Abstract
Background: Published evidence suggests an inverse association between sleep duration and body weight status.
Objective: We examined the association of sleep duration with eating behaviors reported by adult Americans to understand the relation between sleep duration and body weight status.
Design: This cross-sectional study used sleep duration and dietary data from the continuous NHANES conducted from 2005 to 2010 (n = 15,199, age ≥20 y). Eating behaviors examined included the following: reporting of and energy from main meals (breakfast, lunch, and dinner) and snacks (before breakfast, after dinner, and after 2000 h), intermeal intervals, time of day of main meal reporting, and intakes of macronutrients and beverages. Multiple regression methods were used to examine the independent association of hours of sleep duration grouped as short (≤6 h), average (7–8 h), and long (≥9 h) with eating behavior outcomes.
Results: Relative to average-duration sleepers, a smaller percentage of short-duration sleepers mentioned breakfast, lunch (women only), and dinner in the recall (P ≤ 0.04). They also reported a lower mean percentage of energy from main meals but higher energy from all snacks (P ≤ 0.0004) and after 2000 h (P = 0.03). Short-duration sleepers reported the earliest eating time of the first episode and the latest time of the last eating episode. Absolute amounts of sugar and caffeine and percentage of energy from beverages (women only) were higher in short-duration sleepers. However, the total number of eating episodes and energy intake were not related with sleep duration.
Conclusions: Short-duration sleepers began eating earlier and ended their eating later in the day, but despite the longer eating period, they did not report more eating events. Profiles of the relative contribution of main meals and snacks, at or after 2000 h eating, and beverages in short-duration sleepers were suggestive of eating behaviors that may increase energy intake, but 24-h energy intake did not differ among categories of sleep duration.
INTRODUCTION
Recent reviews of published observational studies suggest that the available evidence supports an association of short sleep duration with higher body weight status (1, 2). National surveys report a high prevalence (>35%) of both insufficient sleep (defined as <7 h of nighttime sleep on work or weekdays) and obesity in the adult US population (3, 4). In an attempt to understand the biological basis for the association of sleep duration with obesity, both neuroendocrine factors linked to hunger and satiety as well as aspects of energy balance have been investigated (5–7). Another related area that has received increasing attention concerns the implications of the interaction of the circadian clock with food intake, energy metabolism, and possible health outcomes (8). Recent experimental studies of short-term partial sleep deprivation reported that the nature and time of eating episodes and amounts and types of foods differed between experimental and control conditions (9–14). In these studies (10, 11, 14), sleep-restricted subjects consumed more energy late at night and selected foods of higher fat or carbohydrate content, ie, eating behaviors that may possibly increase energy intake and promote positive energy balance. Although experimental studies are best suited to understand the temporal sequence of changes in food intake after sleep restriction, they provide little information about dietary intakes and eating behaviors of free-living individuals functioning in their typical work, family, and food environments. The few available observational studies found differences in intakes of nutrients, lower fruit and vegetable intakes, more snack and alcohol intakes, and lower dietary variety in free-living individuals who reported their habitual sleep duration (15–22); however, to our knowledge, none have examined whether eating behavior profiles approximate those suggested by experimental studies.
Given the available evidence from experimental and observational studies summarized above, we hypothesized that the type (meals or snacks), timing (time of reporting), and composition (energy and macronutrients) of self-reported eating behaviors of free-living adults may differ by habitual hours of sleep duration. Because changes in one eating behavior in association with sleep duration may be accompanied by concurrent changes in other eating behaviors and dietary intake, we examined several related eating behavior outcomes to provide contextual information for the eating period. We used nationally representative data from recent national surveys to examine weekday sleep duration–associated differences in the following: 1) reporting of main meals (breakfast, lunch, and dinner) and snacks (before breakfast, after dinner, and at or after 2000 h), 2) relative energy contribution of these eating episodes to 24-h energy intake, 3) clock time of reporting of eating episodes and intermeal intervals, and 4) relative contribution of macronutrients and beverages to 24-h energy intake.
SUBJECTS AND METHODS
We used diet and sleep duration data from the continuous NHANES conducted in 2005–2006, 2007–2008, and 2009–2010 for this observational study. (The continuous NHANES fielded from 1999 to 2004 did not include questions on sleep duration.) The documentation and public domain data for these surveys can be downloaded from the NHANES website (23, 24). The study protocol was reviewed by the City University of New York Institutional Review Board with an exempt review. Each NHANES is cross-sectional and includes a stratified, multistage probability sample of the US noninstitutionalized civilian population. The survey is fielded by the National Center for Health Statistics (NCHS)5 of the CDC. Each survey included an at-home interview of the sample person and an examination along with a dietary interview in a specially equipped mobile examination center (MEC). Unweighted response rates for the MEC-examined sample in these surveys were >70% (25).
Analytic sample
All respondents aged ≥20 y with a dietary recall judged as reliable by the NCHS were eligible for inclusion in the analytic sample (n = 15,702). The NCHS considers dietary recalls reliable if, at a minimum, the first 4 of the 5-step multiple-pass recall have been completed and foods reported for each meal are described. We excluded those who were missing information on hours of sleep duration (n = 25), pregnant and lactating women (n = 476), and respondents who reported no energy intake (n = 2) for a final analytic sample of 15,199 (7601 men and 7598 women).
