Abstract
Background: It is important to understand whether eating eggs, which are a major source of dietary choline, results in increased exposure to trimethylamine-N-oxide (TMAO), which is purported to be a risk factor for developing heart disease.
Objective: We determined whether humans eating eggs generate TMAO and, if so, whether there is an associated increase in a marker for inflammation [ie, high-sensitivity C-reactive protein (hsCRP)] or increased oxidation of low-density lipoprotein (LDL).
Design: In a longitudinal, double-blind, randomized dietary intervention, 6 volunteers were fed breakfast doses of 0, 1, 2, 4, or 6 egg yolks. Diets were otherwise controlled on the day before and day of each egg dose with a standardized low-choline menu. Plasma TMAO at timed intervals (immediately before and 1, 2, 4, 8, and 24 h after each dose), 24-h urine TMAO, predose and 24-h postdose serum hsCRP, and plasma oxidized LDL were measured. Volunteers received all 5 doses with each dose separated by >2-wk washout periods.
Results: The consumption of eggs was associated with increased plasma and urine TMAO concentrations (P < 0.01), with ∼14% of the total choline in eggs having been converted to TMAO. There was considerable variation between individuals in the TMAO response. There was no difference in hsCRP or oxidized LDL concentrations after egg doses.
Conclusions: The consumption of ≥2 eggs results in an increased formation of TMAO. Choline is an essential nutrient that is required for normal human liver and muscle functions and important for normal fetal development. Additional study is needed to both confirm the association between TMAO and atherosclerosis and identify factors, microbiota and genetic, that influence the generation of TMAO before policy and medical recommendations are made that suggest reduced dietary choline intake. This trial was registered at clinicaltrials.gov as NCT01906554.
See corresponding article on page 741
INTRODUCTION
Recently, an intriguing and important hypothesis was suggested that dietary choline is converted to trimethylamine by gut bacteria; this trimethylamine is oxidized to trimethylamine-N-oxide (TMAO)5 in the liver, and TMAO acts to increase atherosclerosis in the coronary vasculature (1–4). Choline is present as free choline and choline esters in the diet (5), and these forms of choline are absorbed differently in the intestines. Free choline is absorbed in the small intestine via mediated transport (6), whereas phosphatidylcholine is not converted to choline within the intestinal lumen [it is absorbed intact via the lymphatic system or is hydrolyzed by pancreatic lipases and absorbed as glycerophosphocholine (7)]. On the basis of these differences, dietary free choline (the substrate for trimethylamine formation by bacteria) but not dietary phosphatidylcholine should result in TMAO formation. Although the bacterial degradation of choline in the human gastrointestinal tract has been well documented (8), we previously reported that phosphatidylcholine was not readily used as a substrate for the formation of trimethylamine by gut microbes (9). Furthermore, clinicians who used supplemental choline to treat Huntington's disease noted that patients developed fishy body odor (the odor of trimethylamine) when given large doses of choline (10) but did not develop this fishy odor when molar-equivalent doses of phosphatidylcholine (lecithin) were administered (11). However, Tang et al (3) reported that the ingestion of eggs (which are a source of phosphatidylcholine) resulted in increased plasma TMAO concentrations in human subjects.
Because eggs are an important source of total choline in people's diets, we studied the dose-response relation for TMAO production from dietary egg intake over a dose-range applicable to the human diet. The putative mechanism whereby TMAO causes atherosclerosis involves the upregulation of macrophage scavenger receptors, augmented macrophage cholesterol accumulation, and foam cell formation, which result in increased inflammation and oxidation of cholesterol that is deposited in atherosclerotic plaques (4). In the following egg-dose–TMAO-response studies, we measured high-sensitivity C-reactive protein (hsCRP) (which is a measure of inflammation) and oxidized LDL before and 24 h after egg doses.
SUBJECTS AND METHODS
Subjects
Six healthy volunteers [2 men and 4 nonpregnant women; age range: 28–53 y; BMI (in kg/m2) range: 20–39] were recruited in October 2012 for a protocol approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. Informed consent was obtained from all participants. Inclusion was contingent on good health status with no history of hepatic, renal, cardiovascular, intestinal, or other chronic system diseases as determined by a self-report, physical examination, and standard clinical laboratory tests, such as a complete blood count, blood chemistries, fasting lipid, glucose, and liver- and kidney-function tests. Individuals who used drugs or medications known to alter liver metabolism, cardiovascular, and kidney function, used choline-containing dietary supplements, had food allergies, were eating unusual diets that would interfere with the study, or had taken antibiotics during the 3 mo preceding the study were ineligible for participation.
Design
The study design was a longitudinal, double-blind, randomized dietary intervention in which the equivalent of 0, 1, 2, 4, or 6 egg yolks (a source of phosphatidylcholine) was provided as the dose to each subject. Diets of subjects were controlled on the day before and day of each egg dose by using a standardized menu designed to deliver a standardized low-choline diet with the exception of the varying amount of egg yolks ingested for the dose. A dose event was described as follows: on the day before a dose, subjects were fed the standardized breakfast (an egg substitute with equivalent amounts of calories and macronutrients as on egg-dose days) at the University of North Carolina at Chapel Hill Nutrition Research Institute (NRI) before taking the remainder of the day's meals and snacks with them to eat at home. On the day of an egg dose, subjects arrived in the morning having fasted since 2100 the previous evening. At that time, serum, plasma, and whole blood were collected, and a 24-h urine collection was started. Subjects were served one of the test doses as part of breakfast. Plasma was collected 1, 2, 4, 6, and 8 h after subjects finished eating the egg-dose breakfast. Subjects were required to stay at the NRI during this time and were fed the standardized lunch and snacks. After the 8-h plasma collection, subjects were allowed to leave with their standardized dinner to eat at home. Subjects completed a 24-h urine collection the morning after an egg dose and returned to the NRI having fasted since 2100 the previous evening for the collection of serum and plasma at 24 h after the egg dose. Subjects provided a fecal sample on the first day of the study. Doses were administered in a randomized fashion with the exception of the zero egg dose, which was the first dose of the study to be administered to all subjects. The zero egg-dose event was administered in a dose event as previously described with the standardized diet given on the day before but was followed by one of the other randomly assigned doses on the day after. Otherwise, dose events were separated by a 2–4-wk washout period during which subjects were allowed to return to their normal diets with the exception of seafood. A random-number generator in SAS software (version 9.2; SAS Institute) was used to randomly assign the sequence in which subjects received the doses of 1, 2, 4, or 6 egg yolks.
