Abstract
Background: Adolescents consume more sugar-sweetened beverages than do individuals in any other age group, but it is unknown how the type of sugar-sweetened beverage affects metabolic health in this population.
Objective: The objective was to compare the metabolic health effects of short-term (2-wk) consumption of high-fructose (HF) and high-glucose (HG)–sweetened beverages in adolescents (15–20 y of age).
Design: In a counterbalanced, single-blind fashion, 40 male and female adolescents completed two 2-wk trials that included 1) an HF trial in which they consumed 710 mL of a sugar-sweetened beverage/d (equivalent to 50 g fructose/d and 15 g glucose/d) for 2 wk and 2) an HG trial in which they consumed 710 mL of a sugar-sweetened beverage/d (equivalent to 50 g glucose/d and 15 g fructose/d) for 2 wk in addition to their normal ad libitum diet. In addition, the participants maintained similar physical activity levels during each trial. The day after each trial, insulin sensitivity and resistance [assessed via Quantitative Insulin Sensitivity Check Index (QUICKI) and homeostatic model assessment of insulin resistance (HOMA-IR) index] and fasting and postprandial glucose, lactate, lipid, cholesterol, insulin, C-peptide, insulin secretion, and clearance responses to HF or HG mixed meals were assessed.
Results: Body weight, QUICKI (whole-body insulin sensitivity), HOMA-IR (hepatic insulin resistance), and fasting lipids, cholesterol, glucose, lactate, and insulin secretion or clearance were not different between trials. Fasting HDL- and HDL3-cholesterol concentrations were ∼10–31% greater (P < 0.05) in female adolescents than in male adolescents. Postprandial triacylglycerol, HDL-cholesterol, HDL3-cholesterol, and glucose concentrations were not different between HF and HG trials. The lactate incremental area under the curve was ∼3.7-fold greater during the HF trial (P < 0.05), whereas insulin secretion was 19% greater during the HG trial (P < 0.05).
Conclusions: Moderate amounts of HF- or HG-sweetened beverages for 2 wk did not have differential effects on fasting or postprandial cholesterol, triacylglycerol, glucose, or hepatic insulin clearance in weight-stable, physically active adolescents. This trial was registered at clinicaltrials.gov as NCT02058914.
INTRODUCTION
Numerous epidemiologic studies link consumption of high-fructose (HF)4–sweetened beverages [composed of ≥50% fructose, such as HF corn syrup (HFCS) or sucrose] with increased risk of cardiovascular disease and type 2 diabetes in children and adults (1–3). Some short-term (≤10-wk) experimental feeding trials support these findings and showed that consumption of moderate (40 g/d) to high (25% of energy intake, equivalent to 125 g/d for a 2000-kcal diet) amounts of fructose-sweetened beverages contributed to detrimental metabolic perturbations, such as increased de novo lipogenesis (4), uric acid (5), proinflammatory and prothrombotic mediators (6), triacylglycerol concentrations (4, 7–15), fasting cholesterol concentrations (7), hepatic insulin resistance (16–19), increased intrahepatocellular lipids (18, 20–22), and decreased LDL-cholesterol particle size (23) in adults, whereas the effect of high-glucose (HG)–sweetened beverage consumption had little impact on these variables. However, these effects were not universal across all studies because others showed that short-term consumption of a higher fructose diet had little impact on metabolic health in adults (24–27). To date, most experimental trials have used an adult population with a large age range (predominantly >20 y of age), and the findings were inconsistent. Current national estimates indicate that children, adolescents, and young adults between 12 and 22 y of age consume the greatest quantity of HF-sweetened beverages (28, 29). However, limited clinical trials have examined the metabolic health effects of sugar-sweetened beverages in this population. In a series of studies examining different doses of fructose intake in weight-stable prepubertal children and adolescents, increasing fructose intake from 6% to 24% of energy intake (30 to 120 g/d fructose for a 2000-kcal diet) for 7 d had no effect on metabolic health (30–33). To our knowledge, only one study compared the metabolic health effects of HF-sweetened beverages with HG-sweetened beverages. Jin et al (34) showed that over a 2-d period, isocaloric meals with HF-sweetened beverages augmented the triacylglycerol response compared with HG-sweetened beverages, an effect that was exaggerated in children with nonalcoholic fatty liver disease. To date, it is unknown how longer-term consumption of different types of sugar-sweetened beverages (HF compared with HG) alters metabolic health, insulin sensitivity, insulin secretion, or insulin clearance responses in adolescents.
Given the inconsistent findings between studies and limited amount of experimental trials comparing the effect of HF- and HG-sweetened beverages on metabolic health in adolescents, the primary purpose of this study was to compare the effects of short-term (2-wk) consumption of HF- and HG-sweetened beverages in adolescents (15–20 y of age) on insulin sensitivity, insulin secretion, insulin clearance, and triacylglycerol and cholesterol concentrations. Knowledge of how different sugars affect metabolic health in adolescents will provide important clinical information for dietary guidelines for adolescents for the prevention of type 2 diabetes and cardiovascular disease.
SUBJECTS AND METHODS
Participants
The University of Missouri Health Sciences Institutional Review Board approved this study protocol. All participants provided written informed consent before participating. Initially, all of the participants were screened to determine whether they qualified, and this included assessment of height and weight; a fasting blood sample; completion of a health history, dietary, and physical activity questionnaire; and completion of a 3-d dietary record to determine the average daily fructose consumption of the individuals. Inclusion criteria included the following: male and female adolescents between 15 and 20 y of age; not participating in an organized sport (nonathletes); no previous history of heart, lung, kidney, endocrine, or gastrointestinal disease; not taking any medications known to alter glucose or lipid metabolism; normal fasting blood glucose concentrations (<100 mg/dL); normal fasting triacylglycerol concentrations (<150 mg/dL); and habitual average daily fructose consumption <90th percentile for age and sex [according to estimates provided by Marriott et al (28)]. A total of 81 participants were examined for eligibility. Twenty participants did not meet all of the inclusion criteria, and 21 participants dropped out of the study (10 dropped out because the time commitment was too great, 5 were dropped because we were unable to start an intravenous drip for blood sampling, and 6 were dropped for not being compliant with the study protocol). This study was registered at clinicaltrials.gov as NCT02058914.
General study design
This counterbalanced study consisted of 2 trials that included 1) an HF trial and 2) an HG trial. Participants were blinded to trial allocation, and each trial was performed in a random order and was 15 d in length. During days 1–14 of each trial, the participants consumed either 710 mL of an HF-sweetened beverage/d (sweetened with 50 g fructose and 15 g glucose) for 2 wk (HF trial) or 710 mL of an HG-sweetened beverage/d (sweetened with 50 g glucose and 15 g fructose) for 2 wk (HG trial) in addition to their normal ad libitum diet. In addition, the participants were instructed to maintain their normal physical activity levels during each trial (which were measured with an accelerometer). On day 15 of each trial, the participants reported to the laboratory, after an overnight fast of ∼11 h, for metabolic testing. During this testing day, the participants remained in the laboratory for 12 h and consumed 3 liquid meals (one meal every 4 h and HF meals during the HF trial and HG meals during the HG trial), and blood samples were taken every 15 or 30 min throughout the 12-h testing day. During testing, the participants remained physically inactive (<3000 steps).