Information on the exposure (sleep duration)
During the household interview, an interviewer administered a computer-assisted personal interview to collect information about duration of sleep. Respondents were asked “How much sleep do you usually get at night on weekdays or workdays?” Reported sleep duration of ≥12 h was top coded as 12 by the NCHS. We used this information to categorize the duration of sleep as ≤6 h (short), 7–8 h (average), and ≥9 h (long). This categorization reflects prevailing definitions of short sleep duration in the published literature. For example, national surveys have defined <7 h of sleep duration as short (3), and the Healthy People 2020 defines sleep duration of a minimum of 7 h for adults aged ≥22 y and 8 h for those aged 18–21 y as adequate. The surveys did not include questions about hours of sleep duration on weekends.
Dietary assessment method
During the MEC interview, an interviewer administered a computer-assisted 24-h dietary recall via the Automated Multiple-Pass Method developed and validated by the USDA (26, 27). Each dietary recall collected information on the clock time an eating episode began, name of the eating episode, and description, amounts, and source of all foods and beverages reportedly consumed by the respondent in the preceding 24 h. A second recall, 3–10 d after the first recall, was obtained by telephone. The present study used the first dietary recall for all analyses reported here.
Dietary outcomes examined
As discussed in the Introduction, we examined a number of related eating behavior outcomes to provide contextual understanding of overall eating profiles in association with sleep duration.
Reporting of main meal and snack episodes
Survey respondents named each eating episode reported in the 24-h recall by choosing mutually exclusive labels from a list. The mention of eating episodes named by the respondent as breakfast, brunch, lunch, dinner, and supper, or their equivalents in Spanish, were considered main meals. All other mentions of eating episodes (eg, drink, snack, or extended consumption or their Spanish equivalents) were considered snack events. Eating episodes named as extended consumption by the respondent included foods and beverages consumed over a long duration (eg, a carbonated beverage may be ingested over 3–4 h). Every unique time of report of an eating episode, regardless of the number of foods or the amounts of foods reported, was considered an eating episode. However, eating episodes in which the only reported item was plain tap or bottled water were excluded from this count. From this information, for each respondent, we created 3 types of variables: whether or not a named eating event was mentioned in the recall, the number of these events, and the relative contribution of these events to 24-h energy intake. The derived variables thus included the number of all main meal and snack events reported in the recall and the percentage of 24-h energy intake from these events; the percentage of the population reporting each individual main meal (breakfast, lunch, and dinner), a snack before breakfast, after dinner, or at or after 2000 h; and the percentage of 24-h energy from these events. We previously used these methods to assess meal- and snack-related eating behaviors (28–31).
Intermeal intervals and time of reporting of main meals
The length of the eating period in the recall was defined as the interval between the reported time of the first and the last eating episodes. The average interval between eating episodes was determined by dividing the length of the eating period by the total number of eating episodes in the recall. The clock time of when each main meal episode was reported was also determined.
Other dietary outcomes
Other dietary outcomes included intakes of energy, percentage of energy from macronutrients and beverages, and amounts of sugar, dietary fiber, alcohol, and caffeine. The NHANES public release data include estimates of 24-h intake of these dietary components (except for percentage of energy from beverages) for each respondent. To compute the percentage of 24-h energy from beverages, foods reported in the recall were grouped into beverage and nonbeverage items by using previously published methods (32). Briefly, all types of beverages, including milk, caffeinated and noncaffeinated beverages, energy drinks, alcoholic drinks, and fruit and vegetable juices and drinks were considered beverages. Plain tap or bottled water was not included in the beverage category.
Statistical methods
For descriptive purposes, we estimated mean hours of sleep duration by categories of covariates mentioned. The independent association of each covariate with sleep duration was examined by using multiple regression models with hours of sleep duration as a continuous dependent variable.
To understand the independent association of hours of sleep with eating behavior outcomes examined in this study, we adjusted for a number of covariates known to be related with diet and sleep behaviors. These included age in years (20–39, 40–59, 60–69, or ≥70 y), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, and all others), family income relative to the poverty threshold (≤1.3, >1.3–3.5, >3.5, or unknown), level of education (<12 y, 12 y, some college, or college or greater), employment status in past 2 wk (yes or no), smoking status (never, former, or current smoker), alcohol use status (never, former, or current drinker or unknown), any self-reported current chronic disease condition (yes or no), BMI (in kg/m2; <25, 25 to <30, or ≥30), day of recalled intake (Monday–Thursday or Friday–Sunday), and month of MEC examination (November–April or May–October). Information on most of these covariates (except for BMI or dietary recall–related variables) was collected during the household interview. Because of change in the type and form of physical activity information collected in the 2007–2010 surveys relative to the 2005–2006 survey, we adjusted for physical activity in analyses that were limited to the 2007–2010 survey cycles. (Discussed below in “Sensitivity analysis.”)
The independent association of hours of sleep duration (categorized as ≤6, 7–8, and ≥9 h) with dietary variables was examined with each dietary outcome as a continuous or as a dichotomous dependent variable by using linear and logistic regression procedures, respectively. All covariates mentioned above were included in these models. Respondents with unknown information on education (n = 18), employment status (n = 2), smoking status (n = 4), and BMI (n = 186) were excluded from regression models due to small numbers. For family income relative to poverty threshold and alcohol drinking status, respondents with unknown information (n = 1177 and 1046, respectively) were retained (as a separate category) due to large numbers to maintain representativeness of the sample.
To assess whether the diet and sleep duration associations differed by sex, we examined sleep by sex interactions in multiple regression models mentioned above. When the sleep by sex interaction term was significant (P < 0.05), we present sex-specific estimates of eating behaviors. Our tables of results include covariate-adjusted means or proportions and their 95% CIs obtained from multiple linear or logistic regression models (33).
