.1 y and were 54.5% female and 26.5% African American. The mean ± SD concentration was 1.63 ± 0.16 mEq/L for serum magnesium, 3.43 ± 0.49 mg/dL for serum phosphorus, and 9.88 ± 0.42 mg/dL for serum calcium. These biomarkers were weakly correlated (although all P < 0.0001 given ARIC's large sample size): r = 0.05 for magnesium and phosphorus, r = −0.06 for magnesium and calcium, and r = 0.16 for phosphorus and calcium.
Baseline characteristics of participants stratified by categories of serum magnesium, phosphorus, and calcium are presented in Table 1 and Supplemental Tables 1 and 2 (under “Supplemental data” in the online issue), respectively. In brief, participants with higher concentrations of serum magnesium tended to be male, tended to be white, and overall had a more favorable cardiovascular risk factor profile. Conversely, participants with higher concentrations of serum phosphorus and serum calcium tended to be female, tended to be African American, and overall had a worse cardiovascular risk profile.
TABLE 1.
Participant characteristics by serum magnesium category: the ARIC Study, 1987–1989 (N = 14,709)1
| Category |
||||||
| Characteristic | 1 (n = 1719) | 2 (n = 2375) | 3 (n = 3770) | 4 (n = 3660) | 5 (n = 3185) | P-trend |
| Magnesium (mEq/L)2 | 1.4 (0.5–1.4) | 1.5 (1.5–1.5) | 1.6 (1.6–1.6) | 1.7 (1.7–1.7) | 1.8 (1.8–3.1) | |
| Demographic charateristics | ||||||
| Age (y) | 54.1 ± 5.9 | 53.8 ± 5.8 | 53.9 ± 5.8 | 54.1 ± 5.7 | 54.3 ± 5.6 | 0.01 |
| Female | 1012 (58.9) | 1385 (58.3) | 2038 (54.1) | 1942 (53.0) | 1664 (52.2) | <0.0001 |
| African American | 904 (52.6) | 782 (32.9) | 959 (25.4) | 697 (19.0) | 560 (17.6) | <0.0001 |
| Education | <0.0001 | |||||
| <High school | 585 (34.1) | 610 (25.7) | 815 (21.7) | 768 (21.0) | 624 (19.6) | |
| High school | 615 (35.9) | 992 (41.8) | 1564 (41.6) | 1511 (41.3) | 1320 (41.5) | |
| >High school | 514 (30.0) | 770 (32.5) | 1385 (36.8) | 1377 (37.7) | 1236 (38.9) | |
| Behavioral characteristics | ||||||