Sugar-sweetened beverages
During days 1–14 of each trial, the participants were supplied with an HF- or HG-sweetened beverage. The beverages were made by mixing pure fructose (NOW Foods) and pure glucose (NOW Foods) into water; to add flavor to the drink, sugar-free artificial sweetener (aspartame and acesulfame potassium) was added (Great Value; Wal-Mart Stores Inc). The participants consumed 710 mL of this drink per day, which during the HF trial comprised 50 g fructose/d and 15 g glucose/d and during the HG trial comprised 50 g glucose/d and 15 g fructose/d. In addition, 2.4 g of the artificial sweetener was added to the 710 mL to provide a specific flavor.
Mixed-meal shakes
During the 15th day of each trial, the participants were given 3 liquid shakes. Each shake (∼355 mL) contained 450 kcal, and the energy from each macronutrient was 45% carbohydrate [25% simple sugar (with 14.8% fructose during the HF trial or 4% fructose during the HG trial) and 20% complex carbohydrate as maltodextrin], 40% fat, and 15% protein. Each shake contained 27.5 g whey protein complex (General Nutrition Corporation), 59.1 mL (4 tablespoons) of heavy whipping cream (Great Value; Wal-Mart Stores Inc), 19.5 g of maltodextrin (Carbo Gain; NOW Foods), and 16.7 g fructose and 8.4 g glucose during the HF trial (thus, 50 g fructose over the entire day) or 20.1 g glucose and 5 g fructose during the HG trial (thus, 15 g of fructose over the entire day).
Dietary records
Participants kept 3 to 6 detailed written dietary records with the exact timing of all meals, snacks, and beverages consumed during days 1–14 of each trial. The participants were required to keep 3 of these records during days 12, 13, and 14 of each trial; to control for diet, copies of these records were given to the participants and they were asked to precisely replicate these records during the subsequent trial. The macronutrient composition of each meal was determined by the Nutrition Data System for Research (University of Minnesota).
Free-living physical activity levels
A BodyMedia armband (BodyMedia Inc) was used to measure free-living physical activity levels. The participants were instructed to wear the armband every day for 23 h/d during each intervention, as suggested by the manufacturer. The participants took the armband off to shower and during this time would charge the armband if the battery was low.
Height, weight, and body composition
Standing height was measured with a wall-mounted stadiometer (Seca) while participants were barefoot. Body weight was measured by using a digital scale (Health 0 m; Jarden Corporation). The Bod Pod was used to measure body composition following the manufacturer's instructions (Cosmed USA). Body composition was estimated by using the Siri equation for white participants, whereas for African Americans, body composition was estimated by using the race- and sex-specific formulas provided (35, 36).
Blood collection and analysis
On arrival for mixed-meal testing, a venous catheter was inserted into participants’ antecubital vein, which was kept patent with a saline drip. Venous blood samples were taken at baseline and at 15- to 30-min intervals thereafter. At each time point a blood sample of ∼4 mL was taken and transferred immediately into EDTA-coated tubes and immediately put into an ice bath. Within 30 min from taking the sample, the blood was separated by centrifugation by using an Eppendorf 5702R refrigerated centrifuge at 3000 rpm for 10 min at 4°C and frozen at −80°C until analysis.
Glucose and lactate concentrations were determined by using an automatic analyzer (YSI 2700 Select; YSI Inc). Plasma insulin and C-peptide concentrations were determined from the same sample by using a Milliplex magnetic bead–based quantitative multiplex immunoassay with the MAGPIX instrumentation (Millipore). The intraassay CV was 7.3% and 10% for insulin and C-peptide, respectively, and the interassay CV was 14.5% and 7.9% for insulin and C-peptide, respectively. To eliminate the interassay CV, plasma triacylglycerol, total cholesterol (TC), HDL cholesterol, and HDL3 cholesterol from the same subject were analyzed in the same run. Plasma triacylglycerol and TC concentrations were determined by using a colorimetric assay (Fisher Scientific), and intraassay CVs were 2.7% and 2.0%, respectively. A modified heparin-MnCl2-dextran sulfate method (37) was used to measure plasma HDL cholesterol and HDL3 cholesterol. Intraassay CVs were 3.6% and 2.6% for HDL cholesterol and HDL3 cholesterol, respectively.
Insulin sensitivity, insulin resistance, and postprandial insulin secretion and clearance
The Quantitative Insulin Sensitivity Check Index (QUICKI) was used as a surrogate measure of whole-body insulin sensitivity (38). The HOMA-IR was used as a surrogate measure of hepatic insulin resistance (39). Basal and postprandial prehepatic insulin secretion rates (ISRs) were calculated with the use of C-peptide concentrations by using the insulin secretion deconvolution software program (40). Basal and postprandial insulin clearance was calculated as the molar ratio of insulin to C-peptide at each time point, and this was calculated as a percentage (41): (1 − [insulin (pmol)/C-peptide (pmol)]) × 100.
Power and statistical analysis
G*Power 3 software (version 3.1.3; Universität Kiel, Germany) was used to calculate a priori power. Effect sizes for statistically significant differences found in several other studies (4, 7–14) comparing HF and HG diets were determined, and the mean of these effect sizes was used in the power analysis. It was estimated that with a repeated measures design and α = 0.05, a sample size of 34 would yield a power of 0.80. We recruited additional participants (n = 40) to ensure adequate power.
The Statistical Package for the Social Sciences statistical software, version 18.0 (IBM Inc), was used to perform the statistical analyses. For baseline characteristics, 1-factor ANOVA with follow-up Bonferroni-adjusted post hoc tests were used to compare characteristics between groups. For all other variables, a 2 (trial) by 2 (sex) by 2 (adiposity) mixed-model repeated-measures ANOVA was used to test for statistical significance. For significant main effects, interaction effects, or between-subjects effects, follow-up Bonferroni-adjusted post hoc tests were used to determine specific differences. Significance was set at P ≤ 0.05. All values are reported as means ± SEMs. Lean male and female adolescents were defined as those with ≤22% and ≤32% body fat, respectively, whereas overweight/obese male and female adolescents were defined as those with >22% and >32% body fat, respectively. These classifications are based on what the Bod Pod recommends, which is that men have a body fat <22% and women have a body fat <32% for optimal health.
RESULTS
Participant characteristics and dietary records
Baseline characteristics of the 40 participants who completed the study are listed in Table 1. Baseline body weight, BMI, and body fat percentage were significantly lower (P < 0.05) in lean male and female adolescents compared with overweight/obese male and female adolescents. In addition, average body fat percentage was significantly greater (P < 0.05) in lean female adolescents than in lean male adolescents. For fructose and glucose intake, there was a significant main effect of trial (P < 0.05), indicating that during the HF trial, total fructose intake (73 ± 2 g/d) was significantly greater, whereas total glucose intake was significantly lower (40 ± 2 g/d), compared with the HG trial (fructose intake: 41 ± 2 g/d, glucose intake: 75 ± 2 g/d). For average daily kilocalories consumed (HF: 1942 ± 72 kcal/d; HG: 2025 ± 87 kcal/d) and intakes of fat (HF: 73 ± 4 g/d; HG: 77 ± 5 g/d), protein (HF: 77 ± 5 g/d; HG: 81 ± 5 g/d), and carbohydrate (HF: 246 ± 9 g/d, HG: 251 ± 11 g/d), there was no significant main effect of trial or between-subjects effect for adiposity or sex (P > 0.05).
