Abstract
Background: After the oral administration of iron, the production of circulating non–transferrin-bound iron may contribute to an increased risk of illness in malaria-endemic areas that lack effective medical services.
Objective: In healthy women with a range of body iron stores, we aimed to determine effects on the production of circulating non–transferrin-bound iron resulting from the oral administration of 1) a supplemental dose of iron (60 mg) with water, 2) a supplemental dose of iron (60 mg) with a standard test meal, and 3) a fortification dose of iron (6 mg) with a standard test meal.
Design: With the use of serum ferritin as the indicator, healthy women with replete iron stores (ferritin concentration >25 μg/L; n = 16) and reduced iron stores (ferritin concentration ≤25 μg/L; n = 16) were enrolled in a prospective, randomized, crossover study. After the oral administration of aqueous solutions of ferrous sulfate isotopically labeled with 54Fe, 57Fe, or 58Fe, blood samples were collected for 8 h, and iron absorption was estimated by erythrocyte incorporation at 14 d.
Results: At 4 h, serum non–transferrin-bound iron reached peaks with geometric mean (95% CI) concentrations of 0.81 μmol/L (0.56, 1.1 μmol/L) for 60 mg Fe with water and 0.26 μmol/L (0.15, 0.38 μmol/L) for 60 mg Fe with food but was at assay limits of detection (0.1 μmol Fe/L) for 6 mg Fe with food. For the 60 mg Fe without food, the area under the curve over 8 h for serum non–transferrin-bound iron was positively correlated with the amount of iron absorbed (R = 0.49, P < 0.01) and negatively correlated with serum ferritin (R = −0.39, P < 0.05).
Conclusions: In healthy women, the production of circulating non–transferrin-bound iron is determined by the rate and amount of iron absorbed. The highest concentrations of non–transferrin-bound iron resulted from the administration of supplemental doses of iron without food. Little or no circulating non–transferrin-bound iron resulted from the consumption of a meal with a fortification dose of iron. This trial was registered at clinicaltrials.gov as NCT01404533.
INTRODUCTION
The available evidence (1–4) and current WHO guidance (5) indicate that iron supplementation and fortification for the prevention and correction of iron deficiency are safe in settings without endemic malaria and, with adequate health care, in regions with a high transmission of malaria and other infections. Without regular surveillance and treatment of malaria and other infections, results from a variety of studies have suggested that the oral administration of iron may increase risk of severe illness and death (6). The mechanisms responsible are unknown. A WHO consultation identified circulating non–transferrin-bound iron as a potential source of these apparent adverse effects of iron (7).
Circulating non–transferrin-bound iron consists of forms of iron, which are not complexed with heme or ferritin, that are present in the bloodstream independently of the iron-transport protein transferrin (8). More than 3 decades ago, circulating non–transferrin-bound iron was first identified in sera in which the plasma iron concentration exceeded the iron-binding capacity of transferrin. Circulating non–transferrin-bound iron is now known to appear despite the presence of available binding sites on transferrin if the rate of iron influx into plasma exceeds the rate of iron acquisition by transferrin (9, 10). Concentrations of circulating non–transferrin-bound iron associated with adverse effects have not been established. For non-malarial infections, circulating non–transferrin-bound iron provides a readily available source of iron to enhance the virulence of pathogens reaching the bloodstream (11). For malarial infections, the mechanisms of harmful effects remain to be determined, and a variety of possibilities are under investigation (6, 12–19).
Iron supplementation uses iron preparations, usually in doses of 1–3 mg/kg body weight to treat or prevent iron deficiency in individuals or population groups. The production of circulating non–transferrin-bound iron by supplemental doses of iron has been described by other investigators (9, 20–23). Amounts of additional iron provided by fortification are generally lower than those from supplementation. To our knowledge, the effects of fortification doses of iron on production of circulating non–transferrin-bound iron have not been reported previously.
In a large, randomized, controlled trial in Pemba, Tanzania, which is an area of high malaria transmission, oral iron supplementation (at ∼1 mg/kg per day without food) seemed to benefit iron-deficient children but harm those who were iron replete (24). Circulating non–transferrin-bound iron was not measured. The current study examined healthy women with replete or reduced iron stores who were given aqueous solutions of ferrous sulfate labeled with stable isotopes of iron. Our aim was to compare the effects on production of circulating non–transferrin-bound iron resulting from oral administration of a supplemental dose of iron (∼1 mg/kg) with water, a supplemental dose of iron (∼1 mg/kg) with food, and a fortification dose of iron (∼0.1 mg/kg) with food.
SUBJECTS AND METHODS
Subjects
Apparently healthy women, 18–39 y of age, were recruited from the student and staff population at ETH Zürich and the University of Zürich (Zürich, Switzerland) between May and October 2009. Exclusion criteria were pregnancy and lactation, known metabolic disorders, chronic diseases, eating disorders and allergies, abnormal BMI (in kg/m2; <18.5 or >25) and inflammation [C-reactive protein (CRP) concentration >10 mg/L]. No medication (except oral contraceptives) was allowed, and vitamin and mineral supplements were not allowed for 2 wk before and during the entire study. Women with blood donations <4 mo before the start of the study were not included.
The total sample size was 32 subjects, 16 of whom had replete iron stores, which were evaluated as a serum ferritin concentration >25 μg/L, and 16 of whom had reduced iron stores, which were indicated by a serum ferritin concentration ≤25 μg/L. The sample size of 16 subjects for each subgroup of iron stores was estimated to provide a power of 80% (β = 0.20) to detect an intrasubject difference of 30% in fractional iron absorption with α = 0.05. Fractional iron absorption was used to estimate the sample size in the absence of data with respect to formation of non–transferrin-bound iron.
The study protocol was approved by the ETH Zürich (Zürich, Switzerland) ethics committee. Written informed consent was obtained from all subjects. The study was registered at clinicaltrials.gov as NCT01404533.