| Sport index | 2.31 ± 0.76 | 2.36 ± 0.77 | 2.46 ± 0.81 | 2.49 ± 0.80 | 2.49 ± 0.80 | <0.0001 |
| Smoking status | 0.06 | |||||
| Current | 503 (29.3) | 608 (25.6) | 989 (26.3) | 942 (25.7) | 772 (24.3) | |
| Former | 514 (30.0) | 748 (31.6) | 1195 (31.7) | 1188 (32.5) | 1076 (33.8) | |
| Never | 699 (40.7) | 1015 (42.8) | 1583 (42.0) | 1529 (41.8) | 1335 (41.9) | |
| Alcohol (drinks/wk) | 7.6 ± 13.0 | 5.7 ± 9.3 | 5.5 ± 8.9 | 5.6 ± 8.4 | 5.6 ± 8.5 | 0.001 |
| Physiologic characteristics | ||||||
| BMI (kg/m2) | 29.2 ± 6.2 | 28.3 ± 5.7 | 27.4 ± 5.2 | 27.1 ± 4.8 | 26.9 ± 4.7 | <0.0001 |
| Prevalent diabetes | 476 (29.4) | 345 (14.6) | 383 (10.2) | 250 (6.8) | 170 (5.3) | <0.0001 |
| Prevalent hypertension | 900 (52.5) | 936 (39.5) | 1169 (31.0) | 977 (26.8) | 839 (26.4) | <0.0001 |
| Systolic BP (mm Hg) | 127 ± 21 | 123 ± 19 | 121 ± 19 | 119 ± 18 | 119 ± 18 | <0.0001 |
| Hypertension medication | 700 (40.8) | 665 (28.1) | 811 (21.5) | 657 (18.0) | 548 (17.2) | <0.0001 |
| Diuretic use | 428 (24.9) | 389 (16.4) | 514 (13.6) | 403 (11.0) | 347 (10.9) | <0.0001 |
| Lipid-lowering medication | 42 (2.5) | 69 (2.9) | 101 (2.7) | 94 (2.6) | 90 (2.8) | 0.78 |
| HDL cholesterol (mg/dL) | 51.1 ± 18.7 | 51.4 ± 17.0 | 51.9 ± 17.3 | 52.1 ± 16.8 | 51.9 ± 16.3 | 0.06 |
| LDL cholesterol (mg/dL) | 133 ± 42 | 135 ± 39 | 137 ± 40 | 139 ± 38 | 141 ± 39 | <0.0001 |
| Triglycerides (mg/dL) | 158 ± 130 | 135 ± 91 | 129 ± 84 | 123 ± 76 | 122 ± 76 | <0.0001 |
| eGFR (mL · min–1 · 1.73 m–2) | 100.1 ± 18.2 | 97.9 ± 15.3 | 96.3 ± 14.5 | 94.8 ± 13.6 | 93.3 ± 14.4 | <0.0001 |
| eGFR category | <0.0001 | |||||
| >90 mL · min–1 · 1.73 m–2 | 1196 (74.9) | 1752 (73.8) | 2639 (70.0) | 2493 (68.1) | 2066 (65.1) | |
| 60–90 mL · min–1 · 1.73 m–2 | 355 (22.2) | 597 (24.7) | 1084 (28.8) | 1132 (30.9) | 1042 (32.8) | |
| <60 mL · min–1 · 1.73 m–2 | 46 (2.9) | 35 (1.5) | 45 (1.2) | 34 (0.9) | 66 (2.1) | |
| Calcium3 (mg/dL) | 9.97 ± 0.46 | 9.89 ± 0.42 | 9.87 ± 0.40 | 9.86 ± 0.40 | 9.87 ± 0.41 | <0.0001 |