TABLE 1.
Baseline characteristics of participants1
| Male adolescents |
Female adolescents |
||||
| All (n = 40) | ≤22 BF% (n = 9) | >22 BF% (n = 11) | ≤32 BF% (n = 11) | >32 BF% (n = 9) | |
| Age (y) | 17.9 ± 0.3 | 18.3 ± 0.6 | 17.1 ± 0.5 | 18.3 ± 0.4 | 17.8 ± 0.6 |
| Height (m) | 1.7 ± 0.02 | 1.8 ± 0.02 | 1.7 ± 0.03 | 1.7 ± 0.03 | 1.6 ± 0.03 |
| Weight (kg) | 79.2 ± 2.6 | 75.6 ± 3.2 | 92.5 ± 5.4* | 66.3 ± 3.4 | 82.4 ± 4.7* |
| BMI (kg/m2) | 27.3 ± 0.9 | 23.5 ± 0.8 | 30.6 ± 1.8* | 24.2 ± 1.2 | 31.0 ± 2.2* |
| BF% | 27.4 ± 1.6 | 13.3 ± 1.8 | 32.4 ± 1.7* | 24.4 ± 2.2** | 36.1 ± 1.2* |
All values are means ± SEMs. A 1-factor ANOVA and follow-up Bonferroni-adjusted post hoc tests were used to determine specific differences between groups. Significance was set at P ≤ 0.05. Unless otherwise noted, the between-subjects effects for adiposity or sex were not significant (P > 0.05). For weight, BMI, and BF%, there was a significant between-subjects effect for adiposity (P < 0.05). For BF%, there was a significant between-subjects effect for sex (P < 0.05). *P < 0.05 compared with the lean group within the same sex; **P < 0.05 compared with the lean male adolescent group. BF%, percentage of body fat.
Free-living physical activity
Because of a malfunction in some of the monitors during testing, data for only 35 of the 40 participants could be used in the analysis of free-living physical activity. For steps, there was no significant main effect of trial (P > 0.05), indicating that participants maintained similar free-living physical activity patterns during both interventions (HF: 9256 ± 537 steps; HG: 9221 ± 631 steps). In addition, there was no significant trial × adiposity, trial × sex, or trial × adiposity × sex interaction effect (all P > 0.05) or between-subjects effects for adiposity (P > 0.05), but there was a significant between-subjects effect for sex (P < 0.05), indicating that female adolescents (HF: 7979 ± 626 steps; LF: 7927 ± 747 steps) compared with male adolescents (HF: 10,462 ± 773 steps; LF: 10,444 ± 934 steps) had ∼27% lower free-living physical activity levels (P < 0.05).
Lipids and cholesterol
Fasting triacylglycerol concentrations were not significantly different between trials or between sexes (P > 0.05), but fasting triacylglycerol concentrations were 15–49% greater (P < 0.05) in overweight/obese adolescents than in lean adolescents (Table 2). Similarly, the 12-h triacylglycerol incremental AUC (iAUC) was not significantly different between trials (P > 0.05), but postprandial triacylglycerol concentrations were significantly greater (P < 0.05) in overweight/obese male adolescents compared with lean male adolescents and both of the female groups (Table 3).
TABLE 2.
Body weight, insulin sensitivity and resistance, and fasting glucose, lipid, cholesterol, and insulin kinetic measures1
| Male adolescents |
Female adolescents |
||||
| All (n = 40) | ≤22 BF% (n = 9) | >22 BF% (n = 11) | ≤32 BF% (n = 11) | >32 BF% (n = 9) | |
| Body weight (kg) | |||||
| HF | 79.7 ± 2.7 | 76.9 ± 3.5 | 93.1 ± 6.3* | 65.0 ± 3.5 | 82.2 ± 4.8* |
| HG | 79.8 ± 2.7 | 77.0 ± 3.5 | 92.9 ± 6.2* | 65.2 ± 3.8 | 83.1 ± 4.8* |
| Triacylglycerol (mg/dL) | |||||
| HF | 65 ± 4.5 | 56 ± 9 | 74 ± 6* | 53 ± 6 | 75 ± 12* |
| HG | 63 ± 5.0 | 48 ± 5 | 71 ± 9* | 60 ± 11 | 70 ± 12* |
| Total cholesterol (mg/dL) | |||||
| HF | 132 ± 3 | 126 ± 7 | 133 ± 7 | 127 ± 6 | 131 ± 8 |
| HG | 133 ± 3 | 127 ± 7 | 133 ± 5 | 141 ± 8 | 139 ± 8 |
| HDL cholesterol (mg/dL) | |||||
| HF | 53 ± 2 | 47 ± 3 | 48 ± 2 | 60 ± 3** | 57 ± 4** |
| HG | 54 ± 2 | 47 ± 3 | 48 ± 2 | 64 ± 5** | 55 ± 3** |
| HDL3 cholesterol (mg/dL) | |||||
| HF | 32 ± 1 | 29 ± 2 | 30 ± 1 | 36 ± 2** | 33 ± 3** |
| HG | 32 ± 1 | 30 ± 2 | 30 ± 1 | 35 ± 2** | 34 ± 2** |
| Glucose (mg/dL) | |||||
| HF | 76 ± 1 | 77 ± 2 | 78 ± 2 | 73 ± 3 | 74 ± 3 |
| HG | 78 ± 2 | 82 ± 4 | 79 ± 2 | 77 ± 3 | 75 ± 3 |
| Lactate (mg/dL) | |||||
| HF | 5 ± 1 | 5 ± 1 | 5 ± 1 | 5 ± 1 | 5 ± 1 |
| HG | 6 ± 1 | 5 ± 1 | 5 ± 1 | 6 ± 1 | 6 ± 1 |
| Insulin (pmol/L) | |||||
| HF | 86 ± 7 | 70 ± 15 | 110 ± 13* | 69 ± 9 | 94 ± 12* |
| HG | 91 ± 9 | 67 ± 12 | 115 ± 22* | 79 ± 9 | 101 ± 20* |
| C-peptide (pmol/L) | |||||
| HF | 447 ± 22 | 388 ± 55 | 508 ± 37 | 413 ± 34 | 471 ± 52 |
| HG | 472 ± 33 | 405 ± 42 | 558 ± 69 | 442 ± 61 | 473 ± 86 |
| QUICKI (insulin sensitivity) | |||||
| HF | 0.35 ± 0.01 | 0.36 ± 0.01 | 0.33 ± 0.01* | 0.36 ± 0.01 | 0.34 ± 0.01* |
| HG | 0.34 ± 0.01 | 0.35 ± 0.01 | 0.33 ± 0.01* | 0.35 ± 0.01 | 0.34 ± 0.01* |
| HOMA-IR (insulin resistance) | |||||
| HF | 2.32 ± 0.18 | 2.02 ± 0.40 | 2.94 ± 0.37* | 1.98 ± 0.36 | 3.22 ± 0.59* |
| HG | 2.54 ± 0.24 | 1.83 ± 0.29 | 2.49 ± 0.33* | 2.18 ± 0.27 | 2.73 ± 0.57* |
| Insulin secretion (pmol · kg−1 · min−1) | |||||
| HF | 1.49 ± 0.06 | 1.30 ± 0.15 | 1.53 ± 0.07 | 1.54 ± 0.13 | 1.57 ± 0.15 |
| HG | 1.58 ± 0.10 | 1.36 ± 0.12 | 1.68 ± 0.17 | 1.64 ± 0.23 | 1.62 ± 0.22 |