Study design
Subjects were screened for iron status, inflammation, and body height and weight before assignment to one of the subgroups according to serum ferritin. Subjects were asked to refrain from eating after 2000 and from drinking after 2400 on the days preceding study days. Each subject was given the 3 following iron preparations in a randomized order on 3 study days: 60 mg Fe as ferrous sulfate (54 mg unlabeled Fe and 6 mg 54Fe) in 100 g deionized water (preparation A), 60 mg Fe as ferrous sulfate (54 mg unlabeled Fe and 6 mg 58Fe) with a standardized meal (preparation B), and 6 mg Fe as ferrous sulfate (6 mg 57Fe) with a standardized meal (preparation C). The sequence of the 3 iron preparations (ABC, ACB, BAC, BCA, CAB, and CBA) was randomly allocated to each subject by a random-number assignment by one of the study investigators (JT). Blood samples (8 mL) were collected before administering the iron preparation (0 h) and at 1, 2, 4, 6, and 8 h after administration for a total of 48 mL. Participants were not allowed to eat and drink until the 4-h blood sampling after which they were given a sandwich of cheese and unfortified white bread and allowed to drink deionized water ad libitum. After the 6-h blood sampling, subjects were given an apple, and after the 8-h blood sample, subjects were allowed to eat their self-selected diet. Participants were carefully supervised by a nurse during the 8 h of repeated blood sampling. The protocol was repeated with each iron preparation, and 14 d after the third iron administration, a final blood sample was collected to determine iron absorption.
Test meals
Test meals used in iron preparations B and C consisted of boiled white rice (50 g dry weight) and 25 g (fresh weight) pureed vegetable sauce that was based on cabbage, carrots, zucchini, and onions. Meals were prepared in batches according to a standardized recipe and stored frozen until use. Immediately before consumption, meals were heated in a microwave oven, and the iron preparation was added. The iron preparation A was added to 100 mL deionized water and consumed with an additional 200 mL deionized water. Test meals with iron preparations B and C were consumed with 300 mL deionized water. In the subgroup with replete iron stores, iron preparations A–C were given on 3 consecutive days, and in the subgroup with reduced iron stores, iron preparations were given on the same weekdays of 3 consecutive weeks.
Stable isotope labels and iron preparations
Isotopically labeled 54FeSO4, 57FeSO4, and 58FeSO4 were prepared from isotopically enriched elemental iron (Chemgas) by dissolution in dilute sulfuric acid. Isotopic enrichment was 99.9% for 54Fe, 97.8% for 57Fe, and 99.5% for 58Fe. For iron preparations A and C, 54 mg unlabeled Fe as ferrous sulfate (Sigma-Aldrich) dissolved in water was added together with the isotopic label.
Blood analysis
Hemoglobin was measured in whole blood by using an automated Coulter counter (AcT8 Counter; Beckman Coulter). Ferritin and CRP were measured in serum using an IMMULITE 2000 automatic system (Siemens Healthcare Diagnostics). External 3-level commercial quality-control materials were used for the measurement of hemoglobin, ferritin, and CRP (Beckman Coulter; Siemens Healthcare Diagnostics). Anemia was defined as a hemoglobin concentration <12 g/dL. With the use of serum ferritin as the indicator, replete iron stores were defined as a serum ferritin concentration >25 μg/L, and reduced iron stores were defined as a serum ferritin concentration ≤25 μg/L. A CRP concentration >10 mg/L suggested the presence of inflammation. One subject had an elevated CRP concentration >10 mg/L at baseline, but because the serum ferritin concentration appeared unaffected (ferritin concentration <15 μg/L), the subject was included in the study.
Serum iron and the total iron-binding capacity (TIBC) were determined by using the methods recommended by the International Committee for Standardization in Hæmatology (25), and transferrin saturation was calculated as a percentage as
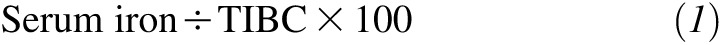 |
Non–transferrin-bound-iron was determined as previously described (10, 26) with minor modifications. In brief, the serum aliquot for non–transferrin-bound iron determination was pretreated with tris-carbonatocobaltate(III) trihydrate to saturate-free transferrin sites and stored frozen until analysis. Non–transferrin-bound iron was chelated with nitriloacetic acid (pH 7.0 at room temperature for 30 min) and separated from the protein fraction by centrifugation with the use of an ultracentrifugation filtration device [Microcon Ultracel YM-30, 30-kDa cutoff, low-binding regenerated cellulose type (Millipore) at 14,000 rpm for 40 min]. The iron content of the ultrafiltrate was measured by using graphite furnace atomic absorption spectrometry (AA240Z, Varian Inc). The lower limit of detection was 0.1 μmol Fe/L.
A hepcidin analysis in serum samples was performed at Intrinsic LifeSciences LLC by using an enzyme-linked immunosorbent assay (27). The lower limit of detection was 5 ng/mL.
Serum aliquots of blood samples collected at time points 1, 2, 4, 6, and 8 h and the whole blood sample collected 14 d after the third iron administration were analyzed for the iron isotopic composition under chemical blank monitoring. Samples were mineralized by microwave digestion with the use of a HNO3/H2O2 mixture followed by separation of the sample iron from the matrix by using anion-exchange chromatography and the precipitation of iron as ferric hydroxide (28). An isotopic analysis was performed by using a high-resolution multicollector inductively coupled plasma mass spectrometer (Neptune; Thermo-Finnigan) (29).
Calculation of iron absorption
Circulating iron was calculated on the basis of a hemoglobin concentration and blood volume estimated on height and weight according to Brown et al (30). The calculation of iron absorption was based on the shift in the isotopic ratios after a 14-d incorporation period according to Walczyk et al (31). Serum iron isotope concentrations were calculated according to principles of isotope dilution (31, 32).