| Phosphorus (mg/dL) | 3.43 ± 0.52 | 3.39 ± 0.48 | 3.42 ± 0.49 | 3.42 ± 0.48 | 3.47 ± 0.51 | <0.0001 |
| Prevalent CHD | 92 (5.4) | 99 (4.2) | 144 (3.8) | 138 (3.8) | 127 (4.0) | 0.04 |
Values are means ± SDs for continuous variables and n (%) for categorical variables unless indicated otherwise. ARIC, Atherosclerosis Risk in Communities; BP, blood pressure; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate.
Values are medians; ranges in parentheses.
Corrected for serum albumin concentration.
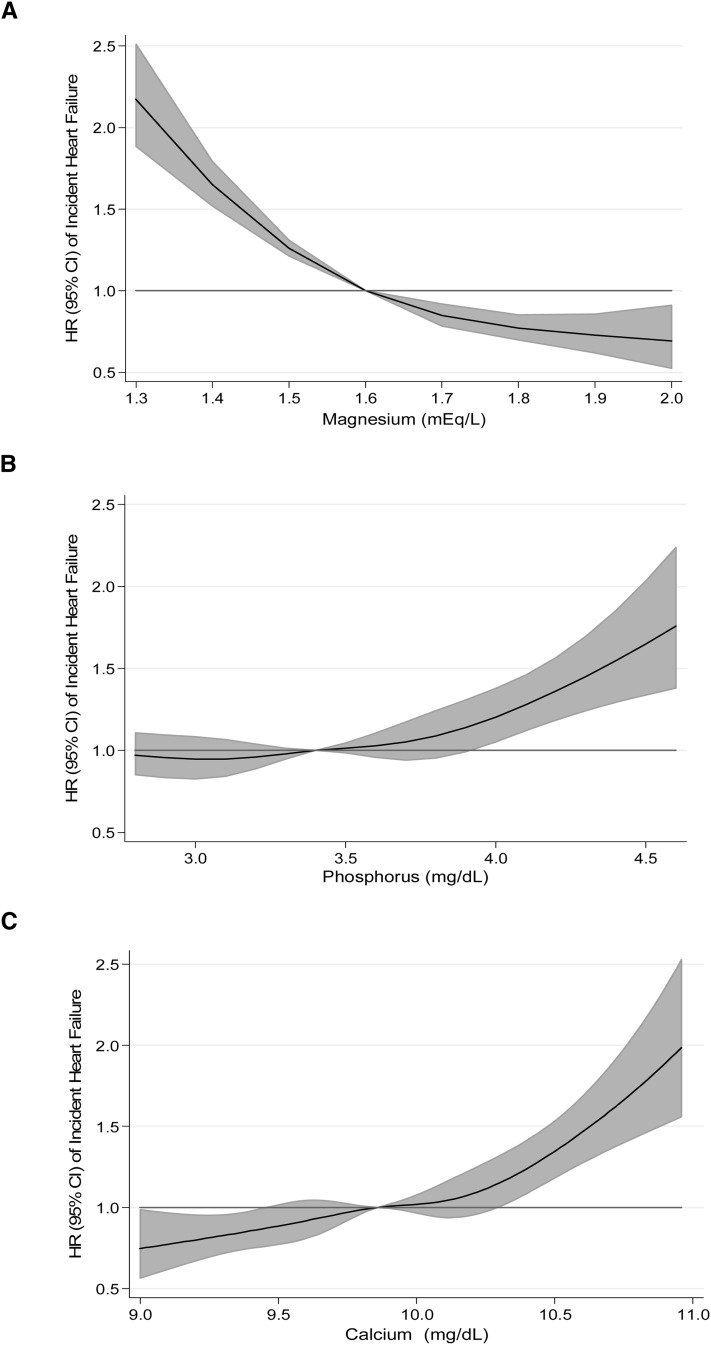
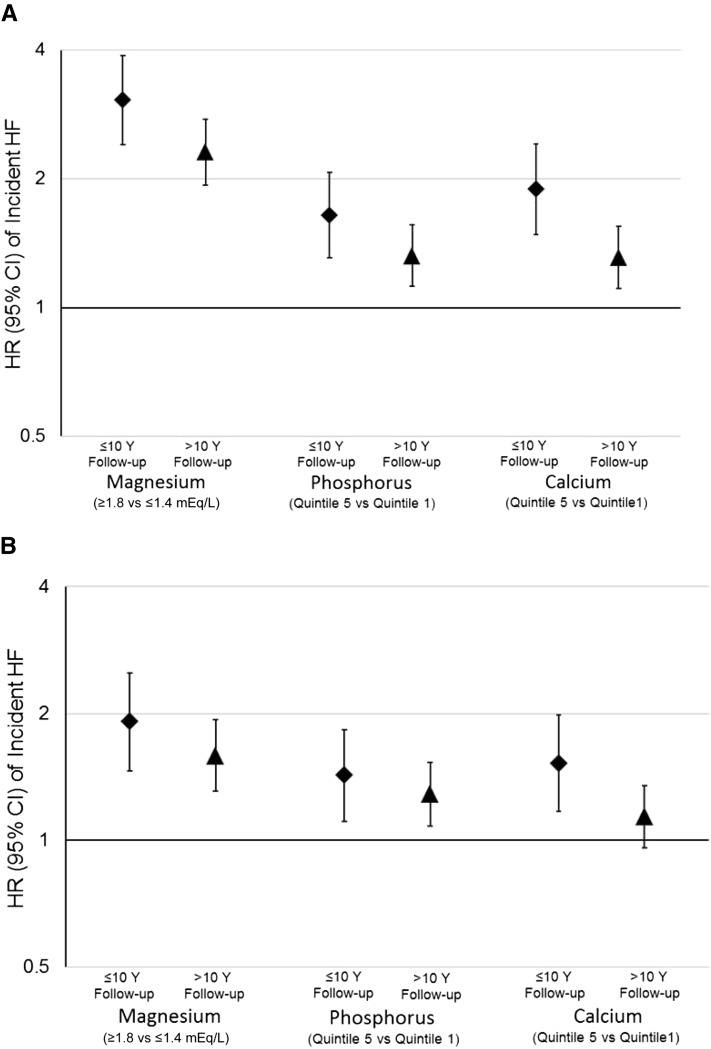
During a median of 20.6 y of follow-up (maximum: 23.1 y), a total of 2250 incident HF events accrued. Serum magnesium showed a linear inverse association with the risk of incident HF (Table 2, Figure 1A). Relative to those in the highest category of serum magnesium, those in the lowest category were at 2.58 (95% CI: 2.23, 2.97) times greater risk of incident HF after demographic adjustments. This association was modestly attenuated but remained statistically significant, with adjustment for behaviors (HR: 2.15; 95% CI: 1.86, 2.48) and further adjustment for CVD risk factors (HR: 1.71; 95% CI: 1.46, 1.99) and incident CHD as a time-varying covariate (HR: 1.66; 95% CI: 1.42, 1.95). The proportional hazards assumption was statistically significantly violated for model 1 [P value for magnesium categories*ln(time) = 0.01]. Overall, associations were stronger earlier in follow-up as opposed to later in follow-up [eg, for model 1, the HR (95% CI) for extreme categories in the first 10 y was 3.06 (2.40, 3.89), whereas for follow-up after 10 y, it was 2.31 (1.93, 2.76)]. Associations between magnesium categories and incident HF stratified by follow-up time are presented in Supplemental Table 3 (under “Supplemental data” in the online issue) and Figure 2. In sensitivity analyses, results were similar when we added alcohol intake to model 2 and when we excluded 2114 participants who reported using diuretics at baseline (data not shown).
TABLE 2.
