Abstract
Background: Concerns have been raised about the concurrent temporal trend between simple sugar intakes, especially of fructose or high-fructose corn syrup (HFCS), and rates of nonalcoholic fatty liver disease (NAFLD) in the United States.
Objective: We examined the effect of different amounts and forms of dietary fructose on the incidence or prevalence of NAFLD and indexes of liver health in humans.
Design: We conducted a systematic review of English-language, human studies of any design in children and adults with low to no alcohol intake and that reported at least one predetermined measure of liver health. The strength of the evidence was evaluated by considering risk of bias, consistency, directness, and precision.
Results: Six observational studies and 21 intervention studies met the inclusion criteria. The overall strength of evidence for observational studies was rated insufficient because of high risk of biases and inconsistent study findings. Of 21 intervention studies, 19 studies were in adults without NAFLD (predominantly healthy, young men) and 1 study each in adults or children with NAFLD. We found a low level of evidence that a hypercaloric fructose diet (supplemented by pure fructose) increases liver fat and aspartate aminotransferase (AST) concentrations in healthy men compared with the consumption of a weight-maintenance diet. In addition, there was a low level of evidence that hypercaloric fructose and glucose diets have similar effects on liver fat and liver enzymes in healthy adults. There was insufficient evidence to draw a conclusion for effects of HFCS or sucrose on NAFLD.
Conclusions: On the basis of indirect comparisons across study findings, the apparent association between indexes of liver health (ie, liver fat, hepatic de novo lipogenesis, alanine aminotransferase, AST, and γ-glutamyl transpeptase) and fructose or sucrose intake appear to be confounded by excessive energy intake. Overall, the available evidence is not sufficiently robust to draw conclusions regarding effects of fructose, HFCS, or sucrose consumption on NAFLD.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD)5 is characterized by hepatic steatosis, which is the abnormal triglyceride accumulation in liver cells. It has been suggested that NAFLD should be recognized as the hepatic component of metabolic syndrome (1). NAFLD was formerly called nonalcoholic steatohepatitis (NASH), which was defined as steatosis with inflammation and hepatocyte necrosis. More recently, clinical practice guidelines have defined NAFLD as hepatic steatosis that is not caused by significant alcohol consumption, other competing causes such as the use of a steatogenic medication or genetic disorders, and coexisting causes of chronic liver disease such as Wilson's disease (2). NAFLD now refers to a spectrum of pathologic disorders that range from simple steatosis and steatohepatitis to advanced fibrosis and cirrhosis (2, 3). The cause, pathophysiology, and pathogenesis of NAFLD and NASH remain poorly understood (4, 5).
Lipid accumulation in hepatocytes may lead to oxidative stress in the endoplasmic reticulum and mitochondria, which can promote inflammation in the liver (6, 7). An assessment of hepatic steatosis requires either radiologic measures (eg, ultrasound, computed tomography, magnetic resonance imaging, proton magnetic resonance spectroscopy, or transient elastography) or a liver biopsy to provide histologic evidence of triglyceride accumulation. Excessive triglyceride accumulation is defined as >5% of hepatocytes that contained detectable triglyceride (4, 8). Some studies have used a combination of noninvasive measures, including BMI, waist circumference, plasma triglyceride concentrations, and liver enzymes as indicators for the potential diagnosis of NAFLD (9, 10). There is currently no consensus for a noninvasive reference standard for the diagnosis of NAFLD. A systematic review of published studies related to NAFLD prevalence and incidence concluded that definitions for the diagnosis of NAFLD are heterogeneous and cautioned that the estimated prevalence varies on the basis of diagnostic technique (11). Despite these caveats, there appears to be an increasing trend in the prevalence of NAFLD in adults and adolescents living in the United States over the past 20 y (12, 13).
It has been suggested that there is a causative relation between the increased prevalence of NAFLD and related disorders (ie, obesity, diabetes, cardiovascular disease, hypertension, cancer and metabolic syndrome) and intakes of sweeteners, particularly fructose (14). The “fructose hypothesis” in part has been driven by data from animal studies and in part by historical trends (15, 16). Specifically, animal studies have shown that high-fructose, compared with glucose, diets result in an increased hepatic triglyceride content (17–21). Of note, although they are useful for elucidating the potential mechanisms for the development and progression of NAFLD, animal models cannot confirm the cause and pathophysiology in humans. Historical dietary consumption trends have suggested an 18% increase in daily energy intake between the 1977–1978 Nationwide Food Consumption Survey and the 1999–2004 NHANES (22). Mean total fructose intake as a percentage of carbohydrate followed a similar trend pattern. More recent NHANES data have suggested a decreasing trend in the average per capita and population mean energy intake of added sugar (14, 23).
The aim of this study was to perform a systematic literature review to assess data related to fructose intake as a monoglyceride or diglyceride and indexes of liver health in humans. Also examined was the potential modifying factors for any associations if identified.
METHODS
In this systematic review, we defined dietary fructose to include the monosaccharide forms high-fructose corn syrup (HFCS) and honey and the disaccharide form sucrose. We formulated initial key questions on the basis of a generic analytic framework for assessments of nutrient and outcome relations(24). We presented the initial key questions and study eligibility criteria to a technical expert panel (TEP) who served in an advisory capacity to help refine questions, review literature search terms and strategies, identify potential confounding factors, and appraise variables for the review of evidence. During the review process, we consulted the TEP regarding technical details (eg, refinements of quality appraisal items and study eligibility criteria). Neither the sponsor nor TEP members participated in our research meetings or reviewed or synthesized the evidence.
We followed the methods for conducting a systematic review outlined in the Institute of Medicine's Standards for Systematic Reviews (25) with the exception of small modifications as indicated. We reported our study results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (26).
Data sources and searches
We conducted literature searches of studies in Ovid MEDLINE (1946 to March 2014 week 2), the Cochrane Central Register of Controlled Trials (through January 2014), CAB Abstracts (1973–2014 week 11), and Global Health (1910–2014 week 11) databases (gateway.ovid.com). All studies published in the English language with human subjects were screened to identify articles relevant to our key questions. Our search strategy used the National Library of Medicine's Medical Subject Headings (MeSH) keyword nomenclature developed for MEDLINE. See Supplemental Table 1 under “Supplemental data” in the online issue for a description of the full search strategy. The search strategy combined MeSH or search terms for fructose, sucrose, sweetening agents, and selected sugar-rich foods with MeSH or search terms for fatty liver, hepatic triglycerides, diagnostic techniques for NAFLD, and liver enzymes. We confirmed that our search strategy identified key articles provided by the TEP. We also screened reference lists of selected reviews and primary articles for additional publications. We did not search for unpublished studies or articles in the gray literature.
Study selection and eligibility criteria
All abstracts identified through the literature search were screened independently by ≥2 investigators on the basis of predetermined eligibility criteria with a low threshold to exclude irrelevant abstracts such as animal or in vitro studies and studies that did not investigate diet and disease associations. Full-text articles were retrieved for abstracts that were accepted by at least one investigator. Articles were evaluated independently by teams of 2 investigators for eligibility. Disagreement on eligibility was resolved in consultation with a third team member. In addition, we screened full-text articles of included studies in published systematic reviews and meta-analyses that investigated effects of fructose on body weight (27), uric acid (28), glycemic effects (29), blood pressure (30), and blood lipids (31) for our outcomes of interest.
We included studies of any design in adults and children (≥4 y of age) with low (as defined by original articles) to no alcohol intake. Our interventions or exposures of interest were free (pure) fructose, total fructose, sucrose, HFCS, and sugar-sweetened beverages (if the absolute amount of fructose or sucrose was quantified and reported in the original article). The term “(total) fructose intake”, which is commonly used in epidemiologic studies, refers to fructose consumption from all sources or forms. Outcomes of interests were an NAFLD or NASH diagnosis as defined by original studies and predetermined indexes of liver health including intrahepatocellular lipids (IHCLs) (liver fat), hepatic de novo lipogenesis (DNL), liver enzymes [alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and γ-glutamyl transpeptase (GGT)], additional indicators of liver function (eg, bilirubin), and liver fibrosis markers (eg, collagen IV or procollagen III). We excluded studies of patients with fructose intolerance, infection-related liver disease, metabolic diseases that affect the liver, Wilson's disease, cirrhosis, and hepatocellular carcinoma. We also excluded studies that provided fructose intravenously, investigated oligofructose (not our interventions or exposures of interest), or did not report the fructose, sucrose, or HFCS dose. Because of a scarcity of data, we did not exclude studies on the basis of a small sample size or short study duration although these factors were taken into consideration when we evaluated the strength of the body of the evidence.
Data extraction and quality assessment
Data were extracted by using standardized forms, and all extracted data are available at the Systematic Review Data Repository (http://srdr.ahrq.gov/projects/64). We extracted information on study characteristics, baseline population characteristics, background diet, dietary assessment methods for fructose or sucrose intake, interventions (for interventional studies only), confounders and effect modifiers that were adjusted for in statistical analysis, biological outcomes, and information used to assess the risk of bias (ROB).
We assessed the ROB (or methodologic quality) for each individual study by using standardized assessment instruments that were modified to include nutrition-specific quality items (32). See Supplemental Table 2 under “Supplemental data” in the online issue for a description of details of the ROB assessment for each study. Briefly, we rated each study as being of high, medium, or low ROB by using a modified Cochrane ROB tool (33) for controlled intervention studies, which was used previously in a systematic review for Dietary Reference Intake values (34), and a modified Newcastle-Ottawa scale (35) for observational studies. We consulted the TEP on methodologic quality items and added 2 quality items on dietary assessment methods to the Newcastle-Ottawa scale (35). The grading was outcome-specific such that a given study that reported its primary outcome well but conducted an incomplete analysis of a secondary outcome was graded as having different qualities for the 2 outcomes. The quality assessment was performed by an investigator and confirmed by another investigator. Disagreement was resolved in consultation with a third team member.
