Abstract
Background: The vitamin D–endocrine system is thought to play a role in physiologic processes that range from mineral metabolism to immune function. Serum 25-hydroxyvitamin D [25(OH)D] is the accepted biomarker for vitamin D status. Skin color is a key determinant of circulating 25(OH)D concentrations, and genes responsible for melanin content have been shown to be under strong evolutionary selection in populations living in temperate zones. Little is known about the effect of latitude on mean concentrations of 25(OH)D in dark-skinned populations.
Objective: The objective was to describe the distribution of 25(OH)D and its subcomponents in 5 population samples of African origin from the United States, Jamaica, Ghana, South Africa, and the Seychelles.
Design: Participants were drawn from the Modeling of the Epidemiologic Transition Study, a cross-sectional observational study in 2500 adults, ages 25–45 y, enrolled between January 2010 and December 2011. Five hundred participants, ∼50% of whom were female, were enrolled in each of 5 study sites: Chicago, IL (latitude: 41°N); Kingston, Jamaica (17°N); Kumasi, Ghana (6°N); Victoria, Seychelles (4°S); and Cape Town, South Africa (34°S). All participants had an ancestry primarily of African origin; participants from the Seychelles trace their history to East Africa.
Results: A negative correlation between 25(OH)D and distance from the equator was observed across population samples. The frequency distribution of 25(OH)D in Ghana was almost perfectly normal (Gaussian), with progressively lower means and increasing skewness observed at higher latitudes.
Conclusions: It is widely assumed that lighter skin color in populations outside the tropics resulted from positive selection, driven in part by the relation between sun exposure, skin melanin content, and 25(OH)D production. Our findings show that robust compensatory mechanisms exist that create tolerance for wide variation in circulating concentrations of 25(OH)D across populations, suggesting a more complex evolutionary relation between skin color and the vitamin D pathway. This trial was registered at clinicaltrials.gov as NCT02111902.
INTRODUCTION
The vitamin D–endocrine system is thought to play multiple roles in physiologic pathways, such as bone mineral metabolism and the modulation of immune response; and potential links to several chronic conditions, such as hypertension, cancer, and obesity have been described (1–5). Vitamin D is acquired from dietary intake and endogenous synthesis after sun exposure, with the latter mechanism accounting for ∼90% of body stores in most regions of the world. In the liver, vitamin D is converted to 25-hydroxyvitamin D [25(OH)D]4, which circulates in both bound and unbound fractions and is the metabolite usually measured to determine vitamin D status. Serum 25(OH)D reflects the combination of exposure to sunlight and diet. Part of 25(OH)D is converted by the kidneys to 1,25-hydroxyvitamin D, the biologically active form of vitamin D that acts as a hormone.
Although adequate 25(OH)D serum concentrations can be maintained by endogenous synthesis with fairly short periods of exposure to the sun, recommendations on how to maintain healthy concentrations of 25(OH)D must take into account the competing risk of skin cancer from UV radiation. Therefore, recommendations for the Dietary Reference Intake assume that no endogenous synthesis occurs and that a person's source of vitamin D comes entirely from their diet, although this rarely occurs in practice.
Because of the complexity of the metabolism of vitamin D, and the scarcity of definitive data, a recent Institute of Medicine (IOM) advisory panel recognized the public health significance associated with specification of serum 25(OH)D cutoffs for sufficiency. Although the panel neither recommended the designation of “abnormal” concentrations at the low end of the 25(OH)D distribution nor supplementation on the basis of measured serum concentrations (6), it suggested that concentrations of 25(OH)D <30 nmol/L (12 ng/mL) will put persons at risk of deficiency and that some, but not all, will be at risk of inadequacy at serum 25(OH)D concentrations between 30 and 50 nmol/L (12 and 20 ng/mL). Other recommendations, such as those offered by the Endocrine Society, propose cutoffs to categorize concentrations of serum 25(OH)D as inadequate (7). Policy guidance with regard to 25(OH)D concentrations is further complicated by large variation between racial-ethnic groups in many countries in the temperate latitudes and by the dearth of information on the concentrations of unbound or the bioavailable fraction of 25(OH)D. Migrations in the modern era from equatorial regions have resulted in lower concentrations of 25(OH)D among persons with darker skin in North America. In the United States, data from the NHANES indicate that ∼73% of African Americans have 25(OH)D concentrations <20 ng/mL, whereas 42% and 21% of Mexican Americans and non-Hispanic whites, respectively, had concentrations below this level (8). Concern therefore exists as to whether these relatively low concentrations are associated with adverse health effects.
The objectives of this article were to describe the distribution of 25(OH)D and its key subcomponents in 5 African-origin populations at varying distance from the equator and to confirm the expected relation to latitude-specific ambient levels of UV radiation.