| Insulin clearance (%) | |||||
| HF | 81 ± 1 | 83 ± 2 | 79 ± 2 | 83 ± 2 | 80 ± 2 |
| HG | 80 ± 1 | 82 ± 3 | 78 ± 3 | 81 ± 2 | 77 ± 3 |
All values are means ± SEMs. A 2 (trial) by 2 (sex) by 2 (adiposity) mixed-model repeated-measures ANOVA and follow-up Bonferroni-adjusted post hoc tests were used to determine specific differences. Significance was P ≤ 0.05. Unless otherwise noted, the main effect of trial; the within-subject interaction terms trial × adiposity, trial × sex, or trial × adiposity × sex; or the between-subjects effect for adiposity, sex, or adiposity × sex interaction were not significant (P > 0.05). For body weight, fasting triacylglycerol, fasting insulin, QUICKI, and HOMA-IR, there was a significant between-subjects effect for adiposity (all P < 0.05). For fasting HDL- and HDL3-cholesterol concentrations, there was a significant between-subjects effect for sex (P < 0.05). *P < 0.05 compared with lean individuals within the same sex; **P < 0.05 compared with male adolescents within the HF or HG trial. BF%, percentage of body fat; HF, high fructose; HG, high glucose; QUICKI, Quantitative Insulin Sensitivity Check Index (whole-body insulin sensitivity).
TABLE 3.
The 12-h iAUCs for glucose, triacylglycerol, cholesterol, and insulin kinetics1
| Male adolescents |
Female adolescents |
||||
| All (n = 40) | ≤22 BF% (n = 9) | >22 BF% (n = 11) | ≤32 BF% (n = 11) | >32 BF% (n = 9) | |
| Triacylglycerol (mg/dL per 12 h) | |||||
| HF | 47 ± 16 | 2 ± 35 | 137 ± 25* | 30 ± 18 | 1 ± 29 |
| HG | 19 ± 15 | −17 ± 20 | 106 ± 28* | 0 ± 27 | −27 ± 24 |
| Total cholesterol (mg/dL per 12 h) | |||||
| HF | −9 ± 6** | 4 ± 16** | −19 ± 9 | −14 ± 9 | −6 ± 10** |
| HG | −21 ± 5 | −40 ± 9 | −17 ± 7 | −13 ± 7 | −17 ± 16 |
| HDL cholesterol (mg/dL per 12 h) | |||||
| HF | −11 ± 3 | 0 ± 4 | −11 ± 5 | −15 ± 5 | −17 ± 5 |
| HG | −11 ± 3 | −15 ± 7 | −16 ± 3 | −11 ± 5 | −3 ± 5 |
| HDL3 cholesterol (mg/dL per 12 h) | |||||
| HF | −9 ± 2 | −1 ± 5 | −11 ± 2 | −13 ± 6 | −8 ± 3 |
| HG | −11 ± 2 | −15 ± 5 | −10 ± 3 | −11 ± 4 | −8 ± 4 |
| Glucose (mg/dL per 12 h) | |||||
| HF | 4706 ± 859 | 6973 ± 2554 | 2430 ± 1018 | 5762 ± 1784 | 3929 ± 1,156 |
| HG | 3838 ± 893 | 1872 ± 2938 | 2010 ± 846 | 5847 ± 1593 | 5584 ± 1,291 |
| Lactate (mg/dL per 12 h) | |||||
| HF | 1088 ± 190** | 1234 ± 393** | 716 ± 228** | 1502 ± 497** | 890 ± 343** |
| HG | 295 ± 129 | 494 ± 190 | 21 ± 142 | 462 ± 330 | 227 ± 334 |
| Insulin (pmol/L per 12 h) | |||||
| HF | 93,628 ± 6432** | 58,975 ± 5648** | 100,947 ± 10,009** | 87,804 ± 10,686** | 126,452 ± 15,457** |
| HG | 115,472 ± 8356 | 74,730 ± 7022 | 111,305 ± 11,292 | 121,996 ± 13,167 | 153,334 ± 24,660 |
| C-peptide (pmol/L per 12 h) | |||||
| HF | 273,183 ± 19,275** | 192,183 ± 17,540 | 293,066 ± 33,665 | 263,220 ± 42,343** | 342,060 ± 42,220** |
| HG | 328,959 ± 22,698 | 226,592 ± 29,306 | 291,584 ± 27,122 | 356,066 ± 37,977 | 443,876 ± 60,748 |
| Insulin secretion (pmol · kg−1 · min−1 per 12 h) | |||||
| HF | 899 ± 70** | 617 ± 55 | 879 ± 118 | 963 ± 169** | 1125 ± 146** |
| HG | 1089 ± 87 | 736 ± 94 | 881 ± 109 | 1306 ± 152 | 1430 ± 238 |
| Insulin clearance (% over 12 h) | |||||
| HF | −4093 ± 404 | −2851 ± 816 | −3489 ± 634 | −5028 ± 977 | −4929 ± 567 |
| HG | −4759 ± 663 | −5576 ± 1528 | −5184 ± 1417 | −5032 ± 887 | −3090 ± 1568 |
All values are means ± SEMs. A 2 (trial) by 2 (sex) by 2 (adiposity) mixed-model repeated-measures ANOVA with follow-up Bonferroni-adjusted post hoc tests were used to determine specific differences. Significance was P ≤ 0.05. Unless otherwise noted, the main effect of trial; the within-subject interaction terms trial × adiposity, trial × sex, or trial × adiposity × sex; or the between-subjects effect for adiposity, sex, or adiposity × sex interaction were not significant (P > 0.05). For the 12-h triacylglycerol iAUC, there was a significant between-subjects effect for adiposity, sex, and adiposity × sex interaction (P < 0.05). For the 12-h total cholesterol iAUC, there was a significant main effect of trial (P < 0.05) and a significant within-subject trial × adiposity × sex interaction effect (P < 0.05). For the 12-h lactate iAUC, there was a significant main effect of trial (P < 0.05). For the 12-h insulin and C-peptide iAUC, there was a significant main effect of trial (P < 0.05), a significant within-subject trial × sex interaction effect (P < 0.05), and a significant between-subjects effect for sex and adiposity (P < 0.05). For the 12-h insulin secretion iAUC, there was a significant main effect of trial (P < 0.05) and a significant within-subject sex × trial interaction (P < 0.05). *P < 0.05 compared with lean male adolescents and lean and overweight/obese female adolescents within the HF or HG trial; **P < 0.05 compared with the HG trial within the same group. BF%, percentage of body fat; HF, high fructose; HG, high glucose; iAUC, incremental AUC.