The diet was designed and prepared under the supervision of a registered dietitian in the Metabolic Research Kitchen at the NRI. Menus were designed and analyzed by using ProNutra software (version 3.4.0.0; Viocare) to provide ∼30% fat, ∼55% carbohydrate, and ∼15% protein. Standardized meal menus at the following 2 calorie amounts were prepared: 1500 and 2000 kcal. Macronutrient equivalent units that could be removed to reduce calories or added to increase calories were built into the menus for the 2 calorie amounts. Snacks of similar macronutrient composition and that were low in total choline were provided for subjects as requested. Caloric requirements were estimated by using the Mifflin–St Jeor equation. A high-yolk scrambled egg recipe was developed by using pasteurized liquid egg yolks and egg whites so that the volume of egg yolk could be controlled. These products contained only 100% egg white or egg yolk. The recipe consisted of a ratio of 1.7 to 1 egg yolk to egg white. The proportion of egg yolk used was based on the expected egg yolk size in a large egg (17 g) per the USDA National Nutrient Database for Standard Reference (release 25; http://ndb.nal.usda.gov/ndb/search/list). Although doses varied in the volume of egg, calories and macronutrients were held constant. Subjects could add a small quantity of ketchup, hot sauce, salt, or pepper to scrambled eggs as desired. A single-batch, high-yolk, scrambled egg recipe was prepared and weighed after cooking and cooling to provide the appropriate egg yolk equivalent at each dose. All foods provided to subjects were documented. Dietary intake was assessed with a 3-d food record before the start of the study and during ≥2 of the remaining 4 doses. Food records were reviewed with subjects by trained interviewers and analyzed by using the Nutrition Data Systems for Research (version 2011; Nutrition Coordinating Center, University of Minnesota). These food records were used to determine whether dietary habits of subjects, particularly in terms of trimethylamine substrates, influenced their responses to the egg dose and to confirm that subjects did not consume foods high in TMAO.
By our assay, the choline content of the experimental diets was 168 and 208 mg total choline/d for the 1500- and 2000-kcal diets, respectively, in addition to the total choline in the egg dose. Each egg dose delivered 1.14 mmol (119 mg) total choline. Therefore, the 0, 1, 2, 4, and 6 egg doses provided 0, 1.1, 2.3, 4.6, and 6.8 mmol (0, 119, 238, 476, and 714 mg) total choline, respectively. TMAO and trimethylamine were not detectable in the egg yolks or standardized diets.
Sample collection
Plasma
Whole blood was collected in tubes containing EDTA, gently inverted, immediately put on wet ice, and centrifuged at 2000 × g at 4°C for 10 min. Samples were kept in ice while plasma (0.5 and 1 mL) was delivered into 1.50-mL screw cap tubes. Plasma aliquots for the analysis of TMAO were acidified with formic acid to a final concentration of 1%. All aliquots of plasma were stored at −80°C until analyzed.
Serum
After whole blood was collected in serum separator tubes, it was gently inverted and allowed to clot at room temperature before centrifugation at 1500 × g at 4°C for 15 min. The serum was stored at 4°C until analysis.
24-h urine
Urine was collected into a collection hat and immediately poured into a 3-L plastic jug that contained 20 mL 99% formic acid. The plastic jug was kept on wet ice during the 24-h collection. On completion, the total volume was noted, and aliquots were stored at −80°C.
Food
Three homogenates of the study food were prepared for analysis of choline and its metabolites as follows: 1) the egg equivalent used as the egg dose, 2) the egg substitute used on the predose day, and 3) all foods from the standardized diet (with the exclusion of the egg equivalent) served to a subject in 1 d. Food samples were homogenized in a prechilled commercial blender and frozen at −80°C until analysis. Similarly, for the analysis of TMAO, each food sample previously described was homogenized in a prechilled blender with 99% formic acid for a final concentration of 1%, and aliquots were taken and frozen at −80°C until analysis.
Clinical assessment
A panel of routine clinical laboratory tests was performed on each subject at screening and before and 24 h after each dose. These laboratory analyses, which were conducted at the clinical laboratory of the Carolina's Medical Center that is accredited by the College of American Pathologists, were done by using a DXC 800 Synchron Clinical System (Beckman-Coulter). The Glomerular filtration rate was calculated by using the Chronic Kidney Disease Epidemiology Collaboration equation (12).
Choline and metabolites in the diet and egg dose
Choline and metabolites were extracted from homogenates by using the method of Bligh and Dyer (13). Aqueous and organic compounds were separated, analyzed, and quantified directly by using liquid chromatography–stable-isotope dilution–multiple-reaction monitoring mass spectrometry after the addition of internal standards labeled with stable isotopes that were used to correct for recovery (14). The phosphatidylcholine content of the egg-dose recipe was confirmed by using the same Bligh and Dyer extraction process, thin-layer chromatography and phosphorus determination of the band co-migrating with authentic standards (15).
Measurement of TMAO in plasma and urine
Acidified plasma, urine, and food samples were extracted with 3 vol extraction solvent [acetonitrile for plasma and urine; methanol:water (1:1; vol:vol) for food] that containing 1% formic acid and 20 μmol TMAO-d9/L (DLM-4779–1; Cambridge Isotope Laboratories) and 10 μmol creatinine-d3/L (D-3689; CDN Isotopes Inc). The 2 stably labeled compounds were internal standards used to correct for recovery. Samples were then centrifuged at 15,000 × g for 2 min, and supernatant fluid was collected for liquid chromatography.