All analyses were conducted by using SAS, version 9.2 (SAS Institute), and SAS-callable SUDAAN, release 11.0.0 (RTI International), with due consideration for the complex survey design of the NHANES (34), per analytic guidelines from the NCHS (35). Day 1 dietary sample weights calculated by the NCHS to account for unequal probability of day of week allocation and nonresponse were used in all analyses. For each dietary outcome, our tables present results (P values) of Wald's global F test for differences among categories of sleep duration and contrasts of ≤6 h and ≥9 h with 7–8 h (the reference category). Two-sided P values <0.05 were considered as significant. We alert the reader that several different dietary outcomes were examined in this study; the P values were not adjusted for multiple comparisons and are provided as a guide to the reader when comparing results across the dietary outcomes.
Because of the acknowledged problem of reports of low-energy intakes in most methods of dietary assessment and national survey (36, 37), we also examined whether the prevalence of underreporting differed by categories of sleep duration. For each respondent, a ratio of reported energy intake to energy required for basal energy expenditure (BEE) was computed as an indicator of energy reporting. The energy requirement for BEE was computed by using sex- and BMI-specific equations derived by the Committee on Dietary Reference Intakes (38). The ratio was operationalized as both a continuous variable and as a categorical variable. A ratio of <1.2 was considered to indicate low-energy-intake reporting (30, 36).
Sensitivity analysis
Because of the possibility that physical activity may be a confounder in understanding the association of eating behaviors and sleep duration, we first explored whether a comparable variable to assess physical activity across all 3 survey waves could be operationalized to use for statistical adjustment in regression models. This was necessitated by differences in the information collected to determine the level of physical activity in the 2005–2006 survey from that collected in the 2007–2010 surveys. For example, the 2007–2010 surveys used the Global Physical Activity Questionnaire, which queried about transportation, recreational activities, and duration, intensity, and frequency of work (23). However, a comparable variable for use with all survey waves did not appear to be appropriate; therefore, we repeated all analysis restricting the sample to 2007–2010 surveys (n = 11,041). Along with all of the covariates mentioned above, these models included an estimate of physical activity measured as metabolic equivalent task minutes per week as an independent variable.
RESULTS
Short (≤6 h), average (7–8 h), and long (≥9 h) durations of sleep were reported by 36.4%, 56.1%, and 7.5% of American adults, respectively (Table 1). Sex, age, race-ethnicity, ratio of family income to poverty threshold, years of education, employment status, smoking status, BMI, self-reported chronic disease, and day of recalled intake were significant independent correlates of hours of sleep duration (P ≤ 0.01) (Supplemental Table 1 under “Supplemental data” in the online issue). The distribution of covariates into categories of hours of sleep duration (Table 1) followed patterns similar to those for mean hours of sleep duration by categories of covariates (Supplemental Table 1 under “Supplemental data” in the online issue).
TABLE 1.
Characteristics of the surveyed population by categories of weekday/workday duration of nighttime sleep reported by adult Americans: NHANES 2005–20101
| Categories of sleep duration |
|||||
| n | All (n = 15,199) | ≤6 h (n = 5994) | 7–8 h (n = 8053) | ≥9 h (n = 1152) | |
| All | 15,199 | — | 36.4 ± 0.8 | 56.1 ± 0.7 | 7.5 ± 0.3 |
| Sex | |||||
| Men | 7601 | 48.6 ± 0.4 | 51.0 ± 0.9 | 48.5 ± 0.6 | 37.8 ± 2.1 |
| Women | 7598 | 51.4 ± 0.4 | 49.0 ± 0.9 | 51.5 ± 0.6 | 62.2 ± 2.1 |
| Age | |||||
| 20–39 y | 4950 | 36.4 ± 0.8 | 36.9 ± 1.1 | 36.0 ± 0.9 | 37.9 ± 2.5 |