Fasting TC concentrations were not significantly different (P < 0.05) between trials, sexes, or adiposity groups (Table 2). The 12-h TC iAUC was 80% higher during the HF trial compared with the HG trial (indicating the decrease in TC was less during the HF trial), and this response was most pronounced in lean male and overweight/obese female adolescents (P < 0.05; Table 3).
Fasting HDL-cholesterol and HDL3-cholesterol concentrations were not significantly different between trials or adiposity groups (P > 0.05), but female compared with male adolescents had 14–31% and 10–22% greater fasting HDL- and HDL3-cholesterol concentrations, respectively (P < 0.05; Table 2). The 12-h HDL- and HDL3-cholesterol iAUCs were not significantly different (P < 0.05) between trials, sexes, or adiposity groups (Table 3).
Glucose and lactate
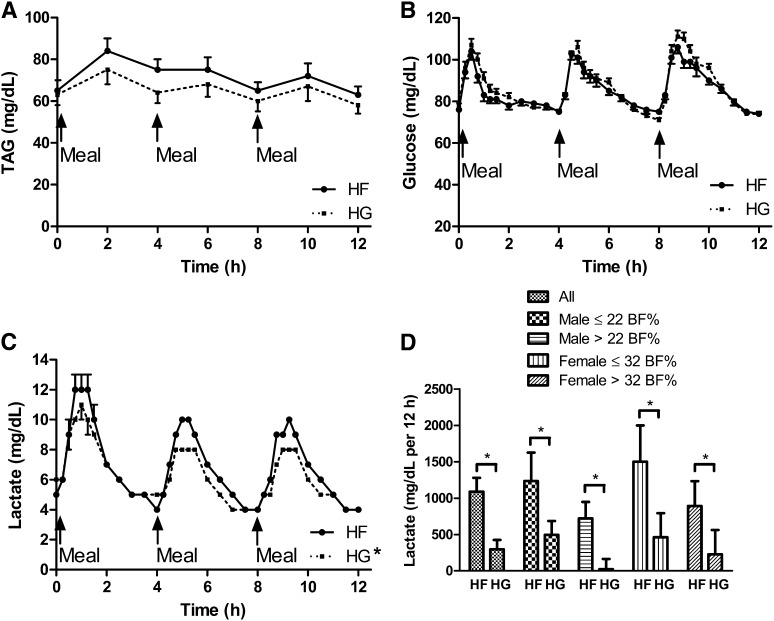
Fasting glucose or lactate concentrations or the 12-h glucose iAUC were not significantly different (P > 0.05) between trials, sexes, or adiposity groups (Table 2 and Table 3, Figure 1). The 12-h lactate iAUC was ∼3.7-fold greater (P < 0.05) during the HF trial than during the HG trial (Figure 1).
FIGURE 1.
Time course of fasting and postprandial concentrations of triacylglycerol (A), glucose (B), and lactate (C) and lactate incremental AUC (D) during each trial. Values are means ± SEMs. Unless otherwise noted, the main effect of trial; the within-subject interaction terms trial × adiposity, trial × sex, or trial × adiposity × sex; and the between-subjects effect for adiposity, sex, or adiposity × sex interaction were not significant (P > 0.05). For lactate, there was a significant main effect of trial (P < 0.05). *The 12-h lactate incremental AUC was significantly lower (P < 0.05) during the HG trial than during the HF trial. BF%, body fat percentage; HF, high fructose; HG, high glucose; TAG, triacylglycerol.
Insulin sensitivity and insulin resistance
Insulin sensitivity (QUICKI) (P > 0.05) was not significantly different between trials or sexes (P > 0.05), but the QUICKI was 3–9% lower (P < 0.05) in overweight/obese adolescents than in lean adolescents (Table 2). Hepatic insulin resistance (HOMA-IR) was not significantly different between trials or sexes (P > 0.05), but the HOMA-IR was ∼22–48% higher (P < 0.05) in overweight/obese adolescents than in lean adolescents (Table 2).
Insulin and C-peptide concentrations
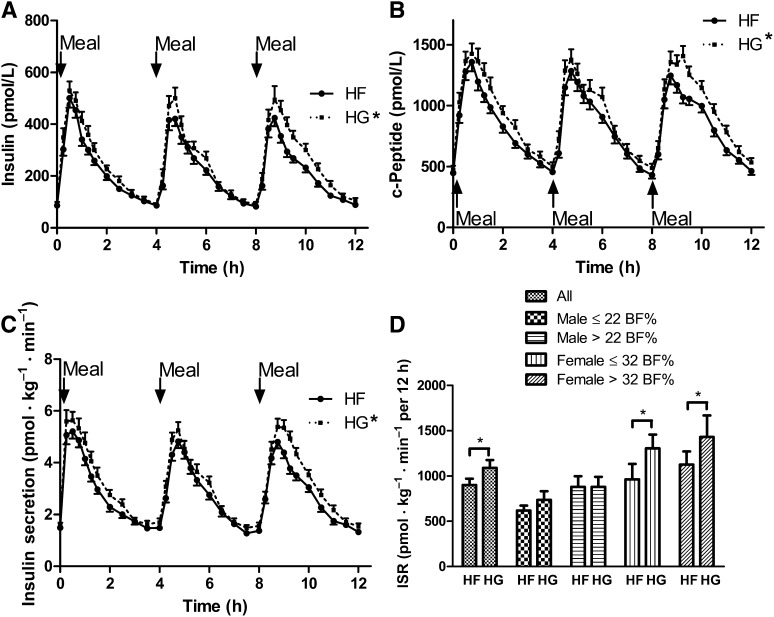
Fasting insulin concentrations were not significantly different (P > 0.05) between trials or between sexes (Table 2), but fasting insulin concentrations were significantly greater (P < 0.05) in overweight/obese adolescents than in lean adolescents (Table 2). Fasting C-peptide concentrations were not significantly different (P < 0.05) between trials, sexes, or adiposity groups (Table 2). The 12-h insulin and C-peptide iAUCs were 21% and 19% lower (P < 0.05), respectively, during the HF trial compared with the HG trial, an effect that was most pronounced in female adolescents (Table 3, Figure 2). Independent of diet, the 12-h insulin and C-peptide iAUCs were 23–52% and 22–42% greater (P < 0.05), respectively, in overweight/obese adolescents than in lean adolescents and 22–48% and 15–41% greater (P < 0.05), respectively, in female adolescents than in male adolescents (Table 3).
FIGURE 2.