SUBJECTS AND METHODS
Participants were drawn from the Vitamin D Ancillary Study as an extension of the Modeling of the Epidemiologic Transition Study; the design and recruitment procedures for this study were described in detail elsewhere (9). In brief, 2500 adults, ages 25–45 y, were enrolled between January 2010 and December 2011. Five hundred participants, ∼50% of whom were female, were enrolled in each of 5 study sites: Victoria, Seychelles; Kumasi, Ghana; Kingston, Jamaica; Cape Town, South Africa; and Chicago, IL. All participants had an ancestry primarily of African origin; participants from the Seychelles trace their history to East Africa. The variations in latitude for the study sites are as follows: 41°N (Chicago, IL) to 17°N (Kingston, Jamaica) to 6°N (Kumasi, Ghana) to 4°S (Victoria, Seychelles) and 34°S (Cape Town, South Africa). The sites were chosen to cover communities in a broad range of social and economic development but were not intended to be representative of the entire country.
The protocol was approved by the Institutional Review Board of Loyola University (Chicago, IL), the Committee on Human Research Publication and Ethics of Kwame Nkrumah University of Science and Technology (Kumasi, Ghana), the Research Ethics Committee of the University of Cape Town (South Africa), the Board for Ethics and Clinical Research of the University of Lausanne (Switzerland), the Ethics Committee of the University of the West Indies (Kingston, Jamaica), and the Health Sciences Institutional Review Board of the University of Wisconsin (Madison, WI). Written informed consent was obtained from all participants.
Basic health history information, with a focus on cardiovascular conditions and diabetes, was collected including age of first diagnosis, where applicable. Questions were included that covered general household characteristics, participant and significant other's occupation, parental education, and household assets and amenities. These questions were based on the Core Welfare Indicators Questionnaire from the World Bank, designed to monitor social indicators in Africa (10).
All measurements were taken at outpatient clinics located in the communities. Standardized protocols were used for blood pressure, anthropometric variables, activity monitoring, and blood sampling. Accrual and examinations were carried out during winter and summer months to ensure an even seasonal distribution of the biologic samples collected. Overnight fasting was solicited from the participants before the baseline clinic examination. Specimens were immediately processed to separate serum, frozen, and shipped to the Coordinating Center in Chicago. When all samples were available, they were sent to the Department of Laboratory Medicine at the University of Washington for analysis. The liquid chromatography–tandem mass spectrometric assay of vitamin D metabolites was performed as described previously (11, 12). Briefly, vitamin D metabolites are dissociated from binding proteins by using sodium hydroxide, internal standard is added, and the analytes are extracted by using heptane. Liquid chromatography was performed by using an Acquity chromatograph (Waters) equipped with a pentafluorophenyl propyl column (Restek), which was developed with an isocratic aqueous methanol:ammonium acetate mobile phase. The transitions monitored for 25(OH)D2 and 25(OH)D3 were 413.3→355.3 and 401.3→365.3, respectively, and 419.3→355.3 and 407.3→371.3 for the hexadeuterated internal standards, respectively. The calibration of the assay was verified by using the National Institute of Standards and Technology standard reference material SRM 972. Interassay variability was 6.0% at 11.5 ng/mL and 5.6% at 12.3 ng/mL for 25(OH)D2 and 25(OH)D3, respectively. The limits of detection were 0.2 and 0.5 ng/mL for 25(OH)D2 and 3-epimer-25(OH)D3, respectively.
Although the IOM Committee on Vitamin D and Calcium Dietary Reference Intakes was not charged with specifying cutoffs for serum 25(OH)D concentrations, they suggested that an individual is at “risk of deficiency” if his/her serum 25(OH)D concentrations is <12 ng/mL and concluded that concentrations of serum 25(OH)D >20 ng/mL are “sufficient” for practically all persons (6). Furthermore, the IOM report noted that some, but not all, persons with concentrations between 12 and 20 ng/mL are potentially at “risk of inadequacy.” In addition, serum 25(OH)D concentrations >30 ng/mL do not provide increased benefit. For consistency with the IOM report and to allow comparability with other recommendations, such as those developed by the Endocrine Society (7), we chose to report prevalence estimates in 4 serum 25(OH)D groups, following the terminology used in a recent National Center for Health Statistics Data Brief (8) but without implying that these categories are necessarily associated with abnormal health outcomes in some or all populations: 1) <12 ng/mL (at risk of deficiency), 2) ≥12 and <20 ng/mL (at risk of inadequacy), 3) ≥20 ng/mL (sufficiency), and 4) ≥30 ng/mL. The proportion of the population with measured serum total 25(OH)D concentrations below the individual required level is also reported. Beaton (13) recommended estimation of this quantity as the proportion of the population below 16 ng/mL (the Estimated Average Requirement as defined by the IOM), which approximates the probability approach introduced by Carriquiry (14) and later applied to vitamin D by Taylor et al (15).
A subsample of individuals was selected from Ghana (n = 235) and the United States (n = 148) to measure skin pigmentation from a non–sun-exposed area of the body (ie, the axillary fossa) using the DermaSpectrometer Monitor (Cortex Technology). The data are expressed in terms of melanin index (M-index) and the degree of skin redness (erythema) index (E-index). The performance of the DermaSpectrometer monitor was described elsewhere (16).
Data management was centralized at the Coordinating Center at Loyola University Chicago. All data forms and questionnaires were scanned at study sites and were sent via secure file transfer protocol (17) to the data manager at the Coordinating Center.