Data synthesis
We qualitatively synthesized all included studies in summary tables by considering their study design, population characteristics, sources and forms of dietary fructose, energy balance of the dietary fructose or sucrose intervention and its comparison, background diet, and types of outcome measure or definitions. Studies were first grouped by the fructose exposure forms, monosaccharide fructose (including pure fructose sweeteners, honey, and HFCS) or sucrose (1:1 fructose-glucose disaccharide), and results were evaluated separately on the basis of the overall energy balance (hypercaloric, hypocaloric, or isocaloric) of diets and on the energy balance of the sugar intervention and its comparison (a positive, neutral, or negative comparison). The term isocaloric diet is used to describe a weight-maintenance diet and hypercaloric and hypocaloric diets were used to describe diets that resulted in a weight gain or weight lost, respectively. A neutral comparison was defined as equivalent energy content between the sugar intervention and its comparison intervention, and a positive or negative comparison was defined as a higher or lower energy content in the sugar intervention than its comparison intervention, respectively. We evaluated the strength of the body of evidence for each exposure-comparison pair within each outcome of interest with respect to the following 4 domains: ROB, consistency, directness, and precision (36). The strength of the body of evidence rating (ie, insufficient, low, moderate, or high level of evidence) was based on the consensus of all team investigators. When only one study was available for each exposure-comparison pair within each outcome of interest, we rated the strength of the body of evidence as insufficient.
We graphically presented quantitative data by using weighted scatterplots to summarize sample sizes, the exposure-comparison pair, study designs, and specific indexes of liver health investigated in the studies that we reviewed. This approach aimed to provide investigators with information about the type and amount of research available, characteristics of that research, and topics on which sufficient evidence had accumulated for synthesis (37). In addition, when more than 2 controlled intervention studies in the same exposure-comparison pair reported sufficient quantitative data on the same outcome, we pooled study results by using the DerSimonian-Laird random-effects model meta-analysis (38). The effect size and its variance for crossover trials were calculated according to methods recommended in the Cochrane Handbook (39). Briefly, effect sizes were calculated as the difference between sugar-intervention and comparison groups at the end of each intervention. The SD of the mean differences (SDdiff) was calculated by using SDs for each intervention (SDE = sugar intervention; SDC = comparison) and an imputed correlation coefficient (Corr) for prepost measurements by using this formula
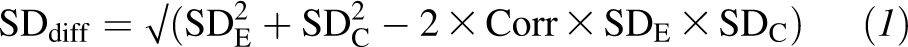 |
An imputed correlation (Corr) value of 0.50 was used to provide a conservative estimate on the basis of the assumption that this value would minimize the error of effect-size estimates. When studies reported data in figures that were not provided numerically, we extracted data points from figures with DataThief III software (www.datathief.org). We tested for heterogeneity with the Cochran Q statistic (considered significant when the P value was less than 0.10) and quantified the extent of heterogeneity with the I2 index. We defined low, moderate, and high heterogeneity as I2 values of 25%, 50%, and 75%, respectively. These cutoffs were arbitrary and used for descriptive purposes only (40).
Analyses were conducted with Stata SE 12 software (Stata Corp). All P values were 2 tailed, and a P value less than 0.05 was considered to indicate a statistically significant difference.
RESULTS
Search results
Our literature search yielded 2137 citations of which 104 citations were considered potentially eligible and retrieved in full text (Figure 1). After full-text screening, 27 publications met our study eligibility criteria. Two additional articles were identified from reference lists of published systematic reviews. In total, 27 unique studies in 29 publications were included in this systematic review (41–69).
FIGURE 1.
Summary of evidence search and selection. *Full-text articles of included studies in published systematic reviews and meta-analyses investigating effects of free fructose on body weight (27), uric acid (28), glycemic effects (29), blood pressure (30), and blood lipids (31) for our outcomes of interest. †Main rejection reasons were as follows: no exposures of interest (38 articles), no outcomes of interest (13 articles), fructose amount in the interventions cannot be quantified (8 articles), review article or letter to the editor (8 articles), liver cirrhosis patients (6 articles), animal study (1 article), alcoholic fatty liver disease (1 article), and not relevant (2 articles). **Two studies investigated both pure fructose and sucrose. All databases are available from gateway.ovid.com. CCRC, Cochrane Central Register of Controlled Trials; HFCS, high-fructose corn syrup.
Observational studies
Three case-control studies (41–43) and one prospective cohort study (66) in adults and 2 cross-sectional studies in children and adolescents (44, 45) were included. Characteristics of these 6 observational studies are summarized in Table 1. We did not conduct the meta-analysis to pool results from the 3 case-control studies because each study only reported unadjusted or underadjusted estimates. Thus, the pooling of unadjusted estimates would have aggregated the confounding bias in studies and may have led to an inappropriate interpretation of meta-analysis results. Findings from the observational studies are qualitatively synthesized in Table 2.
TABLE 1.
Characteristics of observational studies that examined associations of dietary fructose and/or sucrose consumption with risks of developing or the progression of NAFLD1
| First author, pub year (ref) [country]; study design | Participants (ethnicity) | Total sample size | M | Baseline age, (range)2 | BMI or body weight3 | Dietary assessment methods | Dietary fructose and/or sucrose exposures | Outcomes definitions | Confounders adjusted | Funding source | Risk of bias |
| n | % | y | kg/m2 | ||||||||
| Adults | |||||||||||
| Goletzke, 2013 (66) [Australia]; prospective cohort | Participants from the Blue Mountains Eye Study who had provided a complete and plausible FFQ and a fasting blood specimen at follow-up. Participants who consumed >20 g alcohol/d were excluded (predominantly white) | 886 | 37 | 67 (NR) | 26.8 | FFQ | Sugar intake (percentage of total carbohydrate intake) | ALT and GGT were determined by using commercial kits performed on an automated analyzer4 | Sex, energy (use of the multivariate energy-density model), and fiber intake from fruit (g/MJ) | Government | Low |
| Ouyang, 2008 (41) [United States]; case control | Cases were patients with biopsy-proven NAFLD in liver clinics. Controls were patients with biopsy-proven liver disease with no identifiable cause (NR) | 73 (49 cases; 24 controls) | NR | NR | NR | Dietary recall/history over 3-mo period | Daily consumption of HFCS or sugar-containing beverages (kcal/d) | NAFLD case definition: liver biopsy, abnormal liver aminotransferases, and hyperechoic liver by using ultrasound imaging | Matching NAFLD cases with controls by age, sex, and BMI | Government and industry | High |
| Thuy, 2008 (42) [Germany]; case control | Patients undergoing liver biopsy for liver metastasis (NR) | 18 (12 NAFLD cases; 6 controls) | Cases: 75; controls: 34 | Cases: 47 (NR); controls: 55 (NR) | Cases: 27.8; controls: 22.5 | Assessment by a nutritionist | Total fructose and sucrose intake (g/d) | NAFLD case definition: liver biopsy | None | NR | High |
| Volynets, 2012 (43) [Germany]; case control | Patients from Tubingen University Hospital, cases with NAFLD, and controls with no NAFLD (NR) | 30 (20 NAFLD cases; 10 controls) | Cases: 45; controls: 30 | Cases: 41.9 (NR) Controls: 39.6 (NR) | Cases: 33.1; controls: 23.1 | FFQ | Total fructose and sucrose intake (g/d) | NAFLD case definition: ultrasound and blood variables (eg, ALT, AST, and GGT)5 | None | Nonprofit | High |
| Children | |||||||||||
| Davis, 2010 (44) [United States]; cross-sectional | Children from community (Hispanic) | 153 | 25 | NR (8-18) | 31.6 (1.95) | Three 24-h dietary recalls (n = 30); 3-d diet records (n = 123)6 | Total sugar intake (kcal/d) | Hepatic fat fraction measured by using MRI image analysis | Sex, age, energy, BMI, and visceral adipose tissue | Government and industry | Medium |
| Mager, 2010 (45) [Canada]; cross-sectional | Children from a liver clinic showing fatty liver (48% white. 28% Hispanic. 21% Asian. and 3% other) | 38 or 35 (inconsistent reporting) | 68 | 14.1 (5.5–19.9) | 30.2 (1.99) | 3-d food-intake records (2 weekdays and 1 weekend) (n = 35) | Total fructose intake | ALT, AST, and GGT from blood test after 12-h fast | Adiponectin and triglycerides | Nonprofit and industry | High |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; FFQ, food-frequency questionnaire; GGT, γ-glutamyl transpeptase; HFCS, high-fructose corn syrup; NAFLD, nonalcoholic fatty liver disease; NR, not reported; pub, publication; ref, reference.
All values are means.
All values are means; BMI z scores in parentheses.
OCD Fusion 5.1; Ortho Clinical.
Grade scale used for NAFLD diagnosis was as follows: grade 0 = steatosis, grade 1 = mild steatosis, grade 2 = moderate steatosis, and grade 3 = severe steatosis.
No difference in macronutrients or energy between methods.
TABLE 2.