Time course of insulin (A) and C-peptide concentrations (B), insulin secretion (C), and insulin secretion iAUC (D) during each trial. All values are means ± SEMs. Unless otherwise noted, the main effect of trial; the within-subject interaction terms trial × adiposity, trial × sex, or trial × adiposity × sex; or the between-subjects effect for adiposity, sex, or adiposity × sex interaction were not significant (P > 0.05). For the 12-h insulin and C-peptide iAUC, there was a significant main effect of trial (P < 0.05), a significant within-subject trial × sex interaction effect (P < 0.05), and a significant between-subjects effect for sex and adiposity (P < 0.05). For the 12-h insulin secretion iAUC, there was a significant main effect of trial (P < 0.05) and a significant within-subject sex × trial interaction (P < 0.05). *The 12-h insulin, C-peptide, and insulin secretion iAUC were significantly greater (P < 0.05) during the HG trial than during the HF trial. BF%, body fat percentage; HF, high fructose; HG, high glucose; iAUC, incremental AUC; ISR, insulin secretion rate.
Insulin secretion and clearance
Basal ISRs and insulin clearance were not significantly different between trials, sexes, or adiposity groups (P > 0.05; Table 2). The 12-h ISR iAUC was 19% greater (P < 0.05) during the HG trial compared with the HF trial (Figure 2). This difference in ISR between trials was more pronounced in female adolescents (∼25–56% greater) than in male adolescents (P < 0.05; Figure 2). The 12-h insulin clearance iAUC was not significantly different (P > 0.05) between trials, sexes, or adiposity groups (Table 3).
DISCUSSION
To our knowledge, this is the first study to determine how the type of sugar-sweetened beverage (HF compared with HG) influences insulin sensitivity and resistance (assessed via QUICKI and HOMA-IR), traditional markers of metabolic health (triacylglycerol, cholesterol, glucose), and insulin secretion and clearance in adolescents (aged 15–20 y), a population who consumes more sugar-sweetened beverages than does any other age group (28, 29). The primary findings of this study are as follows: 1) HF- and HG-sweetened beverages do not differentially alter insulin sensitivity (assessed via QUICKI), hepatic insulin resistance (assessed via HOMA-IR), or traditional fasting markers of metabolic health (triacylglycerol, cholesterol, glucose, insulin secretion, or insulin clearance) or postprandial markers of metabolic health (triacylglycerol, HDL cholesterol, HDL3 cholesterol, glucose, and insulin clearance); 2) HF meals, compared with HG meals, resulted in greater postprandial lactate concentrations; 3) HG meals resulted in greater postprandial insulin secretion; 4) despite similar whole-body insulin sensitivity and hepatic insulin resistance and independent of the type of sugar-sweetened beverage consumed, female adolescents had greater insulin secretion and HDL-cholesterol concentrations than did male adolescents; 5) female adolescents had lower free-living physical activity levels compared with male adolescents; and 6) independent of the type of sugar-sweetened beverage consumed or previous physical activity levels, overweight/obese adolescents showed lower whole-body insulin sensitivity and greater hepatic insulin resistance than did their lean counterparts. These findings expand on previous data showing that high fructose intake is not associated with deleterious metabolic consequences in adolescents (30–33) and suggest that short-term (2-wk) consumption of moderate amounts (50 g/d) of HF- or HG-sweetened beverages do not detrimentally alter metabolic health in weight-stable, physically active adolescents. Collectively, the data suggest that fructose or glucose consumption per se is not detrimental to an adolescent's health, and perhaps factors other than fructose intake (obesity, physical activity levels) should be targeted to prevent chronic disease in this population.
Triacylglycerol responses
Previous research has shown that acute or chronic ingestion of fructose with mixed meals, compared with glucose with mixed meals, augments the postprandial triacylglycerol response in adults (4, 7–14). This effect may be mediated via reduced triacylglycerol clearance (as a result of reduced insulin secretion; thus, a reduced insulin-mediated increase in adipose tissue lipoprotein lipase activity resulting in reduced triacylglycerol hydrolysis in adipose tissue) and increased de novo lipogenesis and hepatic triacylglycerol secretion (4, 10, 42). Unexpectedly, we found that HF mixed meals, compared with HG mixed meals, did not augment the postprandial triacylglycerol response in adolescents despite greater insulin concentrations with HG mixed meals (although there was a trend for postprandial triacylglycerol to be greater during the HF trial). In addition, overweight/obese male adolescents had a greater postprandial triacylglycerol response compared with lean male adolescents and lean and overweight/obese female adolescents, an effect that was independent of previous diet. This effect was expected, because previous work has shown that obese adults, particularly those with greater visceral fat (typically males), have greater postprandial triacylglycerol responses compared with lean adolescents (43) with lower visceral fat.
Cholesterol responses
The data from the present study show that sugar-sweetened beverages do not alter fasting TC concentrations, and this finding is similar to some (26, 31), but not all (4, 16), research. In the current investigation, cholesterol concentrations decreased in the postprandial period, which has been documented before in adults (44, 45). The decline in postprandial TC was greater when HG meals were ingested, particularly in lean male adolescents and overweight/obese female adolescents. It is possible that the greater insulin concentrations during the HG trial resulted in greater reverse cholesterol transport.
Chronic consumption (≥2 wk) of glucose-, fructose-, sucrose-, or HFCS-sweetened beverages did not alter fasting HDL-cholesterol concentrations in adults (7, 16, 26). Likewise, in our study, chronic consumption of sugar-sweetened beverages did not alter fasting or postprandial HDL-cholesterol concentrations in adolescents. For the first time, we examined how sugar-sweetened beverages affect HDL3-cholesterol concentrations, and similar to total HDL cholesterol, fasting or postprandial HDL3-cholesterol concentrations were not differentially altered by HF- or HG-sweetened beverages in adolescents. Regardless of adiposity or type of sugar-sweetened beverage consumed, female adolescents had greater HDL- and HDL3-cholesterol concentrations than did male adolescents. Previous work in adults has shown that females typically have greater HDL-cholesterol concentrations compared with males, which is attributable to greater secretion rates of apolipoprotein A-I (a major protein component of HDL cholesterol) and not to differences in the catabolic rate of HDL cholesterol between sexes (46).
Glucose and lactate responses
The impact of sugar-sweetened beverages on fasting glucose concentrations is mixed and may depend on the age of the subjects, energy status, and duration of consuming the drinks. For instance, in the present study, fasting glucose concentrations were unaltered after consuming HF- or HG-sweetened beverages for 2 wk in adolescents (aged 15–20 y), and Stanhope et al (7) also reported no changes in fasting glucose after 2-wk consumption of glucose, fructose, or HFCS in adolescents and young adults (aged 18–40 y). However, 10-wk consumption of fructose-sweetened beverages, but not glucose-sweetened beverages, increased fasting glucose values in older adults (aged 40–72 y) (4).
Previous work has shown that glucose-sweetened beverages, compared with fructose or HFCS-sweetened beverages, result in greater postprandial glucose responses (AUC and glucose peak) in adults (7, 10, 12, 47). On the contrary, we did not find differences in postprandial glucose concentrations between HF- and HG-sweetened beverages. The greater insulin concentrations induced by HG mixed meals may have helped to maintain euglycemia. It is also possible that the difference in the amount of glucose ingested (20.1 g glucose with each meal during the HG trial compared with 8.4 g glucose with each meal during HF trial) between trials was not large enough to evoke different postprandial glucose responses.