Statistical analysis
The primary outcome measure of the study was the formation of circulating non–transferrin-bound iron after oral iron administration. Secondary outcome measures were the proportion (%) of iron absorbed from each test meal, amounts of iron (mg) absorbed from each test meal, serum transferrin saturation, and serum hepcidin concentration. The appearance of stable isotopes of iron in the circulation, serum iron, and TIBC were also measured in subjects with reduced iron stores (n = 16).
BMI was calculated as weight divided by height squared. Non–transferrin-bound iron, serum isotope appearance, and serum iron at each sample time point were reported as the increase from baseline after the administration of iron (Δ change). The increase in serum transferrin saturation from baseline was also calculated. Overall responses of transferrin saturation, non–transferrin-bound iron, serum isotope appearance, and serum iron for each test meal were evaluated as the AUC, which was calculated by using the trapezoidal integration rule (33).
The normality of data was assessed before analysis by using the Shapiro-Wilk test and graphically by evaluating histograms and Q-Q plots. Data not normally distributed were log transformed for analysis. A standard value was added before log transformation to variables with negative values. Log-transformed data were back transformed for reporting. Normally distributed data are presented as means (±SDs, ±95% CIs, or both), data normally distributed after log transformation are presented as geometric means (±95% CIs), and non-normally distributed data are presented as medians and ranges.
Differences in baseline characteristics and iron absorption between groups with reduced and replete iron stores were tested by using the unpaired t test for normally distributed variables and the Mann-Whitney test for non-parametric variables. Differences in the fractional (%) and amount (mg) of iron absorbed between test meals were compared by using a repeated-measures ANOVA followed by pairwise comparisons with the Bonferroni correction for multiple comparisons. Associations between iron stores, as evaluated by serum ferritin, and both fractional iron absorption and amounts of iron absorbed were examined by using Pearson's correlation for each meal.
Differences between the different test meals at each respective sample time point as well as for the AUC over 8 h were compared by using a repeated-measures ANOVA followed by pairwise comparisons with Bonferroni correction (transferrin saturation, non–transferrin-bound iron, serum isotope appearance, and serum iron). Levene's test was used to examine the homogeneity of variances.
The serum hepcidin response over 8 h was evaluated by using a linear mixed-effects model with the use of time, the test meal, and the time × test meal interaction as fixed effects. Random effects of the test meal were introduced for the intercept and slope for each subject. A pairwise post hoc evaluation for the effect of time was done by Bonferroni correction to determine changes that occurred over time. The association between the overall AUC for non–transferrin-bound iron and the amount of iron absorption (mg) was examined by using Pearson's correlation with residuals tested for normality.
Statistical analyses were conducted with IBM SPSS software (version 20.0; SPSS Inc). Differences were considered statistically significant at P < 0.05.
RESULTS
All participants completed the study. Age, anthropometric data, and selected laboratory measurements of study participants at baseline are shown in Table 1. The 2 groups of women differed only with respect to mean (±SD) serum ferritin, which was moderately higher in the group classified as having replete iron stores (39.1 ± 10.1 μg/L) than in the group with reduced iron stores (16.7 ± 6.6 μg/L). In the group with lower serum ferritin concentrations, the degree of iron depletion was modest; only 4 of 16 subjects had a serum ferritin concentration <12.0 μg/L; none of the subjects were anemic. No adverse effects of iron administration were reported or observed.
TABLE 1.
Baseline characteristics of 32 women who participated in the study
| Reduced iron stores (serum ferritin concentration ≤25 μg/L; n = 16) | Replete iron stores (serum ferritin concentration >25 μg/L; n = 16) | All (n = 32) | |
| Demographics | |||
| Age (y) | 21.7 (18.9–32.9)1 | 22.2 (19.1–27.4) | 21.9 (18.9–32.9) |
| Anthropometric measures | |||
| Weight (kg) | 57.9 ± 6.52 | 59.9 ± 4.6 | 58.9 ± 5.6 |
| Height (m) | 1.67 ± 0.06 | 1.68 ± 0.04 | 1.68 ± 0.05 |
| BMI (m/kg2) | 20.7 ± 2.0 | 21.2 ± 1.6 | 21.0 ± 1.8 |
| Laboratory measures | |||
| Hemoglobin (g/dL) | 13.3 ± 0.6 | 13.4 ± 0.6 | 13.3 ± 0.6 |
| Serum ferritin (μg/L) | 16.7 ± 6.63 | 39.1 ± 10.1 | 27.9 ± 14.2 |
| C-reactive protein (mg/L) | 2.1 (0.3–22.6) | 0.5 (0.3–2.2) | 0.9 (0.3–22.6) |
Median; range in parentheses (all such values).
Mean ± SD (all such values).
Significantly different from replete iron stores, P < 0.001 (independent t test).
Iron absorption from the 3 test meals in the 2 study groups is summarized in Table 2, which shows both the geometric mean proportions (%) and amounts (mg) of iron absorbed from each of the 3 test meals. Overall, the fractional iron absorption from the 60-mg supplemental iron dose was higher when given without a meal (P < 0.0001). When iron was given with a meal, the overall fractional iron absorption was higher from the 6-mg fortification dose that the 60-mg supplemental dose (P < 0.01). From each test meal, the geometric mean fractional iron absorption was greater in women with reduced iron stores than in replete subjects (all P < 0.05). For the absolute amounts of iron absorbed, the overall absorption was greatest from the 60-mg supplemental dose given without a meal, intermediate when given with a meal, and least from the 6-mg fortification dose with a meal (P < 0.0001). From each test meal, the geometric mean amount of iron absorbed was greater in women with reduced iron stores than in replete subjects (all P < 0.05). Notably, in women with reduced iron stores, the reduction in iron absorption from the 60-mg supplemental dose with a meal, relative to that without a meal, was not significant (P = 0.06). In these women, the geometric mean amount of iron absorbed from the 6-mg fortification dose was substantially less than that from either supplemental dose (P < 0.0001 for both).
TABLE 2.