Summary of evidence from 6 observational studies that examined the associations of dietary fructose and/or sucrose consumption with risks of developing or progression of NAFLD1
| Outcome of interest; population | Studies (total sample size) | Risk of bias (ref), consistency, directness, precision | Key findings |
| NAFLD (various diagnostic criteria); adults | Three case-control studies (81 cases; 40 hospital controls) | Three high risk (41–43), consistent, direct, imprecise | Unadjusted analyses showed significantly higher mean (±SE) daily fructose intake in NAFLD cases (n = 32) than controls (n = 16) (42, 43); NAFLD cases: 52 ± 5.2 to 58 ± 4.4 g/d (=208–232 kcal/d from dietary fructose); controls: 40 ± 3.8 to 41 ± 3.2 g/d (=160–164 kcal/d from dietary fructose) |
| NAFLD cases (n = 49) reported significantly higher mean (SE) daily consumption of HFCS or sugar-containing beverages than controls (matched by age, sex, and BMI) (n = 24) (41); NAFLD cases: 365 ± NR kcal/d; controls: 170 ± NR kcal/d | |||
| Hepatic fat fraction (MRI); Hispanic children from community | One cross-sectional study (n = 153) | One medium risk (44), NA | Hepatic fat fraction (%) was not significantly associated with total dietary sucrose intake (percentage of kcal/d or g/d with energy as a covariate) |
| Liver enzymes; children with fatty liver and older adults | One cross-sectional study in children (n = 38); one prospective cohort study in older adults (n = 886) | One high risk (45) and one low risk (66), inconsistent, indirect, precise | “In the ‘best fit’ multivariate models (r2 = 0.96, P=0.001), …(omitted)…higher intakes of fructose were associated significantly with higher GGT levels (P=0.03)” in children with fatty liver (45) |
| Sugar intake (percentage of total carbohydrate intake) was not associated with ALT or GGT in multivariable concurrent change analyses from baseline to 5-y follow-up in older adults (66) |
ALT, alanine aminotransferase; GGT, γ-glutamyl transpeptase; HFCS, high-fructose corn syrup; NA, not applicable; NAFLD, nonalcoholic fatty liver disease; NR, not reported; ref, reference.
Three case-control studies (all rated at high ROB) compared dietary fructose intakes in a total of 81 adult NAFLD case patients and 40 hospital control patients without NAFLD (41–43). NAFLD was defined by a liver biopsy in 2 studies (41, 42) and by ultrasound and blood variables in the third study (43). One study compared the daily consumption of HFCS- or sugar-containing beverages (kcal/d) in NAFLD cases with that in age-sex-BMI–matched controls (41). The other 2 studies performed unadjusted analyses by comparing total fructose or sucrose intakes (g/d) between NAFLD cases and control patients (42, 43). All 3 case-control studies showed higher mean daily fructose (from all sources, including sugar-sweetened beverages) or sucrose intakes in NAFLD patients compared with hospital control patients. However, the validity of these findings was questionable because of potential biases (eg, recall and selection biases) and unadjusted confounding. One prospective cohort study (low ROB) examined associations between carbohydrate quality (the glycemic index and intakes of sugar, starch, and fiber) and liver enzymes in 866 older adults. The study showed that sugar intake (percentage of total carbohydrate intake) was not associated with ALT or GGT in multivariable, concurrent-change analyses from baseline to 5 y of follow-up (66).
One cross-sectional study (rated at medium ROB) examined associations between dietary sucrose intake and the hepatic fat fraction (HFF) measured by using liver magnetic resonance imaging in 153 overweight or obese children and adolescents with Hispanic ancestry who were living in the United States (44). The study showed that the HFF was not significantly associated with total sugar intake after controlling for demographic and anthropometric characteristics and energy intake but reported significant interactions between the HFF, PNPLA genotype and total sugar intake (44). Another cross-sectional study (rated at high ROB) examined lifestyle patterns (including diet) and metabolic variables in 38 overweight or obese children and adolescents with NAFLD diagnosed at a liver clinic and reported that “higher intakes of fructose were associated significantly with higher GGT concentrations (P=0.03)” in “best fit” multivariable models (45). Other liver enzymes such as ALT and AST were also assessed, but there were no results reported in relation to dietary fructose intakes, which indicated a high potential for a selected outcome reporting bias.
Together, we identified 6 observational studies that examined associations between dietary fructose or sucrose intake and risks of developing or the progression of NAFLD. Four studies rated at high ROB consistently reported that higher dietary fructose and sucrose intakes were associated with higher risks of developing or progression of NAFLD. However, sugar intake was not associated with indexes of liver health (HFF, ALT, and GGT) in one cross-sectional study (medium ROB) in children and one prospective cohort study (low ROB) in adults. Thus, the overall strength of evidence was rated insufficient because of high risk of biases and inconsistent study findings (Table 2).
Intervention studies
Twenty-one studies (in 23 publications (46–65, 67–69) that reported data on effects of fructose and/or sucrose consumption on indexes of prespecified indexes of liver health were included. Of these, 12 and 6 studies investigated effects of pure fructose and sucrose, respectively, and one study each investigated effects of HFCS or honey on at least one of the following liver health outcome: liver fat (48–50, 54, 56, 58, 59, 61, 64, 67), hepatic DNL (51, 55), liver enzymes (46, 48, 52–54, 57, 58, 60, 62, 63, 67, 68), and bilirubin outcomes (47, 63). Two studies investigated effects of both fructose (pure fructose or HFCS) and sucrose (46, 59). These 19 studies were in adults without NAFLD (mostly healthy, young men). In addition, 2 studies investigated effects of a fructose-reduction diet on IHCLs or liver enzymes in patients with NAFLD (69) or children with NAFLD (65). An evidence map of these 21 intervention studies by intervention-comparator pairs and outcomes of interest is shown in Figure 2. Of these publications, 13 trials were randomized controlled studies, 2 trials were nonrandomized controlled studies, and 6 trials were before-and-after studies without a concurrent control. Study characteristics are shown in Table 3.
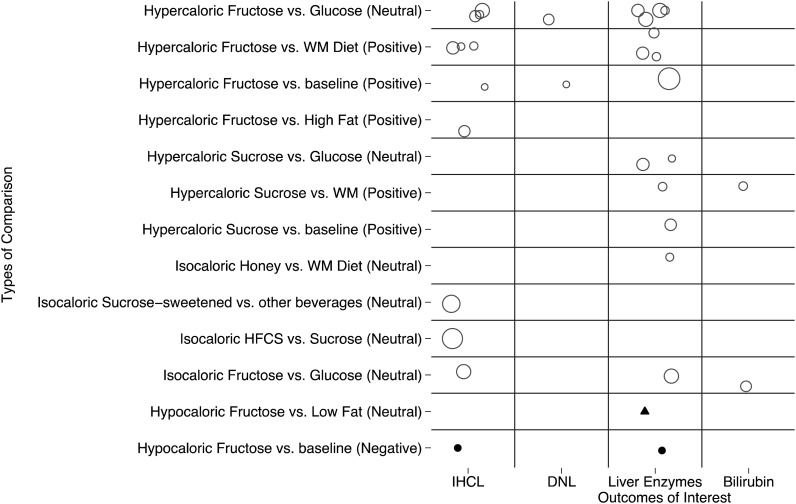
FIGURE 2.
Evidence map of intervention studies that examined effects of fructose or sucrose on indexes of liver health. Open circles represent studies in adults without NAFLD, solid circles represent studies in adults with NAFLD, and a triangle represents a study in children with NAFLD. The size of each symbol (open circle, solid circle, or triangle) is proportional to the sample size (sample size in each study ranged from 7 to 64). See Table 3 for more-detailed characteristics of included studies represented here. DNL, de novo lipogenesis; HFCS, high-fructose corn syrup; IHCL, intrahepatocellular lipid; NAFLD, nonalcoholic fatty liver disease; WM, weight maintenance; (Negative), negative energy comparison; (Neutral), neutral energy comparison; (Positive), positive energy comparison.
TABLE 3.
