Abstract
Background: Recent posttrial analysis of a completed randomized trial found an increased risk of prostate cancer among healthy men taking high-dose vitamin E supplements. Trials that examined the effect of vitamin C supplements on cancer risk are few.
Objective: We examined whether vitamin E or vitamin C supplementation affects the risk of cancer events during posttrial follow-up of the Physicians’ Health Study II.
Design: Beginning in 1997, a total of 14,641 US male physicians aged ≥50 y were randomly assigned to receive 400 IU of vitamin E every other day, 500 mg of vitamin C daily, or their respective placebos. The vitamin E and vitamin C treatment ended in 2007, and observational follow-up continued through June 2011.
Results: This study included an additional 356 cases of incident prostate cancer and 771 total cancers that developed during a mean (maximum) of 2.8 (3.8) y of posttrial observation. During an overall mean of 10.3 (13.8) y, there were a total of 1373 incident prostate cancers and 2669 total cancers documented. In comparison with placebo, vitamin E supplementation had no effect on the incidence of prostate cancer (HR: 0.99; 95% CI: 0.89, 1.10) or total cancers (HR: 1.02; 95% CI: 0.95, 1.10). There was also no effect of vitamin C supplementation on total cancers (HR: 1.02; 95% CI: 0.94, 1.10) or incident prostate cancer (HR: 1.03; 95% CI: 0.93, 1.15). Neither vitamin E nor vitamin C supplementation had effects on other site-specific cancers overall. Stratification by known cancer risk factors, history of cancer, other randomized treatment, and follow-up time showed no significant interactions.
Conclusion: In this large-scale randomized trial in men, vitamin E and C supplementation had no immediate or long-term effects on the risk of total cancers, prostate cancer, or other site-specific cancers. This trial was registered at clinicaltrials.gov as NCT00270647.
INTRODUCTION
Antioxidant micronutrients such as vitamin E and vitamin C are hypothesized to protect against carcinogenesis (1, 2). Nevertheless, randomized trials of vitamin supplements have provided little evidence for major effects of high-dose vitamin E on the risk of total or site-specific cancers (3–8), except for one trial that suggested a possible benefit for prostate (9) and colorectal (10) cancers. Vitamin C is less well studied in randomized trials, with current evidence showing neither benefit nor harm for total or site-specific cancers (5, 7).
Cancer is a multistage process that takes years to develop and progress (11, 12). Both carcinogenic and potential chemopreventive factors require a long exposure period for the effects to emerge. However, randomized trials typically have limited duration of intervention because of cost constraints and challenges of long-term adherence. As a result, a latent effect of treatment may not be detected during the intervention period but could appear with additional posttrial follow-up. Of note, the Selenium and Vitamin E Cancer Prevention Trial (SELECT)5, which examined the effect of selenium and vitamin E on prostate cancer prevention, initially reported that the HRs of prostate cancer were 1.13 for vitamin E, 1.04 for selenium, and 1.05 for vitamin E plus selenium supplementation (all P > 0.05) over a median treatment and follow-up of 5.5 y (4). A recent study in the SELECT added 54,464 person-years of posttrial follow-up and found a significant increase in the risk of prostate cancer with vitamin E supplementation (HR: 1.17; 99% CI: 1.004, 1.36) (13). This finding raised questions about long-term safety of vitamin E supplement use.
The Physicians’ Health Study (PHS) II was a randomized trial that examined, in part, the effects of vitamin E and vitamin C supplementation on total and prostate cancer in men (14). The primary report of the trial period showed no effect of vitamin E or C supplementation on total or site-specific cancers during a mean treatment and follow-up of 8 y (5). Since the end of vitamin E and C components on 31 August 2007, follow-up of the PHS II continued in parallel with the recently completed multivitamin component (15). In this report, we extend analyses to posttrial follow-up and further examine the effects of vitamin E and C supplementation on the risk of total, prostate, and other site-specific cancers.
SUBJECTS AND METHODS
Study design
The PHS II was a randomized, double-blind, placebo-controlled 2 × 2 × 2 × 2 factorial trial evaluating the risks and benefits of vitamin E (400 IU synthetic α-tocopherol on alternate days; BASF Corporation), vitamin C (500 mg synthetic ascorbic acid daily; BASF Corporation), and a multivitamin (Centrum Silver daily; Wyeth Pharmaceuticals) in the prevention of cancer and cardiovascular disease among 14,641 male physicians aged ≥50 y. Vitamin E and C components ended as scheduled on 31 August 2007. The multivitamin component ended as scheduled on 1 June 2011. A fourth randomized component, β-carotene (50 mg Lurotin on alternate days; BASF Corporation), ended as scheduled in 2003.
The design of the PHS II has been described previously (14). In brief, recruitment, enrollment, and randomization of men into the PHS II occurred in 2 phases. First, starting in July 1997, participants in the PHS I (16, 17) were invited to participate in the PHS II. Men had to be willing to forgo any use of multivitamins or individual supplements containing ≥100% of the recommended daily allowance of vitamins E, C, or A or β-carotene during the course of the trial. Men with a history of cancer, myocardial infarction, or stroke remained eligible to enroll. Of 18,763 PHS I participants invited, 7641 (41%) willing and eligible men were randomly assigned and retained their original β-carotene treatment assignment. Second, invitational letters and baseline questionnaires were mailed to 254,597 US male physicians aged ≥50 y identified from a mailing list provided by the American Medical Association. Between July 1999 and July 2001, 42,165 men completed a baseline questionnaire, of which 11,128 were willing and eligible to participate in the PHS II. After a placebo run-in period, 7000 willing and eligible men (63%) who took at least two-thirds of the run-in pills were randomly assigned.