Iron absorption from 3 test meals1
| Reduced iron stores (serum ferritin concentration ≤25 μg/L; n = 16) | Replete iron stores (serum ferritin concentration >25 μg/L; n = 16) | All (n = 32) | |
| Iron absorption (mg) | |||
| 6 mg with meal | 1.22 (0.87, 1.72)2,a,* | 0.37 (0.25, 0.51)2,a | 0.66 (0.48, 0.91)2,a |
| 60 mg with meal | 6.00 (4.47, 8.06)b,† | 3.17 (2.47, 4.07)b | 4.36 (3.51, 5.41)b |
| 60 mg without meal | 8.82 (6.75, 11.53)b,‡ | 6.34 (5.54, 7.25)c | 7.48 (6.41, 8.71)c |
| Fractional iron absorption (%) | |||
| 6 mg with meal | 20.4 (14.5, 28.7)2,a,* | 5.9 (4.1, 8.5)2,a | 11.0 (7.9, 15.2)2,a |
| 60 mg with meal | 10.0 (7.4, 13.4)b,† | 5.3 (4.1, 6.8)a | 7.3 (5.9, 9.0)b |
| 60 mg without meal | 14.7 (11.2, 19.2)a,b,‡ | 10.6 (9.2, 12.1)b | 12.5 (10.7, 14.5)a |
All values are geometric means; 95% CIs in parentheses. *,†,‡Significantly greater than in women with replete iron stores (independent t test): *P < 0.001, †P < 0.01, ‡P < 0.05.
Significant difference between group means, P < 0.0001 (repeated-measures ANOVA with Bonferroni correction). Post hoc analysis: labeled means in a column without a common letter differed, P < 0.01.
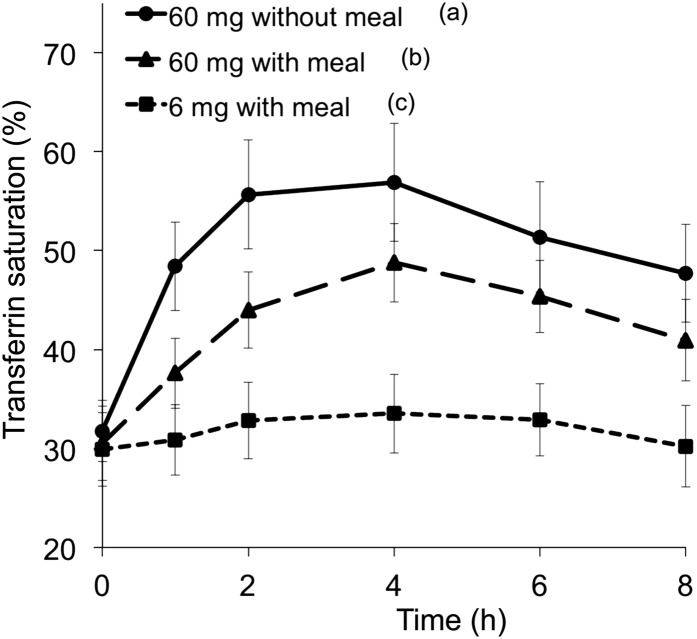
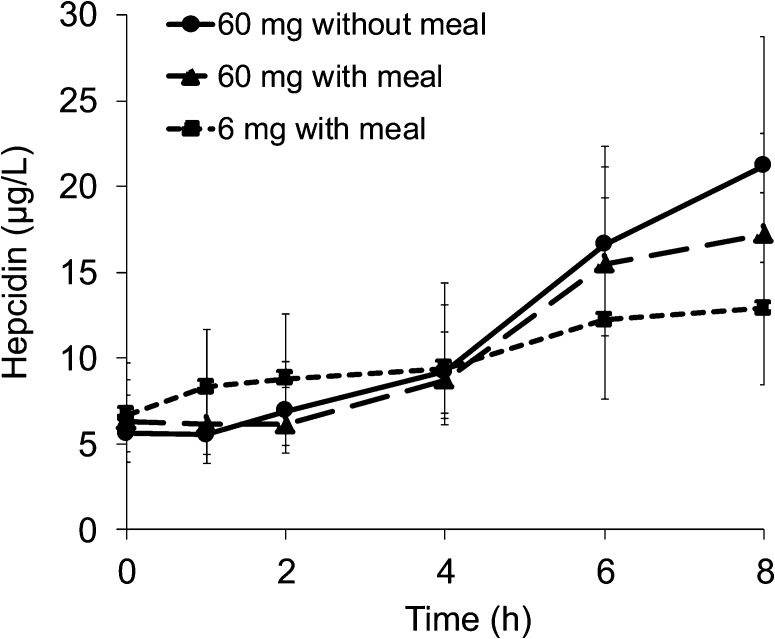
After the administration of the 60-mg supplemental doses of iron, both with and without food, transferrin saturation promptly increased to reach peaks at 2–4 h and progressively declined but had not returned to baseline by 8 h (Figure 1). The rate of increase in transferrin saturation, which was assessed as the ratio of the increase in saturation to the time since ingestion, was greatest for the 60-mg supplemental dose of iron without a meal, less for the supplemental iron with a meal, and least for the 6-mg fortification dose. The increase in transferrin saturation from baseline differed between the 3 meals after 1 h (P < 0.0001). For serum hepcidin concentrations, there was a significant overall effect of the time and test meal interaction (P-time × test meal interaction < 0.01, P-time < 0.001, P-test meal = 0.710; Figure 2). The serum hepcidin increase from baseline was significant at 4 h (P < 0.05) and remained elevated at 8 h (P < 0.001).
FIGURE 1.
Mean (95% CI) transferrin saturation during 8 h after an oral dose of 6 mg Fe with a meal, 60 mg Fe with a meal, or 60 mg Fe without a meal as FeSO4. Curves display values at each time point for the respective meal for 25–29 subjects; transferrin saturation data were incomplete at some time points for 7 iron-replete subjects. Labeled legends indicate that the AUC for meals without a common letter differed, P < 0.0001 (repeated-measures ANOVA with Bonferroni correction).