Serum magnesium, phosphorus, and calcium and risk of incident heart failure: the ARIC Study, 1987–2009 (N = 14,709)1
| Quintile |
||||||
| Category | 1 | 2 | 3 | 4 | 5 | P-trend |
| Magnesium (mEq/L) | ||||||
| Median (range) | 1.4 (0.5–1.4) | 1.5 (1.5–1.5) | 1.6 (1.6–1.6) | 1.7 (1.7–1.7) | 1.8 (1.8–3.1) | |
| No. of events/total | 448/1753 | 421/2423 | 538/3839 | 481/3736 | 362/3242 | |
| Incidence rate2 | 15.89 | 9.90 | 7.79 | 7.04 | 6.00 | |
| Model 1 | 2.58 (2.23, 2.97)3 | 1.68 (1.46, 1.94) | 1.32 (1.15, 1.50) | 1.18 (1.03, 1.35) | 1.00 | <0.0001 |
| Model 2 | 2.15 (1.86, 2.48) | 1.52 (1.32, 1.76) | 1.25 (1.09, 1.43) | 1.14 (1.00, 1.31) | 1.00 | <0.0001 |
| Model 3 | 1.71 (1.46, 1.99) | 1.41 (1.21, 1.63) | 1.24 (1.08, 1.42) | 1.17 (1.02, 1.35) | 1.00 | <0.0001 |
| Model 4 | 1.66 (1.42, 1.95) | 1.37 (1.18, 1.59) | 1.22 (1.06, 1.40) | 1.16 (1.01, 1.34) | 1.00 | <0.0001 |
| Phosphorus (mg/dL) | ||||||
| Median (range) | 2.8 (1.0–3.0) | 3.2 (3.1–3.2) | 3.4 (3.3–3.5) | 3.7 (3.6–3.8) | 4.1 (3.9–9.1) | |
| No. of events/total | 489/3253 | 314/2114 | 530/3728 | 422/3059 | 467/2703 | |
| Incidence rate2 | 8.47 | 8.22 | 7.86 | 7.61 | 9.78 | |
| Model 1 | 1.00 | 1.04 (0.90, 1.19) | 1.01 (0.89, 1.15) | 1.03 (0.90, 1.17) | 1.42 (1.24, 1.63) | <0.0001 |
| Model 2 | 1.00 | 1.08 (0.93, 1.24) | 1.05 (0.92, 1.19) | 1.01 (0.88, 1.16) | 1.43 (1.25, 1.64) | <0.0001 |
| Model 3 | 1.00 | 1.08 (0.93, 1.24) | 1.04 (0.91, 1.18) | 1.02 (0.89, 1.17) | 1.34 (1.16, 1.54) | <0.0001 |
| Model 4 | 1.00 | 1.16 (1.00, 1.34) | 1.09 (0.96, 1.24) | 1.10 (0.96, 1.26) | 1.36 (1.18, 1.56) | 0.0005 |
| Calcium4 (mg/dL) | ||||||
| Median (range) | 9.4 (7.3–9.5) | 9.7 (9.5–9.8) | 9.9 (9.8–10.0) | 10.1 (10.0–10.2) | 10.4 (10.2–13.6) | |
| No. of events/total | 376/2869 | 415/3090 | 426/2964 | 468/2970 | 538/2964 | |
| Incidence rate2 | 7.06 | 7.33 | 7.95 | 8.92 | 10.61 | |
| Model 1 | 1.00 | 1.07 (0.93, 1.23) | 1.12 (0.98, 1.29) | 1.28 (1.11, 1.46) | 1.48 (1.29, 1.70) | <0.0001 |
| Model 2 | 1.00 | 1.06 (0.92, 1.22) | 1.09 (0.95, 1.25) | 1.22 (1.06, 1.40) | 1.36 (1.19, 1.56) | 0.002 |
| Model 3 | 1.00 | 1.09 (0.94, 1.25) | 1.12 (0.97, 1.29) | 1.13 (0.98, 1.31) | 1.24 (1.07, 1.43) | 0.004 |
| Model 4 | 1.00 | 1.15 (1.00, 1.33) | 1.21 (1.05, 1.40) | 1.21 (1.05, 1.40) | 1.41 (1.22, 1.62) | <0.0001 |
Model 1 was adjusted for age, sex, race, and center. Model 2 was adjusted as for model 1 plus for education, physical activity, smoking status, and BMI. Model 3 was adjusted as for model 2 plus for prevalent diabetes, systolic blood pressure, hypertension medication use, lipid-lowering medication use, prevalent CHD, estimated glomerular filtration rate, LDL cholesterol, HDL cholesterol, and triglycerides. Model 4 was adjusted as for model 3 plus for incident CHD as a time-varying covariate. ARIC, Atherosclerosis Risk in Communities; CHD, coronary heart disease.
Unadjusted incidence rate per 1000 person-years.
HR; 95% CI in parentheses (all such values).
Corrected for serum albumin concentration.
FIGURE 1.