TMAO and creatinine were quantified by using liquid chromatography–stable-isotope dilution–multiple-reaction monitoring mass spectrometry. Chromatographic separations were performed on an Atlantis Silica HILIC 3μm 4.6 × 50mm column (Waters Corp) using a Waters ACQUITY UPLC system (Waters Corp). The column was heated to 40°C, and the flow rate was maintained at 1 mL/min. The gradient was increase from 5% A for 0.05 min to 15% A in 0.35 min to 20% A in 0.6 min to 30% A in 1 min to 45% A in 0.55 min to 55% A in 0.05 min at 55% A for 0.9 min to 5% A in 0.05 min and at 5% A for 1.45 min, where A was 10% acetonitrile and 90% water with 10 mmol ammonium formate/L and 0.125% formic acid, and B was 90% acetonitrile and 10% water with 10 mmol ammonium formate/L and 0.125% formic acid. Mass spectrometry was performed with and electrospray ionization probe operated in positive-ion mode with the capillary voltage at 1.2 kV. Temperatures of source and desolvation gases were set at 130°C and 350°C, respectively. Nitrogen was used as the cone and desolvation gas with flow rates at 1 and 600 L/h, respectively. Argon was used as collision gas with a flow rate of 0.1 mL/min. Metabolites and their corresponding isotopes were monitored on a Waters TQ detector (Waters Corp) by using characteristic precursor-product ion transitions as follows: 76→58 for TMAO, 85→66 for TMAO-d9, 114→86 for creatinine, and 117→89 for creatinine-d3, and concentrations were determined from a calibration curve by using the peak area ratio of the metabolite to its isotope.
Data from 24-h urine collections were included in analyses only if the amount of creatinine was >75% of the mean of all collections for that subject. Acidified samples of the diet and egg dose were checked for the presence of TMAO by using the assay previously described and for trimethylamine by using capillary gas chromatography–mass spectrometry (16).
Oxidized LDL
Oxidized LDL was measured in samples of plasma collected from each subject immediately before and at 24 h after each egg dose. A solid-phase 2-site enzyme immunoassay was used according to the manufacturer's protocol (Mercodia Inc).
hsCRP
Serum samples collected from each subject immediately before and at 24 h after each egg dose were analyzed for hsCRP by the clinical laboratory at the Carolina's Medical Center by using a latex-enhanced turbidimetric immunoassay method (Diazyme).
Statistical analyses
The total AUC was computed for each egg dosage (0, 1, 2, 4, and 6 doses) by using plasma TMAO concentrations at 0, 1, 2, 6, 8, and 24 h for each subject. A repeated-measures regression model was run with these areas as the response variable and the egg yolk dosage as the predictor. For 24-h urine collections, a repeated-measures regression model was run with the amount of TMAO in urine collections as the response variable and the egg yolk dosage as the predictor. For both models, variable estimates and their SEs were used to make pairwise comparisons for the various egg yolk dosages. Because multiple measures were taken on each subject, the correlation between observations that came from the same subject was accounted for in both models. For each model, correlation matrix structures considered were compound symmetric, autoregressive, and unstructured, and the one that fit the data best was used. P < 0.05 was considered statistically significant.
Paired t tests were used to test for differences in oxidized LDL concentrations and differences in hsCRP concentrations in plasma and serum, respectively, collected before and after each dose. All analyses were performed with SAS software (version 9.2; SAS Institute).
RESULTS
Characteristics of subjects studied and their clinical laboratory values on admission to the study are described in Table 1. All subjects had normal clinical laboratory tests and glomerular filtration rates. Cholesterol concentrations, on average, were at the upper concentration of the normal range (201 mg/dL).
TABLE 1.
Subject characteristics and clinical laboratory test results on admission to the study1
| Characteristic | Values |
| Age (y) | 42.5 ± 3.5 |
| BMI (kg/m2) | 30.7 ± 3.1 |
| Alanine transaminase (IU/L) | 24 ± 3.7 |
| Aspartate transaminase (IU/L) | 25 ± 1.2 |
| Blood urea nitrogen (mg/dL) | 11 ± 0.8 |
| Creatine (mg/dL) | 0.85 ± 0.07 |
| Fasting glucose (mg/dL) | 93 ± 11 |
| Cholesterol (mg/dL) | 201 ± 32 |
| HDL (mg/dL) | 56 ± 18 |
| LDL (mg/dL) | 123 ± 35 |
| Non-HDL cholesterol (mg/dL) | 144 ± 48 |
| Triglycerides (mg/dL) | 107 ± 79 |
| High-sensitivity C-reactive protein (mg/L) | 4 ± 5 |
| Oxidized LDL (U/L) | 42 ± 21 |
| Glomerular filtration rate (mL · min −1 · 1.73 m−2) | 97 ± 19 |
All values are means ± SEs. On the morning of the first dose day of the study, height and weight were measured, and blood was collected from 6 healthy volunteers who had been fasting since 2100 the previous night. All blood chemistries were conducted on serum except oxidized LDL, which was conducted on plasma samples. n = 6.
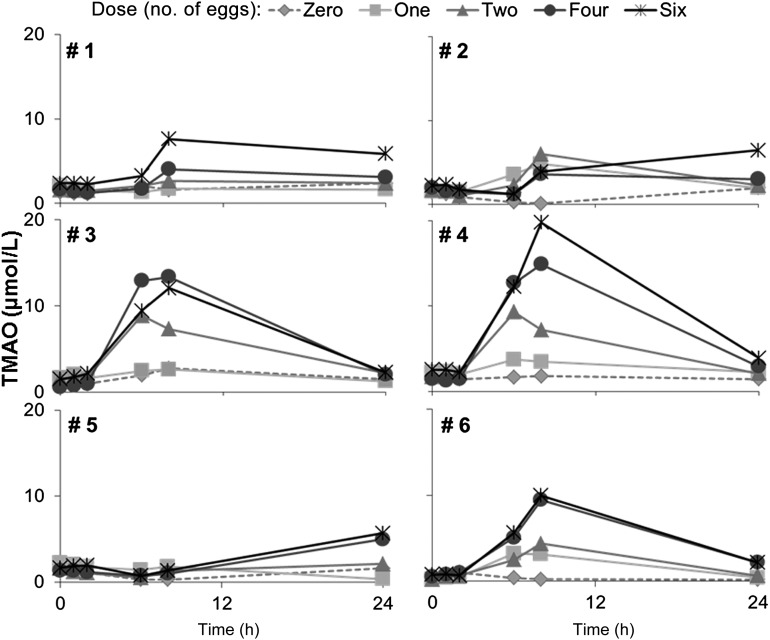
We observed that a dose of egg yolks with a breakfast meal resulted in increased plasma concentrations of TMAO, with peak concentrations achieved at 6–8 h after the egg dose (Figure 1). Shapes of plasma TMAO curves varied between individuals after egg dosing at equivalent dietary total choline intakes with a ≥4-fold difference in TMAO production between subjects (Figure 1). We showed that the intestinal microbial community membership varied across subjects (see Supplementary Figure 1 under “Supplemental data” in the online issue for phyla level abundance data). Subjects were also polymorphic in the FMO3 gene that encodes the enzyme that metabolizes trimethylamine to form TMAO (see Supplementary Table 1 under “Supplemental data” in the online issue).