FIGURE 2.
Geometric mean (95% CI) hepcidin concentrations during 8 h after an oral dose of 6 mg Fe with a meal, 60 mg Fe with a meal, or 60 mg Fe without a meal as FeSO4. Curves display values at each time point for the respective meal for all 32 subjects. P values for the linear mixed effects model were as follows: P-time × meal effect < 0.01, P-time < 0.001, and P-meal = 0.71. Note that hepcidin concentrations continued to increase after peaks of transferrin saturation (Figure 1) and non–transferrin-bound iron (Figure 3) had been reached at ∼4 h.
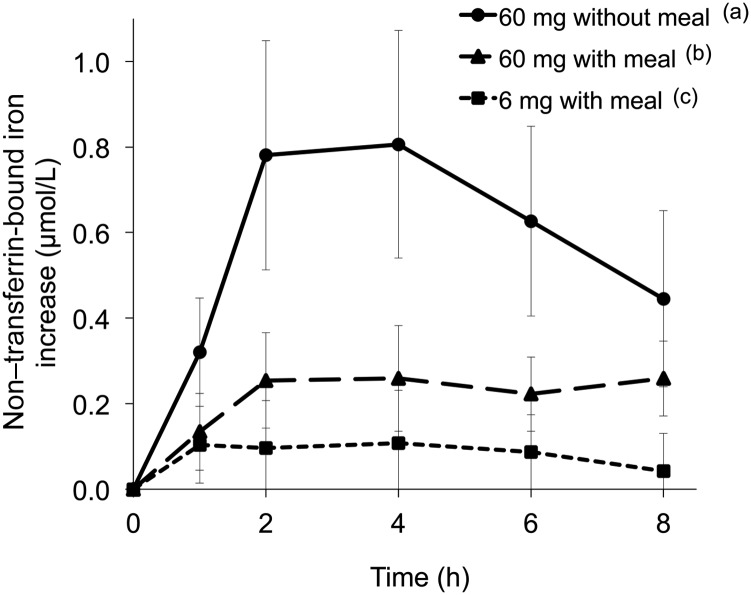
With 60-mg supplementation doses of iron, serum non–transferrin-bound iron increased rapidly over the first 2 h to reach peaks at 4 h and progressively declined but was still present at 8 h (see Figure 3 for geometric means with 95% CIs; see Supplemental Figure 1 under “Supplemental data” in the online issue for plots of individual values for each test meal for both study groups). At each time point from 1 to 6 h after baseline, the 60-mg supplementation dose without a meal resulted in a greater increase in the geometric mean serum non–transferrin-bound iron than that of the same supplemental dose with a meal (P < 0.05). The difference was not different at 8 h (P = 0.279). Similarly, the AUC of the increase in serum non–transferrin-bound iron with the 60-mg supplementation dose without a meal was greater than that of the same supplemental dose with a meal (P < 0.0001). With the 6-mg fortification dose of iron with a meal, increases in serum non–transferrin-bound iron were at or near lower limits of detection (0.1 μmol/L) of the non–transferrin-bound iron assay at all time points.
FIGURE 3.
Geometric mean (95% CI) increases in concentrations of non–transferrin-bound iron from baseline (Δ change) over 8 h after an oral dose of 6 mg Fe with a meal, 60 mg Fe with a meal, or 60 mg Fe without a meal as FeSO4. Curves display values at each time point for the respective meal for all 32 subjects. Labeled legends indicate that the AUC for meals without a common letter differed, P < 0.05 (repeated-measures ANOVA with Bonferroni correction).
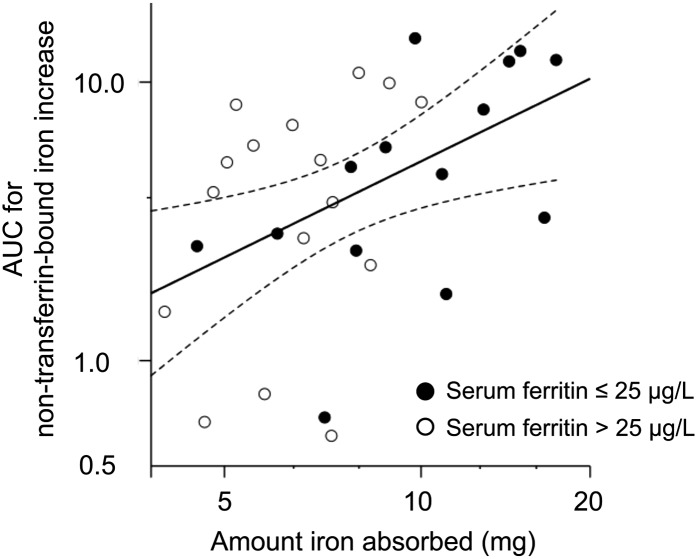
For the 60-mg supplemental iron dose without a meal, the amount of iron absorbed and AUC for the increase in serum non–transferrin-bound iron were correlated (Figure 4; R = 0.49, P < 0.01). In addition, serum ferritin was negatively correlated with both the peak concentration for the increase in serum non–transferrin-bound iron at 4 h (R = −0.43, P < 0.05) and the AUC (R = −0.39, P < 0.05).
FIGURE 4.
Correlation between the AUC for non–transferrin-bound iron increase from baseline (Figure 3) and the amount (mg) of iron absorbed for the test meal administering 60 mg without a meal (R = 0.49, P < 0.01; Pearson's correlation). Data for non–transferrin-bound iron increases and the amount of iron absorbed were analyzed on a log scale. Axis values are back transformed data presented on a log scale. Dotted lines indicate 95% CIs of the regression line.