| 40–59 y | 5015 | 38.9 ± 0.6 | 41.8 ± 0.9 | 38.4 ± 0.8 | 28.0 ± 2.1 |
| 60–69 y | 2458 | 12.7 ± 0.4 | 11.2 ± 0.5 | 13.7 ± 0.6 | 12.4 ± 1.3 |
| ≥70 y | 2776 | 12.0 ± 0.5 | 10.0 ± 0.5 | 11.9 ± 0.6 | 21.7 ± 1.8 |
| Race-ethnicity | |||||
| Non-Hispanic white | 7408 | 70.8 ± 1.9 | 64.7 ± 2.3 | 74.4 ± 1.8 | 73.2 ± 2.6 |
| Non-Hispanic black | 3089 | 11.3 ± 1.0 | 16.7 ± 1.5 | 7.9 ± 0.7 | 10.8 ± 1.4 |
| Mexican American | 2740 | 8.1 ± 0.9 | 7.6 ± 0.9 | 8.4 ± 0.9 | 8.0 ± 1.5 |
| Other | 1962 | 9.8 ± 0.8 | 10.9 ± 0.9 | 9.3 ± 0.9 | 8.0 ± 1.1 |
| Poverty-income ratio | |||||
| ≤1.3 | 4212 | 19.1 ± 0.8 | 21.8 ± 1.0 | 16.5 ± 0.7 | 25.0 ± 2.0 |
| >1.3–3.5 | 5434 | 33.6 ± 0.9 | 34.7 ± 1.0 | 32.3 ± 1.1 | 38.2 ± 2.4 |
| >3.5 | 4376 | 41.2 ± 1.2 | 36.4 ± 1.3 | 45.5 ± 1.3 | 32.0 ± 2.5 |
| Unknown | 1177 | 6.11 ± 0.4 | 7.0 ± 0.6 | 5.7 ± 0.4 | 4.8 ± 0.8 |
| Education | |||||
| <12 y | 4388 | 18.6 ± 0.8 | 19.7 ± 0.8 | 16.8 ± 0.9 | 26.5 ± 2.1 |
| 12 y | 3641 | 24.6 ± 0.7 | 26.9 ± 1.0 | 23.1 ± 0.9 | 24.4 ± 1.5 |
| Some college | 4165 | 30.6 ± 0.5 | 33.9 ± 1.0 | 28.7 ± 0.8 | 28.4 ± 2.0 |
| College | 2987 | 25.2 ± 1.1 | 19.4 ± 0.9 | 31.3 ± 1.5 | 20.6 ± 2.0 |
| Employment | |||||
| Yes | 8449 | 63.0 ± 0.8 | 64.8 ± 0.9 | 65.0 ± 1.0 | 39.0 ± 2.1 |
| No | 6748 | 37.0 ± 0.8 | 35.2 ± 0.9 | 35.0 ± 1.0 | 61.0 ± 2.1 |
| Smoking status | |||||
| Never smoked | 7992 | 52.4 ± 0.9 | 48.4 ± 1.1 | 54.9 ± 1.1 | 53.3 ± 2.1 |
| Former smoker | 3835 | 24.5 ± 0.6 | 22.6 ± 0.7 | 26.1 ± 0.9 | 20.9 ± 1.7 |
| Current smoker | 3368 | 23.1 ± 0.7 | 28.9 ± 0.9 | 18.9 ± 0.7 | 25.7 ± 2.0 |
| Alcohol drinking status | |||||
| Never drinker | 1898 | 10.1 ± 0.6 | 9.8 ± 0.7 | 9.7 ± 0.6 | 15.1 ± 1.8 |
| Former drinker | 2125 | 12.2 ± 0.5 | 12.9 ± 0.7 | 11.7 ± 0.6 | 12.0 ± 1.2 |
| Current drinker | 10,130 | 71.2 ± 0.9 | 70.4 ± 1.1 | 72.2 ± 1.0 | 66.9 ± 2.4 |
| Unknown | 1046 | 6.5 ± 0.4 | 6.9 ± 0.6 | 6.4 ± 0.5 | 6.0 ± 0.8 |
| Any current self-reported chronic disease? | |||||
| Yes | 6935 | 40.4 ± 0.8 | 45.3 ± 0.9 | 36.9 ± 1.0 | 43.1 ± 2.1 |
| No | 8264 | 59.6 ± 0.8 | 54.7 ± 0.9 | 63.1 ± 1.0 | 56.9 ± 2.1 |
| BMI | |||||
| <25 kg/m2 | 4327 | 31.4 ± 0.8 | 28.4 ± 0.8 | 32.5 ± 1.0 | 38.0 ± 2.1 |
| 25 to <30 kg/m2 | 5141 | 33.2 ± 0.6 | 32.7 ± 0.9 | 34.2 ± 0.9 | 28.0 ± 1.7 |
| ≥30 kg/m2 | 5545 | 34.4 ± 0.7 | 37.9 ± 0.8 | 32.4 ± 1.0 | 31.6 ± 2.2 |
| Day of recalled intake | |||||
| Monday–Thursday | 5795 | 57.1 ± 0.6 | 55.5 ± 1.1 | 57.0 ± 0.9 | 64.9 ± 1.7 |
| Friday–Sunday | 9404 | 42.9 ± 0.6 | 44.5 ± 1.1 | 43.0 ± 0.9 | 35.1 ± 1.7 |
| Month of MEC examination | |||||
| November–April | 6883 | 39.8 ± 3.4 | 40.6 ± 3.5 | 38.8 ± 3.4 | 44.2 ± 4.2 |
| May–October | 8316 | 60.1 ± 3.4 | 59.4 ± 3.5 | 61.2 ± 3.4 | 55.9 ± 4.2 |
| Physical activity level (MET-min/wk)2 (NHANES 2007–2010 only) | 11,041 | ||||
| First tertile | 4249 | 32.2 ± 1.2 | 31.6 ± 1.2 | 31.3 ± 1.2 | 43.5 ± 2.3 |
| Second tertile | 3486 | 34.7 ± 0.7 | 31.2 ± 0.8 | 37.2 ± 0.9 | 32.4 ± 2.1 |
| Third tertile | 3304 | 33.1 ± 0.8 | 37.2 ± 1.0 | 31.6 ± 1.1 | 24.0 ± 1.8 |
All values are weighted percentages ± SEs unless otherwise indicated. MEC, mobile examination center; MET-min, metabolic equivalent task-minutes.
Computed from minutes per week of self-reported work-, transportation-, household-, and recreation-related physical activity.
Reporting of main meal and snack eating episodes
Compared with average-duration sleepers, a smaller percentage of short-duration sleepers reported breakfast and dinner in the recall (P < 0.04) (Table 2). Lunch and all 3 main meals were reported by a smaller percentage of women who reported short sleep duration (P ≤ 0.0001). In the ≤6 h sleep-duration category, a higher percentage of breakfast reporters also reported a snack before breakfast (P ≤ 0.004). However, the percentage reporting an after-dinner snack (among dinner reporters) did not differ among categories of hours of sleep duration. Relative to average-duration sleepers, a higher percentage of short-duration sleepers reported ≥50% of energy from snack episodes (P = 0.002).