In total, 14,641 men were randomly assigned into the PHS II in blocks of 16 and stratified by age, previous diagnosis of cancer, previous diagnosis of cardiovascular disease, and, for the 7641 PHS I participants, by their original β-carotene treatment assignment. Men were randomly assigned to receive vitamin E, vitamin C, β-carotene, multivitamin, or their respective placebos. All participants provided written informed consent. The institutional review board at Brigham and Women's Hospital approved the research protocol.
Study treatment, follow-up, and confirmation of endpoints
Study treatment and follow-up were reported previously (5). Every 6 mo in the first year and annually thereafter, the participants received monthly calendar packs containing the study interventions and questionnaires asking about adherence to the assigned regimen, potential adverse events, occurrence of new endpoints, and updated risk factors. Follow-up and validation of reported cancer endpoints continued by using the same methods during the vitamin E and C intervention (through August 2007) and the multivitamin intervention (through June 2011). Morbidity and mortality follow-ups were 98.2% and 99.9% complete, respectively.
For each reported cancer endpoint, we obtained relevant medical records after the participant provided consent. Records were reviewed by the Endpoints Committee of physicians blinded to the treatment assignment. The vast majority (96.9%) of cancers were confirmed with pathology or cytology reports. Rarely, a reported cancer was confirmed on the basis of strong clinical and radiologic or laboratory evidence. Mortality was confirmed by the Endpoints Committee or by obtaining a death certificate. A National Death Index search was performed for the participants with unknown vital status.
For the vitamin E component, the primary endpoint was prostate cancer, with total cancers (excluding nonmelanoma skin cancer) and colorectal cancer as prespecified secondary endpoints. For the vitamin C component, total cancers was the primary endpoint. Other site-specific cancers and cancer mortality were also assessed and confirmed.
Statistical analyses
All analyses were conducted on the basis of an intention-to treat principle, in which the participants were classified according to their treatment assignments and followed up until occurrence of a confirmed cancer endpoint, death, or 1 June 2011, whichever came first. SAS version 9.1 (SAS Institute) was used for all analyses, with significance set at P < 0.05 for 2-sided tests.
We first compared participant baseline characteristics by treatment assignment. We then used Cox proportional hazards models to calculate HRs and 95% CIs of cancer in treatment groups compared with their respective placebo groups. The proportional hazards assumption was tested by modeling interaction terms with the logarithm of time and was not violated (P > 0.05). For each prespecified endpoint, models were stratified on the presence of cancer at randomization and adjusted for the following study design variables: age, PHS cohort (PHS I participants, new participants), and other randomized assignments. For analyses of total cancers, all new cancers were included, regardless of whether or not the participant had a history of cancer at baseline. For analyses of each site-specific cancer, the participants were excluded if they had a history of cancer of the same site at baseline. For analyses of total mortality, any cancer mortality, and site-specific cancer mortality, we included all participants. Analyses were repeated for 3 time periods: intervention, posttrial observation, and overall. For analyses of site-specific cancers during posttrial observation, the participants were excluded if they developed cancer of the same site that was confirmed or unrefuted by the end of intervention. To explore possible effect modifiers for vitamins E and C, we stratified analyses by known cancer risk factors (age, BMI, smoking status, exercise, alcohol consumption, aspirin use, parental history of cancer), history of cancer, other randomized treatment, and follow-up time and assessed the interaction by using product terms between subgroup indicators and treatment assignment.
RESULTS
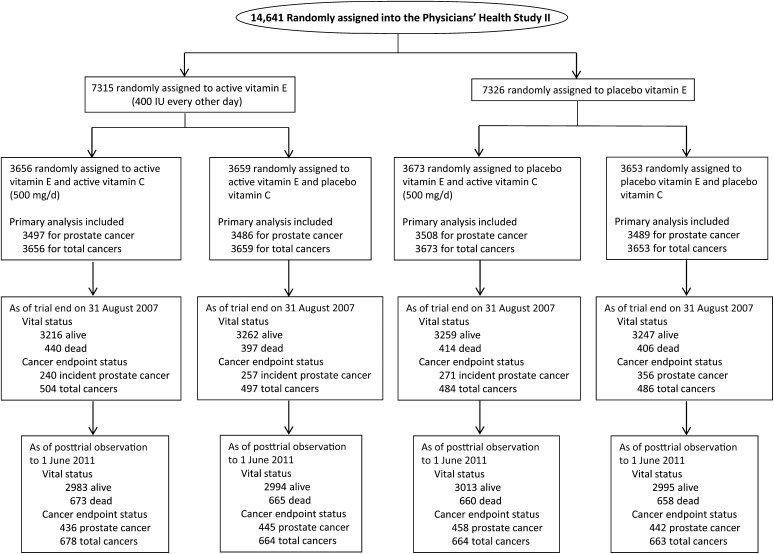
The 14,641 men who were randomly assigned into the PHS II had a mean (±SD) age of 64.3 ± 9.2 y. All baseline characteristics were equally distributed between the vitamin E or vitamin C groups and their placebo groups (all P > 0.05) (Table 1). Active vitamin E and C treatments lasted for a mean (maximum) of 7.6 (10.0) y, and posttrial observation continued for a mean of 2.8 (3.8) additional years. The numbers of confirmed posttrial cancers were 771 for total cancers, 356 for incident prostate cancer, 46 for incident colorectal cancer, and 53 for incident lung cancer. The flow diagram of the PHS II from randomization through the end of intervention and posttrial observation is shown in Figure 1.
TABLE 1.