Characteristics of intervention studies that examined effects of monosaccharide fructose, sucrose, HFCS, other fructose-supplemented, or fructose-reduced diets on indexes of liver health1
| First author, pub year (ref) [country]; study design | Participants (ethnicity) | Participants enrolled/analyzed | M | Baseline age, (range)2 | BMI or body weight3 | Source of dietary fructose in interventions | Interventions (participants) | Energy balance of study design | Duration of intervention (washout duration) | Outcomes assessed (primary or secondary endpoint) | Funding source | Risk of bias |
| n | % | y | ||||||||||
| Adults | ||||||||||||
| Aeberli, 2011 (46) [Switzerland]; crossover RCT | Healthy M living in Zurich (NR) | 29/24 | 100.0 | 26.3 (NR) | 22.4 kg/m2 | Fructose, sucrose, or glucose sweetened SSBs | Fructose in SSB, 40, 80 g/d compared with sucrose in SSB, 40, 80 g/d, compared with glucose in SSB, 40, 80 g/d (n = 25–27) | Hypercaloric | 3 wk (4 wk) | AST, ALT (secondary) | Nonprofit and industry | Low |
| Al-Waili, 2003 (52) [United Arab Emirates]; before-and-after trial | Healthy volunteers from medical staff (NR) | 10/10 | 70.0 | 31.2 (20–45) | NR | Honey, 1.2 g/kg body weight | Fructose, 0.46 g fructose/kg body weight (n = 10) | Hypercaloric | 2 wk | ALT, AST, ALP (NR) | NR | Medium |
| Bravo, 2013 (59) [United States]; parallel RCT | Healthy men and women, not taking any prescription medicine or over-the counter products for weight loss (NR) | 80/64 | 56 | 38.7 (20–60) | 23–35 kg/m2 | HFCS-55 or sucrose-sweetened low-fat (1%) milk | HFCS at 8% (n = 8), 18% (n = 12), or 30% (n = 11) of energy requirement compared with sucrose at 8% (n = 13), 18% (n = 10), or 30% (n = 10) of energy requirement | Isocaloric (on the basis of lack of significant differences in total energy intake or body weight during study) | 10 wk | Liver fat by unenhanced computed tomography (primary) | Industry | Medium |
| Couchepin, 2008 (53) [Switzerland]; crossover RCT | Healthy young M and F adults (white) | 16/16 | 50.0 | 22.7 (NR) | 21.8 kg/m2 | NR | Fructose, 3.5 g/kg fat-free mass/d (+38% of daily energy) compared with isocaloric diet (n = 16) | Hypercaloric | 6 d (28 d) | ALT (NR) | Nonprofit | Medium |
| Faeh, 2005 (55) [Switzerland]; before-and-after trial | Healthy, young adult M (NR) | 7/7 | 100.0 | NR (22–31) | 71.5 kg | D-Fructose (Fluka Chemie Gmbh) in as a 20% fructose solution with 3 main meals | Fructose, 3 g/kg body weight (n = 7)4 | Hypercaloric | Fructose, 6 d (isocaloric diet given 3 d before intervention and acted as washout between interventions) | Hepatic de novo lipogenesis (NR) | Nonprofit | High |
| Johnston, 2013 (67) [United Kingdom]; parallel RCT | Healthy centrally overweight men, aged 18–50 y (NR) | 32/32 | 100.0 | 34 (NR) | 29 kg/m2 | Monosaccharaides were consumed 4 times/d in divided amounts mixed with 500 mL H2O | Fructose, 25% total energy requirements compared with glucose 25% total energy requirements | Isocaloric (period 1) and hypercaloric (period 2) | 2 wk | IHCLs (primary); ALT, AST, GGT (secondary) | Nonprofit | Low |
| Kelsay, 1974 (60) [United States]; crossover RCT | Healthy F students at the University of Maryland (NR) | 8/8 | 0.0 | NR (18–23) | 43.6–65.3kg | Uncooked fondant patties made with sucrose | Sucrose, 850 kcal/d compared with glucose, 850 kcal/d compared with isocaloric diet (n = 8) | Isocaloric | Sucrose and glucose, 4 wk (control consumed 1 wk before each period and 2 wk washout between sucrose and glucose) | ALKP, ALT, AST (NR) | NR | Medium |
| Koh, 1988 (47) [United States]; Non-RCT | IGT adults. Controls: University and faculty staff (NR) | 18/18 | IGT: 33; control: 33 | IGT: 54 (NR); control: 50 (NR) | IGT: 164 lb; control: 145 lb | Free fructose in packets, mixed with unsweetened fruit juice, milk, and water | Fructose, 15% total energy compared with glucose packets, 15% total energy (n = 18)5 | Isocaloric | 4 wk | Bilirubin (NR) | NR | Medium |
| Lê, 2006 (56) [Switzerland]; before-and-after trial | Healthy, moderately physically active, young adult M | 7/7 | 100 | 24.7 (NR) | 69.3 kg | Pure fructose in 20% solution with the 3 main meals | Fructose, 1.5 g · kg−1 · d−1 representing an excess of 18% of the daily energy requirement (n = 7) | Hypercaloric | 4 wk | IHCLs (NR) | Nonprofit | High |
| Lê, 2009 (54) [Switzerland]; crossover RTC | Healthy, M adult offspring of type 2 diabetes patients and healthy M adults | 24/24 | 100 | 24.7 (NR) | NR | Pure fructose in 20% solution | Fructose, 3.5 g · kg−1 · d−1 compared with isocaloric diet (n = 24) | Hypercaloric | 7 d (4–5-wk washout) | IHCLs (primary), ALT (secondary) | Nonprofit and industry | High |
| Maersk, 2012 (61) [Denmark]; parallel RCT | Healthy, nondiabetic, middle-aged adults | 60/47 | 37 | 38.7 (20-50) | 32 kg/m2 | Sucrose-sweetened cola (Coca Cola) | Sucrose 106 g/d (n = 10) compared with diet cola (n = 12) compared with semi-skimmed milk (n = 12) compared with water (n = 13) | Isocaloric (on the basis of dietary questionnaire and weight change) | 6 mo | IHCLs (primary) | Government, nonprofit, and industry | High |
| Ngo Sock, 2010 (48) [Switzerland]; crossover RCT | Healthy, moderately physically active adult M | 11/11 | 100 | 24.6 (NR) | NR | Pure fructose in 20% solution | Fructose, 3.5 g · kg−1 · d−1 compared with glucose, 3.5 g · kg−1 · d−1 compared with weight-maintenance diet (n = 11) | Hypercaloric | 7 d (2–3-wk washout) | IHCLs (primary); ALT, AST (secondary) | Nonprofit | Medium |
| Perez-Polo, 2010 (57) [Spain]; before-and-after trial | Healthy, older adult M (NR) | 74 | 100.0 | 51 (NR) | 28.5 kg/m2 | Free fructose in 10% solution | Fructose, 200 g/d (n = 36); fructose with allopurinol, 200 g/d (n = 38) | Hypercaloric | 2 wk | ALT, AST, GGT (secondary) | Nonprofit | Medium |
| Porikos, 1983 (62) [United States]; before-and-after trial | Middle-aged adult M (NR) | 21 | 100.0 | NR (24-45) | NR6 | Snacks and food with sucrose | Sucrose, 25–30% kcal (n = 21) | Hypercaloric | 30 d | ALT, AST (primary) | Government and industry | High |
| Purkins, 2004 (63) [UK]; crossover RCT | Healthy adult M (NR) | 12 /12 | 100.0 | NR (20-41) | NR | Readily available sucrose-containing food | High-carbohydrate diet (sucrose, 1408 kcal/d; 4500 kcal/d), compared with high-fat diet (sucrose 180 kcal/d; 4500 kcal/d) compared with standard diet (1900 kcal/d) (n = 12) | Hypercaloric | 8 d (14-d washout) | ALP, ALT, AST, GGT, bilirubin (primary) | Industry | High |
| Silbernagel, 2011 (50); Silbernagel, 2012 (49) [Germany]; parallel RCT | Healthy, middle-aged adults | 25/20 | 60 | 30.5 (20–50) | 25.9 kg/m2 | Pure fructose powder dissolved in water | Fructose, 150 g/d (n = 10) compared with glucose, 150 g/d (n = 10) | Hypercaloric | 4 wk | IHCLs (primary) | Nonprofit | High |
| Sobrecases, 2010 (58) [Switzerland]; non-RCT | Healthy adult M | 30/30 | 100 | 23.9 (NR) | 22.6 kg/m2 | NR | Fructose, 3.5 g/kg (n = 12) compared with high fat, 30% total energy as saturated fat (n = 10) compared with fructose plus high fat (n = 8) | Hypercaloric | Fructose: 7 d, high fat and fructose and high fat: 4 d | IHCLs (primary), ALT (secondary) | Nonprofit | High |
| Stanhope, 2009 (51); Cox, 2012 (68) [United States]; parallel RCT | Healthy, older adults (NR) | First 23 enrolled people/18 (DNL outcome); 39/32 (liver enzyme outcomes) | 50.0 | 53.7 (NR) | 29.3 kg/m2 | Free fructose added to unsweetened beverage (Kool-Aid; Kraft) | Fructose (+25% of daily energy) (n = 10) compared with glucose SSB (+25% of daily energy) (n = 8) | Hypercaloric | 10 wk7 | Hepatic de novo lipogenesis, ALT, AST, GGT (NR) | Government | High (DNL outcome); medium (liver enzyme outcomes) |
| Theytaz, 2012 (64) [Switzerland]; crossover RCT | Healthy, nonobese, nonsmokers, and sedentary M | 9/9 | 100 | 23.3 (NR) | 22.6 kg/m2 | Pure fructose provided as drinks 5 times/d | Fructose, 3 g/kg, + essential amino acid (+38% of daily energy) compared with Fructose, 3 g/kg plus placebo (+36% of daily energy) compared with weight-maintenance diet (n = 9) | Hypercaloric | 6 d (4–10-wk washout) | IHCLs (primary) | Industry | Medium |
| Volynets, 2012 (69) [Germany]; before-and-after trial | Patients with NAFLD, diagnosed by ultrasound and blood variables (NR) | 15/10 | 40 | 45.5 (34.5–51.5) | 31.1 | Reduction in the consumption of fructose-rich foods (eg, to avoid sweets, lemonades, fruit juices) and to prefer foods with a lower content of fructose | Nutritionists advised patients to reduce their daily fructose intake by 50% | Hypocaloric | 6 mo | IHCLs (primary); ALT, AST, GGT (secondary) | Nonprofit | High |
| Children | ||||||||||||
| Vos, 2009 (65) [United States]; parallel RCT | Children with NAFLD (NR)8 | 10 | NR | 13.0 | (2.1) | Elimination of sugar-containing beverages, fruit juice, and HFCS | Low fructose (−20 g/d fructose intake) (n = 6) compared with low fat (−15 g/d fructose intake) (n = 4)9 | Hypocaloric | 6 mo | ALT, AST (secondary) | Nonprofit and government | High |
ALT, alanine aminotransferase; ALKP, alkanine phosphatase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; DNL, de novo lipogenesis; GGT, γ-glutamyl transpeptase; HFCS, high-fructose corn syrup; HFCS-55, high-fructose corn syrup (55% fructose and 45% glucose); IGT, impaired glucose intolerant; IHCL, intrahepatocellular lipid; NR, not reported; pub, publication; RCT, randomized controlled trial; ref, reference; SSB, sugar sweetened beverage.
All values are means; ranges in parentheses.
All values are means or ranges; BMI z score in parentheses.
Study design included fish oil compared with fish oil and fructose compared with fructose. Fish-oil interventions were not included in the analysis.
A nonrandomized crossover trial. Ranges of fructose intake reported were as follows: fructose and glucose = for IGT, 45–83 g/d; for control, 45–112 g/d.
Fifteen normal-weight and 6 overweight subjects.
Ten-week duration included an 8-wk hypercaloric diet and 2-wk isocaloric diet.
Seven children (70%) with a confirmatory liver biopsy that showed nonalcoholic steatohepatitis.
Low fructose denoted the elimination of sugar-containing beverages, fruit juice, and food items that were high in high-fructose corn syrup. Low fat according to the American Heart Association recommendation.
Effects of monosaccharide fructose on liver fat and other indexes of liver health in adults
Six short-term (≤4 wk) intervention studies (2 at medium, 4 at high ROB) investigated effects of a hypercaloric fructose diet (the majority delivered as fructose-sweetened drinks) on IHCLs by using proton magnetic resonance spectroscopy (a liver fat measure) (48–50, 54, 56, 58, 64). Of these publication, 3 studies also examined liver enzymes as secondary outcomes (48, 56, 58). In addition, 4 other short-term intervention studies (1 at low, 2 at medium, and 1 at high ROB) investigated effects of a hypercaloric fructose diet (delivered as fructose-sweetened drinks) on liver enzyme (AST, ALT, and/or GGT) (46, 53, 57) or hepatic DNL (55) outcomes.