FIGURE 1.
Concentration of TMAO in plasma. Six healthy volunteers (subjects) consumed a standardized low-choline diet on the day before each of 5 randomly assigned doses of 0, 1, 2, 4, or 6 egg yolks. Subjects consumed the egg dose for breakfast followed by the same standardized low-choline lunch, dinner, and snacks as consumed the previous day. Each egg dose delivered 1.14 mmol (119 mg) total choline. Therefore, the 0, 1, 2, 4, and 6 egg doses provided 0, 1.1, 2.3, 4.6, and 6.8 mmol (0, 119, 238, 476, and 714 mg) total choline, respectively, in addition to the standardized diet. TMAO was measured in plasma collected from each volunteer immediately before and 1, 2, 6, 8, and 24 h after the egg dose was consumed by using liquid chromatography and electrospray ionization–isotope dilution mass spectrometry. Doses were separated by a 2–4-wk washout period. Doses are represented as follows: 0, diamond with dotted line; 1, square with solid light-gray line; 2, triangle with solid-gray line; 4, circle with solid dark-gray line; and 6, asterisks with black solid line. TMAO, trimethylamine-N-oxide.
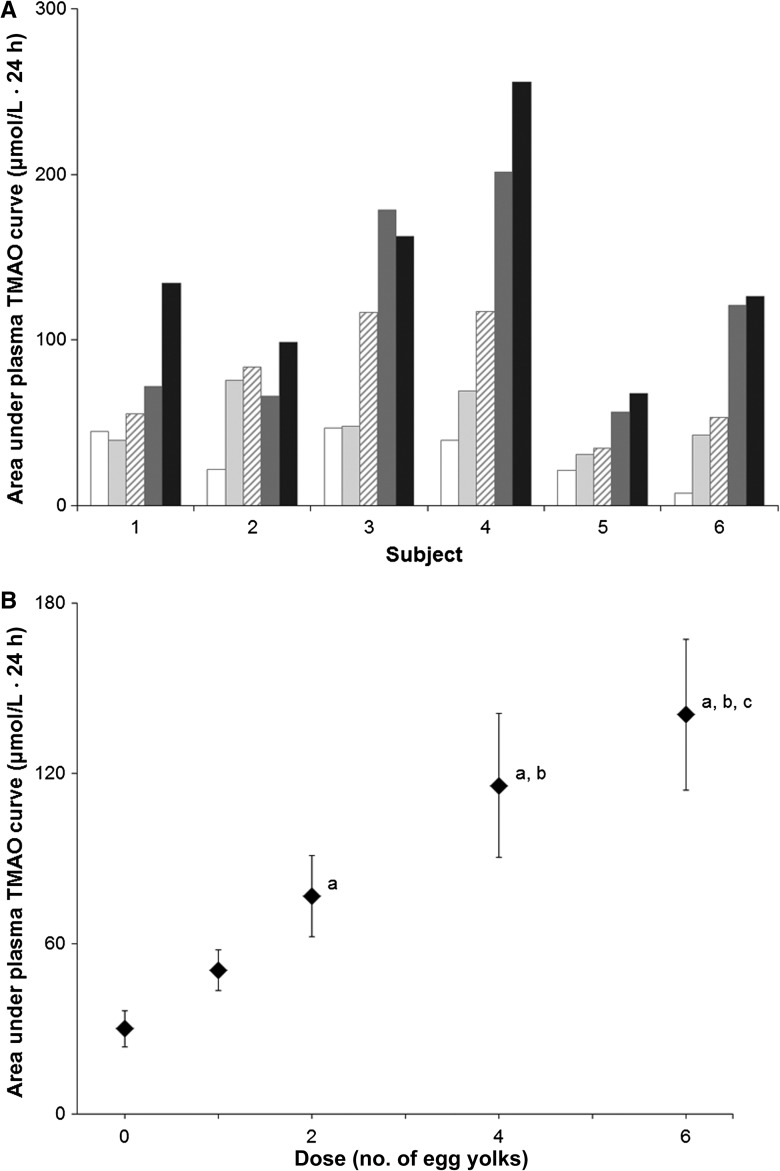
AUCs (which reflected the flux of TMAO through the plasma pool) increased with the increasing dose of egg yolk (Figure 2). According to the pairwise comparisons conducted on variable estimates and their SEs that resulted from the repeated-measures regression model, the response to one egg yolk did not differ from the response to zero yolks. The response to 2 yolks was significantly greater than the response to zero yolks (P < 0.05) but did not differ from that for 1 and 4 yolks. The response to4 yolks was significantly greater than the responses to zero and one egg yolk (P < 0.05) but did not differ from that for 6 yolks. Finally, the response to 6 yolks was significantly greater than the responses to 0 (P < 0.01), 1, and 2 egg yolks (P < 0.05).
FIGURE 2.