Baseline characteristics according to vitamin E and vitamin C treatment assignment in 14,641 men from the Physicians’ Health Study II1
| Vitamin E |
Vitamin C |
|||
| Self-reported baseline characteristics | Active (n = 7315) | Placebo (n = 7326) | Active (n = 7329) | Placebo (n = 7312) |
| Age (y) | 64.2 ± 9.12 | 64.3 ± 9.2 | 64.3 ± 9.2 | 64.3 ± 9.1 |
| Age [n (%)] | ||||
| 50–59 y | 2940 (40.2) | 2951 (40.3) | 2953 (40.3) | 2938 (40.2) |
| 60–69 y | 2349 (32.1) | 2346 (32.0) | 2347 (32.0) | 2348 (32.1) |
| ≥70 y | 2026 (27.7) | 2029 (27.7) | 2029 (27.7) | 2026 (27.7) |
| BMI (kg/m2) | 26.0 ± 3.6 | 26.0 ± 3.7 | 26.0 ± 3.7 | 26.0 ± 3.7 |
| Cigarette smoking [n (%)] | ||||
| Never | 4104 (56.1) | 4148 (56.7) | 4135 (56.5) | 4117 (56.4) |
| Former | 2967 (40.6) | 2885 (39.4) | 2908 (39.7) | 2944 (40.3) |
| Current | 239 (3.3) | 285 (3.9) | 280 (3.8) | 244 (3.3) |
| Exercise ≥1 time/wk [n (%)] | ||||
| No | 2739 (38.4) | 2766 (38.7) | 2759 (38.5) | 2746 (38.6) |
| Yes | 4389 (61.6) | 4383 (61.3) | 4408 (61.5) | 4364 (61.4) |
| Alcohol consumption [n (%)] | ||||
| Rarely/never | 1372 (18.9) | 1358 (18.7) | 1364 (18.7) | 1366 (18.8) |
| ≥1 drink/mo | 5893 (81.1) | 5923 (81.4) | 5920 (81.3) | 5896 (81.2) |
| Current aspirin use [n (%)] | ||||
| No | 1627 (22.6) | 1634 (22.6) | 1638 (22.6) | 1623 (22.6) |
| Yes | 5578 (77.4) | 5589 (77.4) | 5605 (77.4) | 5562 (77.4) |
| Parental history of cancers3 [n (%)] | ||||
| No | 2906 (46.5) | 2931 (46.5) | 2927 (46.5) | 2910 (46.5) |
| Yes | 3344 (53.5) | 3377 (53.5) | 3371 (53.5) | 3350 (53.5) |
| Parental history of prostate cancer3 [n (%)] | ||||
| No | 5713 (89.6) | 5792 (89.8) | 5755 (89.5) | 5750 (89.9) |
| Yes | 663 (10.4) | 661 (10.2) | 678 (10.5) | 646 (10.1) |
| Parental history of colorectal cancer3 [n (%)] | ||||
| No | 5492 (88.0) | 5552 (88.5) | 5535 (88.5) | 5509 (88.1) |
| Yes | 748 (12.0) | 719 (11.5) | 721 (11.5) | 746 (11.9) |
| Self-reported history of cancers3 [n (%)] | ||||
| No | 6678 (91.3) | 6689 (91.3) | 6691 (91.3) | 6676 (91.3) |
| Yes | 637 (8.7) | 637 (8.7) | 638 (8.7) | 636 (8.7) |
| Self-reported history of prostate cancer [n (%)] | ||||
| No | 6983 (95.5) | 6997 (95.5) | 7005 (95.6) | 6975 (95.4) |
| Yes | 332 (4.5) | 329 (4.5) | 324 (4.4) | 337 (4.6) |
| Self-reported history of colorectal cancer [n (%)] | ||||
| No | 7252 (99.1) | 7267 (99.2) | 7270 (99.2) | 7249 (99.1) |
| Yes | 63 (0.9) | 59 (0.8) | 59 (0.8) | 63 (0.9) |
The numbers of subgroups do not always sum to totals because of missing information for some variables. P > 0.05 for all comparisons between active and placebo groups of vitamin E and vitamin C, with chi-square tests used for categorical variables and t tests used for continuous variables.
Mean ± SD (all such values).
Excludes men with missing information on parental history of cancer, prostate cancer, and colorectal cancer.
FIGURE 1.
Flow diagram of participants from random assignment through the end of the vitamin E and vitamin C interventions and posttrial observation in the Physicians’ Health Study II.
Vitamin E and cancer
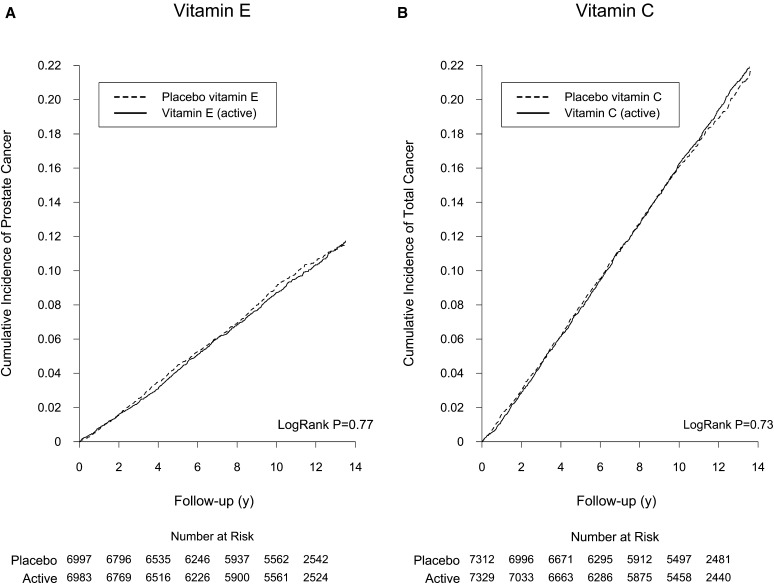
During a mean (maximum) of 10.3 (13.8) y total follow-up, there were 680 incident prostate cancers (9.10 per 1000 person-years) in the active vitamin E group and 693 (9.25 per 1000 person-years) in the placebo group. Compared with placebo, vitamin E had no effect on the incidence of prostate cancer during the intervention (HR: 0.96; 95% CI: 0.85, 1.09), during posttrial observation (HR: 1.06; 95% CI: 0.86, 1.30), or overall (HR: 0.99; 95% CI: 0.89, 1.10) (Table 2). The cumulative incidence curves indicate the lack of effect over the intervention as well as the posttrial observation period (overall crude log-rank P = 0.77) (Figure 2A). For total cancers, the overall rates were 17.8 and 17.5 per 1000 person-years in the active and placebo vitamin E groups, respectively. There was no effect of vitamin E on total cancer risk during the intervention (HR: 1.04; 95% CI: 0.96, 1.14), during posttrial observation (HR: 0.98; 95% CI: 0.85, 1.13), or overall (HR: 1.02; 95% CI: 0.95, 1.10). We also found no effect of vitamin E on any site-specific cancers, including colorectal (overall HR: 0.92), lung (HR: 0.92), bladder (HR: 1.15), and pancreatic (HR: 1.22) cancer, and total cancer mortality (see Supplemental Figure 1 under “Supplemental data” in the online issue) and site-specific cancer mortality (all P > 0.05). We found no significant effect modification of vitamin E on prostate cancer and total cancers by cancer risk factors, other PHS II randomized treatment, or periods of follow-up (all P-interaction > 0.05) (see Supplemental Table 1 under “Supplemental data” in the online issue).