Furthermore, 2 other short-term intervention studies (both at medium ROB) examined the effects of an isocaloric fructose diet on bilirubin (47) or liver enzyme (52) outcomes. Neither study specified a primary outcome.
Hypercaloric fructose compared with weight-maintenance diets (positive energy comparison)
Four studies from a research group in Switzerland compared effects of a hypercaloric fructose diet with a weight-maintenance diet on IHCLs in a total of 81 healthy, male adults (48, 54, 56, 58, 64). The earliest study assessed the effect of a 4-wk high-fructose diet (1.5 g fructose · kg body weight−1 · d−1 added to a weight-maintenance diet) on IHCLs in 7 healthy, male adults (56). This before-and-after trial (at high ROB) showed no significant changes in IHCLs (∼6 mmol/kg; reported in figure only) and body weight after the 4-wk high-fructose hypercaloric diet. Note that the small sample size might have limited the statistical power to detect changes. The other 4 studies (2 studies at medium and 2 studies at high ROB) from this research group assessed effects of 1-wk (6 or 7 d) high-fructose intake (3.0 or 3.5 g fructose · kg body weight−1 · d−1 added to the same weight-maintenance diet previously mentioned by providing ∼35% of energy above the energy requirement) on IHCLs in a total of 74 healthy, male adults (48, 54, 58, 64). Our random-effects meta-analysis showed that hypercaloric fructose diets significantly increased IHCLs by an average of 54% (pooled mean percentage change: 54%; 95% CI: 29%, 79%; I2 = 0%) compared with the consumption of a weight-maintenance diet) (Figure 3). Note that baseline IHCLs concentrations were low, that is much less than 5.5% (the common definition of NAFLD).
FIGURE 3.
Random-effects meta-analysis of the comparison of effects of a hypercaloric fructose diet with a weight-maintenance diet (positive energy comparison) on liver fat measured by IHCLs 1H MRS. Each black box represents the individual study's effect estimate, and the horizontal line represents the 95% CI of the effect estimate. The diamond shape represents the meta-analysis pooled effect estimate and its CI. A vertical dashed line displays the location of the meta-analysis pooled effect estimate. DM, diabetes mellitus; IHCL, intrahepatocellular lipid; IHCLs 1H MRS, intrahepatocellular lipids by proton magnetic resonance spectroscopy; NR, not reported; RCT, randomized controlled trial; ROB, risk of bias; ww, wet weight; %Change, net percentage change in intrahepatocellular lipids from baseline between groups; %vol, percent volume.
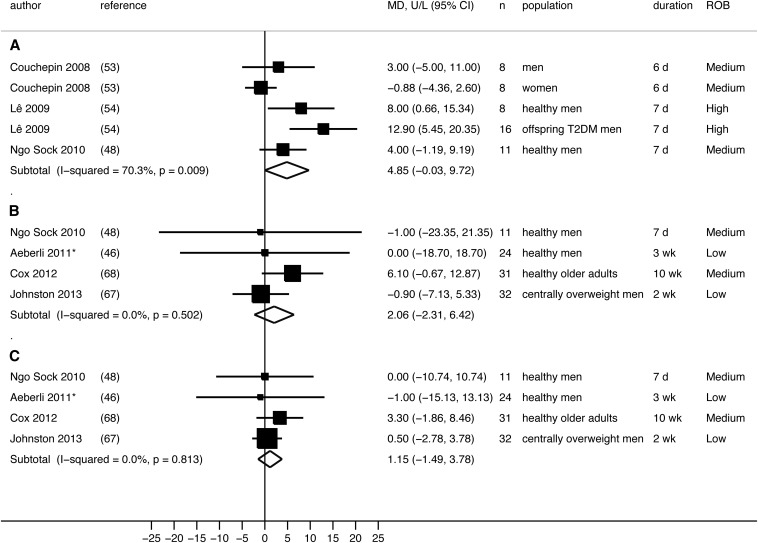
For liver enzyme outcomes, 3 randomized controlled trials (RCTs) (2 at medium and 1 at high ROB) could be meta-analyzed (48, 53, 54), and one nonrandomized controlled trial (at high ROB) reported insufficient quantitative data to be included in our analysis (58). Our random-effects meta-analysis of the 3 RCTs (48, 53, 54) showed that high-fructose diets (3.5 g · kg fat-free mass−1 · d−1; 30% or 35% of energy above the energy requirement) marginally increased ALT concentrations (pooled mean difference: 4.85 U/L; 95% CI: −0.03, 9.72 U/L; P = 0.05) compared with consumption of a weight-maintaining diet in a total of 43 healthy men and 8 women, with significant heterogeneity across studies (I2 = 70%, P = 0.009) (Figure 4A). Of these studies, only one RCT included a subgroup of adult women (53). With the exclusion of women, our meta-analysis reached significance with reduced heterogeneity (pooled mean difference: 6.70 U/L; 95% CI: 2.44, 10.96 U/L; P = 0.002, I2 = 35%). A nonrandomized controlled trial investigated the same high amount of fructose diet (3.5 g fructose · kg body weight−1 · d−1 added to the weight-maintenance diet) in healthy, young adult men by comparing it with a high-fat diet (low fructose) and a high-fructose plus high–saturated fat diet (58). The study concluded that “only the FruFat diet [high fructose plus high saturated fat diet] increased ALT (by +70%)”. Furthermore, a before-and-after trial (medium ROB) showed that all liver enzyme concentrations increased after a high-fructose diet (200 g/d) for 2 wk in 74 healthy, older adult men (57): AST increased by 9 IU/L (P < 0.001), ALT increased by 3 IU/L (P < 0.01), and GGT increased by 13 IU/L (P < 0.001).
FIGURE 4.
Random-effects meta-analysis of RCTs reporting liver enzyme outcomes. A: Hypercaloric fructose compared with WM diet: ALT outcome. B: Hypercaloric fructose compared with glucose: ALT outcome. C: Hypercaloric fructose compared with glucose: AST outcome. Each black box represents the individual study's effect estimate, and the horizontal line represents the 95% CI of the effect estimate. Within each panel, the diamond shape represents the meta-analysis pooled effect estimate and its CI. *Because the same 24 men were randomly assigned to receive 2 different doses of fructose or glucose, only results from one dose can be included in the meta-analysis. Results from 80 g fructose/d compared with glucose diets were included in the current meta-analysis. The use of results from 40 g fructose/d compared with glucose diets produced similar pooled-effect estimates. ALT, alanine aminotransferase; AST, aspartate aminotransferase; MD, mean difference between groups; ROB, risk of bias; T2DM, type 2 diabetes; WM, weight maintenance.
For the hepatic DNL outcome, one before-and-after trial (high ROB) showed that a hypercaloric high-fructose diet (3 g/kg body weight adding to a weight-maintenance diet) significantly increased hepatic DNL by 7.8% (95% CI: 5.8%, 9.8%) in 7 healthy men (55).
Together, there was a low level of evidence that a hypercaloric fructose diet compared with a weight-maintenance diet could increase liver fat and AST concentrations in healthy men. However, there was a lack of independent repetition of study findings outside of a single research group in Switzerland and a high potential for a selected outcome reporting bias that could have led to a false-positive meta-analysis finding. There was insufficient evidence to draw a conclusion for effects on hepatic DNL on the basis of results from a single RCT.
Hypercaloric diets high in fructose or glucose (neutral energy comparison)
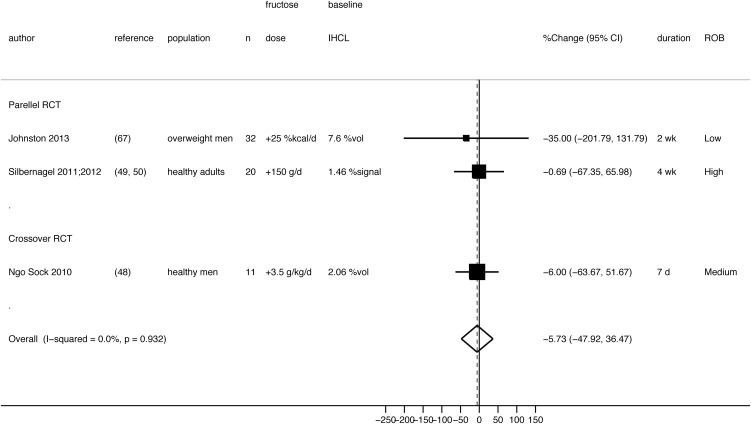
Three short-term RCTs (one crossover and 2 parallel RCTs) examined effects of fructose compared with glucose overfeeding on IHCLs (48–50, 67). The crossover RCT (medium ROB) was conducted by the Swiss research group and compared hypercaloric high-fructose with high-glucose diets (3.5 g fructose or glucose · kg body weight−1 · d−1 added to a weight-maintenance diet) in 11 healthy, moderately physically active, young adult men (48). One parallel RCT (high ROB), the Tuebingen Fructose or Glucose study, was conducted in 20 healthy, German, middle-aged adult men and women (49, 50), and another parallel RCT (low ROB) was conducted in 32 centrally overweight men living in the United Kingdom (67). In all 3 studies, fructose or glucose (dissolved in water) was consumed 3 or 4 times/d with main meals. Mean IHCL concentrations were significantly increased by both fructose and glucose diets (48–50, 67), but there was no significant difference between the 2 monosaccharides (pooled mean percentage change: −5.7%; 95% CI: −50%, 36%; I2 = 0%) (Figure 5).
FIGURE 5.