Association of serum magnesium (A), phosphorus (B), and calcium (C) (corrected for serum albumin concentration) with risk of incident heart failure: the Atherosclerosis Risk in Communities Study, 1987–2009 (N = 14,709). Biomarkers modeled as restricted cubic splines with knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles, with adjustment for age, race, sex, and study center.
FIGURE 2.
HRs and their 95% CIs for extreme categories of serum magnesium, phosphorus, and calcium and risk of incident heart failure, stratified by follow-up time: the Atherosclerosis Risk in Communities Study, 1987–2009 (N = 14,709). A: Demographics adjusted for age, sex, race, and center (model 1). B: Adjusted for demographics, behaviors, and cardiovascular disease risk factors, including age, sex, race, center, education, physical activity, smoking status, BMI, prevalent diabetes, systolic blood pressure, hypertension medication use, lipid-lowering medication use, prevalent coronary heart disease, estimated glomerular filtration rate, LDL cholesterol, HDL cholesterol, and triglycerides (model 3 in other analyses). HF, heart failure.
Serum phosphorus was positively associated with the risk of incident HF, although the association was nonlinear, with most of the excess risk observed at higher concentrations of phosphorus (Table 2, Figure 1B). Individuals in the highest quintile of phosphorus were 1.42 (95% CI: 1.24, 1.63) times more likely to develop incident HF relative to their counterparts in the lowest quintile. The association was attenuated only slightly with the addition of behaviors (model 2: HR: 1.43; 95% CI: 1.25, 1.64), CVD risk factors (model 3: HR: 1.34; 95% CI: 1.16, 1.54), and time-dependent CHD as covariates (model 4: HR: 1.36; 95% CI: 1.18, 1.56). When comparing the top decile of serum phosphorus with the lowest quintile, the HR for incident HF was 1.52 (95% CI: 1.30, 1.78) for model 1 and 1.37 (95% CI: 1.16, 1.62) for model 3. Although the proportional hazards assumptions were not statistically violated for serum phosphorus models, associations between phosphorus and incident HF were slightly stronger during the first 10 y of follow-up as opposed to later in follow-up (Supplemental Table 3 under “Supplemental data” in the online issue and Figure 2).
Serum calcium was positively associated with the risk of incident HF (Table 2, Figure 1C). The HR for those in the highest compared with the lowest quintile of serum calcium was 1.48 (95% CI: 1.29, 1.70) after adjustment for demographic characteristics. Additional adjustment for behaviors, CVD risk factors, and CHD incidence had little impact on the estimates (model 4: HR: 1.41; 95% CI: 1.22, 1.62). Across all models, the proportional hazards assumption was violated: P values for calcium quintiles (modeled linearly)*ln(time) were 0.0009 for model 1, 0.001 for model 2, 0.01 for model 3, and 0.02 for model 4. As shown in Supplemental Table 3 (under “Supplemental data” in the online issue) and Figure 2, magnitudes of associations were greater in the first 10 y of follow-up relative to later in follow-up. Restricting follow-up to an even shorter period (5 y) resulted in estimates of slightly greater magnitude, although the CIs were wide because only 293 events occurred during the first 5 y of follow-up [HR(Q5 vs Q1) (95% CI): 2.71 (1.80, 4.08) for model 1, 2.52 (1.66, 3.81) for model 2, 2.09 (1.33, 3.27) model 3, and 2.45 (1.56, 3.86) for model 4].
In additional analyses, we included all 3 biomarkers simultaneously in model 3. The estimates were virtually identical to those when the biomarkers were individually included in model 3. HRs (95% CIs) for extreme categories were as follows: 1.72 (1.47, 2.01) for magnesium, 1.35 (1.17, 1.56) for phosphorus, and 1.20 (1.04, 1.38) for calcium. Results were also similar in sensitivity analyses when visit 1 and visit 2 biomarker values were averaged (data not shown). For all 3 biomarkers, there was no evidence of effect modification of their associations to incident HF by race, sex, hypertension, diabetes, kidney function, or prevalent MI. Furthermore, there was no evidence of interactions between the 3 biomarkers on the risk of incident HF.
DISCUSSION
In this large, biracial, population-based cohort study, lower concentrations of serum magnesium and higher concentrations of serum phosphorus and calcium were independently associated with the risk of incident HF. These associations were only modestly attenuated with adjustment for additional CVD risk factors, and there was no evidence of interactions by sex, race, kidney function, or prevalent CHD. For all 3 biomarkers, associations were somewhat stronger early in the follow-up period relative to later in follow-up. This is not surprising because these biomarkers were measured at baseline, which was often many years before HF events accrued.