TABLE 2.
Association between randomly assigned vitamin E supplementation and the risks of total cancers, site-specific cancers, and mortality in the PHS II1
| Intervention |
Posttrial observation3 |
Overall4 |
|||||
| Outcome | No. in analysis2 | Active/placebo | HR (95% CI) | Active/placebo | HR (95% CI) | Active/placebo | HR (95% CI) |
| No. of events | No. of events | No. of events | |||||
| Total cancers | 14,641 | 1001/970 | 1.04 (0.96, 1.14) | 381/390 | 0.98 (0.85, 1.13) | 1342/1327 | 1.02 (0.95, 1.10) |
| Total epithelial cell cancers5 | 14,641 | 892/887 | 1.02 (0.93, 1.12) | 344/347 | 1.00 (0.86, 1.16) | 1198/1204 | 1.01 (0.93, 1.09) |
| Prostate cancer | 13,980 | 497/520 | 0.96 (0.85, 1.09) | 183/173 | 1.06 (0.86, 1.30) | 680/693 | 0.99 (0.89, 1.10) |
| Prostate cancer deaths | 14,641 | 40/40 | 1.03 (0.66, 1.60) | 37/31 | 1.27 (0.78, 2.04) | 77/71 | 1.13 (0.82, 1.56) |
| Colorectal cancer | 14,519 | 76/88 | 0.88 (0.64, 1.19) | 24/22 | 1.11 (0.62, 1.97) | 100/110 | 0.92 (0.70, 1.21) |
| Colorectal cancer deaths | 14,641 | 22/33 | 0.69 (0.40, 1.18) | 13/8 | 1.67 (0.69, 4.03) | 35/41 | 0.88 (0.56, 1.38) |
| Lung cancer | 14,610 | 50/59 | 0.86 (0.59, 1.26) | 27/26 | 1.06 (0.62, 1.82) | 77/85 | 0.92 (0.68, 1.26) |
| Lung cancer deaths | 14,641 | 46/48 | 0.98 (0.65, 1.47) | 22/22 | 1.04 (0.57, 1.87) | 68/70 | 1.00 (0.71, 1.39) |
| Bladder cancer | 14,570 | 38/33 | 1.16 (0.73, 1.85) | 14/13 | 1.10 (0.52, 2.34) | 52/46 | 1.15 (0.77, 1.70) |
| Bladder cancer deaths | 14,641 | 9/14 | 0.66 (0.28, 1.52) | 5/5 | 1.08 (0.31, 3.75) | 14/19 | 0.76 (0.38, 1.52) |
| Pancreatic cancer | 14,638 | 32/25 | 1.30 (0.77, 2.20) | 11/11 | 1.02 (0.44, 2.35) | 43/36 | 1.22 (0.78, 1.90) |
| Pancreatic cancer deaths | 14,641 | 31/23 | 1.38 (0.80, 2.36) | 11/13 | 0.87 (0.39, 1.93) | 42/36 | 1.20 (0.77, 1.87) |
| Lymphoma | 14,595 | 74/61 | 1.23 (0.88, 1.73) | 22/31 | 0.72 (0.41, 1.24) | 96/92 | 1.06 (0.79, 1.41) |
| Lymphoma deaths | 14,641 | 25/21 | 1.23 (0.69, 2.20) | 9/16 | 0.58 (0.26, 1.31) | 34/37 | 0.95 (0.60, 1.51) |
| Leukemia | 14,612 | 46/34 | 1.38 (0.88, 2.14) | 15/15 | 1.01 (0.50, 2.07) | 61/49 | 1.26 (0.87, 1.84) |
| Leukemia deaths | 14,641 | 22/12 | 1.87 (0.93, 3.79) | 12/13 | 0.95 (0.43, 2.08) | 34/25 | 1.38 (0.83, 2.32) |
| Melanoma | 14,483 | 75/63 | 1.20 (0.86, 1.68) | 32/34 | 0.95 (0.59, 1.54) | 107/97 | 1.11 (0.85, 1.46) |
| Melanoma deaths | 14,641 | 7/7 | 1.04 (0.36, 2.95) | 3/2 | 1.57 (0.26, 9.40) | 10/9 | 1.15 (0.47, 2.83) |
| Total mortality | 14,641 | 843/823 | 1.07 (0.97, 1.18) | 540/551 | 1.03 (0.92, 1.16) | 1383/1374 | 1.06 (0.98, 1.14) |
| Cancer mortality | 14,641 | 295/265 | 1.14 (0.97, 1.35) | 151/148 | 1.05 (0.84, 1.32) | 446/413 | 1.11 (0.97, 1.27) |
HRs (95% CIs) were obtained from Cox proportional hazards models that adjusted for age, the PHS cohort (original PHS I participant, new PHS II participant), and randomized treatment assignment (β-carotene, multivitamin, and either vitamin E or vitamin C) and stratified on baseline cancer. PHS, Physicians’ Health Study.