Random-effects meta-analysis comparing effects of a hypercaloric fructose diet with a hypercaloric glucose diet (neutral energy comparison) on liver fat measured by IHCLs 1H MRS. Each black box represents the individual study's effect estimate, and the horizontal line represents the 95% CI of the effect estimate. The diamond shape represents the meta-analysis pooled effect estimate and its CI. A vertical dashed line displays the location of the meta-analysis pooled effect estimate. IHCL, intrahepatocellular lipid; IHCLs 1H MRS, intrahepatocellular lipids by proton magnetic resonance spectroscopy; RCT, randomized controlled trial; ROB, risk of bias; %Change, net percentage change in intrahepatocellular lipids from baseline between groups; %signal, percent signal; %vol, percent volume.
For liver enzyme outcomes, 2 crossover RCTs (1 at low and 1 at high ROB) and 2 paralleled RCTs (both at medium ROB) that compared hypercaloric high-fructose with high-glucose diets could be meta-analyzed (46, 48, 67, 68). Our random-effects meta-analysis showed no significant difference in effects on ALT and AST [pooled mean differences (95% CIs): 2.06 U/L (−2.31, 6.42 U/L) and 1.15 U/L (−1.49, 3.78 U/L); I2 = 0%] between hypercaloric high-fructose and -glucose diets (doses ranged from 40 g/d to 3.5 g fructose or glucose · kg body weight−1 · d−1 added to a weight-maintenance diet) (Figure 4, B and C).
For the hepatic DNL outcome, one 10-wk RCT (high ROB) showed that postprandial DNL, but not fasting hepatic DNL, was significantly increased by 48% (95% CI: −8.7%, 104%) when hypercaloric high-fructose and high-glucose diets (+25% of daily energy) were compared in 18 healthy older adults (51).
Together, there was a low level of evidence that hypercaloric fructose and glucose diets have similar effects on liver fat and liver enzymes in healthy adults. There was insufficient evidence to draw a conclusion for effects on hepatic DNL on the basis of results from a single RCT. Note that the hypercaloric nature of the diets may have obscured any differences between the 2 monosaccharides were any to exist.
Effects of isocaloric fructose intake in adults
Three short-term intervention studies (1 at low and 2 at medium ROB) investigated effects of isocaloric fructose intake (47, 52, 67). Studies could not be synthesized together because they investigated different comparisons. One 2-wk parallel RCT (low ROB) examined effects of an isocaloric diet with 25% of the daily energy requirement from fructose or glucose on IHCLs and liver enzyme outcomes (ALT, AST, and GGT) in 32 centrally overweight men who were living in the United Kingdom (67). The 2 isocaloric monosaccharide diets did not alter IHCLs (+0.11% ± 2.1%) but reduced liver enzyme concentrations slightly. However, the 2 isocaloric fructose and glucose diets did not differ in any hepatic outcome measure. One 4-wk controlled trial compared effects of an energy-balanced diet with 15% of total calories (45–83 g/d) from fructose or glucose (both sugars were given in packets and mixed with unsweetened fruit juice, milk, or water) in 9 adults with impaired glucose intolerance and 9 healthy adults (47). The study showed that total bilirubin concentrations increased significantly in adults with impaired glucose intolerance after glucose consumption (+0.45 mg/dL) but not fructose consumption (−0.9 mg/dL). Total bilirubin concentrations did not change in healthy adults with normal glucose-tolerance. Another 2-wk before-and-after study (medium ROB) showed that a controlled regular diet supplemented with the daily consumption of 1.2 g honey/kg body weight dissolved in 250 mL water (containing 38 g% fructose, 30 g% glucose, and some vitamins and minerals) decreased concentrations of AST by 22% and ALT by 18% in 10 healthy volunteers from the medical staff (52).
Together, there was insufficient evidence to draw a conclusion on effects of isocaloric fructose intake (from pure fructose or honey) on indexes of liver health on the basis of results from a single study per exposure-comparison pair.
Effects of HFCS compared with sucrose on liver fat in adults
A 10-wk RCT (medium ROB) compared effects of a HFCS-sweetened beverage and sucrose-sweetened low-fat (1%) milk on liver fat deposition measured by using computed tomography (59). Eighty healthy adult men and women were challenged with the 3 amounts of HFCS-55 (ie, 55% fructose and 45% glucose) or sucrose-sweetened low-fat milk at 8%, 18%, or 30% of the energy requirement for weight maintenance. These amounts are equivalent to the 25th, 50th, and 90th percentiles, respectively, for fructose consumption in the general population. All participants were instructed to substitute test beverages for food or other beverages usually consumed. No additional instruction was given. Noncompliance (defined as the cumulative consumption of test beverages <80% or 5 consecutive days without the consumption of a single serving of the test beverage) led to automatic withdrawal from the study. Analyses were done in 64 participants who completed the study (dropout: 20%). During the10-wk intervention period, energy intake increased across the entire cohort and was driven by an increase in intake of total sugar (mean: 97 compared with 216 g/d; P < 0.001), which resulted in a mean weight gain of 0.8 kg independent of the diet group. Despite the weight gain, there were no significant changes in liver fat (mean: 13.32% compared with 13.21%) regardless of the type or amount of beverage.
No other studies investigated effects of HFCS. Thus, there was insufficient evidence to draw a conclusion for effects of HFCS on liver fat on the basis of results from a single RCT.
Effects of sucrose on liver fat and other indexes of liver health in adults
For the liver fat outcome, one 6-mo parallel RCT (high ROB) evaluated effects of 4 commercially available beverages [sucrose-sweetened regular cola (Coca Cola), aspartame-sweetened diet cola (Coca Cola), semi-skimmed milk (Arla Foods), and still mineral water (AQUA D'OR)] on ectopic fat accumulation in the liver in 60 healthy, normoglycemic individuals who were living in Denmark (61). IHCL was determined by using proton magnetic resonance spectroscopy. Study participants were provided with 1 L test drink/d and allowed to drink water, tea, coffee, and their usual amounts of alcohol. The 1 L sucrose-sweetened regular cola (106 g sucrose/d = 53 g bound fructose/d), semi-skimmed milk, aspartame-sweetened diet cola, and mineral water provided 430, 451, 4, and 0 kcal/d, respectively. The dropout or termination (all women) was 22%. Analyses were performed in 47 individuals who completed the study and were adjusted for sex. At the end of the intervention period, IHCL was significantly increased in the sucrose-sweetened regular cola group (n = 10; +132%) but not in the 3 other beverage groups. IHCL was similar in the semi-skimmed milk, diet cola, and water groups (61). There were no significant differences in total energy intake or body weight in the 4 groups during the study, which suggested energy compensation in response to increased intake of the 2 energy-containing beverages regular cola and semi-skimmed milk.
Three crossover RCTs (46, 60, 63) and one before-and-after study (62) examined effects of hypercaloric diets high in sucrose on liver enzymes or other indexes of liver health outcomes. These 4 studies could not be synthesized together because of different comparisons. One crossover RCT (low ROB) reported no significant differences in AST and ALT between hypercaloric high-sucrose (40 or 80 g/d) and high-glucose diets in 24 healthy men (46). Another crossover RCT (at medium ROB) also showed no significant differences in AST and ALT after energy-balance diets with high sucrose (850 kcal/d) or high glucose in 8 healthy, female university students (60). On the contrary, a crossover RCT (high ROB) showed that all liver function test indexes (alkaline phosphatase, ALT, AST, GGT, and bilirubin) were significantly raised after a hypercaloric high-sucrose (double energy requirement with 32% of energy from sucrose) diet compared with a standard energy-balanced diet in 12 healthy men (63). The before-and-after trial (high ROB) also showed that a hypercaloric sucrose-containing food-supplemented diet (25–30% kcal) significantly increased ALT and AST concentrations (62).
Together, there was insufficient evidence to draw a conclusion for effects of sucrose on liver fat and other indexes of liver health in adults on the basis of results from a single study for each exposure-comparison pair within each outcome of interest as well as high risks for biases.
Effects of hypocaloric fructose diets in NAFLD patients
One parallel RCT (high ROB) in 10 obese children with NAFLD (65) and one before-and-after trial (high ROB) in 10 overweight adults with NAFLD (69) examined the effects of a fructose-reduction diet on IHCLs or liver enzymes. The RCT in obese children evaluated diet education of a low-fructose (eliminating sugar-containing beverages, fruit juice, and food items with HFCS) and compared it with diet education of a low-fat diet (American Heart Association recommendations), and results showed no significant changes in ALT and AST concentrations in either group after 6 mo (65). However, children's BMI z scores did not change significantly during the study. The before-and-after trial in overweight adults assessed the effect of a dietary intervention to reduce fructose intake by 50% on IHCLs and liver enzyme concentrations (69). Patients had reduced their fructose intakes by an average of 61% along with significant reductions in average daily intake of total calories (−36%), total fat (−31%), and saturated fat (−24%) after the 6-mo intervention. After 6 mo, the mean IHCL content was significantly reduced (−36%), and AST and ALT concentrations were within the normal range, whereas GGT concentrations were lower in 7 of 10 patients. Patients’ body weight and BMI were significantly lowered after 6 mo as result of the hypocaloric dietary intervention.
Together, there was insufficient evidence to draw a conclusion for effects of a hypocaloric fructose diet on the progression of NAFLD in patients with NAFLD because of high risks for biases, although both studies did not find that a hypocaloric fructose diet had a significant effect on ALT and AST concentrations.