CHD is believed to underlie more than half of the incident HF cases in the general population younger than 75 y (32). Therefore, we adjusted for CHD as a time-varying covariate to evaluate whether our findings were simply the result of these biomarkers being associated with a greater risk of CHD and CHD associated with a greater risk of HF. The fact that our results were only modestly attenuated after more carefully accounting for CHD by using incident CHD as a time-varying covariate suggests that these biomarkers may be associated with the risk of HF at least partly independently of CHD.
Magnesium and HF
Observational studies have linked low serum magnesium to more adverse CVD risk factor profiles (9–11) and greater risk of CVD events (14–19). In the present analysis, participants in the lowest quintile of magnesium were at 2.5 times greater risk of incident HF after adjustment for demographic factors. With additional adjustment for behaviors and CVD risk factors (some of which may be on the causal pathway between magnesium and HF), individuals in the lowest quintile of magnesium were at 66% greater risk of developing incident HF than those in the highest quintile. Serum magnesium concentrations were lower among African Americans than among whites (mean: 1.58 compared with 1.65 mEq/L), and thus low serum magnesium may be an additional factor underlying the higher risk of HF among African Americans (2, 29). We were unable to locate any previous articles exploring the association between serum magnesium and incidence of HF. Patients with HF are, however, more likely to have low serum magnesium than are other older individuals (33), and among patients with HF, low serum magnesium has been associated with all-cause mortality (34). In addition, small randomized clinical trials of patients with HF have suggested that magnesium supplementation improves left ventricular function (35) and heart rate variability (36). The idea that magnesium may have cardioprotective properties is not new. Serum magnesium deficiency is thought to elevate the risk of atrial fibrillation after cardiac surgery (37, 38), and in that setting, magnesium is sometimes used prophylactically to prevent atrial fibrillation events, although evidence for this intervention is inconclusive (37, 38). Previous work by our group and others has also identified low serum magnesium to be associated with an increased risk of incident atrial fibrillation (39, 40). Atrial fibrillation and heart failure are closely related; they often co-occur as a cause of each other (41, 42).
Phosphorus and HF
Individuals in the highest quintile of serum phosphorus were at ∼40% greater risk of developing incident HF than those in those in the lowest quintile, regardless of degree of adjustment. The association was nonlinear, with excess risk present only among those in the upper quintile of serum phosphorus. Our finding is concordant with previous epidemiologic investigations that have shown high phosphorus concentrations to be associated with elevated risk of CVD (13, 20, 21, 24), atrial fibrillation (also in ARIC) (39), and poorer outcomes among patients with chronic kidney disease (43). Only the Framingham Study (13) and a post hoc analysis of the Cholesterol and Recurrent Events clinical trial (24) have found positive associations of serum phosphorus to HF risk. There is presently heightened scientific interest regarding the role of phosphorus homeostasis in HF risk, given recent findings from basic science (44) and epidemiology (45, 46) linking fibroblast growth factor 23, a marker of phosphorus homeostasis, to cardiac disease.
Calcium and HF
Serum calcium was positively and linearly associated with the risk of incident HF. During the full follow-up, individuals in the highest quintile were at ∼40% greater risk of incident HF than their counterparts in the lowest serum calcium quintile. However, when restricted to the first 5 y of follow-up, individuals in the highest quintile were at ∼2.5-fold greater risk, indicating that perhaps serum calcium may be a marker of more proximal risk. The role of calcium in cardiovascular risk is currently under intense scientific scrutiny, given controversial [and at times conflicting (47)] reanalysis (48) and meta-analyses (48, 49) of clinical trial data, which have suggested that calcium supplementation is associated with an increased risk of MI. It has been speculated that large doses of calcium supplements transiently increase serum calcium concentrations, even above the normal range, for several hours after ingestion, thereby promoting vascular calcification and increased coagulability (7, 8). A different but related question was examined here: whether serum calcium concentrations within the general population are associated with HF. Most (21–23), although not all (20), observational studies of serum calcium concentrations have shown a positive association with the risk of MI and combined CVD endpoints but have not specifically examined HF incidence.
Strengths and limitations
Strengths of this study are the use of data from a large population-based prospective cohort and resulting high number of events that enabled subgroup analyses. Limitations of this study warrant consideration. First, measurements of serum magnesium, phosphorus, and calcium took place at the baseline visit. As such, concentrations may not have been representative of those more proximal to the development of most HF events. That said, results were similar in sensitivity analyses, which averaged biomarker concentrations from visit 1 and visit 2 (data not shown; these biomarkers are not available at any other ARIC visit). Second, these are observational data. Despite our attempts at adjustment, it is possible that confounding (due to both residual confounding and unmeasured confounders) remained. Last, ARIC HF events were identified through ICD codes from hospital discharge and death certificates, and thus some cases may have been missed. However, ARIC has shown HF ICD codes to have high validity (30).