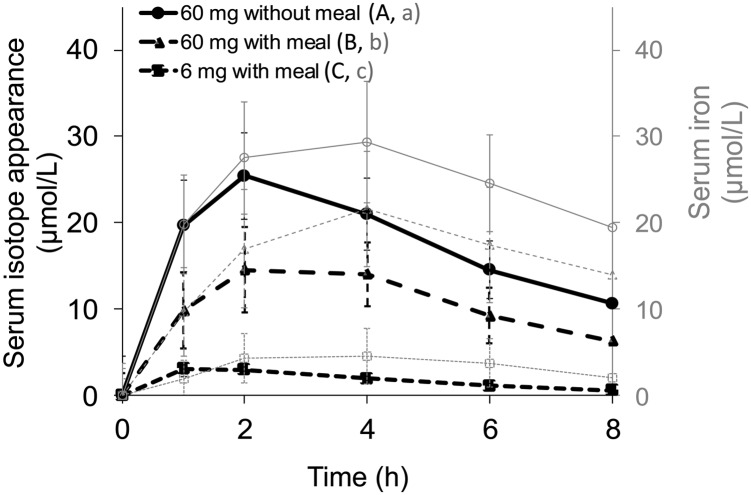
The labeling of test meals with stable isotopes of iron (54Fe, 57Fe, and 58Fe) allowed the comparison of the appearance of isotopes in serum with changes in serum iron for women with reduced iron stores (Figure 5). For 60-mg supplemental doses of iron, both with and without a meal, peak serum isotope concentrations were observed at 2 h and declined progressively but were still elevated above baseline at 8 h. For serum iron, peak concentrations were reached 2 h later at 4 h and also steadily decreased but remained above baseline at 8 h. For 60-mg supplemental doses of iron given with and without food, the AUC for both serum isotope appearance and serum iron increase differed significantly (P < 0.0001 for both). For the 6-mg fortification dose of iron, mean (95% CI) peak concentrations for the serum iron isotope were reached at 1 h [3.1 μmol/L (2.2, 4.1 μmol/L)] and those for serum iron at 4 h [4.5 μmol/L (1.7, 7.4 μmol/L)].
FIGURE 5.
Mean (95% CI) increases in serum isotope appearance and serum iron from baseline (Δ change) during 8 h after an oral dose of 6 mg Fe with a meal, 60 mg Fe with a meal, or 60 mg Fe without a meal as FeSO4 in subjects with a serum ferritin concentration <25 μg/L. Curves display values at each time point for the respective meal for 16 subjects. Labeled legends indicate that the AUC for meals without a common letter differed, P < 0.05 (repeated-measures ANOVA with Bonferroni correction).
DISCUSSION
These results provide evidence that the production of circulating non–transferrin-bound iron after the oral administration of ferrous sulfate is determined by the rate and amount of iron absorbed (Figures 3 and 4). The rate and magnitude of the influx of ingested iron into the systemic circulation are influenced by the amount of iron consumed (Table 2), the presence of food (Figures 1 and 5), and body iron stores (Table 2). Supplemental doses of iron ∼1 mg/kg body weight, with or without food, produced circulating non–transferrin-bound iron despite the presence of unoccupied binding sites on transferrin (Figures 1 and 3) as observed previously (9, 20–23), evidently as a consequence of iron entering plasma at a rate that surpasses the rate of iron uptake by plasma transferrin. The greatest amounts of circulating non–transferrin-bound iron arose from the administration of supplemental doses of iron without food (Figure 3). Little or no circulating non–transferrin-bound iron resulted from the consumption of a meal with a fortification dose of iron (Figure 3). In subjects with the greatest need for iron, the administration of supplemental doses of iron with food produced modest, non-significant reductions in the total amount of iron absorbed (Table 2) but substantially decreased the production of circulating non–transferrin-bound iron (Figure 3; see Supplemental Figure 1 under “Supplemental data” in the online issue).
Circulating non–transferrin-bound iron is almost certainly a heterogeneous mixture of iron complexes of uncertain chemical composition that vary according to the specific cause and circumstances of formation (8). Although incompletely characterized, the pathologic importance of circulating non–transferrin-bound iron is well established in a variety of disorders (8). No reference method for the measurement of circulating non–transferrin-bound iron has been developed. Our measurements (10, 26) were made by using an adaptation of a chelation-ultrafiltration method (34) similar to those methods used in the initial reports of the appearance of circulating non–transferrin-bound iron after the oral administration of iron supplements (9, 20).
At steady state during fasting, serum iron is derived predominantly from the recycling of iron from senescent erythrocytes by reticuloendothelial macrophages (35). After the oral administration of the labeled iron preparations, the appearance of the iron isotopes coincided with the rapid increase in serum iron and transferrin saturation during the initial 2 h (Figure 5). With supplemental doses of iron, non–transferrin-bound iron emerged, and concentrations rose more rapidly than when the same dose was given with food. These patterns suggested that the ingested iron was rapidly absorbed and entered the portal circulation where any available transferrin would have become saturated and any remaining absorbed iron would have formed circulating non–transferrin-bound iron. Much of the circulating non–transferrin-bound iron within the portal circulation would have been removed efficiently by hepatocytes (36), but any remaining non–transferrin-bound iron and saturated transferrin would have entered the systemic circulation. Over the subsequent 2 h, serum iron isotopes declined even as concentrations of circulating non–transferrin-bound iron remained relatively constant, and the serum iron and transferrin saturation continued to rise (Figures 3 and 5). These patterns suggested that the bolus of labeled iron in the systemic circulation was being cleared even as macrophage-derived iron continued to enter the bloodstream. After 4 h, serum iron, transferrin saturation, and iron isotopes all fell, approximately in parallel. All of these remained raised in the last blood samples taken at 8 h, which suggested that the clearance of the iron bolus was still incomplete. Between 4 and 8 h, circulating non–transferrin-bound iron formed with the supplemental dose of iron given without food also declined but was still elevated in the last blood sample. With the fortification dose of iron, any circulating non–transferrin-bound iron formed was at the limits of detection of our assay. Over the 8 h after iron administration, serum hepcidin, which is the chief controller of iron homeostasis, increased significantly from baseline by 4 h and remained elevated at 8 h (Figure 2). Hepcidin concentrations may have continued to increase subsequently; in an previous study, peak concentrations were reached at ∼12 h (27).