For total cancers, site-specific mortality, total mortality, and cancer mortality, analyses included all 14,641 participants. For the incidence of site-specific cancers, analyses were restricted to men without that site-specific cancer at baseline.
For analyses of site-specific cancers during posttrial observation, participants were excluded if they developed cancer of the same site that was confirmed or unrefuted by the end of intervention.
The sum of the number of total cancers during the intervention and posttrial observation periods may exceed the number of total cancers overall because of multiple cancers in the same individual.
Epithelial cell cancers were limited to carcinomas, which included all cancers except for lymphoma and leukemia.
FIGURE 2.
Cumulative incidence rate of cancer by random assignment during overall follow-up (active intervention plus posttrial observation) in the Physicians’ Health Study II. A: Cumulative incidence rate of prostate cancer by randomized vitamin E assignment. B: Cumulative incidence rate of total cancer by randomized vitamin C assignment.
Vitamin C and cancer
During the total follow-up period, there were 1342 confirmed total cancers (17.8 per 1000 person-years) in the active vitamin C group and 1327 (17.5 per 1000 person-years) in the placebo group. There was no effect of vitamin C on the risk of total cancers during the intervention (HR: 1.01; 95% CI: 0.92, 1.10), during posttrial observation (HR: 1.05; 95% CI: 0.91, 1.21), or overall (HR: 1.02; 95% CI: 0.94, 1.10) (Table 3). The cumulative incidence curves showed no difference in total cancer risk between vitamin C treatment groups during the intervention and posttrial observation (overall crude log-rank P = 0.73) (Figure 2B). There was a marginally significant reduction in incidence of colorectal cancer in the active vitamin C group compared with the placebo group during the posttrial observation (n = 46; HR: 0.54; 95% CI: 0.29, 0.99; P = 0.045), but the risk reduction was not significant during the intervention (HR: 0.88; 95% CI: 0.65, 1.20) or overall (HR: 0.79; 95% CI: 0.61, 1.04) (see Supplemental Figure 2 under “Supplemental data” in the online issue). Vitamin C had no effect on other site-specific cancers during all time periods, including prostate (overall HR: 1.03), lung (HR: 1.00), bladder (HR: 1.05), and pancreatic (HR: 0.89) cancers (all P > 0.05). In addition, vitamin C had no effect on total cancer mortality or site-specific cancer mortality. We also found no effect modification of vitamin C on total cancers by cancer risk factors, other randomized treatment, or periods of follow-up time (all P-interaction > 0.05) (see Supplemental Table 2 under “Supplemental data” in the online issue).
TABLE 3.
Association between randomized vitamin C supplementation and the risks of total cancers, site-specific cancers, and mortality in the PHS II1
| Intervention |
Posttrial observation3 |
Overall4 |
|||||
| Outcome | No. in analysis2 | Active/placebo | HR (95% CI) | Active/placebo | HR (95% CI) | Active/placebo | HR (95% CI) |
| No. of events | No. of events | No. of events | |||||
| Total cancers | 14,641 | 988/983 | 1.01 (0.92, 1.10) | 394/377 | 1.05 (0.91, 1.21) | 1342/1327 | 1.02 (0.94, 1.10) |
| Total epithelial cell cancers5 | 14,641 | 887/892 | 1.00 (0.91, 1.10) | 353/338 | 1.05 (0.90, 1.22) | 1203/1199 | 1.01 (0.93, 1.09) |
| Prostate cancer | 13,980 | 511/506 | 1.01 (0.89, 1.14) | 186/170 | 1.09 (0.89, 1.35) | 697/676 | 1.03 (0.93, 1.15) |
| Prostate cancer deaths | 14,641 | 47/33 | 1.40 (0.90, 2.19) | 33/35 | 0.95 (0.59, 1.53) | 80/68 | 1.17 (0.84, 1.61) |
| Colorectal cancer | 14,519 | 77/87 | 0.88 (0.65, 1.20) | 16/30 | 0.54 (0.29, 0.99) | 93/117 | 0.79 (0.61, 1.04) |
| Colorectal cancer deaths | 14,641 | 28/27 | 1.03 (0.61, 1.76) | 6/15 | 0.40 (0.16, 1.04) | 34/42 | 0.81 (0.52, 1.27) |
| Lung cancer | 14,610 | 53/56 | 0.95 (0.65, 1.38) | 28/25 | 1.12 (0.66, 1.93) | 81/81 | 1.00 (0.74, 1.36) |
| Lung cancer deaths | 14,641 | 41/53 | 0.77 (0.52, 1.16) | 25/19 | 1.32 (0.73, 2.40) | 66/72 | 0.92 (0.66, 1.28) |
| Bladder cancer | 14,570 | 33/38 | 0.87 (0.55, 1.39) | 17/10 | 1.71 (0.78, 3.73) | 50/48 | 1.05 (0.70, 1.56) |
| Bladder cancer deaths | 14,641 | 10/13 | 0.76 (0.33, 1.74) | 4/6 | 0.67 (0.19, 2.36) | 14/19 | 0.73 (0.37, 1.46) |
| Pancreatic cancer | 14,638 | 28/29 | 0.97 (0.58, 1.63) | 9/13 | 0.70 (0.30, 1.64) | 37/42 | 0.89 (0.57, 1.38) |
| Pancreatic cancer deaths | 14,641 | 25/29 | 0.87 (0.51, 1.49) | 8/16 | 0.50 (0.22, 1.18) | 33/45 | 0.74 (0.47, 1.16) |
| Lymphoma | 14,595 | 70/65 | 1.08 (0.77, 1.52) | 27/26 | 1.04 (0.61, 1.78) | 97/91 | 1.07 (0.80, 1.42) |
| Lymphoma deaths | 14,641 | 21/25 | 0.84 (0.47, 1.51) | 12/13 | 0.92 (0.42, 2.02) | 33/38 | 0.87 (0.55, 1.39) |
| Leukemia | 14,612 | 44/36 | 1.23 (0.79, 1.91) | 15/15 | 1.01 (0.49, 2.06) | 59/51 | 1.16 (0.80, 1.69) |
| Leukemia deaths | 14,641 | 18/16 | 1.13 (0.58, 2.21) | 11/14 | 0.79 (0.36, 1.74) | 29/30 | 0.97 (0.58, 1.61) |
| Melanoma | 14,483 | 63/75 | 0.84 (0.60, 1.18) | 35/31 | 1.13 (0.70, 1.83) | 98/106 | 0.93 (0.70, 1.22) |
| Melanoma deaths | 14,641 | 10/4 | 2.50 (0.78, 7.96) | 3/2 | 1.52 (0.25, 9.10) | 13/6 | 2.17 (0.83, 5.72) |
| Total mortality | 14,641 | 858/808 | 1.06 (0.97, 1.17) | 526/565 | 0.93 (0.83, 1.05) | 1384/1373 | 1.01 (0.94, 1.09) |
| Cancer mortality | 14,641 | 286/274 | 1.04 (0.89, 1.23) | 142/157 | 0.91 (0.73, 1.14) | 428/431 | 1.00 (0.87, 1.14) |
HRs (95% CIs) were obtained from Cox proportional hazards models that adjusted for age, the PHS cohort (original PHS I participant, new PHS II participant), and randomized treatment assignment (β-carotene, multivitamin, and either vitamin E or vitamin C) and stratified on baseline cancer. PHS, Physicians’ Health Study.