It is also important to be cognizant of the fact that concentrations of serum magnesium, phosphorus, and calcium are influenced by numerous metabolic factors as well as by dietary intake. For example, ∼40–60% of magnesium consumed is absorbed, although absorption efficiency can rise to ∼80% when intakes are low (50). In addition to dietary intake, serum magnesium concentrations also reflect intracellular concentrations and renal handling, which may have a stronger effect on serum magnesium than dietary intake. Importantly, however, less than 25% of US adults meet the Recommended Daily Allowance for dietary magnesium intake (50).
Conclusions
Within the large, prospective, population-based ARIC cohort, we found that, as hypothesized, serum magnesium was inversely and independently associated with the development of HF, whereas serum calcium and phosphorus were positively and independently associated with the risk of HF. Additional work is needed to elucidate whether these associations are causal and, if so, to clarify the specific mechanisms that underlie the associations. Whether these biomarkers will be useful candidates for HF risk prediction or targets for prevention remains to be seen.
Supplementary Material
Acknowledgments
We thank the staff and participants of the ARIC Study for their important contributions.
The authors’ responsibilities were as follows—PLL and ARF: designed the research; PLL: performed the statistical analysis and had primary responsibility for the final content; and PLL, AA, EDM, LRL, BCA, JC, and ARF: wrote the manuscript. All authors read and approved the final manuscript. No conflicts of interest were reported.
Footnotes
Abbreviations used: ARIC, Atherosclerosis Risk in Communities; CHD, coronary heart disease; CVD, cardiovascular disease; HF, heart failure; MI, myocardial infarction.
REFERENCES
- 1.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure. Circulation 2002;106:3068–72. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013;127:e6–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rude RK. Magnesium. In: Coates PM, Betz JM, Blackman MR, Cragg GM, eds. Encyclopedia of dietary supplements. New York, NY: Informa Healthcare, . 2010:527–37. [Google Scholar]
- 4.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 2009;20:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amann K, Tornig J, Kugel B, Gross M-L, Tyralla K, El-Shakmak A, Szabo A, Ritz E. Hyperphosphatemia aggravates cardiac fibrosis and microvascular disease in experimental uremia. Kidney Int 2003;63:1296–301. [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphate and left ventricular hypertrophy in young adults: the Coronary Artery Risk Development in Young Adults Study. Kidney Blood Press Res 2009;32:37–44. [DOI] [PubMed] [Google Scholar]
- 7.Reid IR, Bolland M, Avenell A, Grey A. Cardiovascular effects of calcium supplementation. Osteoporos Int 2011;22:1649–58. [DOI] [PubMed] [Google Scholar]
- 8.Reid IR, Bolland MJ. Calcium supplements: bad for the heart? Heart 2012;98:895–6. [DOI] [PubMed] [Google Scholar]
- 9.Jee SH, Miller ER, Guallar E, Singh VK, Appel LJ, Klag MJ. The effect of magnesium supplementation on blood pressure: a meta-analysis of randomized clinical trials. Am J Hypertens 2002;15:691–6. [DOI] [PubMed] [Google Scholar]
- 10.He K, Liu K, Daviglus ML, Morris SJ, Loria CM, Van Horn L, Jacobs DR, Savage PJ. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 2006;113:1675–82. [DOI] [PubMed] [Google Scholar]
- 11.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabetes Med 2006;23:1050–6. [DOI] [PubMed] [Google Scholar]
- 12.Onufrak SJ, Bellasi A, Shaw LJ, Herzog CA, Cardarelli F, Wilson PW, Vaccarino V, Raggi P. Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis 2008;199:424–31. [DOI] [PubMed] [Google Scholar]
- 13.Dhingra R, Gona P, Benjamin EJ, Wang TJ, Aragam J, D'Agostino RB, Kannel WB, Vasan RS. Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur J Heart Fail 2010;12:812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohira T, Peacock JM, Iso H, Chambless LE, Rosamond WD, Folsom AR. Serum and dietary magnesium and risk of ischemic stroke. Am J Epidemiol 2009;169:1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 1998;136:480–90. [DOI] [PubMed] [Google Scholar]
- 16.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2010;160:464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraborti S, Chakraborti T, Mandal M, Mandal A, Das S, Ghosh S. Protective role of magnesium in cardiovascular diseases: a review. Mol Cell Biochem 2002;238:163–79. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg MJ. Magnesium deficiency and sudden death. Am Heart J 1992;124:544–9. [DOI] [PubMed] [Google Scholar]
- 19.Misialek JR, Lopez FL, Lutsey PL, Huxley RR, Peacock JM, Chen LY, Soliman EZ, Agarwal SK, Alonso A. Serum and dietary magnesium and incidence of atrial fibrillation in whites and in African Americans; Atherosclerosis Risk in Communities (ARIC) Study. Circ J 2013;77:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Sr, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007;167:879–85. [DOI] [PubMed] [Google Scholar]