Means and SDs were used to summarize continuous variables and percentages applied to categorical variables. The distribution of 25(OH)D was estimated via kernel density estimators with the Epanechnikov kernel function (18). ANOVA was applied to compare means of the continuous characteristics, whereas the chi-square test was used for categorical characteristics. When applicable, the Scheffe approach was implemented to adjust for multiple comparisons. CIs based on bootstrap sampling were calculated for the Pearson correlation coefficient (19, 20). The statistical package STATA (STATA version 12.1; StataCorp) was used to perform all statistical analyses and to estimate the frequency distributions of 25(OH)D.
RESULTS
The descriptive characteristics of the participants are presented in Table 1, stratified by sex and site. An approximate monotonic increase in relative weight was observed among women, with mean BMIs (in kg/m2) ranging from 26 to 34, whereas men in Chicago and Victoria exhibited higher mean BMIs than did men at the other 3 sites. Blood pressure trended upward with increasing mean BMI, with the exception of South Africa, where higher levels were noted relative to the degree of obesity, especially among men.
TABLE 1.
Baseline characteristics of participants in the VIDA study, which included participants of African descent from 5 countries1
| Chicago, IL (41°N) | Cape Town, South Africa (34°S) | Kingston, Jamaica (17°N) | Kumasi, Ghana (6°N) | Victoria, Seychelles (4°S) | |
| Men | |||||
| Sample size (n) | 245 | 232 | 249 | 207 | 230 |
| Age (y) | 35.6 ± 6.22a | 33.7 ± 5.6b | 34.0 ± 5.9b | 34.6 ± 6.7b | 36.5 ± 5.1a |
| BMI (kg/m2) | 29.7 ± 7.5a | 22.4 ± 4.3b | 23.6 ± 4.5b | 22.2 ± 2.7b | 26.5 ± 4.9c |
| Systolic blood pressure (mm Hg) | 127.9 ± 14.5a | 129.0 ± 17.1a | 121.5 ± 12.8b | 118.9 ± 13.1b | 122.7 ± 14.6b |
| Diastolic blood pressure (mm Hg) | 81.0 ± 12.1a | 79.6 ± 13.2a | 71.2 ± 11.1b | 68.5 ± 11.4b | 75.0 ± 11.4c |
| Education (y) | 12.7 ± 1.6 | 9.5 ± 2.6 | 10.6 ± 2.1 | 9.2 ± 3.8 | 10.6 ± 2.1 |
| Employed (%) | 85.2 | 91.4 | 90.8 | 98.6 | 97.8 |
| Manual laborer (%) | 67.1 | 90.3 | 61.8 | 60.9 | 54.4 |
| Women | |||||
| Sample size (n) | 257 | 268 | 251 | 293 | 270 |
| Age (y) | 35.0 ± 6.3a | 33.1 ± 6.0b | 34.7 ± 6.2b | 34.0 ± 6.6b | 35.8 ± 6.0c |
| BMI (kg/m2) | 34.1 ± 8.8a | 31.9 ± 8.2b | 29.5 ± 6.7c | 25.5 ± 5.2d | 27.6 ± 6.2c |
| Systolic blood pressure (mm Hg) | 117.5 ± 16.1a | 118.2 ± 18.6a | 115.2 ± 14.7a | 110.5 ± 15.2b | 110.8 ± 12.8b |
| Diastolic blood pressure (mm Hg) | 79.6 ± 13.2a | 76.3 ± 11.8b | 72.1 ± 11.4c | 66.2 ± 11.4d | 71.2 ± 9.9c |
| Education (y) | 13.8 ± 2.6 | 10.0 ± 2.1 | 10.7 ± 2.0 | 7.5 ± 4.1 | 13.0 ± 2.5 |
| Employed (%) | 83.7 | 81.7 | 72.1 | 89.4 | 95.6 |
| Manual laborer (%) | 28.9 | 89.3 | 68.8 | 90.4 | 26.4 |
ANOVA (chi-square) tests used to compare means or proportions of all continuous or categorical variables, respectively, across countries. Post hoc tests were performed with the use of a Scheffe adjustment. Values not sharing a common superscript letter are significantly different, P < 0.05. VIDA, Vitamin D Ancillary.
Mean ± SD (all such values).