For total cancers, site-specific mortality, total mortality, and cancer mortality, analyses included all 14,641 participants. For the incidence of site-specific cancers, analyses were restricted to men without that site-specific cancer at baseline.
For analyses of site-specific cancers during posttrial observation, participants were excluded if they developed cancer of the same site that was confirmed or unrefuted by the end of intervention.
The sum of the number of total cancers during the intervention and posttrial observation periods may exceed the number of total cancers overall because of multiple cancers in the same individual.
Epithelial cell cancers were limited to carcinomas, which included all cancers except for lymphoma and leukemia.
Finally, we conducted 2 sensitivity analyses. First, we censored participants at the last date of follow-up. Second, we examined site-specific cancers during posttrial observation by excluding only participants who developed confirmed cancer of the same site by the end of the intervention. The results did not materially change (see Supplemental Tables 3–5 under “Supplemental data” in the online issue).
DISCUSSION
In this analysis of PHS II with an additional 3.8 y of posttrial observation beyond 8 y of intervention, there remained no effect of vitamin E or C supplementation on prostate cancer, total cancers, or other cancer endpoints. We also did not find modification of the treatment effect by participant baseline characteristics, concurrent randomized treatment, or duration of follow-up time. Our findings contrast with those from the SELECT, which reported an increased risk of prostate cancer in men randomly assigned to receive vitamin E supplementation that emerged during posttrial follow-up.
Given the widespread use of vitamin supplements in the United States (18), knowledge of their benefits and risks remains of paramount public health importance. Cancer development is a prolonged process (11, 12). Carcinogenic factors such as smoking cause residual excess risk of lung and other cancers decades after cessation. Potential chemopreventive factors such as aspirin show similar latent beneficial effect (19, 20). The plausible latent effect provides an alternative explanation for discrepant findings from observational studies and randomized trials, in which the former usually examine exposures lasting years or even a lifetime whereas the latter evaluate intervention effects during relatively short period of time. Because of the long developing period for many cancers, continued posttrial follow-up is necessary in cancer prevention trials to assess long-term treatment effects, either beneficial or harmful, that may emerge after the intervention ends.
The hypothesis that high intake of vitamin E may lower the risk of cancer was initially suggested by observations from epidemiologic studies (21, 22) and also supported by laboratory study findings (2, 23). Evaluation of the chemopreventive potential of vitamin E in randomized trials obtained largely null results. Early trials (24, 25) found that antioxidant combinations including vitamin E significantly reduced cancer incidence, particularly in men. However, the effect of each individual agent in the combined supplement could not be separated in these trials. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) study examined α-tocopherol (50 mg/d) and β-carotene (20 mg/d) on lung cancer risk in 29,133 Finnish male smokers (26). After 6.1 y of treatment, vitamin E supplementation had no effect on the primary endpoint of lung cancer (27) but was associated with a significant 34% reduction in the secondary endpoint of prostate cancer (9). The beneficial effect appeared ∼18 mo after the start of the treatment and was substantially attenuated during posttrial follow-up (28). A nonsignificant reduction in colorectal cancer with vitamin E supplementation was also found in the ATBC study (10). In contrast to the ATBC trial, the Heart Outcomes Protection Evaluation (HOPE) and HOPE–The Ongoing Outcomes trials found no effect of long-term vitamin E supplementation of 400 IU/d on cancer in 9541 UK men aged 40–80 y with vascular disease or diabetes (3). Over 7 y of follow-up, the RRs of total cancers, prostate cancer, and cancer deaths in the vitamin E group compared with the placebo group were 0.94, 0.98, and 0.88, respectively (all P > 0.05) (8). Two trials in US women, the Women's Health Study (6) and the Women's Antioxidant Cardiovascular Study (WACS) (7), also found no effect of vitamin E (600 IU every other day) on total cancers, site-specific cancers, or cancer deaths. Of note, posttrial follow-up of the vitamin E component in the HOPE, Women's Health Study, and WACS has yet to be reported.