- 21.Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008;156:556–63. [DOI] [PubMed] [Google Scholar]
- 22.Lind L, Skarfors E, Berglund L, Lithell H, Ljunghall S. Serum calcium: a new, independent, prospective risk factor for myocardial infarction in middle-aged men followed for 18 years. J Clin Epidemiol 1997;50:967–73. [DOI] [PubMed] [Google Scholar]
- 23.Jorde R, Sundsfjord J, Fitzgerald P, Bønaa KH. Serum calcium and cardiovascular risk factors and diseases: the Tromsø Study. Hypertension 1999;34:484–90. [DOI] [PubMed] [Google Scholar]
- 24.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005;112:2627–33. [DOI] [PubMed] [Google Scholar]
- 25.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 26.The ARIC Investigators. ARIC: Atherosclerosis Risk in Communities Study: operations manual 10. Clinical chemistry determinations. Bethesda, MD: National Heart, Lung, and Blood Institute, 1987. [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eriksson H, Caidaul K, Larsson B, Ohlson L-O, Welin L, Wilhelmsen L, Svardsudd K. Cardiac and pulmonary causes of dyspnoea—validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J 1987;8:1007–14. [DOI] [PubMed] [Google Scholar]
- 29.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities Study). Am J Cardiol 2008;101:1016–22. [DOI] [PubMed] [Google Scholar]
- 30.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) Study: a comparison of diagnostic criteria. Circ Heart Fail 2012;5:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011;57:1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox KF, Cowie MR, Wood DA, Coats AJS, Gibbs JSR, Underwood SR, Turner RM, Poole-Wilson PA, Davies SW, Sutton GC. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J 2001;22:228–36. [DOI] [PubMed] [Google Scholar]
- 33.Arinzon Z, Peisakh A, Schrire S, Berner YN. Prevalence of hypomagnesemia (HM) in a geriatric long-term care (LTC) setting. Arch Gerontol Geriatr 2010;51:36–40. [DOI] [PubMed] [Google Scholar]
- 34.Adamopoulos C, Pitt B, Sui X, Love TE, Zannad F, Ahmed A. Low serum magnesium and cardiovascular mortality in chronic heart failure: a propensity-matched study. Int J Cardiol 2009;136:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witte KKA, Nikitin NP, Parker AC, von Haehling S, Volk H-D, Anker SD, Clark AL, Cleland JGF. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J 2005;26:2238–44. [DOI] [PubMed] [Google Scholar]
- 36.Almoznino-Sarafian D, Sarafian G, Berman S, Shteinshnaider M, Tzur I, Cohen N, Gorelik O. Magnesium administration may improve heart rate variability in patients with heart failure. Nutr Metab Cardiovasc Dis 2009;19:641–5. [DOI] [PubMed] [Google Scholar]
- 37.Miller S, Crystal E, Garfinkle M, Lau C, Lashevsky I, Connolly SJ. Effects of magnesium on atrial fibrillation after cardiac surgery: a meta-analysis. Heart 2005;91:618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho KM, Lewis JP. Prevention of atrial fibrillation in cardiac surgery: time to consider a multimodality pharmacological approach. Cardiovasc Ther 2010;28:59–65. [DOI] [PubMed] [Google Scholar]
- 39.Lopez FL, Agarwal SK, Grams ME, Loehr LR, Soliman EZ, Lutsey PL, Chen LY, Huxley RR, Alonso A. Relation of serum phosphorus levels to the incidence of atrial fibrillation (from the Atherosclerosis Risk in Communities [ARIC] Study). Am J Cardiol 2013;111:857–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan AM, Lubitz SA, Sullivan LM, Sun JX, Levy D, Vasan RS, Magnani JW, Ellinor PT, Benjamin EJ, Wang TJ. Low serum magnesium and the development of atrial fibrillation in the community: the Framingham Heart Study. Circulation 2013;127:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–5. [DOI] [PubMed] [Google Scholar]
- 42.Chamberlain AM, Redfield MM, Alonso A, Weston SA, Roger VL. Atrial fibrillation and mortality in heart failure: a community study. Circ Heart Fail 2011;4:740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GFM. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease. JAMA 2011;305:1119–27. [DOI] [PubMed] [Google Scholar]
- 44.Faul C, Amaral AP, Oskouei B, Hu M-C, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011;121:4393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 2012;60:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med 2010;152:640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med 2010;152:315–23. [DOI] [PubMed] [Google Scholar]
- 48.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ 2011;342:d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, Reid IR. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ 2010; 341:c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. Wardlaw’s perspectives in nutrition. 9th ed. New York, NY: McGraw-Hill, 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.