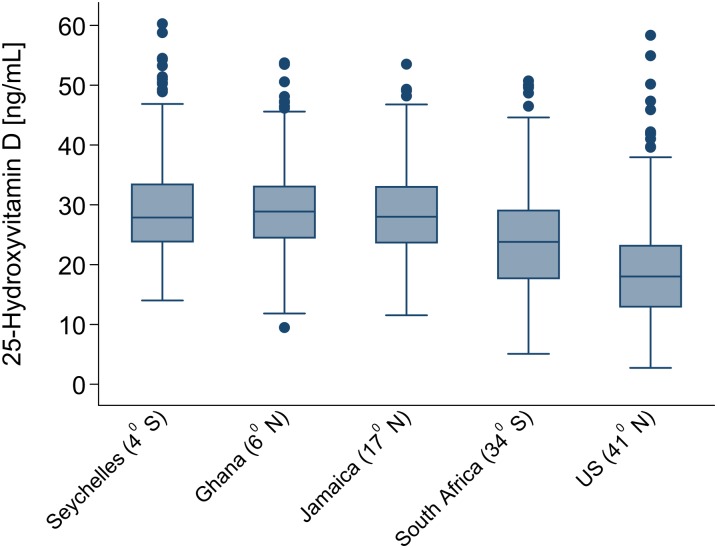
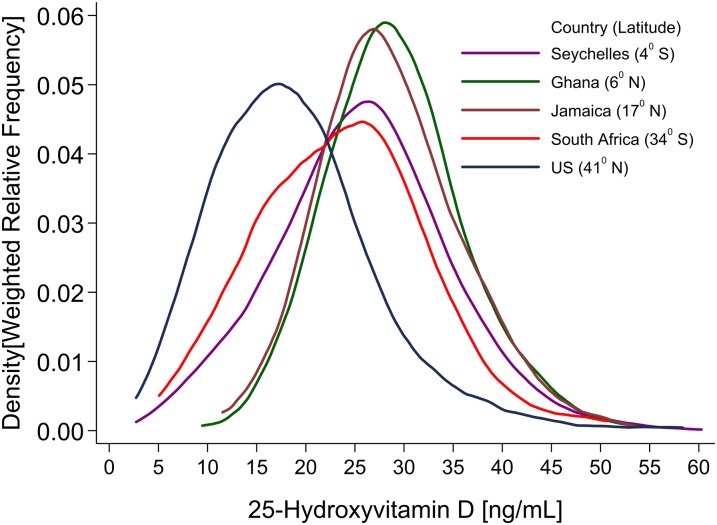
Assays of mean total 25(OH)D and its subcomponents are presented in Table 2. The primary contrast across groups was variation in 25(OH)D according to distance from the equator (latitude), with the following mean concentrations: 29.2 ng/mL in Victoria, Seychelles (4°S); 30.4 ng/mL in Kumasi, Ghana (6°N); 28.9 ng/mL in Kingston, Jamaica (17° N); 23.7 ng/mL in Cape Town, South Africa (34° S); and 17.2 ng/mL in Chicago, IL (41° N). The main contributor to total 25(OH)D was 25(OH)D3, with 25(OH)D2 accounting for between 1.0% (0.3 of 29.2 ng/mL) in the Seychelles and ∼7.6% (1.3 of 17.2 ng/mL) in the United States. The range of 3-epimer-25(OH)D3 varied from 1.2 to 2.0 ng/mL, corresponding to ∼6% of the total 25(OH)D. The corresponding BMI-adjusted values of these data are summarized as a box plot in Figure 1 and as smoothed frequency distributions in Figure 2. The participants from Ghana, as noted, had the highest mean concentrations of total 25(OH)D, and the distribution was approximately normal. In the Seychelles, a modest increase in the variance of total 25(OH)D with associated left-skewness was noted, although mean values were similar to Jamaica. African Americans had substantially lower values, with pronounced leftward skewing.
TABLE 2.
Circulating serum total 25(OH)D concentrations in the 5 study population samples1
| 25(OH)D |
||||
| Country (latitude) | Total 25(OH)D2 | 25(OH)D32 | 25(OH)D23 | 3-Epimer-25(OH)D34 |
| ng/mL | ||||
| United States (41°N) | 17.2 ± 8.0a | 16.5 ± 7.6a | 1.3 ± 4.5a | 1.0 ± 0.6a |
| South Africa (34°S) | 23.7 ± 8.3b | 23.2 ± 8.1b | 0.8 ± 0.7b | 1.4 ± 0.9b |
| Jamaica (17°N) | 28.9 ± 7.1c | 28.9 ± 7.1c | 0.4 ± 0.5c | 1.8 ± 0.8c |
| Ghana (6°N) | 30.4 ± 6.9c | 29.8 ± 6.8c | 1.0 ± 0.6a | 2.0 ± 1.5d |
| Seychelles (4°S) | 29.2 ± 7.8c | 29.2 ± 7.8c | 0.3 ± 0.1c | 1.2 ± 0.8b |
All values are means ± SDs. ANOVA used to compare means across countries resulted in significant differences (P < 0.001). Post hoc tests were performed with the use of a Scheffe adjustment. Values not sharing a common superscript letter are significantly different (P < 0.05), except for mean 25(OH)D2 in Jamaica and South Africa (P = 0.063) and mean 3-epimer-D3 in the Seychelles and United States (P = 0.282). 25(OH)D, 25-hydroxyvitamin D.
Sample size: n = 497, 502, 459, 497, and 494 for the United States, South Africa, Jamaica, Ghana, and Seychelles, respectively.
Sample size: n = 323, 452, 238, 490, and 419 for the United States, South Africa, Jamaica, Ghana, and Seychelles, respectively. Only values above the limit of detection (0.2 ng/mL) are reported.
Sample size: n = 333, 440, 458, 495, and 315 for the United States, South Africa, Jamaica, Ghana, and Seychelles, respectively. Only values above the limit of detection (0.5 ng/mL) are reported.
FIGURE 1.
Box plot of total serum BMI-adjusted 25-hydroxyvitamin D concentrations in the 5 study population samples from the Vitamin D Ancillary Study. The sample consisted of 497, 502, 459, 497, and 494 participants from the United States, South Africa, Jamaica, Ghana, and Seychelles, respectively. The bottom and top sides of the box represent the 25th and 75th percentiles, respectively, and the length of the box corresponds to the IQR. The upper whisker is the 75th percentile plus 1.5 times IQR, whereas the lower whisker is the 25th percentiles minus 1.5 times IQR.