TABLE 2.
Adult Americans who reported main meals and snacks in a 24-h recall by categories of weekday/workday duration of nighttime sleep: NHANES 2005–20101
| Duration of sleep |
|||||
| n | ≤6 h | 7–8 h | ≥9 h | 2 | |
| Reported breakfast | 14,992 | 833 (82, 85) | 86 (84, 87) | 823 (79, 85) | 0.002 |
| Reported lunch4 | |||||
| Men | 7496 | 77 (75, 79) | 78 (75, 80) | 74 (68, 78) | 0.2 |
| Women | 7496 | 763 (74, 78) | 82 (80, 84) | 78 (74, 82) | <0.0001 |
| Reported dinner | 14,992 | 913 (90, 92) | 93 (92, 94) | 93 (90, 94) | 0.04 |
| Reported all 3 main meals in the recall4,5 | |||||
| Men | 7496 | 59 (56, 61) | 61 (58, 63) | 513 (45, 56) | 0.002 |
| Women | 7496 | 603 (57, 62) | 67 (65, 69) | 64 (59, 69) | 0.0001 |
| Reported any snack6 before breakfast (among breakfast reporters) | 11,088 | 153 (13, 17) | 11 (10, 13) | 12 (9, 15) | 0.004 |
| Reported any snack after dinner (among dinner reporters) | 11,632 | 66 (63, 68) | 64 (63, 66) | 64 (59, 68) | 0.5 |
| Reported ≥50% of 24-h energy from snacks | 14,992 | 9.43 (8.6, 10.4) | 6.8 (6.0, 7.8) | 8.4 (6.5, 10.9) | 0.002 |
| EI:BEE7 of ≥1.2 | 14,753 | 52 (50, 54) | 55 (53, 57) | 53 (48, 57) | 0.07 |
Values are adjusted percentages (95% CIs) unless otherwise indicated. Values were derived from logistic regression models with each dietary variable as a dichotomous outcome. Independent variables included hours of sleep duration (≤6, 7–8, or ≥9 h), sex (in models for all), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, or other), poverty-income ratio (<1.3, 1.3–3.5, >3.5, or unknown), years of education (<12, 12, some college, or college), BMI (in kg/m2; <25, 25 to <30, or ≥30), smoking status (never, former, or current smoker), alcohol use status (never, former, current drinker, or unknown), day of recall (Monday–Thursday or Friday–Sunday), month of mobile examination center examination (November–April or May–October), chronic disease (yes or no), and employed (yes or no). BEE, basal energy expenditure; EI, energy intake.
Derived by using Wald's global F test for differences among categories of hours of sleep duration.
Significantly different from the reference category of 7–8 h of sleep, P < 0.05.
Significant sleep by sex interaction (P-interaction ≤ 0.0006).
Main meals included eating episodes that were named as breakfast, brunch, lunch, dinner, and supper or their Spanish equivalents.
“Snack” included all eating episodes that were not main meals as defined above.
Ratio of reported EI to calculated energy requirement for basal needs (BEE). A ratio of <1.2 was used as an indicator of possible low energy reporting; n = 239 were excluded due to a BMI (in kg/m2) of <18.5.
Number of main meal and snack episodes and their relative contribution to 24-h energy intake
Relative to average-duration sleepers, both short- and long-duration sleepers reported lower number and percentage of energy from main meals (P ≤ 0.0004) (Table 3). Sleep duration–related differences in the reported number of snack episodes did not reach statistical significance. However, the percentage of 24-h energy from all snacks or from episodes reported at or after 2000 h was higher in both short- and long-duration sleepers relative to average-duration sleepers. The number of snack episodes reported after dinner was higher in short-duration sleepers (P = 0.03), but the percentage of energy from these after-dinner snacks did not differ from that reported by average-duration sleepers.
TABLE 3.
Main meal and non–main meal episodes and their relative contribution to 24-h energy intake reported by adult Americans by categories of weekday/workday duration of nighttime sleep: NHANES 2005–20101
| Duration of sleep |
||||
| ≤6 h | 7–8 h | ≥9 h | 2 | |
| No. of all eating episodes | 4.99 (4.92, 5.07) | 4.98 (4.91, 5.04) | 4.89 (4.78, 4.99) | 0.2 |
| No. of all main meal3 episodes | 2.734 (2.69, 2.76) | 2.80 (2.77, 2.83) | 2.744 (2.68, 2.79) | 0.0004 |
| Twenty-four-hour energy from main meals (%) | 77.04 (76.3, 77.6) | 78.8 (78.2, 79.4) | 77.24 (75.9, 78.4) | 0.0002 |
| Number of all snack5 episodes | 2.27 (2.20, 2.34) | 2.18 (2.11, 2.24) | 2.15 (2.05, 2.25) | 0.07 |
| Twenty-four-hour energy from snack episodes (%) | 23.04 (22.3, 23.6) | 21.2 (20.6, 21.8) | 22.84 (21.5, 24.1) | 0.0002 |
| No. of snack episodes reported after dinner by dinner reporters (n = 7422) | 1.404 (1.36, 1.43) | 1.35 (1.32, 1.38) | 1.37 (1.30, 1.45) | 0.03 |
| Twenty-four-hour energy from after-dinner snacks by dinner reporters (n = 7422) (%) | 15.2 (14.5, 16.0) | 14.7 (14.1, 15.3) | 15.2 (13.7, 16.7) | 0.4 |
| Twenty-four-hour energy from all eating episodes reported at or after 2000 h (%) | 16.24 (15.2, 17.1) | 15.1 (14.3, 15.9) | 16.84 (15.1, 18.5) | 0.03 |
Values are adjusted means (95% CIs) from linear regression models with each dietary variable as a continuous outcome; n = 14,992, except as noted. Independent variables included hours of sleep duration (≤6, 7–8, or ≥9 h), sex (in models for all), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, or other), poverty-income ratio (<1.3, 1.3–3.5, >3.5, or unknown), years of education (<12, 12, some college, or college), BMI (in kg/m2; <25, 25 to <30, or ≥30), smoking status (never, former, or current smoker), alcohol use status (never, former, current drinker, or unknown), day of recall (Monday–Thursday or Friday–Sunday), month of mobile examination center examination (November–April or May–October), chronic disease (yes or no), and employed (yes or no).