Our short-term study was carried out in healthy women in the absence of infection and inflammation, but the results should be broadly applicable to infants and children (11). Although subjects in the Pemba trial received iron supplements daily for ≤18 mo (24), we considered that the overall patterns of the relation between the formation of circulating non–transferrin-bound iron and iron absorption were likely to have resembled those shown in our study (37). Consequently, our findings seem relevant to those from the Pemba trial, in which a similar supplemental dose of iron (∼1 mg/kg body weight) was given without food (24). The main trial in Pemba showed overall increased risk of illness and death with iron and folic acid supplementation but included no iron-related measures. In a substudy, iron status was evaluated at baseline by the measurement of the erythrocyte zinc protoporphyrin:heme ratio, and additional surveillance and treatment of malaria and other infections were provided (24, 38). In the substudy, risks of hospitalization and death were decreased significantly by almost one-half in iron-deficient children but increased in those who were initially iron replete. The WHO Consultation (7) hypothesized that circulating non–transferrin-bound iron was produced by the bolus doses of iron given without food to children; our results support this supposition. The WHO Consultation also speculated that iron-deficient children might have been protected from the formation of circulating non–transferrin-bound iron by increased tissue iron requirements. Our study did not corroborate this possibility; greater iron use in women with reduced body iron stores was not protective. In our study, after a supplemental dose of iron without food, lower serum ferritin concentrations were correlated with increased amounts of serum non–transferrin-bound iron (R = 0.49, P = 0.006). The highest concentrations of circulating non–transferrin-bound iron were shown in women with the lowest iron stores after supplemental doses of iron without food (see Supplemental Figure 1 under “Supplemental data” in the online issue).
In women with the greatest need for iron, the provision of supplemental doses of iron with food substantially decreased the production of circulating non–transferrin-bound iron while resulting in modest reductions in the total amount of iron absorbed (Table 2). The composition of the meal was a critical factor; in a previous study with a meal low in inhibitors of iron absorption, no significant effect on non–transferrin-bound iron production was observed (9). Still, previous studies with radiolabeled iron showed that rice meals, which are known to be inhibitory (39), produced substantial reductions in iron absorption, but circulating non–transferrin-bound iron was not measured (40). In our study of women with reduced iron stores, a fortification dose of iron (∼0.1 mg/kg) with the test meal produced no significant increase in circulating non–transferrin-bound iron, but the amount of iron absorbed was greatly reduced, again relative to the amounts observed with a supplemental dose without a meal (Table 2). In women with replete iron stores, the provision of supplemental doses of iron with food significantly decreased both the formation of circulating non–transferrin-bound iron and iron absorption.
In conclusion, overall, our results provide evidence that the production of circulating non–transferrin-bound iron was greatly reduced by giving supplemental doses of iron with food and was absent or minimal with fortification doses of iron and food. In areas with a high transmission of malaria and other infections that lack effective medical services, these findings suggest that risks of adverse effects from circulating non–transferrin-bound iron can be reduced by giving supplemental doses of iron with food and virtually eliminated by using fortification doses of iron with staple foods.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—IE, RFH, and GMB: designed the research; IE, JTF, CZ, and MEW: conducted the research; IE, MA, RFH, and GMB: wrote the manuscript; GMB and MA: analyzed data and had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Okebe JU, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev 2011;10:CD006589. [DOI] [PubMed]
- 2.Raiten DJ, Namaste S, Brabin B. Considerations for the safe and effective use of iron interventions in areas of malaria burden - executive summary. Int J Vitam Nutr Res 2011;81:57–71. [DOI] [PubMed] [Google Scholar]
- 3.Brittenham GM. Safety of iron fortification and supplementation in malaria-endemic areas. Nestlé Nutr Workshop Ser 2012;70:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zlotkin S, Newton S, Aimone AM, Azindow I, Amenga-Etego S, Tchum K, Mahama E, Thorpe KE, Owusu-Agyei S. Effect of iron fortification on malaria incidence in infants and young children in Ghana: a randomized trial. JAMA 2013;310:938–47. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization e-Library of Evidence for Nutrition Actions. Intermittent iron supplementation for children in malaria-endemic regions. Version 22. May 2014. Available from: http://www.who.int/elena/titles/iron_infants_malaria/en/ (cited 2 July 2014).