The HF mixed meals in the current study induced greater postprandial lactate concentrations, and this finding has been documented before (48). Unlike glucose, fructose undergoes almost complete hepatic metabolism and bypasses the phosphofructokinase regulatory step in glycolysis, which allows greater saturation of the glycolysis pathway (49). Tracer studies have shown that a significant amount of fructose can be converted to lactate, because ingestion of fructose + glucose increases the lactate rate of appearance to a greater extent than does ingestion of glucose alone (50). Therefore, the greater postprandial lactate concentrations with HF mixed meals in the current study were likely mediated via increased hepatic lactate production.
Insulin responses
In adults, the consumption of fructose-sweetened beverages for ≥6 d has been shown to impair hepatic insulin sensitivity (16–19), but our findings in adolescents did not support this previous work. Other studies in adults showed that consumption of fructose-, glucose-, or HFCS-sweetened beverages for ≥2 wk does not alter whole-body insulin sensitivity (7, 15, 26). Extending on these findings, the present investigation showed that whole-body insulin sensitivity (assessed via QUICKI) was unaltered after consumption of HF- or HG-sweetened beverages for 2 wk in adolescents. It may be that a longer duration of consuming fructose-sweetened beverages, a greater dose of fructose, overnutrition, or lower levels of physical activity are needed to reduce whole-body or hepatic insulin sensitivity with HF- or HG-sweetened beverage consumption.
The present study is the first to our knowledge to compare how HF and HG mixed meals alter insulin secretion or insulin clearance in adolescents. Despite no differences in insulin sensitivity, insulin resistance, or venous postprandial glucose concentrations in the periphery, HG mixed meals resulted in greater postprandial insulin secretion rates compared with HF mixed meals. It is possible that blood glucose concentrations were greater in the hepatic portal vein while consuming the HG-mixed meals, which resulted in greater insulin secretion rates and thus lowered the blood glucose concentrations in the periphery (where we measured blood glucose). In addition, given that insulin secretion is not only potentiated by glucose concentrations but also by other factors (gut hormones and metabolites), it is possible that these other factors contributed to the greater insulin secretion rates with HG mixed meals. One potential factor is differences in the incretin hormone glucose-dependent insulinotropic polypeptide. Although not measured in this study, other work in adults that used solid and liquid foods showed that HG mixed meals, compared with HF mixed meals, resulted in more rapid increases in glucose-dependent insulinotropic polypeptide, a hormone that potentiates insulin release, and this phenomenon may contribute to greater insulin secretion with HG mixed meals (12).
Conclusions
In conclusion, the consumption of moderate amounts of HF- or HG-sweetened beverages for 2 wk in addition to an ad libitum diet has little impact on traditional markers of metabolic health (triacylglycerol, cholesterol, glucose) in weight-stable, physically active adolescents. However, acute ingestion of HF mixed meals, compared with HG mixed meals, resulted in lower postprandial insulin secretion rates but greater postprandial lactate concentrations. Collectively, the data from this study and others (30–33) suggest that fructose or glucose consumption per se is not detrimental to an adolescent's health and perhaps factors other than fructose intake (obesity, physical activity levels) should be modulated to prevent chronic disease.
Acknowledgments
We thank Roman Hovorka for supplying the ISEC program free of charge.
The authors’ responsibilities were as follows—TDH: designed and conducted the research, analyzed data, performed statistical analysis, and wrote the manuscript; YL, YM-P, LMN, and NCW: conducted the research and analyzed data; and JAK: conceived of the study, designed and conducted the research, analyzed data, edited the final version of the manuscript, and had primary responsibility for final content. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: HF, high fructose; HFCS, high-fructose corn syrup; HG, high glucose; iAUC, incremental AUC; ISR, insulin secretion rate; QUICKI, Quantitative Insulin Sensitivity Check Index; TC, total cholesterol.
REFERENCES
- 1.Aeberli I, Zimmermann MB, Molinari L, Lehmann R, l'Allemand D, Spinas GA, Berneis K. Fructose intake is a predictor of LDL particle size in overweight schoolchildren. Am J Clin Nutr 2007;86(4):1174–8. [DOI] [PubMed] [Google Scholar]
- 2.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:1321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric sweetener consumption and dyslipidemia among US adults. JAMA 2010;303:1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Keim NL, et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metab (Lond) 2012;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Keim NL, et al. Circulating concentrations of monocyte chemoattractant protein-1, plasminogen activator inhibitor-1, and soluble leukocyte adhesion molecule-1 in overweight/obese men and women consuming fructose- or glucose-sweetened beverages for 10 weeks. J Clin Endocrinol Metab 2011;96:E2034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, Chen G, Fong TH, Lee V, Menorca RI, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab 2011;96:E1596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egli L, Lecoultre V, Theytaz F, Campos V, Hodson L, Schneiter P, Mittendorfer B, Patterson BW, Fielding BA, Gerber PA, et al. Exercise prevents fructose-induced hypertriglyceridemia in healthy young subjects. Diabetes 2013;62:2259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bortolotti M, Dubuis J, Schneiter P, Tappy L. Effects of dietary protein on lipid metabolism in high fructose fed humans. Clin Nutr 2012;31:238–45. [DOI] [PubMed] [Google Scholar]
- 10.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr 2007;85(6):1511–20. [DOI] [PubMed] [Google Scholar]
- 11.Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr 2008;87(5):1194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D'Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 2004;89:2963–72. [DOI] [PubMed] [Google Scholar]
- 13.Abraha A, Humphreys SM, Clark ML, Matthews DR, Frayn KN. Acute effect of fructose on postprandial lipaemia in diabetic and non-diabetic subjects. Br J Nutr 1998;80(2):169–75. [PubMed] [Google Scholar]
- 14.Cohen JC, Schall R. Reassessing the effects of simple carbohydrates on the serum triglyceride responses to fat meals. Am J Clin Nutr 1988;48:1031–4. [DOI] [PubMed] [Google Scholar]
- 15.Le KA, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr 2006;84(6):1374–9. [DOI] [PubMed] [Google Scholar]
- 16.Aeberli I, Hochuli M, Gerber PA, Sze L, Murer SB, Tappy L, Spinas GA, Berneis K. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care 2013;36(1):150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 2005;54(7):1907–13. [DOI] [PubMed] [Google Scholar]