Derived by using Wald's global F test for differences among categories of hours of sleep duration.
“Main meals” included eating episodes named by the respondent as breakfast, brunch, lunch, dinner, and supper or their Spanish equivalents.
Significantly different from the reference category of 7–8 h of sleep, P < 0.05.
“Snack” included all eating episodes that were not main meals as defined above.
Intermeal intervals and clock time of reporting of main meals
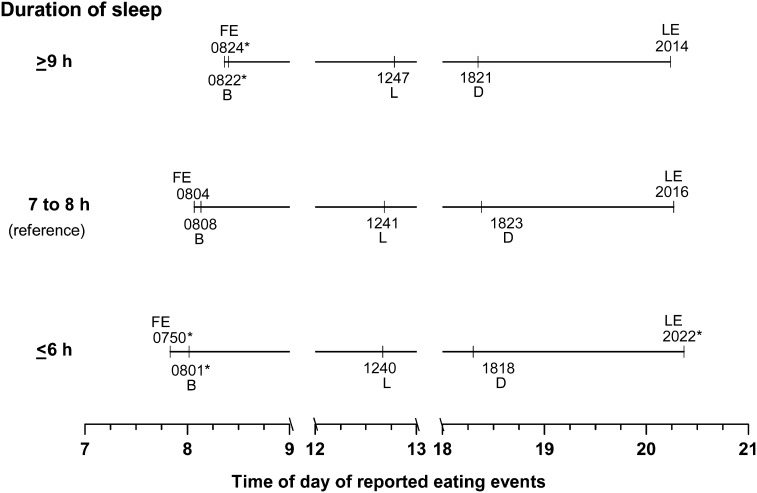
The time interval between the first and the last eating episode in the recall was 12.53, 12.19, and 11.84 h for short-, average-, and long-duration sleepers, respectively (P ≤ 0.0001) (Table 4). Not surprisingly, therefore, the average interval between eating episodes was longest in short-duration sleepers (P < 0.005) (Table 4). The clock time of reporting of the first and the last eating episode and time of breakfast were also significantly different among categories of sleep duration. The earliest time of first eating episode and breakfast, but latest time of the last eating episode of the day were associated with the ≤6 h of sleep duration (Figure 1, Table 4). However, the mean clock times of lunch and dinner reports were not related with sleep duration.
TABLE 4.
Adjusted mean intermeal intervals and clock time (95% CIs) of meals reported by adult Americans in a 24-h recall by categories of weekday/workday duration of nighttime sleep: NHANES 2005–20101
| Duration of sleep |
||||
| ≤6 h | 7–8 h | ≥9 h | 2 | |
| Length of the eating period3 (h) | 12.534 (12.38, 12.67) | 12.19 (12.09, 12.29) | 11.844 (11.64, 12.05) | <0.0001 |
| Average interval between eating episodes (h) | 2.664 (2.63, 2.69) | 2.60 (2.57, 2.63) | 2.57 (2.51, 2.62) | 0.005 |
| Clock time of the first eating episode of the day | 07504 (0741, 0759) | 0804 (0757, 0811) | 08244 (0811, 0836) | 0.0002 |
| Clock time of the last eating episode of the day | 20224 (2017, 2027) | 2016 (2011, 2020) | 2014 (2006, 2022) | 0.04 |
| Clock time of breakfast among breakfast reporters (n = 12,729) | 08014 (7:53, 0808) | 0808 (0803, 0813) | 08224 (8:11, 0832) | 0.006 |
| Clock time of lunch among lunch reporters (n = 11,052) | 1240 (1234, 1246) | 1241 (1236, 1246) | 1247 (1238, 1255) | 0.3 |
| Clock time of dinner among dinner reporters (n = 13,402) | 1818 (1809, 1827) | 1823 (1814, 1831) | 1821 (1811, 1832) | 0.4 |
Values were derived from linear regression models with each dietary variable as a continuous outcome; n = 14,992, except as noted. Independent variables included hours of sleep duration (≤6, 7–8, or ≥9 h), sex (in models for all), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, or other), poverty-income ratio (<1.3, 1.3–3.5, >3.5, or unknown), years of education (<12, 12, some college, or college), BMI (in kg/m2; <25, 25 to <30, or ≥30), smoking status (never, former, or current smoker), alcohol use status (never, former, current drinker, or unknown), day of recall (Monday–Thursday or Friday–Sunday), month of mobile examination center examination (November–April or May–October), chronic disease (yes or no), and employed (yes or no).