- 6.Spottiswoode N, Fried M, Drakesmith H, Duffy PE. Implications of malaria on iron deficiency control strategies. Adv Nutr 2012;3:570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr Bull 2007;28:S621–7. [DOI] [PubMed] [Google Scholar]
- 8.Brissot P, Ropert M, Le Lan C, Loreal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta 2012;1820:403ndash10. [DOI] [PubMed]
- 9.Hutchinson C, Al-Ashgar W, Liu DY, Hider RC, Powell JJ, Geissler CA. Oral ferrous sulphate leads to a marked increase in pro-oxidant nontransferrin-bound iron. Eur J Clin Invest 2004;34:782–4. [DOI] [PubMed] [Google Scholar]
- 10.Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood 2011;118:6675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science 2012;338:768–72. [DOI] [PubMed] [Google Scholar]
- 12.Ratledge C. Iron metabolism and infection. Food Nutr Bull 2007;28:S515–23. [DOI] [PubMed] [Google Scholar]
- 13.Clark M, Fisher NC, Kasthuri R, Cerami Hand C. Parasite maturation and host serum iron influence the labile iron pool of erythrocyte stage Plasmodium falciparum. Br J Haematol 2013;161:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portugal S, Carret C, Recker M, Armitage AE, Goncalves LA, Epiphanio S, Sullivan D, Roy C, Newbold CI, Drakesmith H, et al. Host-mediated regulation of superinfection in malaria. Nat Med 2011;17:732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray C, McCormick C, Turner G, Craig A. ICAM-1 can play a major role in mediating P. falciparum adhesion to endothelium under flow. Mol Biochem Parasitol 2003;128:187–93. [DOI] [PubMed] [Google Scholar]
- 16.Kartikasari AE, Georgiou NA, Visseren FL, van Kats-Renaud H, van Asbeck BS, Marx JJ. Endothelial activation and induction of monocyte adhesion by nontransferrin-bound iron present in human sera. FASEB J 2006;20:353–5. [DOI] [PubMed] [Google Scholar]
- 17.van Tits LJ, Jacobs EM, Swinkels DW, Lemmers HL, van der Vleuten GM, de Graaf J, Stalenhoef AF. Non-transferrin-bound iron is associated with plasma level of soluble intercellular adhesion molecule-1 but not with in vivo low-density lipoprotein oxidation. Atherosclerosis 2007;194:272–8. [DOI] [PubMed] [Google Scholar]
- 18.Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood 2003;102:2670–7. [DOI] [PubMed] [Google Scholar]
- 19.Hershko C. Mechanism of iron toxicity. Food Nutr Bull 2007;28:S500–9. [DOI] [PubMed] [Google Scholar]
- 20.Dresow B, Petersen D, Fischer R, Nielsen P. Non-transferrin-bound iron in plasma following administration of oral iron drugs. Biometals 2008;21:273–6. [DOI] [PubMed] [Google Scholar]
- 21.Schümann K, Kroll S, Romero-Abal ME, Georgiou NA, Marx JJ, Weiss G, Solomons NW. Impact of oral iron challenges on circulating non-transferrin-bound iron in healthy Guatemalan males. Ann Nutr Metab 2012;60:98–107. [DOI] [PubMed] [Google Scholar]
- 22.Schümann K, Solomons NW, Romero-Abal ME, Orozco M, Weiss G, Marx J. Oral administration of ferrous sulfate, but not of iron polymaltose or sodium iron ethylenediaminetetraacetic acid (NaFeEDTA), results in a substantial increase of non-transferrin-bound iron in healthy iron-adequate men. Food Nutr Bull 2012;33:128–36. [DOI] [PubMed] [Google Scholar]
- 23.Schümann K, Solomons NW, Orozco M, Romero-Abal ME, Weiss G. Differences in circulating non-transferrin-bound iron after oral administration of ferrous sulfate, sodium iron EDTA, or iron polymaltose in women with marginal iron stores. Food Nutr Bull 2013;34:185–93. [DOI] [PubMed] [Google Scholar]
- 24.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 2006;367:133–43. [DOI] [PubMed] [Google Scholar]
- 25.ICSH. Revised recommendations for the measurements of the serum iron in human blood. Iron Panel of the International Committee for Standardization in Haematology. Br J Haematol 1990;75:615–6. [DOI] [PubMed] [Google Scholar]
- 26.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 2010;115:4284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood 2008;112:4292–7. [DOI] [PubMed] [Google Scholar]
- 28.Schoenberg R, von Blanckenburg F. An assessment of the accuracy of stable Fe isotope ratio measurements on samples with organic and inorganic matrices by high-resolution multicollector ICP-MS. Int J Mass Spectrom 2005;242:257–72. [Google Scholar]
- 29.Chen Z, Griffin IJ, Plumlee LM, Abrams SA. High resolution inductively coupled plasma mass spectrometry allows rapid assessment of iron absorption in infants and children. J Nutr 2005;135:1790–5. [DOI] [PubMed] [Google Scholar]
- 30.Brown E, Hooper JJ, Hodges JLJ, Bradley B, Wennesland R, Yamauchi H. Red cell, plasma, and blood volume in the healthy women measured by radiochromium cell-labeling and hematocrit. J Clin Invest 1962;41:2182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walczyk T, Davidsson L, Zavaleta N, Hurrell RF. Stable isotope labels as a tool to determine the iron absorption by Peruvian school children from a breakfast meal. Fresenius J Anal Chem 1997;359:445–9. [Google Scholar]
- 32.Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF. A double stable isotope technique for measuring iron absorption in infants. Br J Nutr 1994;71:411–24. [DOI] [PubMed] [Google Scholar]
- 33.Atkinson KE. An introduction to numerical analysis. New York, NY: John Wiley & Sons, 1989. [Google Scholar]
- 34.Gosriwatana I, Loreal O, Lu S, Brissot P, Porter J, Hider RC. Quantification of non-transferrin-bound iron in the presence of unsaturated transferrin. Anal Biochem 1999;273:212–20. [DOI] [PubMed] [Google Scholar]
- 35.Brittenham GM. Pathophysiology of iron homeostasis In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, Heslop H. eds. Hematology: basic principles and practice, chapter 33. New York, NY: Elsevier, 2013:427–36. [Google Scholar]
- 36.Brissot P, Wright TL, Ma WL, Weisiger RA. Efficient clearance of non-transferrin-bound iron by rat liver. Implications for hepatic iron loading in iron overload states. J Clin Invest 1985;76:1463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurrell RF, Davidsson L, Reddy M, Kastenmayer P, Cook JD. A comparison of iron absorption in adults and infants consuming identical infant formulas. Br J Nutr 1998;79:31–6. [DOI] [PubMed] [Google Scholar]
- 38.Stoltzfus RJ, Heidkamp R, Kenkel D, Habicht JP. Iron supplementation of young children: learning from the new evidence. Food Nutr Bull 2007;28:S572–84. [DOI] [PubMed] [Google Scholar]
- 39.Tuntawiroon M, Sritongkul N, Rossander-Hulten L, Pleehachinda R, Suwanik R, Brune M, Hallberg L. Rice and iron absorption in man. Eur J Clin Nutr 1990;44:489–97. [PubMed] [Google Scholar]
- 40.Cook JD, Reddy MB. Efficacy of weekly compared with daily iron supplementation. Am J Clin Nutr 1995;62:117–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.