FIGURE 2.
Frequency distribution of total serum BMI-adjusted 25-hydroxyvitamin D concentrations in the 5 study population samples from the Vitamin D Ancillary Study. The sample consisted of 497, 502, 459, 497, and 494 participants from the United States, South Africa, Jamaica, Ghana, and Seychelles, respectively.
Prevalence estimates for the risk of deficiency [25(OH)D <12 ng/mL], risk of inadequacy [12 ng/mL ≤25(OH)D <20 ng/mL], and sufficiency [25(OH)D ≥20 ng/mL], as well as prevalence of concentrations >30 ng/mL, are depicted in Table 3. There were no participants from Seychelles or Jamaica with values that would place them in the “at risk of deficiency” category. However, 0.2% of participants in Ghana, 6.6% in South Africa, and almost 30% in the United States were classified as “at risk.” The risk of inadequacy subgroup [12 ng/mL ≤25(OH)D <20 ng/mL] included 40% of African Americans and approximately one-third of South Africans. On the contrary, >90% of the participants from Ghana, Seychelles, and Jamaica had sufficient (≥20 ng/mL) concentrations of total 25(OH)D. The proportions of these populations below the Estimated Average Requirement were 0% in Seychelles, 0.8% in Ghana, and 2.8% in Jamaica; whereas, South Africa (19.1%) and the United States (50.9%) fared the worst. Pairwise comparisons of the prevalences across countries (adjusted for multiple comparisons) for each of the 4 serum total 25(OH)D subgroups resulted in statistical differences between the United States and South Africa and all other countries. Prevalences in Jamaica, Ghana, and Seychelles were not different between these 3 countries (P > 0.05 for pairwise comparisons) for risk of deficiency, risk of inadequacy, and sufficiency. However, for 25(OH)D ≥30 ng/mL, Ghana differed from Jamaica and Seychelles (P < 0.01).
TABLE 3.
Prevalence of at risk of deficiency [total 25(OH)D <12 ng/mL], at risk of inadequacy [12 ng/mL ≤ total 25(OH)D <20 ng/mL], sufficiency [total 25(OH)D ≥20 ng/mL], and total 25(OH)D ≥30 ng/mL of mean total 25(OH)D1
| Country (latitude) | At risk of deficiency | At risk of inadequacy | Sufficiency | 25(OH)D ≥30 ng/mL | Proportion below EAR2 |
| United States (41°N) | 28.8 | 39.8 | 31.4 | 7.4 | 50.9 |
| South Africa (34°S) | 6.6 | 28.5 | 65.9 | 21.5 | 19.1 |
| Jamaica (17°N) | 0.0 | 8.3 | 91.7 | 39.7 | 2.8 |
| Ghana (6°N) | 0.2 | 4.6 | 95.2 | 49.9 | 0.8 |
| Seychelles (4°S) | 0.0 | 8.1 | 91.9 | 39.5 | 0.0 |
The chi-square P value was <0.001 for all comparisons across countries. The Bonferroni-adjusted pairwise comparisons resulted in differences between the United States and South Africa and all of the other countries, whereas Jamaica, Ghana, and Seychelles did not differ between one another (P > 0.05 for pairwise comparisons) for risk of deficiency, risk of inadequacy, and sufficiency. For 25(OH)D ≥30 ng/mL, Ghana differed from Jamaica and Seychelles (P < 0.01). EAR, Estimated Average Requirement; 25(OH)D, 25-hydroxyvitamin D.
Proportion of the sample below the total 25(OH)D concentration corresponding to the EAR as defined by the Institute of Medicine, <16 ng/mL.
Melanin content (M-index) and skin redness (E-index, erythema) are summarized in Table 4. The M-index was ∼1.4 times higher in Ghana than in the United States (P < 0.001), whereas the E-index was essentially the same across the 2 sites. Correlation coefficients between total 25(OH)D and skin pigmentation variables (M- and E-indexes) were essentially zero.
TABLE 4.
M- and E-index values for a sample of participants in the United States (Chicago, IL) and Kumasi, Ghana, and Pearson correlation coefficients (95% CIs) for total 25(OH)D and skin pigmentation1
| M-index |
E-index |
||||
| Country (latitude) | n | Mean ± SD | r (95% CI)2 | Mean ± SD | r (95% CI)3 |
| Ghana (6°N) | 235 | 96.3 ± 10.9 | 0.03 (−0.09, 0.15) | 21.0 ± 4.0 | −0.06 (−0.17, 0.06) |
| United States (41°N) | 148 | 66.6 ± 15.1 | −0.08 (−0.23, 0.06) | 19.4 ± 6.4 | −0.01 (−0.09, 0.19) |
E-index, erythema index; M-index, melanin index; 25(OH)D, 25-hydroxyvitamin D.
Pearson correlation coefficients (95% bootstrap-based CIs) between total 25(OH)D and melanin content (M-index).
Pearson correlation coefficients (95% bootstrap-based CIs) between total 25(OH)D and skin redness or erythema (E-index).