Derived by using Wald's global F test for differences among 3 categories of hours of sleep duration.
Interval between the reported times of the first and the last eating episodes in the 24-h recall.
Significantly different from the reference category of 7–8 h of sleep, P < 0.05.
FIGURE 1.
Mean clock time of meals and other eating events reported by American adults in a 24-h recall by categories of hours of sleep duration: NHANES 2005–2010. Estimates are from linear regression models with each eating time as a continuous outcome. Independent variables included duration of sleep (≤6, 7–8, or ≥9 h), sex, race-ethnicity, poverty-income ratio, education, BMI, smoking status, alcohol use status, day of recall, month of mobile examination center examination, chronic disease, and employment status. *Significantly different from the reference category of 7–8 h of sleep, P < 0.05. B, breakfast; D, dinner; FE, first event; L, lunch; LE, last event.
Intake of macronutrients, beverages, and other dietary constituents
Hours of sleep duration was not an independent correlate of 24-h dietary energy, percentage of energy from carbohydrate and fat, or alcohol intake (Table 5). Both short- and long-duration sleepers reported a slightly lower percentage of energy from protein compared with average-duration sleepers (P = 0.007). Intake of total sugar (P = 0.04) and percentage of 24-h energy from beverages (women only; P < 0.0001) were higher in short-duration sleepers. Caffeine intake was highest in short-duration sleepers and lowest in long-duration sleepers (P = 0.0001).
TABLE 5.
Adjusted mean (95% CI) dietary energy and macronutrient intakes reported by adult Americans by categories of weekday/workday duration of nighttime sleep: NHANES 2005–20101
| Duration of sleep |
||||
| ≤6 h | 7–8 h | ≥9 h | 2 | |
| Energy (kcal) | 2157 (2112, 2201) | 2150 (2114, 2186) | 2138 (2060, 2215) | 0.9 |
| Twenty-four-hour energy from carbohydrate (%) | 49 (49, 50) | 49 (48, 49) | 48 (47, 49) | 0.06 |
| Twenty-four-hour energy from protein (%) | 15.73 (15.5, 15.9) | 16.0 (15.8, 16.2) | 15.63 (15.3, 15.9) | 0.007 |
| Twenty-four-hour energy from fat (%) | 33.2 (32.8, 33.6) | 33.5 (33.2, 33.7) | 33.6 (32.8, 34.4) | 0.3 |
| Twenty-four-hour energy from beverages4 (%) | ||||
| Men (n = 7496) | 20.5 (19.7, 21.2) | 20.1 (19.4, 20.7) | 20.8 (18.7, 22.9) | 0.6 |
| Women (n = 7496) | 18.73 (17.7, 19.7) | 16.9 (16.2, 17.5) | 18.3 (16.6, 20.1) | 0.0001 |
| Total sugar (g) | 1203 (116, 124) | 116 (113, 119) | 115 (111, 120) | 0.04 |
| Dietary fiber (g) | 16.2 (15.7, 16.8) | 16.5 (16.1, 16.9) | 15.23 (14.4, 15.9) | 0.001 |
| Alcohol (g) | 11.1 (9.7, 12.4) | 10.9 (9.9, 11.9) | 13.3 (10.5, 16.0) | 0.2 |
| Caffeine (mg) | 2003 (188, 212) | 184 (173, 194) | 1603 (142, 179) | 0.0001 |
| EI:BEE5 (n = 14,753) | 1.33 (1.30, 1.35) | 1.33 (1.31, 1.35) | 1.32 (1.27, 1.37) | 0.9 |
Values were derived from linear regression models with each dietary variable as a continuous outcome; n = 14,992, except as noted. Independent variables included hours of sleep duration (≤6, 7–8, or ≥9 h), sex (in models for all), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, or other), poverty-income ratio (<1.3, 1.3–3.5, >3.5, or unknown), years of education (<12, 12, some college, or college), BMI (in kg/m2; <25, 25 to <30, or ≥30), smoking status (never, former, or current smoker), alcohol use status (never, former, current drinker, or unknown), day of recall (Monday–Thursday or Friday–Sunday), month of mobile examination center examination (November–April or May–October), chronic disease (yes or no), and employed (yes or no). BEE, basal energy expenditure; EI, energy intake.
Derived by using Wald's global F test for differences among categories of hours of sleep duration.
Significantly different from the reference category of 7–8 h of sleep, P < 0.05.
Significant sleep by sex interaction (P-interaction = 0.01). “Beverages” included all types of beverages: milk, caffeinated and noncaffeinated beverages, energy drinks, alcoholic drinks, and fruit and vegetable juices and drinks and excluded plain tap or bottled water.
Ratio of reported EI to calculated energy requirement for basal needs (BEE). Excluded 239 subjects with a BMI (in kg/m2) of <18.5.
Assessment of impact of possible energy underreporting
The percentage of respondents who reported a low ratio of energy intake to BEE (ie, energy intake:BEE of <1.2) was not different among categories of sleep duration (Table 2). Similarly, mean energy intake:BEE ratios did not differ among categories of sleep (Table 5).
Analysis restricted to 2007–2010 survey cycles with adjustment for physical activity
With additional control for physical activity in the sample limited to the 2007–2010 surveys, the associations noted above remained in the same direction and were significant (see Supplemental Tables 2–5 under “Supplemental data” in the online issue). Exceptions included the following: a significant difference among sleep duration categories for number of all eating episodes reported in the recall [ie, long-duration sleepers reported fewer eating episodes (P = 0.0007) and fewer snack episodes (P = 0.02) than did average-duration sleepers].