Plasma TMAO. Six healthy volunteers (subjects) consumed a standardized low-choline diet on the day before each of 5 randomly assigned doses of 0, 1, 2, 4, or 6 egg yolks. Subjects consumed the egg dose for breakfast, followed by the same standardized low-choline lunch, dinner, and snacks as consumed the previous day. Each egg dose delivered 1.14 mmol (119 mg) total choline. Therefore, 0, 1, 2, 4, and 6 egg doses provided 0, 1.1, 2.3, 4.6, and 6.8 mmol (0, 119, 238, 476, and 714 mg) total choline, respectively, in addition to the standardized diet. TMAO was measured in plasma collected from each volunteer immediately before and 1, 2, 6, 8, and 24 h after the egg dose was consumed by using liquid chromatography and electrospray ionization–isotope dilution mass spectrometry. Doses were separated by a 2–4-wk washout period. A: Area under plasma TMAO curves. Plasma TMAO data were used to calculate the total AUC for each dose for all subjects. Values for the different egg doses are represented by bars patterned as follows: 0 eggs, white; 1 egg, light gray; 2 eggs, diagonal hatch; 4 eggs, dark gray; and 6 eggs, black. B: Mean (±SE) area under plasma TMAO curves after egg doses. Data from A were used to calculate areas under plasma TMAO 24-h dose-response curves for all subjects (n = 6) for each dose. A repeated-measures regression model with the plasma TMAO AUC as the response variable and the egg dose as the predictor was used. Variable estimates (±SEs) resulting from the model were used to make pairwise comparisons for various dietary choline intakes (egg dosages). aP < 0.05, different from 0 eggs; bP < 0.05, different from 1 egg; cP < 0.05, different from 2 eggs. TMAO, trimethylamine-N-oxide.
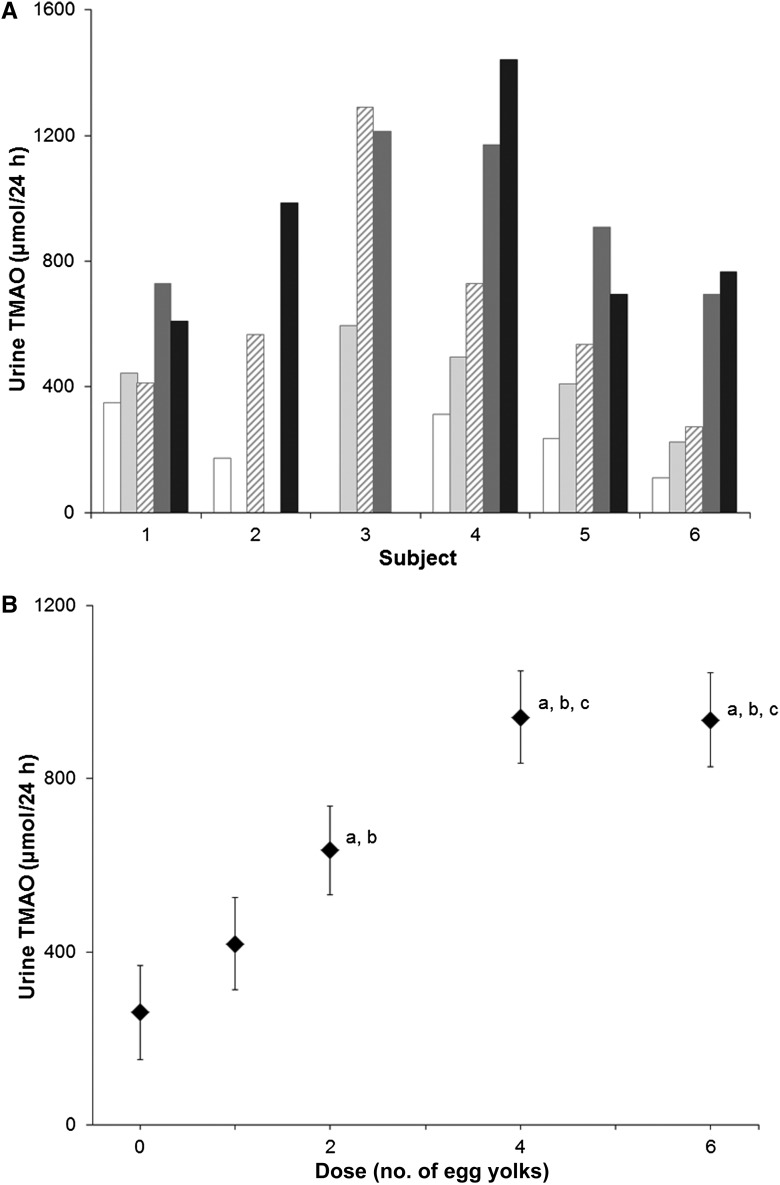
Subjects excreted TMAO into urine (Figure 3), and this amount increased with the egg dose through the 4-egg dose [excreting 236 ± 44 (SE), 433 ± 61, 634 ± 145, 944 ± 108, and 900 ± 149 μmol TMAO/24 h after the ingestion of 0, 1, 2, 4, or 6 egg yolks, respectively; Figure 3). The smallest response was observed in the dose of zero egg yolks, whereas the greatest response was seen in the doses of 4 and 6 yolks. According to pairwise comparisons conducted on variable estimates and their SEs that resulted from the repeated-measures regression model, the response to one egg yolk did not differ from the response to zero yolks. The response to 2 yolks was significantly greater than the response to zero yolks (P < 0.005) and one yolk (P < 0.05). The responses to 4 and 6 yolks did not differ from each other, but both were significantly greater than responses to 0 (P < 0.0005), 1 (P < 0.005), and 2 (P < 0.05) yolks.
FIGURE 3.
Amount of TMAO in 24-h urine collections after egg doses. Six healthy volunteers (subjects) consumed a standardized low-choline diet on the day before each of 5 randomly assigned doses of 0, 1, 2, 4, or 6 egg yolks. Subjects were fed the egg dose for breakfast, followed by the same standardized low-choline lunch, dinner, and snacks as consumed the previous day. Each egg dose delivered 1.14 mmol (119 mg) total choline. Therefore, 0, 1, 2, 4, and 6 egg doses provided 0, 1.1, 2.3, 4.6, and 6.8 mmol (0, 119, 238, 476, and 714 mg) total choline, respectively, in addition to the standardized diet. A: Amount of TMAO in 24-h urine collections after egg doses. Urine was collected for 24 h on the day of each egg dose, and the concentration of TMAO was measured by using liquid chromatography and electrospray ionization–isotope dilution mass spectrometry as described in Subjects and Methods. The amount of TMAO was calculated from the concentration and volume of urine collected in 24 h for each dose for each subject. Some samples were not included in the figure because the amount of creatinine excreted per 24 h fell below 75% of the mean amount in all 24-h urine collections for that subject, suggesting that the individual sample was not a complete 24-h collection. TMAO values for the different egg doses are represented by bars patterned as follows: 0 eggs, white; 1 egg, light gray; 2 eggs, diagonal hatch; 4 eggs, dark gray; and 6 eggs, black. B: Mean (±SE) amount of TMAO excreted into urine in 24 h. Data from A were used to calculate the amount of TMAO in 24-h urine collections for all subjects (n = 6) for each dose. A repeated-measures regression model with urine TMAO as the response variable and the egg dose as the predictor was used. Variable estimates (±SE) resulting from the model were used to make pairwise comparisons for the various dietary choline intakes (egg dosages). aP < 0.05, different from 0 eggs; bP < 0.05, different from 1 egg; cP < 0.05, different from 2 eggs. TMAO, trimethylamine-N-oxide.