The SELECT tested vitamin E supplementation of 400 IU/d on the prevention of prostate cancer in 35,533 men and initially reported no effect (HR: 1.13; 95% CI: 0.95, 1.35) during 5.5 y of treatment (4). The recent report of the SELECT added 54,464 person-years of posttrial follow-up and found that men randomly assigned to take active vitamin E developed significantly more prostate cancer compared with placebo (HR: 1.17; 99% CI: 1.004, 1.36) (13). The increased prostate cancer appeared ∼3 y after randomization and continued throughout follow-up. Although there is no known biological mechanism for this effect, this finding raised concerns about the safety of long-term vitamin E supplementation in generally healthy men. The PHS II findings of no effect for vitamin E supplementation on the risk of prostate cancer during the intervention and posttrial observation contrast with the findings of the SELECT. Several notable differences between the 2 trials should be considered. First, the PHS II randomly assigned participants with previously diagnosed cancers; we excluded those who reported prostate cancer at baseline in analyses of the incident prostate cancer endpoint. In contrast, the SELECT screened for and excluded men with prostate cancer before randomization. Second, the vitamin E dose tested in the SELECT (400 IU daily) is higher than in the PHS II (400 IU every other day). Third, the PHS II was designed and analyzed as a factorial trial; the effect of vitamin E was estimated after stratifying by the other intervention components. The SELECT, on the other hand, was a parallel-arm trial with 4 treatment groups analyzed separately; the increased rate of prostate cancer was observed in vitamin E alone but not in the vitamin E plus selenium group (13). Finally, the PHS II included US male physicians who had an overall high socioeconomic level, healthy lifestyle, and favorable nutritional status, whereas the SELECT enrolled men from the general population.
Epidemiologic studies have shown an inverse association between dietary intake of vitamin C and the risk of various cancers (29, 30). However, the evidence for any effect of vitamin C supplementation on cancer in randomized trials remains limited. Several primary (3, 6, 25) and secondary (31–34) prevention trials evaluated combined antioxidant supplements that included vitamin C for cancer prevention. The results did not support a beneficial effect, particularly in women. The WACS trial tested individual vitamin C supplementation of 500 mg/d in 8171 women initially free of cancer and found RRs of 1.11 for total cancers and 1.28 for cancer mortality (both P > 0.05) (7). To our knowledge, the PHS II remains the only cancer prevention trial of individual vitamin C supplementation in men. The present study confirms our previous finding of no effect of long-term vitamin C supplementation on total and major site-specific cancers (5) with extended follow-up. Our finding of a significant reduction in colorectal cancer with active vitamin C supplementation during the posttrial observation period is intriguing. During the intervention period, there was a 21% nonsignificant reduction in colorectal cancer in the active vitamin C group compared with the placebo group. The reduction in colorectal cancer with vitamin C supplementation increased to 46% and became statistically significant during the posttrial observation, suggesting a possible late effect of vitamin C supplementation. These findings warrant additional studies to confirm or refute and highlight the importance of continued posttrial observation to detect any potential latent effects for vitamin C and other nutritional interventions.
The PHS II has many strengths, including a large number of accrued cancer cases, cumulative follow-up of more than a decade, and excellent adherence of participants to the study regimen. However, the PHS II also has notable limitations. First, the PHS II did not perform a standardized screening for cancers at baseline, and thus some participants may have had subclinical cancers before the intervention. Second, the PHS II tested only a single dosage of vitamin E and C supplements and a selected form of vitamin E (synthetic α-tocopherol). It is possible that other supplement dosages and forms may have different effects. Third, the PHS II is a well-nourished, predominantly white, highly educated cohort. The results of PHS II may not be generalizable to other populations of different socioeconomic and nutritional status. Finally, our study included a relatively short posttrial observation period. We will continue to follow up the PHS II participants to identify any possible late effects of the randomly assigned supplementations.
In conclusion, additional posttrial follow-up of the PHS II did not show any overall or late treatment effect of vitamin E or C supplementation on prostate cancer, other site-specific cancers, or total cancers. These findings confirm our initial trial results and indicate that vitamin E and C supplement use has no immediate or long-term effects on cancer risk.
Supplementary Material
Acknowledgments
We are deeply indebted to the 14,641 physician participants for their long-standing dedication and conscientious collaboration to the PHS II. We also acknowledge the contributions of Gregory Kotler and Natalya Gomelskaya in the development of the manuscript.
The authors’ responsibilities were as follows—LW, HDS, VB, and JMG: designed the study; HDS, JEB, and JMG: obtained funding; RJG, WGC, JEM, JEB, and JMG: acquired data; HDS, RJG, WGC, and VB: provided administrative, technical, or material support; LW, HDS, RJG, and VB: performed statistical analysis; LW, HDS, RJG, WGC, VB, JEM, JEB, and JMG: interpreted the results; LW and HDS: drafted the manuscript, had full access to all the data in the study, and take primary responsibility for the integrity and accuracy of the data and the final content of the manuscript; and RJG, WGC, VB, JEM, JEB, and JMG: provided critical revision of the manuscript for important intellectual content. LW received a career development grant from the NIH. HDS received investigator-initiated research funding from the NIH, the Tomato Products Wellness Council, and Cambridge Theranostics Ltd. RJG reported receiving investigator-initiated research funding from the NIH, Bristol-Meyers Squibb, AstraZeneca, and Novartis and signing a consulting agreement with Merck to give an invited talk. WGC received research funding support from the NIH, Harvard University (Clinical Nutrition Research Center), and DSM Nutritional Products Inc (formerly Roche Vitamins). JEM reported receiving investigator-initiated research funding from the NIH, assistance with study pills and packaging from BASF and Cognis Corporations for the Women's Antioxidant and Folic Acid Cardiovascular Study and from Pronova BioPharma and Pharmavite for the VITamin D and OmegA-3 TriaL, and funding from the nonprofit Aurora Foundation. JEB received investigator-initiated research funding from the NIH and assistance with study pills and packaging from Natural Source Vitamin E Association and Bayer Healthcare for the Women's Health Study. JMG reported receiving investigator-initiated research funding from the NIH, the Veterans Administration, and BASF Corporation to assist in the establishment of this trial cohort; assistance with study agents and packaging from BASF Corporation and Pfizer (formerly Wyeth, American Home Products, and Lederle); and assistance with study packaging provided by DSM Nutritional Products Inc (formerly Roche Vitamins). None of the other authors had disclosures to report.