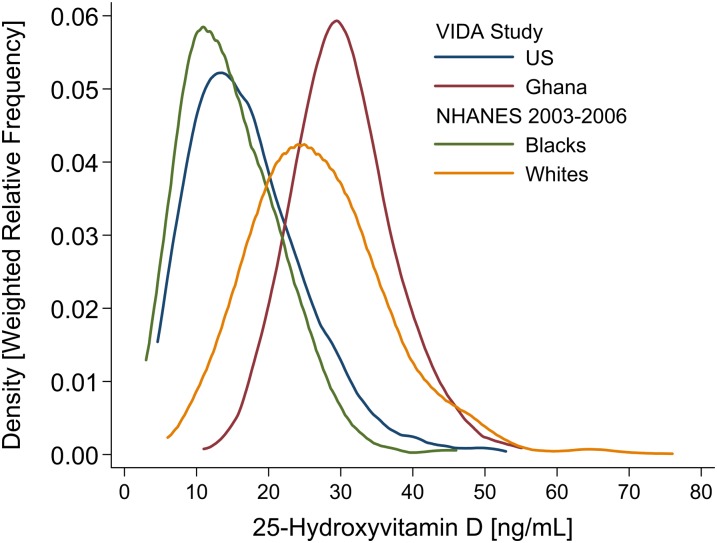
To provide a context for the interpretation of these data, Figure 3 includes the reported 25(OH)D concentrations from the African American and white samples enrolled in NHANES 2003–2006. Despite the fact that the assays were performed in different laboratories, the close correspondence of distributions between Ghanaians and US whites, on the one hand, and both samples of African Americans, on the other, was evident.
FIGURE 3.
Frequency distribution of total serum 25-hydroxyvitamin D concentrations in Chicago (US; 41°N) and Kumasi (Ghana; 6°N) participants from the VIDA Study and non-Hispanic black and non-Hispanic white participants in the NHANES 2003–2006 (37). The NHANES 2003–2006 sample included 776 non-Hispanic blacks and 1577 non-Hispanic whites, whereas each of the 2 countries in the VIDA study, United States and Ghana, included 497 participants. VIDA, Vitamin D Ancillary.
DISCUSSION
In an internally standardized international comparative study we showed that serum concentrations of 25(OH)D in dark-skinned individuals in the Western hemisphere and West, South, and East Africa follow precisely the pattern anticipated on the basis of latitude at the place of residence under the assumption that UV exposure is the primary determinant of circulating concentrations. Skin color was subject to strong positive selection in populations that migrated out of Africa to Europe and Asia in the period 40–80,000 bce, and the transition to lighter skin tone was driven by mutations in at least 2 independent sets of genes (21–23). The clinical correlation between average UV radiation at specified geographical regions and skin color has further been shown to be robust across virtually all populations at their ancestral location before European expansion (22). In our study, the African Americans and the South Africans outside the tropical zone had the lowest concentrations of serum 25(OH)D. The brief interval during which African-origin populations have lived in North America would not be expected to result in large-scale evolutionary change. Likewise, the downward migration of Bantu speakers from the Niger delta region of West Africa into present-day South Africa occurred only during 500–1000 bce, also too short of a time period for large-scale evolutionary change to have been observed. In a small preliminary study we previously confirmed that serum 25(OH)D concentrations among Nigerians were also very similar to those observed among white Americans (21).
It has been suggested in the case of bone health that compensatory mechanisms are brought to bear on vitamin D pathways in darker-skinned populations when 25(OH)D is low to preserve normal development (24). Whether similar plasticity exists in other physiologic pathways involving vitamin D is unknown. An important limitation of our study is the absence of data on the unbound, or bioavailable, fraction. A recent publication showed that, despite large variation in total 25(OH)D, the fraction that is bioavailable is similar in US blacks and whites (25), which may be the most important source of the hypothesized “plasticity.”
Given the evolutionary framework outlined above, the inference would seem unavoidable that the population distribution of 25(OH)D observed in Americans of European descent and Ghanaians represents the physiologic norm for the species. However, recently published data, as noted above, show that variation in vitamin D–binding protein accounts for most of the variation in total serum 25(OH)D between US blacks and whites, with the result that concentrations of bioavailable 25(OH)D are similar in the 2 groups (25). This finding would imply that the Ghanaian sample we studied also has higher concentrations of bound 25(OH)D because it is unlikely that an increase in the bioavailable compound alone could account for the higher total serum 25(OH)D concentrations seen in Ghana. It is not understood why concentrations of binding protein would vary by latitude. Furthermore, it is even more difficult to explain why evolutionary selection on skin color would have been driven by a positive survival advantage from “sufficient” 25(OH)D if the variation by latitude reflects only the bound form of the hormone. Additional studies of the concentrations of bioavailable 25(OH)D in tropical settings are required to clarify how the set point for bound and unbound 25(OH)D is regulated.