The standardized diet delivered 1.9 mmol total choline–containing compounds (mean of the calorie levels for 6 subjects), and each egg yolk added 1.14 mmol total choline. Thus, the portion of total dietary choline converted to TMAO (estimated by using the least-squares mean 24-h urine concentration of TMAO) was ≥14%, 14%, 15%, 15%, and 11% after the ingestion of 0, 1, 2, 4, or 6 egg yolks, respectively.
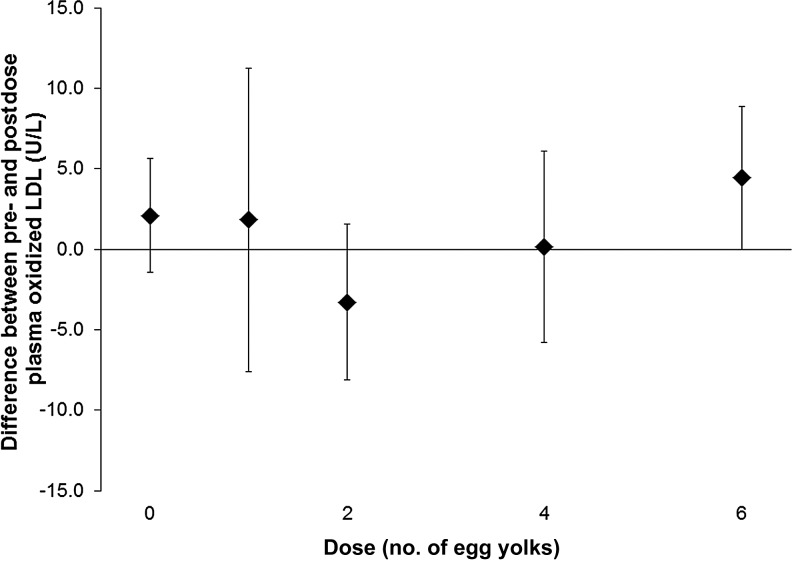
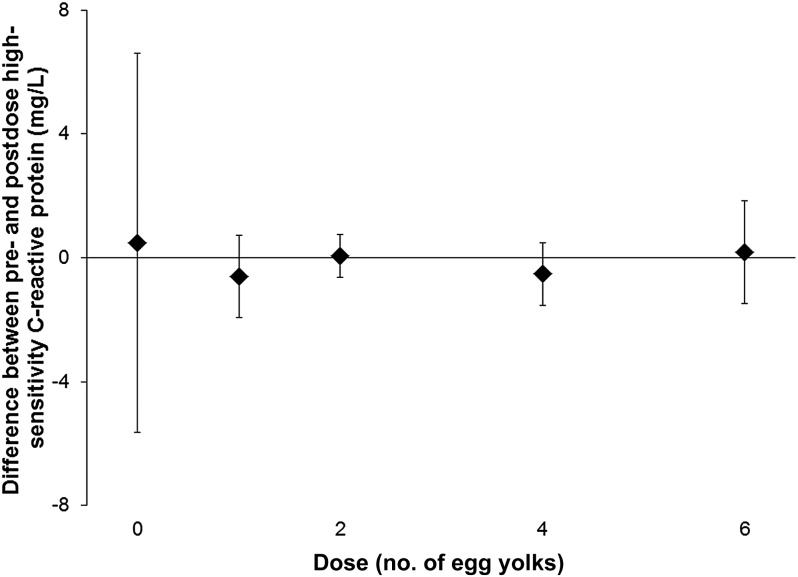
Oxidized LDL and hsCRP are markers used to identify people at risk of cardiovascular disease. For all subjects, there was no difference in oxidized LDL between plasma collected before and 24 h after each dose of eggs (paired t tests, P > 0.05; Figure 4). In addition, there was no difference in hsCRP between serum collected before and 24 h after each dose of eggs (paired t tests, P > 0.05; Figure 5).
FIGURE 4.
Mean (±SD) differences (after dose minus before dose) in plasma oxidized LDL concentrations. Six healthy volunteers (subjects) consumed a standardized low-choline diet on the day before each of 5 randomly assigned doses of 0, 1, 2, 4, or 6 egg yolks. Subjects consumed the egg dose for breakfast, followed by the same standardized low-choline lunch, dinner, and snacks as consumed the previous day. Each egg dose delivered 1.14 mmol (119 mg) total choline. Therefore, 0, 1, 2, 4, and 6 egg doses provided 0, 1.1, 2.3, 4.6, and 6.8 mmol (0, 119, 238, 476, and 714 mg) total choline, respectively, in addition to the standardized diet. Oxidized LDL was measured in plasma collected immediately before and 24 h after each egg dose from each subject, and differences were calculated. According to paired t tests, there was no difference between predose and postdose values.
FIGURE 5.
Mean (±SD) differences (after dose minus before dose) in serum high-sensitivity C-reactive protein concentrations. Six healthy volunteers (subjects) consumed a standardized low-choline diet on the day before each of 5 randomly assigned doses of 0, 1, 2, 4, or 6 egg yolks. Subjects consumed the egg dose for breakfast, followed by the same standardized low-choline lunch, dinner, and snacks as consumed the previous day. Each egg dose delivered 1.14 mmol (119 mg) total choline. Therefore, 0, 1, 2, 4, and 6 egg doses provided 0, 1.1, 2.3, 4.6, and 6.8 mmol (0, 119, 238, 476, and 712 mg) total choline, respectively, in addition to the standardized diet. High-sensitivity C-reactive protein was measured in serum collected immediately before and 24 h after each egg dose from each subject, and differences were calculated. According to paired t tests, there was no difference between predose and postdose values.