DISCUSSION
We found scarce, poor-quality, and heterogeneous data (different exposure comparisons) on effects of different amounts and forms of dietary fructose on risks of developing or the progression of NAFLD and on indexes of liver health in humans (Figure 2). For observational studies, the overall strength of evidence for associations of dietary fructose or sucrose consumption with risks of developing or the progression of NAFLD was rated insufficient because of high risk of biases and inconsistent study findings. Note that the causality between dietary fructose and NAFLD could not be assessed from retrospective case-control and cross-sectional studies. Of the 21 intervention studies identified, 12 studies (57%) investigated effects of a hypercaloric fructose diet (supplemented by pure fructose) in almost exclusively healthy, young, male adults. Of these studies, 8 studies (67%) were conducted by the same group of investigators. Few intervention studies have directly distinguished between effects of increases in energy intake or body weight from high intakes of monosaccharides and disaccharides, particularly of fructose, per se on liver fat or NAFLD. Within the context of current intakes of fructose in the United Stets, there are no studies, to our knowledge, that have compared liquid and solid forms of fructose on liver fat or NAFLD. Only one study directly compared the isocaloric replacement of fructose and glucose (25% kcal) on liver fat and liver enzyme outcomes and showed the 2 monosaccharaides did not differ in any hepatic outcome measure (67). Of studies for which some data were available, common limitations included small sample sizes, short intervention periods, a lack of energy intake or body weight control, the use of pure fructose rather than sucrose or HFCS, and fructose loads that exceed current intakes. With these caveats in mind, we concluded that there was a low level of evidence that a hypercaloric fructose diet increases liver fat and AST concentrations in healthy men compared with a weight-maintenance diet in healthy men. However, it should be stressed that liver fat concentrations observed in healthy men at baseline and after fructose overfeeding were still far below concentrations observed in patients with NAFLD (70). Furthermore, the comparison of the substitution of glucose to fructose in hypercaloric diets may not be directly representative of that encountered in free-living individuals who consume fructose and glucose not only as monosaccharides but also has disaccharides and dextrins. In addition, there was a low level of evidence that hypercaloric fructose and glucose diets have similar effects on liver fat and liver enzymes in healthy adults. Note that the elevation of liver fat or liver enzyme concentrations does not automatically imply the presence of liver disease; the concentrations of liver enzymes do not correlate well with the extent of liver damage nor prognosis (71, 72). Our conclusions are consistent with a recently published systematic review and meta-analysis that examined the effect of fructose on markers of NAFLD in controlled trials although the authors pooled studies that reported IHCL outcomes by using a different metric (standardized mean difference) (73). The consistent findings further support the strengths of our approaches to conducting both qualitative and quantitative syntheses of data in light of the significant clinical and statistical heterogeneity observed in available data as well as the poor quality of the data.
With the potential to progressing to advance liver disorders, NAFLD has been recognized as a major public health concern (74, 75). Diet is considered to play a central role in the development of NAFLD through its effects on hormones, transcription factors, and lipid metabolism (5). However, it difficult to isolate causes to a single dietary cause because of the high prevalence of NAFLD in obese individuals and patients with diabetes (76–79). Furthermore, there is currently no consensus for a noninvasive reference standard for the diagnosis of NAFLD. Limitations in invasively assessing liver fat have resulted in little data on which to base diet recommendations, and data available are, for the most part, inconclusive (80). Currently, there are no specific guidelines for the prevention or treatment of NAFLD or NASH with diet beyond weight loss, which is an independent risk factor for NAFLD (81–83).
This systematic review identified several important research gaps. First, a liver biopsy is the gold standard for diagnosing NAFLD but is unlikely to be used in large population-based studies. Without the use of the same definition of a reference standard, conducting more studies on NAFLD is unlikely to add meaningfully to the body of evidence. Therefore, the establishment of a noninvasive reference standard for NAFLD diagnosis is urgently needed to advance our knowledge in this research area. Second, well-controlled studies are needed to examine factors that may modify effects of fructose or sucrose consumption on NAFLD. For example, a genetic variation may modify effects of fructose or sugar intake on liver fat (44, 84) and determine whether certain subpopulations may be more vulnerable to the development of NAFLD. Last, future studies need to have sufficient statistical power to test hypotheses with prospective observational study designs in the general population or use experimental conditions hat approximated current intakes and chemical forms (pure fructose does not exist in a typical diet), so that results can inform dietary intake recommendations for disease prevention.
Our systematic review had several strengths. Unlike other published systematic reviews on fructose and a variety of health outcomes (27–31), we evaluated all study designs and all sources and forms of fructose intake in relation to liver health with a goal to reach conclusions that are more applicable to general population. We also tailored risk-of-bias tools to include nutrition-specific quality items, which are important for evaluating the quality of nutrition research (85). Our study also had several limitations, which were primarily inherited from poor-quality and heterogeneous studies included in this systematic review. In addition, most of the data were only available in healthy men, which limited the generalizability of our findings. The dearth of data in women could not answer the question of whether sex is an effect modifier for associations between dietary fructose consumption and liver health. Finally, we showed that a selected outcome reporting bias (86) was likely in many studies, which could have led to an overestimation of effects.
In conclusion, on the basis of indirect comparisons across study findings, the apparent association between indexes of liver health (ie, liver fat, hepatic DNL, ALT, AST, and GGT) and fructose or sucrose intake appear to be confounded by excessive energy intake. Current evidence does not allow us to discern the intertwined associations between excess body weight, monosaccharide fructose or sucrose intake, and NAFLD. Therefore, we concluded that, overall, the available evidence is not sufficiently robust to draw conclusions regarding effects of fructose, HFCS, or sucrose consumption on NAFLD.
Supplementary Material
Acknowledgments
We thank the content expertise provided by our TEP (Naomi Fukagawa, Fayez K Ghishan, Bernadette Marriotts, Esther F Myers, and Xavier Pi-Sunyer).
The authors’ responsibilities were as follows—MC, JL, and AHL: conception and design; MC, JM, and AHL: analysis and interpretation of data; MC: drafting of the manuscript; MC, JM, KP, and SB: collection and assembly of data; and all authors: critical revision of the manuscript for important intellectual content and final approval of the article. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DNL, de novo lipogenesis; GGT, γ-glutamyl transpeptase; HFCS, high-fructose corn syrup; HFF, hepatic fat fraction; IHCL, intrahepatocellular lipid; MeSH, Medical Subject Headings; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; RCT, randomized controlled trial; ROB, risk of bias; TEP, technical expert panel.
REFERENCES
- 1.Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28:27–38. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–23. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221–31. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 2011;332:1519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr 2007;86:285–300. [DOI] [PubMed] [Google Scholar]
- 6.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narasimhan S, Gokulakrishnan K, Sampathkumar R, Farooq S, Ravikumar R, Mohan V, Balasubramanyam M. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Clin Biochem 2010;43:815–21. [DOI] [PubMed] [Google Scholar]
- 8.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clinics in Liver Disease 2004;8:521–33, viii. [DOI] [PubMed]
- 9.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedogni G, Kahn HS, Bellentani S, Tiribelli C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol 2010;10:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274–85. [DOI] [PubMed] [Google Scholar]
- 12.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr 2013;162:496–500e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011;9:524–30e1; quiz e60. [DOI] [PubMed] [Google Scholar]
- 14.White JS. Challenging the fructose hypothesis: new perspectives on fructose consumption and metabolism. Adv Nutr 2013;4:246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lustig RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc 2010;110:1307–21. [DOI] [PubMed] [Google Scholar]
- 16.Montonen J, Jarvinen R, Knekt P, Heliovaara M, Reunanen A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr 2007;137:1447–54. [DOI] [PubMed] [Google Scholar]
- 17.Ackerman Z, Oron-Herman M, Grozovski M, Rosenthal T, Pappo O, Link G, Sela BA. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension 2005;45:1012–8. [DOI] [PubMed] [Google Scholar]
- 18.Armutcu F, Coskun O, Gurel A, Kanter M, Can M, Ucar F, Unalacak M. Thymosin alpha 1 attenuates lipid peroxidation and improves fructose-induced steatohepatitis in rats. Clin Biochem 2005;38:540–7. [DOI] [PubMed] [Google Scholar]
- 19.Davail S, Rideau N, Bernadet MD, Andre JM, Guy G, Hoo-Paris R. Effects of dietary fructose on liver steatosis in overfed mule ducks. Horm Metab Res 2005;37:32–5. [DOI] [PubMed]
- 20.Kawasaki T, Igarashi K, Koeda T, Sugimoto K, Nakagawa K, Hayashi S, Yamaji R, Inui H, Fukusato T, Yamanouchi T. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J Nutr 2009;139:2067–71. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World journal of gastroenterology. World J Gastroenterol 2012;18:2300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–43. [DOI] [PubMed] [Google Scholar]
- 23.Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011;94:726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell R, Chung M, Balk EM, Atkinson S, Giovannucci EL, Ip S, Lichtenstein AH, Mayne ST, Raman G, Ross AC, et al. Opportunities and challenges in conducting systematic reviews to support the development of nutrient reference values: vitamin A as an example. Am J Clin Nutr 2009;89:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.IOM. Finding what works in health care: standards for systematic reviews. Washington, DC: National Academy of Sciences, 2011. [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sievenpiper JL, de Souza RJ, Mirrahimi A, Yu ME, Carleton AJ, Beyene J, Chiavaroli L, Di Buono M, Jenkins AL, Leiter LA, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med 2012;156:291–304. [DOI] [PubMed] [Google Scholar]
- 28.Wang DD, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Cozma AI, Mirrahimi A, Yu ME, Carleton AJ, Di Buono M, et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr 2012;142:916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sievenpiper JL, Chiavaroli L, de Souza RJ, Mirrahimi A, Cozma AI, Ha V, Wang DD, Yu ME, Carleton AJ, Beyene J, et al. 'Catalytic’ doses of fructose may benefit glycaemic control without harming cardiometabolic risk factors: a small meta-analysis of randomised controlled feeding trials. Br J Nutr 2012;108:418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ha V, Sievenpiper JL, de Souza RJ, Chiavaroli L, Wang DD, Cozma AI, Mirrahimi A, Yu ME, Carleton AJ, Dibuono M, et al. Effect of fructose on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Hypertension 2012;59:787–95. [DOI] [PubMed] [Google Scholar]
- 31.Sievenpiper JL, Carleton AJ, Chatha S, Jiang HY, de Souza RJ, Beyene J, Kendall CW, Jenkins DJ. Heterogeneous effects of fructose on blood lipids in individuals with type 2 diabetes: systematic review and meta-analysis of experimental trials in humans. Diabetes Care 2009;32:1930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition. J Nutr 2008;138:2297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, Lichtenstein A, Patel K, Raman G, Tatsioni A, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep) 2009;183:1–420. [PMC free article] [PubMed] [Google Scholar]
- 35.Well G, Shea B, O'Connel D, Peterson J, Welch V, Bosos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. 2011. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (cited 3 July 2014).