Footnotes
Abbreviations used: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention; HOPE, Heart Outcomes Protection Evaluation; PHS, Physicians’ Health Study; SELECT, Selenium and Vitamin E Cancer Prevention Trial; WACS, Women's Antioxidant Cardiovascular Study.
REFERENCES
- 1.Ames BN. Dietary carcinogens and anticarcinogens: oxygen radicals and degenerative diseases. Science 1983;221:1256–64. [DOI] [PubMed] [Google Scholar]
- 2.Mamede AC, Tavares SD, Abrantes AM, Trindade J, Maia JM, Botelho MF. The role of vitamins in cancer: a review. Nutr Cancer 2011;63:479–94. [DOI] [PubMed] [Google Scholar]
- 3.Heart Protection Study Collaborative Group. MRC/BH F Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:23–33.12114037 [Google Scholar]
- 4.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009;301:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD, Buring JE. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA 2009;301:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA 2005;294:56–65. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, Buring JE, Manson JE. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst 2009;101:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 2005;293:1338–47. [DOI] [PubMed] [Google Scholar]
- 9.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, Haapakoski J, Malila N, Rautalahti M, Ripatti S, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst 1998;90:440–6. [DOI] [PubMed] [Google Scholar]
- 10.Albanes D, Malila N, Taylor PR, Huttunen JK, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Barrett MJ, Pietinen P, et al. Effects of supplemental alpha-tocopherol and beta-carotene on colorectal cancer: results from a controlled trial (Finland). Cancer Causes Control 2000;11:197–205. [DOI] [PubMed] [Google Scholar]
- 11.Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet 1993;9:138–41. [DOI] [PubMed] [Google Scholar]
- 12.Sugano H. The cancer problem—carcinogenesis and prevention from the viewpoint of the natural history of cancer. Anticancer Res 1999;19(5A):3787–90. [PubMed] [Google Scholar]
- 13.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011;306:1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II—a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol 2000;10:125–34. [DOI] [PubMed] [Google Scholar]
- 15.Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, Schvartz M, Manson JE, Glynn RJ, Buring JE, Gaziano JM. Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA 2012;308:1751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 1996;334:1145–9. [DOI] [PubMed] [Google Scholar]
- 17.Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989;321:129–35. [DOI] [PubMed] [Google Scholar]
- 18.Timbo BB, Ross MP, McCarthy PV, Lin CT. Dietary supplements in a national survey: prevalence of use and reports of adverse events. J Am Diet Assoc 2006;106:1966–74. [DOI] [PubMed] [Google Scholar]
- 19.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 2007;369:1603–13. [DOI] [PubMed] [Google Scholar]
- 20.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741–50. [DOI] [PubMed] [Google Scholar]
- 21.Chan JM, Giovannucci EL. Vegetables, fruits, associated micronutrients, and risk of prostate cancer. Epidemiol Rev 2001;23:82–6. [DOI] [PubMed] [Google Scholar]
- 22.Flagg EW, Coates RJ, Greenberg RS. Epidemiologic studies of antioxidants and cancer in humans. J Am Coll Nutr 1995;14:419–27. [DOI] [PubMed] [Google Scholar]
- 23.Shklar G, Oh SK. Experimental basis for cancer prevention by vitamin E. Cancer Invest 2000;18:214–22. [DOI] [PubMed] [Google Scholar]
- 24.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst 1993;85:1483–92. [DOI] [PubMed] [Google Scholar]
- 25.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briancon S. The SU.VI.MAX study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med 2004;164:2335–42. [DOI] [PubMed] [Google Scholar]
- 26.ATBC Cancer Prevention Study Group. The Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study: design, methods, participant characteristics, and compliance. Ann Epidemiol 1994;4:1–10. [DOI] [PubMed] [Google Scholar]
- 27.Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994;330:1029–35. [DOI] [PubMed] [Google Scholar]
- 28.Virtamo J, Pietinen P, Huttunen JK, Korhonen P, Malila N, Virtanen MJ, Albanes D, Taylor PR, Albert P. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA 2003;290:476–85. [DOI] [PubMed] [Google Scholar]
- 29.Byers T, Guerrero N. Epidemiologic evidence for vitamin C and vitamin E in cancer prevention. Am J Clin Nutr 1995;62(suppl):1385S–92S. [DOI] [PubMed] [Google Scholar]
- 30.Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr 1991;53(suppl):270S–82S. [DOI] [PubMed] [Google Scholar]
- 31.McKeown-Eyssen G, Holloway C, Jazmaji V, Bright-See E, Dion P, Bruce WR. A randomized trial of vitamins C and E in the prevention of recurrence of colorectal polyps. Cancer Res 1988;48:4701–5. [PubMed] [Google Scholar]
- 32.Greenberg ER, Baron JA, Tosteson TD, Freeman DH, Jr, Beck GJ, Bond JH, Colacchio TA, Coller JA, Frankl HD, Haile RW, et al. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. N Engl J Med 1994;331:141–7. [DOI] [PubMed] [Google Scholar]
- 33.Plummer M, Vivas J, Lopez G, Bravo JC, Peraza S, Carillo E, Cano E, Castro D, Andrade O, Sanchez V, et al. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high-risk population. J Natl Cancer Inst 2007;99:137–46. [DOI] [PubMed] [Google Scholar]
- 34.Bonelli L, Puntoni M, Gatteschi B, Massa P, Missale G, Munizzi F, Turbino L, Villanacci V, De Censi A, Bruzzi P. Antioxidant supplement and long-term reduction of recurrent adenomas of the large bowel: a double-blind randomized trial. J Gastroenterol 2013;48:698–705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.