With the exception of studies focused on rickets in children and HIV, data on 25(OH)D in healthy African adult populations are sparse (26). A recent survey that included 5 small samples of rural population groups in East Africa found average 25(OH)D concentrations in adults [∼46 ng/mL (115 nmol/L) (27, 28); ie, ∼50% higher than our results from Ghana]. It is possible that the Masai and the other groups studied in East Africa were receiving more UV radiation to exposed body surfaces. Laboratory standardization of 25(OH)D assays is also an unsolved problem in this field and could account for the difference observed between these 2 African samples near the equator, along with sampling bias (29). Luxwolda et al (28) concluded that values in the range found in their study represent the level of sufficiency that “matches our Paleolithic genome.” It is possible that healthy adult populations in equatorial Africa have mean concentrations of 25(OH)D in the range of 30–45 ng/mL, as these data show; however, as we argued above, there are as yet no vitamin D–specific adverse outcomes that can be identified in populations such as African Americans with significantly lower mean values and their bioavailable compound is not lower than among light-skinned persons. Therefore, we conclude that it is premature to assert that concentrations in the range of 30–45 ng/mL are, in some way, more “genome appropriate” for humans.
Shriver and Parra (16) reported mean (±SD) M-index values of 56.6 ± 14.8 and 30.5 ± 2.8 for African Americans and European Americans, respectively. The corresponding mean (±SD) values for the E-index were 2.7 ± 5.1 and 6.6 ± 1.2. The dissimilarities observed between the mean E-index in our study and those reported by Shriver and Parra are likely attributable to instrument differences. We found no association between the degree of skin pigmentation and baseline 25(OH)D in the African American and Ghanaian samples. This finding may appear counterintuitive; however, detailed experimental studies of the relation between pigmentation and the response of 25(OH)D to UV radiation also found little correlation (30). Other observational epidemiologic studies showed no association between pigmentation and 25(OH)D concentrations (31), a negative correlation (32, 33), and even a positive effect of skin pigmentation on serum concentratons of 25(OH)D (34). Further large studies will be required to answer this question. It is possible that the variance in skin tone within these populations was insufficient to allow identification of a significant correlation with 25(OH)D, given the other sources of variation that were unaccounted for.
The complex metabolic actions of 25(OH)D makes it difficult to identify potential relations between variation in serum concentrations of this hormone and states of ill health in observational studies. Likewise, trials have consistently shown no benefit of supplementation, although admittedly these studies may not have accumulated enough person-years of observation (35). Obesity has been proposed as a potentially important consequence of low 25(OH)D; however, a recent study using Mendelian randomization and genetic markers that influence lifelong vitamin D concentrations suggested that low 25(OH)D is a consequence rather than a cause of obesity (36). As noted, we anticipate that determination of bioavailable concentrations of 25(OH)D in these populations will help resolve the paradox of similar mean concentrations of 25(OH)D in Europeans and Africans and the apparent tolerance for wide variation in mean concentrations among historically recent migrants such as African Americans. The data presented here set the background against which physiologic abnormalities or the role of secondary or compensatory mechanisms for lower 25(OH)D can be studied.
Acknowledgments
The authors’ responsibilities were as follows—RAD-A, AL, and RSC: designed the research and were responsible for the final content; RAD-A, AL, PB, TF, EVL, JP-R, BT, ANH, and LRD: conducted the research; and RAD-A: analyzed the data. All of the authors contributed to the writing of the manuscript. None of the authors had any conflicts of interest to report.
Footnotes
Abbreviations used: E-index, erythema index; IOM, Institute of Medicine; M-index, melanin index; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 2.Michos ED. Vitamin D deficiency and the risk of incident type 2 diabetes. Future Cardiol 2009;5:15–8. [DOI] [PubMed] [Google Scholar]
- 3.Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 2009;6:621–30. [DOI] [PubMed] [Google Scholar]
- 4.Reis AF, Hauache OM, Velho G. Vitamin D endocrine system and the genetic susceptibility to diabetes, obesity and vascular disease: a review of evidence. Diabetes Metab 2005;31:318–25. [DOI] [PubMed] [Google Scholar]
- 5.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross AC. The 2011 report on Dietary Reference Intakes for calcium and vitamin D. Public Health Nutr 2011;14:938–9. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 8.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001-2006. Hyattsville, MD: National Center for Health Statistics; 2011. (NCHS data brief no. 59.). [PubMed]
- 9.Luke A, Bovet P, Forrester TE, Lambert EV, Plange-Rhule J, Schoeller DA, Dugas LR, Durazo-Arvizu RA, Shoham D, Cooper RS, et al. Protocol for the Modeling the Epidemiologic Transition Study: a longitudinal observational study of energy balance and change in body weight, diabetes and cardiovascular disease risk. BMC Public Health 2011;11:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westerterp KR. Assessment of physical activity level in relation to obesity: current evidence and research issues. Med Sci Sports Exerc 1999;31(suppl):S522–5. [DOI] [PubMed] [Google Scholar]
- 11.Hoofnagle AN, Laha TJ, Donaldson TF. A rubber transfer gasket to improve the throughput of liquid-liquid extraction in 96-well plates: application to vitamin D testing. J Chromatogr B Analyt Technol Biomed Life Sci 2010;878:1639–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strathmann FG, Sadilkova K, Laha TJ, LeSourd SE, Bornhorst JA, Hoofnagle AN, Jack R. 3-Epi-25 hydroxyvitamin D concentrations are not correlated with age in a cohort of infants and adults. Clin Chim Acta 2012;413:203–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaton GH. Recommended Dietary Intakes: individuals and populations. In: Shils RE, Olson JA, Shike M, eds. Modern nutrition in health and disease. Philadelphia, PA: Lippincott Williams amp Wilkins, 1999:1705–25.