DISCUSSION
Tang et al (3) and Wang et al (4) reported that dietary choline is in part converted to trimethylamine and oxidized by liver to form TMAO. Although it was well documented that bacterial metabolism of choline to form trimethylamine occurs in the human gastrointestinal tract (8), we previously thought that phosphatidylcholine was not a substrate for TMA-forming bacteria (9). Therefore, eggs, which are a major dietary source of choline in the form of phosphatidylcholine (5), were not expected to result in the formation of TMAO. Recently, Tang et al (3) reported that people who are 2 eggs formed TMAO. In the current study, we confirmed this finding and current additional information on effects of a range of egg doses on plasma and urinary TMAO concentrations and the variability in TMAO responses in individuals. We showed that ∼11–15% of dietary total choline is converted into TMAO after consumption of meal that contained eggs.
Does this increased exposure to TMAO after eating eggs place people at increased risk of heart disease? There is one experiment reported by Wang et al (4) that strongly supported the hypothesis that TMAO is causally linked to the development of atherosclerotic lesions in cardiac blood vessels. In the apolipoprotein E knockout mouse (which is sensitive to developing atherosclerosis), feeding choline or TMAO increased the size of atherosclerotic lesions in the coronary arteries (4). There has been no direct data in humans that showed a causal link, but there are supporting data from observational studies. Increased plasma concentrations of TMAO were associated with increased risk of a major adverse cardiovascular event during 3 y follow-up in 4007 patients who were undergoing elective coronary angiography (3). However, these data are difficult to interpret because the patients with cardiovascular events also had reduced kidney function (glomerular filtration rate: 75 mL · min−1 · 1.73 m−2) compared with that of patients without events (glomerular filtration rate of 83 mL · min−1 · 1.73 m−2; P < 0.001). TMAO is excreted into urine via the kidney, and thus, the plasma TMAO concentration may be a marker for decreased renal function associated with atherosclerosis of the renal vasculature rather than a direct cause of atherosclerosis in humans. Two large studies of cardiovascular risk factors in humans, the Atherosclerosis Risk in Communities (14,430 men and women) study (17) and the PROSPECT-European Prospective Investigation into Cancer and Nutrition (16,165 women) study (18) observed no significant increase in cardiovascular risk with increasing dietary intake of choline. Note that both studies reported, when compared with the lowest quartile of intake, that incident cardiovascular disease risk was slightly and nonsignificantly higher in the highest quartile of choline intake (1.2-fold increase with CIs that overlapped zero). Fish are an important source of trimethylamine (the immediate precursor for TMAO formation) in the diet (19), and therefore, if TMAO is a risk factor, we would expect that fish intake would be positively associated with risk of cardiovascular disease. However, no evidence of an association between dietary intake of fish and any cardiovascular endpoint was observed in the Physicians’ Health Study (20), and a meta-analysis of 11 available cohort studies concluded that fish consumption is inversely associated with fatal coronary heart disease (21). Additional investigation is needed to understand the underlying genetic, environmental, or microbiota factors that influenced these disparate results.
In our study, the amount of TMAO in plasma or urine varied between individuals by ≤4-fold from the equivalent dietary choline intake. People are different from one another in types of microbes that live in the gastointestinal tract (22), as were our subjects (see Supplementary Figure 1 under “Supplemental data” in the online issue), which is a characteristic that can profoundly affect metabolism (23) and may mediate differences in the trimethylamine formation from dietary choline. Alternatively, these differences in TMAO formation from dietary eggs may be due to differences in hepatic oxidation of trimethylamine mediated by 2 flavin-containing monooxygenase (FMO) family members FMO1 and FMO3 (1). Hepatic FMO3 expression in mice is reduced in males compared with females because of downregulation by androgens (1). In our study, men (subjects 3 and 4) produced more TMAO than did women after each egg dose (Figures 2 and 3), which perhaps indicated a profound difference in the regulation of FMO3 activity in humans. Furthermore, mutations in FMO genes can affect the conversion of trimethylamine to TMAO. The exonic FMO3 G566A single-nucleotide polymorphism (rs2266782) results in an ∼25% reduction in the catalytic activity of the enzyme that converts trimethylamine to TMAO (24). We genotyped our subjects’ DNA for this single-nucleotide polymorphism (see Supplementary Table 1 under “Supplemental data” in the online issue), but it was not possible to relate this genotype with TMAO production because of the small sample size.
The putative mechanism whereby TMAO causes atherosclerosis involves the upregulation of macrophage scavenger receptors, augmented macrophage cholesterol accumulation, and foam cell formation, which result in increased inflammation and the oxidation of LDL (4). We observed that increased plasma TMAO concentrations in subjects seen after a single dose of eggs, ranging from 1 to 6 eggs, was not associated with an increase in hsCRP (which is a measure of inflammation) or oxidized LDL. It remains possible that inflammation-associated changes in these biomarkers might occur after chronic exposure to a diet high in eggs. However, the consumption of 3 eggs/d for 12 wk, in conjunction with a moderately carbohydrate-restricted diet, led to improvements in atherogenic dyslipidemia variables, including oxidized LDL (25). In addition, an abstract presented at Experimental Biology 2013 (26) reported that a 6-wk intervention with 2 eggs/d did not have adverse effects on endothelial function, blood pressure, and body weight. These 2 studies suggested that, although the consumption of eggs may increase the generation of TMAO, this increase is not necessarily associated with an increase in cardiovascular risk markers.
The current study was designed as a pilot study and, therefore, had a number of limitations, including a small sample size. A larger study is necessary to be able to examine all factors that may be involved in the production of TMAO from dietary choline intake. Studies on mechanisms with which TMAO purportedly affects cardiovascular function also are necessary. However, our study offers initial evidence of variable TMAO-production responses to different amounts of dietary choline intake, which did not appear to be associated with short-term changes in 2 markers of cardiovascular risk.