- 18.Lecoultre V, Egli L, Carrel G, Theytaz F, Kreis R, Schneiter P, Boss A, Zwygart K, Le KA, Bortolotti M, et al. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obesity (Silver Spring) 2013;21:782–5. [DOI] [PubMed] [Google Scholar]
- 19.Couchepin C, Le KA, Bortolotti M, da Encarnacao JA, Oboni JB, Tran C, Schneiter P, Tappy L. Markedly blunted metabolic effects of fructose in healthy young female subjects compared with male subjects. Diabetes Care 2008;31(6):1254–6. [DOI] [PubMed] [Google Scholar]
- 20.Sobrecases H, Le KA, Bortolotti M, Schneiter P, Ith M, Kreis R, Boesch C, Tappy L. Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab 2010;36(3):244–6. [DOI] [PubMed] [Google Scholar]
- 21.Theytaz F, Noguchi Y, Egli L, Campos V, Buehler T, Hodson L, Patterson BW, Nishikata N, Kreis R, Mittendorfer B, et al. Effects of supplementation with essential amino acids on intrahepatic lipid concentrations during fructose overfeeding in humans. Am J Clin Nutr 2012;96(5):1008–16. [DOI] [PubMed] [Google Scholar]
- 22.Le KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr 2009;89(6):1760–5. [DOI] [PubMed] [Google Scholar]
- 23.Aeberli I, Gerber PA, Hochuli M, Kohler S, Haile SR, Gouni-Berthold I, Berthold HK, Spinas GA, Berneis K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 2011;94(2):479–85. [DOI] [PubMed] [Google Scholar]
- 24.Bravo S, Lowndes J, Sinnett S, Yu Z, Rippe J. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl Physiol Nutr Metab 2013;38:681–8. [DOI] [PubMed] [Google Scholar]
- 25.Black RN, Spence M, McMahon RO, Cuskelly GJ, Ennis CN, McCance DR, Young IS, Bell PM, Hunter SJ. Effect of eucaloric high- and low-sucrose diets with identical macronutrient profile on insulin resistance and vascular risk: a randomized controlled trial. Diabetes 2006;55(12):3566–72. [DOI] [PubMed] [Google Scholar]
- 26.Heden TD, Liu Y, Kearney ML, Kanaley JA. Weight classification does not influence the short-term endocrine and metabolic effects of high-fructose corn syrup sweetened beverages. Appl Physiol Nutr Metab 2014;39(5):544–52. [DOI] [PubMed] [Google Scholar]
- 27.Johnston RD, Stephenson MC, Crossland H, Cordon SM, Palcidi E, Cox EF, Taylor MA, Aithal GP, Macdonald IA. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology 2013;145(5):1016–25. [DOI] [PubMed] [Google Scholar]
- 28.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 2009;139(6 suppl):1228S–35S. [DOI] [PubMed] [Google Scholar]
- 29.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- 30.Sunehag AL, Toffolo G, Treuth MS, Butte NF, Cobelli C, Bier DM, Haymond MW. Effects of dietary macronutrient content on glucose metabolism in children. J Clin Endocrinol Metab 2002;87:5168–78. [DOI] [PubMed] [Google Scholar]
- 31.Sunehag AL, Toffolo G, Campioni M, Bier DM, Haymond MW. Short-term high dietary fructose intake had no effects on insulin sensitivity and secretion or glucose and lipid metabolism in healthy, obese adolescents. J Pediatr Endocrinol Metab 2008;21:225–35. [DOI] [PubMed] [Google Scholar]
- 32.Treuth MS, Sunehag AL, Trautwein LM, Bier DM, Haymond MW, Butte NF. Metabolic adaptation to high-fat and high-carbohydrate diets in children and adolescents. Am J Clin Nutr 2003;77:479–89. [DOI] [PubMed] [Google Scholar]
- 33.Mohammad MA, Sunehag AL, Haymond MW. Effect of dietary macronutrient composition under moderate hypocaloric intake on maternal adaptation during lactation. Am J Clin Nutr 2009;89(6):1821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin R, Le NA, Liu S, Farkas Epperson M, Ziegler TR, Welsh JA, Jones DP, McClain CJ, Vos MB. Children with NAFLD are more sensitive to the adverse metabolic effects of fructose beverages than children without NAFLD. J Clin Endocrinol Metab 2012;97(7):E1088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. Am J Clin Nutr 2000;71:1392–402. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz O, Russell M, Daley TL, Baumgartner RN, Waki M, Lichtman S, Wang J, Pierson RN, Jr, Heymsfield SB. Differences in skeletal muscle and bone mineral mass between black and white females and their relevance to estimates of body composition. Am J Clin Nutr 1992;55:8–13. [DOI] [PubMed] [Google Scholar]
- 37.Zhang JQ, Thomas TR, Ball SD. Effect of exercise timing on postprandial lipemia and HDL cholesterol subfractions. J Appl Physiol 1998;85:1516–22. [DOI] [PubMed] [Google Scholar]
- 38.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–10. [DOI] [PubMed] [Google Scholar]
- 39.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 40. Hovorka R, Soons PA, Young MA. ISEC: a program to calculate insulin secretion. Comput Methods Programs Biomed 1996;50(3):253ndash64. [DOI] [PubMed]
- 41.Polonsky KS, Pugh W, Jaspan JB, Cohen DM, Karrison T, Tager HS, Rubenstein AH. C-peptide and insulin secretion: relationship between peripheral concentrations of C-peptide and insulin and their secretion rates in the dog. J Clin Invest 1984;74:1821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr 2008;138(6):1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreno LA, Quintela I, Fleta J, Sarria A, Roda L, Giner A, Bueno M. Postprandial triglyceridemia in obese and non-obese adolescents: importance of body composition and fat distribution. J Pediatr Endocrinol Metab 2001;14:193–202. [DOI] [PubMed] [Google Scholar]
- 44.Bourdon I, Yokoyama W, Davis P, Hudson C, Backus R, Richter D, Knuckles B, Schneeman BO. Postprandial lipid, glucose, insulin, and cholecystokinin responses in men fed barley pasta enriched with beta-glucan. Am J Clin Nutr 1999;69:55–63. [DOI] [PubMed] [Google Scholar]
- 45.Heden TD, Liu Y, Sims LJ, Whaley-Connell AT, Chockalingam A, Dellsperger KC, Kanaley JA. Meal frequency differentially alters postprandial triacylglycerol and insulin concentrations in obese women. Obesity (Silver Spring) 2013;21:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tilly-Kiesi M, Lichtenstein AH, Joven J, Vilella E, Cheung MC, Carrasco WV, Ordovas JM, Dolnikowski G, Schaefer EJ. Impact of gender on the metabolism of apolipoprotein A-I in HDL subclasses LpAI and LpAI:AII in older subjects. Arterioscler Thromb Vasc Biol 1997;17:3513–8. [DOI] [PubMed] [Google Scholar]
- 47.Stanhope KL, Griffen SC, Bremer AA, Vink RG, Schaefer EJ, Nakajima K, Schwarz JM, Beysen C, Berglund L, Keim NL, et al. Metabolic responses to prolonged consumption of glucose- and fructose-sweetened beverages are not associated with postprandial or 24-h glucose and insulin excursions. Am J Clin Nutr 2011;94(1):112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chong MF, Fielding BA, Frayn KN. Metabolic interaction of dietary sugars and plasma lipids with a focus on mechanisms and de novo lipogenesis. Proc Nutr Soc 2007;66(1):52–9. [DOI] [PubMed] [Google Scholar]
- 49.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr 1993;58(suppl):754S–65S. [DOI] [PubMed] [Google Scholar]
- 50.Lecoultre V, Benoit R, Carrel G, Schutz Y, Millet GP, Tappy L, Schneiter P. Fructose and glucose co-ingestion during prolonged exercise increases lactate and glucose fluxes and oxidation compared with an equimolar intake of glucose. Am J Clin Nutr 2010;92(5):1071–9. [DOI] [PubMed] [Google Scholar]