- 14.Carriquiry AL. Assessing the prevalence of nutrient inadequacy. Public Health Nutr 1999;2:23–33. [DOI] [PubMed] [Google Scholar]
- 15.Taylor CL, Carriquiry AL, Bailey RL, Sempos CT, Yetley EA. Appropriateness of the probability approach with a nutrient status biomarker to assess population inadequacy: a study using vitamin D. Am J Clin Nutr 2013;97:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shriver MD, Parra EJ. Comparison of narrow-band reflectance spectroscopy and tristimulus colorimetry for measurements of skin and hair color in persons of different biological ancestry. Am J Phys Anthropol 2000;112:17–27. [DOI] [PubMed] [Google Scholar]
- 17.Nagy KA, Girard IA, Brown TK. Energetics of free-ranging mammals, reptiles, and birds. Annu Rev Nutr 1999;19:247–77. [DOI] [PubMed] [Google Scholar]
- 18.Salgado-Ugarte IM, Shimizu M, Taniuchi T. snp6: Exploring the shape of univariate data using kernel density estimators. Stata Tech Bull 1993;16:8–19. [Google Scholar]
- 19.Haukoos JS, Lewis RJ. Advanced statistics: bootstrapping confidence intervals for statistics with “difficult” distributions. Acad Emerg Med 2005;12:360–5. [DOI] [PubMed] [Google Scholar]
- 20.Walsh JF, Reznikoff M. Bootstrapping: a tool for clinical research. J Clin Psychol 1990;46:928–30. [DOI] [PubMed] [Google Scholar]
- 21.Durazo-Arvizu RA, Aloia JF, Dugas LR, Tayo BO, Shoham DA, Bertino AM, Yeh JK, Cooper RS, Luke A. 25-Hydroxyvitamin D levels in African American and Nigerian women. Am J Hum Biol 2013;25:560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norton HL, Kittles RA, Parra E, McKeigue P, Mao X, Cheng K, Canfield VA, Bradley DG, McEvoy B, Shriver MD. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol Biol Evol 2007;24:710–22. [DOI] [PubMed] [Google Scholar]
- 23.Rogers AR, Iltis D, Wooding S. Genetic variation at the MC1R locus and the time since loss of human body hair. Curr Anthropol 2004;45:105–8. [Google Scholar]
- 24.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr 2008;88(suppl):545S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013;369:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahl DA, Cooper C, Ebeling PR, Eggersdorfer M, Hilger J, Hoffmann K, Josse R, Kanis JA, Mithal A, Pierroz DD, et al. A global representation of vitamin D status in healthy populations. Arch Osteoporos. 2012;7:155–72. [DOI] [PubMed] [Google Scholar]
- 27.Luxwolda MF, Kuipers RS, Kema IP, Dijck-Brouwer DA, Muskiet FA. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr 2012;108:1557–61. [DOI] [PubMed] [Google Scholar]
- 28.Luxwolda MF, Kuipers RS, Kema IP, van der Veer E, Dijck-Brouwer DA, Muskiet FA. Vitamin D status indicators in indigenous populations in East Africa. Eur J Nutr 2013;52:1115–25. [DOI] [PubMed] [Google Scholar]
- 29.Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM, Vitamin DSP. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl 2012;243:32–40. [DOI] [PubMed] [Google Scholar]
- 30.Bogh MK, Schmedes AV, Philipsen PA, Thieden E, Wulf HC. Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation. J Invest Dermatol 2010;130:546–53. [DOI] [PubMed] [Google Scholar]
- 31.Ginter JK, Krithika S, Gozdzik A, Hanwell H, Whiting S, Parra EJ. Vitamin D status of older adults of diverse ancestry living in the greater Toronto area. BMC Geriatr 2013;13:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gozdzik A, Barta JL, Wu H, Wagner D, Cole DE, Vieth R, Whiting S, Parra EJ. Low wintertime vitamin D levels in a sample of healthy young adults of diverse ancestry living in the Toronto area: associations with vitamin D intake and skin pigmentation. BMC Public Health 2008;8:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Libon F, Cavalier E, Nikkels AF. Skin color is relevant to vitamin d synthesis. Dermatology 2013;227:250–4. [DOI] [PubMed] [Google Scholar]
- 34.Glass D, Lens M, Swaminathan R, Spector TD, Bataille V. Pigmentation and vitamin D metabolism in Caucasians: low vitamin D serum levels in fair skin types in the UK. PLoS ONE 2009;4:e6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jorde R, Strand Hutchinson M, Kjaergaard M, Sneve M, Grimnes G. Supplementation with high doses of vitamin D to subjects without vitamin D deficiency may have negative effects: pooled data from four intervention trials in Tromso. ISRN Endocrinol 2013;2013:348705. [DOI] [PMC free article] [PubMed]
- 36.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, Cooper JD, Dastani Z, Li R, Houston DK, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 2013;10:e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey data (NHANES). Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2003–2006. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2003-4/L06VID_C.htm and http://www.cdc.gov/nchs/nhanes/nhanes5-6/VID_D.htm (cited 8 January 2013).