Choline is an essential nutrient required for normal human liver and muscle function (27), and it is important for normal fetal development (28). Eggs are an important dietary source of total choline. There are considerable consequences that will arise if policy and medical recommendations are made suggesting reduced dietary choline intake. It is important that future studies be conducted to determine whether the association between plasma TMAO concentrations and risk of cardiovascular disease in humans persists after correction for differences in renal function between groups and, if so, whether a similar risk can be shown between dietary total choline intake and risk of cardiovascular disease. In addition, it is important that future studies be conducted to determine whether the increase in atherosclerosis observed after TMAO administration (4) can be replicated in other mouse models and laboratories. Until additional data are available, it would be unwise to recommend that dietary intake of total choline be reduced below the recommended Adequate Intake (29).
Supplementary Material
Acknowledgments
Anna Vaughan assisted with the experimental diet design, preparation and dispensing of study diets, and input of diet records. Leslie Fischer and Jana Harrison assisted with the Institutional Review Board application process. Carolyn Harris, Sheena Cunningham, and Brandi Childers assisted with the meal preparation. Jennifer Owen assisted with the processing of peripheral white blood cells, sample collection, and genotyping.
The authors’ responsibilities were as follows—SHZ: designed the research and had primary responsibility for the final content of the manuscript; CAM, TB, SZ, XZ, KDC, K-AdC, BJB, and AO: conducted the research; CAM and JAG: analyzed data; CAM and SHZ: wrote the manuscript; and all authors: read and approved the final manuscript. SHZ serves on advisory boards for Dupont, American Pistachio Growers, and Metabolon, and this service presented no conflict of interest. CAM, KDC, K-AdC, SZ, XZ, JAG, TB, BJB, and AO had no conflicts of interest.
Footnotes
Abbreviations used: FMO, flavin-containing monooxygenase; hsCRP, high-sensitivity C-reactive protein; NRI, Nutrition Research Institute; TMAO, trimethylamine-N-oxide.
REFERENCES
- 1.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 2013;17:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 2003;133:1302–7. [DOI] [PubMed] [Google Scholar]
- 6.Sheard NF, Zeisel SH. An in vitro study of choline uptake by intestine from neonatal and adult rats. Pediatr Res 1986;20:768–72. [DOI] [PubMed] [Google Scholar]
- 7.Fox JM, Betzing H, Lekim D. Pharmacokinetics of orally ingested phosphatidylcholine. In: Barbeau A, Growdon JH, Wurtman RJ, eds. Nutrition and the brain. New York, NY: Raven Press, . 1979:95–108. [Google Scholar]
- 8.Simenhoff ML, Saukkonen JJ, Burke JF, Wesson LG, Schaedler RW. Amine metabolism and the small bowel in uraemia. Lancet 1976;2:818–21. [DOI] [PubMed] [Google Scholar]
- 9.Zeisel SH, Wishnok JS, Blusztajn JK. Formation of methylamines from ingested choline and lecithin. J Pharmacol Exp Ther 1983;225:320–4. [PubMed] [Google Scholar]
- 10.Growdon JH, Cohen EL, Wurtman RJ. Effects of oral choline administration on serum and CSF choline levels in patients with Huntington's disease. J Neurochem 1977;28:229–31. [DOI] [PubMed] [Google Scholar]
- 11.Growdon JH, Gelenberg AJ. Choline and lecithin administration to patients with tardive dyskinesia. Trans Am Neurol Assoc 1978;103:95–9. [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–7. [DOI] [PubMed] [Google Scholar]
- 14.Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem 2002;74:4734–40. [DOI] [PubMed] [Google Scholar]
- 15.Svanborg A, Svennerholm L. Plasma total lipids, cholesterol, triglycerides, phospholipids and free fatty acids in a healthy Scandanavian population. Acta Med Scand 1961;169:43–9. [Google Scholar]
- 16.daCosta KA, Vrbanac JJ, Zeisel SH. The measurement of dimethylamine, trimethylamine, and trimethylamine N-oxide using capillary gas chromatography-mass spectrometry. Anal Biochem 1990;187:234–9. [DOI] [PubMed] [Google Scholar]
- 17.Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord 2007;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalmeijer GW, Olthof MR, Verhoef P, Bots ML, van der Schouw YT. Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur J Clin Nutr 2008;62:386–94. [DOI] [PubMed] [Google Scholar]
- 19.Zeisel SH, DaCosta KA. Increase in human exposure to methylamine precursors of N-nitrosamines after eating fish. Cancer Res 1986;46:6136–8. [PubMed] [Google Scholar]
- 20.Morris MC, Manson JE, Rosner B, Buring JE, Willett WC, Hennekens CH. Fish consumption and cardiovascular disease in the physicians’ health study: a prospective study. Am J Epidemiol 1995;142:166–75. [DOI] [PubMed] [Google Scholar]
- 21.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation 2004;109:2705–11. [DOI] [PubMed] [Google Scholar]
- 22.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 2005;102:11070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol 2009;587:4153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki H, Shimizu M. Survey of variants of human flavin-containing monooxygenase 3 (FMO3) and their drug oxidation activities. Biochem Pharmacol 2013;85:1588–93. [DOI] [PubMed] [Google Scholar]
- 25.Blesso CN, Andersen CJ, Barona J, Volek JS, Fernandez ML. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism 2013;62:400–10. [DOI] [PubMed] [Google Scholar]
- 26.Katz DL, Ma Y, Kavak Y, Njike V. Effects of egg ingestion on endothelial function in adults with coronary artery disease: a randomized, controlled, crossover trial. FASEB J 2013;27:225.6. [DOI] [PubMed] [Google Scholar]
- 27.Fischer LM, daCosta K, Kwock L, Stewart P, Lu T-S, Stabler S, Allen R, Zeisel S. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr 2007;85:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeisel SH. Nutrition in pregnancy: the argument for including a source of choline. Int J Womens Health 2013;5:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Institute of Medicine, National Academy of Sciences USA. Choline. Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotin, and choline. Washington DC: National Academy Press, 1998:390–422. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.