- 36.Agency for Healthcare Research and Quality. Methods guide for effectiveness and comparative effectiveness reviews. AHRQ publication no. 10(14)-EHC063-EF. Rockville, MD: Agency for Healthcare Research and Quality, 2014. [PubMed] [Google Scholar]
- 37.Dahabreh IJ, Hadar N, Chung M. Emerging magnetic resonance imaging technologies for musculoskeletal imaging under loading stress: scope of the literature. Ann Intern Med 2011;155:616–24. [DOI] [PubMed] [Google Scholar]
- 38.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from: www.cochrane-handbook.org.
- 40.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 2008;48:993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Konigsrainer A, Maier KP, Bischoff SC, Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr 2008;138:1452–5. [DOI] [PubMed] [Google Scholar]
- 43.Volynets V, Kuper MA, Strahl S, Maier IB, Spruss A, Wagnerberger S, Konigsrainer A, Bischoff SC, Bergheim I. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (NAFLD). Dig Dis Sci 2012;57:1932–41. [DOI] [PubMed] [Google Scholar]
- 44.Davis JN, Le K A, Walker RW, Vikman S, Spruijt-Metz D, Weigensberg MJ, Allayee H, Goran MI. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am J Clin Nutr 2010;92:1522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mager DR, Patterson C, So S, Rogenstein CD, Wykes LJ, Roberts EA. Dietary and physical activity patterns in children with fatty liver. Eur J Clin Nutr 2010;64:628–35. [DOI] [PubMed] [Google Scholar]
- 46.Aeberli I, Gerber PA, Hochuli M, Kohler S, Haile SR, Gouni-Berthold I, Berthold HK, Spinas GA, Berneis K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 2011;94:479–85. [DOI] [PubMed] [Google Scholar]
- 47.Koh ET, Ard NF, Mendoza F. Effects of fructose feeding on blood parameters and blood pressure in impaired glucose-tolerant subjects. J Am Diet Assoc 1988;88:932–8. [PubMed] [Google Scholar]
- 48.Ngo Sock ET, Le KA, Ith M, Kreis R, Boesch C, Tappy L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr 2010;103:939–43. [DOI] [PubMed] [Google Scholar]
- 49.Silbernagel G, Lutjohann D, Machann J, Meichsner S, Kantartzis K, Schick F, Haring HU, Stefan N, Fritsche A. Cholesterol synthesis is associated with hepatic lipid content and dependent on fructose/glucose intake in healthy humans. Exp Diabetes Res 2012;2012:361863. [DOI] [PMC free article] [PubMed]
- 50.Silbernagel G, Machann J, Unmuth S, Schick F, Stefan N, Haring HU, Fritsche A. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. Br J Nutr 2011;106:79–86. [DOI] [PubMed] [Google Scholar]
- 51.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Waili NS. Effects of daily consumption of honey solution on hematological indices and blood levels of minerals and enzymes in normal individuals. J Med Food 2003;6:135–4049. [DOI] [PubMed] [Google Scholar]
- 53.Couchepin C, Le KA, Bortolotti M, da Encarnacao JA, Oboni JB, Tran C, Schneiter P, Tappy L. Markedly blunted metabolic effects of fructose in healthy young female subjects compared with male subjects. Diabetes Care 2008;31:1254–6. [DOI] [PubMed] [Google Scholar]
- 54.Lê K A, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr 2009;89:1760–56. [DOI] [PubMed] [Google Scholar]
- 55.Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 2005;54:1907–13 (Published erratum appears in Diabetes 2006;55:563.) [DOI] [PubMed] [Google Scholar]
- 56.Lê K A, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr 2006;84:1374–9. [DOI] [PubMed] [Google Scholar]
- 57.Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34:454–61. [DOI] [PubMed] [Google Scholar]
- 58.Sobrecases H, Le KA, Bortolotti M, Schneiter P, Ith M, Kreis R, Boesch C, Tappy L. Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab 2010;36:244–6. [DOI] [PubMed] [Google Scholar]
- 59.Bravo S, Lowndes J, Sinnett S, Yu Z, Rippe J. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl Physiol Nutr Metab 2013;38:681–8. [DOI] [PubMed]
- 60.Kelsay JL, Behall KM, Holden JM, Prather ES. Diets high in glucose or sucrose and young women. Am J Clin Nutr 1974;27:926–36. [DOI] [PubMed] [Google Scholar]
- 61.Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–9. [DOI] [PubMed] [Google Scholar]
- 62.Porikos KP, Van Itallie TB. Diet-induced changes in serum transaminase and triglyceride levels in healthy adult men. Role of sucrose and excess calories. Am J Med 1983;75:624–30. [DOI] [PubMed] [Google Scholar]
- 63.Purkins L, Love ER, Eve MD, Wooldridge CL, Cowan C, Smart TS, Johnson PJ, Rapeport WG. The influence of diet upon liver function tests and serum lipids in healthy male volunteers resident in a phase I unit. Br J Clin Pharmacol 2004;57:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Theytaz F, Noguchi Y, Egli L, Campos V, Buehler T, Hodson L, Patterson BW, Nishikata N, Kreis R, Mittendorfer B, et al. Effects of supplementation with essential amino acids on intrahepatic lipid concentrations during fructose overfeeding in humans. Am J Clin Nutr 2012;96:1008–16. [DOI] [PubMed] [Google Scholar]
- 65.Vos MB, Weber MB, Welsh J, Khatoon F, Jones DP, Whitington PF, McClain CJ. Fructose and oxidized low-density lipoprotein in pediatric nonalcoholic fatty liver disease: a pilot study. Arch Pediatr Adolesc Med 2009;163:674–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goletzke J, Buyken AE, Gopinath B, Rochtchina E, Barclay AW, Cheng G, Brand-Miller JC, Mitchell P. Carbohydrate quality is not associated with liver enzyme activity and plasma TAG and HDL concentrations over 5 years in an older population. Br J Nutr 2013;110:918–25. [DOI] [PubMed] [Google Scholar]
- 67.Johnston RD, Stephenson MC, Crossland H, Cordon SM, Palcidi E, Cox EF, Taylor MA, Aithal GP, Macdonald IA. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology 2013;145:1016–25e2. [DOI] [PubMed] [Google Scholar]
- 68.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Keim NL, et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metab (Lond) 2012;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Volynets V, Machann J, Kuper MA, Maier IB, Spruss A, Konigsrainer A, Bischoff SC, Bergheim I. A moderate weight reduction through dietary intervention decreases hepatic fat content in patients with non-alcoholic fatty liver disease (NAFLD): a pilot study. Eur J Nutr 2013;52:527–35. [DOI] [PubMed] [Google Scholar]
- 70.Cuthbertson DJ, Irwin A, Pugh CJ, Jones H, Sprung VS, Daousi C, Adams VL, Bimson WE, Shojaee-Moradie F, Umpleby AM, et al. Ectopic lipid storage in Non-Alcoholic Fatty Liver Disease is not mediated by impaired mitochondrial oxidative capacity in skeletal muscle. Clin Sci (Epub ahead of print 16 April 2014). [DOI] [PubMed] [Google Scholar]
- 71.Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician 1999;59:2223–30. [PubMed] [Google Scholar]
- 72.Thapa BR, Walia A. Liver function tests and their interpretation. Indian J Pediatr 2007;74:663–71. [DOI] [PubMed] [Google Scholar]
- 73.Chiu S, Sievenpiper JL, de Souza RJ, Cozma AI, Mirrahimi A, Carleton AJ, Ha V, Di Buono M, Jenkins AL, Leiter LA, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 2014;68:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanyal AJ. NASH: a global health problem. Hepatol Res 2011;41:670–4. [DOI] [PubMed] [Google Scholar]
- 75.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology 2008;134:1682–98. [DOI] [PubMed] [Google Scholar]
- 76.Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ 2011;343:d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schindhelm RK, Heine RJ, Diamant M. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007;30:e94; author reply e95. [DOI] [PubMed] [Google Scholar]
- 78.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005;288:E462–8. [DOI] [PubMed] [Google Scholar]
- 79.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–31. [DOI] [PubMed] [Google Scholar]
- 80.Mensink RP, Plat J, Schrauwen P. Diet and nonalcoholic fatty liver disease. Curr Opin Lipidol 2008;19:25–9. [DOI] [PubMed] [Google Scholar]
- 81.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 2005;100:1082–90. [DOI] [PubMed] [Google Scholar]
- 82.Bugianesi E, Marzocchi R, Villanova N, Marchesini G. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): treatment. Best Pract Res Clin Gastroenterol 2004;18:1105–16. [DOI] [PubMed] [Google Scholar]
- 83.Clark JM. Weight loss as a treatment for nonalcoholic fatty liver disease. J Clin Gastroenterol 2006;40(suppl 1):S39–43a. [DOI] [PubMed] [Google Scholar]
- 84.Wagenknecht LE, Palmer ND, Bowden DW, Rotter JI, Norris JM, Ziegler J, Chen YD, Haffner S, Scherzinger A, Langefeld CD. Association of PNPLA3 with non-alcoholic fatty liver disease in a minority cohort: the Insulin Resistance Atherosclerosis Family Study. Liver Int 2011;31:412–6. [DOI] [PMC free article] [PubMed]
- 85.Chung M, Balk EM, Ip S, Lee J, Terasawa T, Raman G, Trikalinos T, Lichtenstein AH, Lau J. Systematic review to support the development of nutrient reference intake values: challenges and solutions. Am J Clin Nutr 2010;92:273–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, Williamson PR. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.