DISCUSSION
The study findings provide a distinct picture of eating behavior profile of short-duration sleepers characterized by skipping main meals with higher contribution of snacks to energy intake, earlier eating time of the first eating episode, and later time of the last eating episode, but few differences in macronutrient composition or energy content of the diet. Many of the observed eating behaviors of short-duration sleepers (breakfast skipping, snacking, and more beverages) have long been investigated as possible at-risk eating behaviors for promoting positive energy balance (39). Long-duration (≥9 h) sleepers also reported eating behaviors that differed from average-duration sleepers.
The most remarkable differences in eating behaviors of short-duration sleepers in our study concerned the reported clock time of eating events recalled. Hours of sleep duration were related directly with time of reporting of the first eating episode (and breakfast) and inversely with the last eating episode of the day, resulting in a longer period of food consumption. One may speculate that a longer eating period possibly presents more opportunities for food intake. Despite the longer eating period, we found little meaningful variation in the number of all eating episodes due to sleep duration. However, each main meal was less likely to be reported by short-duration sleepers. As a result, the contribution of main meal energy to total energy intake was slightly lower (∼1.8%) in short-duration sleepers. Given that energy intake did not differ by sleep duration, these results suggest some displacement of main meal energy by snack energy in short-duration sleepers. In an experimental study (14), however, sleep restriction was associated with the addition of snack energy to unchanged main meal energy and consequently higher energy intake. Another recent experimental study found eating frequency to be associated with sleep restriction only when sleep time was delayed (10). Kim et al (20) also found that women with short-duration sleep had a snack-dominated eating pattern with negative loadings for each main meal but positive loadings for snacks in factor analysis. Report of higher snack energy intakes by adolescents, however, was not associated with actigraphy-measured sleep duration after multivariate adjustment (19). Although the magnitude of the increase in energy contribution of snacks in our study was small, such displacement may affect micronutrient intake of short-duration sleepers. Depending on foods selected, snacks can make important contributions to daily nutrient intake; however, overall, foods commonly consumed as snacks tend to be energy dense and provide a smaller proportion of micronutrients relative to their energy content (40).
Sleep restriction under experimental conditions was reported to increase energy intake late in the evening, especially after dinner (10, 11, 14). In our study, neither the likelihood of reporting an after-dinner eating episode nor the percentage of energy from these episodes differed by sleep duration. However, there was a suggestion of increase in late-day eating, because the percentage of 24-h energy reported at or after 2000 h was higher in short-duration sleepers. The potential contribution of late-night eating to energy balance remains equivocal; some studies reported a direct association between late-night eating and body weight status (41, 42), but other findings were null (29, 43). Experimental and observational studies also suggest a preference for foods with higher fat and carbohydrate content at night (11, 14, 44).
In our study, differences in dietary macronutrient composition of intakes of short-duration sleepers relative to adequate-duration sleepers were small in magnitude. Published evidence on this topic presents a mixed picture. Two earlier observational studies in adults (18, 45) also found that the association of fat intake with sleep duration was not significant after covariate adjustment. However, actigraphy-assessed sleep duration was an inverse correlate of percentage of fat energy reported by adolescents (19) and women (46). Experimental studies reported higher intakes of carbohydrate (11, 14), protein (11), and fat (12) in the sleep-restricted condition.
In women, beverages contributed a higher percentage of 24-h energy in short-duration sleepers. Higher energy from beverages was shown to relate to higher frequency of snacking, beverage-only episodes, longer ingestive periods, and higher energy intakes from nonbeverage foods (32). Moreover, it has been suggested that energy in the beverage form is poorly compensated in subsequent intake. Thus, high beverage consumption is a potentially weight-influencing eating behavior. The caffeine intake of short-duration sleepers was also higher relative to that of average-duration sleepers. Major dietary sources of caffeine in the American diet include coffee, carbonated beverages, and tea (47). Caffeine is known to affect sleep latency and sleep time under experimental conditions, and it has been suggested that caffeine consumption in population surveys is associated with sleep problems (48).
Our results suggest sex differences in the association of sleep duration with some eating behaviors. Associations of main meal reporting and beverage contribution to the diet with sleep duration were noted only in women. Our study cannot provide information about the reasons for this sex differential; however, we note that sex differences in eating behaviors, satiation, and energy metabolism have been reported in the literature (49–52).
Long-duration sleepers (≥9 h) also reported eating behavior profiles that differed from average-duration sleepers and for several dietary behaviors were more like those of short-duration sleepers. For example, long-duration sleepers were also less likely to mention breakfast or all 3 main meals and reported a lower percentage of energy from main meals but a higher percentage from snacks and lower fiber intake.
Although the large nationally representative study sample and available information on multiple potential confounders of the sleep duration and diet association are strengths of our study, the results should be interpreted with due attention to the following limitations. First, given the cross-sectional study design, we limited our narrative to associations and make no causal inferences about the observed relations. Second, both the sleep duration and the dietary information were self-reported. Some reports have suggested poor concordance of objectively assessed and self-reported sleep duration (53, 54), and the association with diet differed by how sleep duration was assessed (46). Moreover, our study provides no information on hours of weekend sleep or sleep quality and their possible associations with dietary outcomes. Thus, although the dietary and eating behavior outcomes average both weekdays and weekend days, the sleep duration information was limited to weekday sleep.
All self-reported methods of dietary assessment, including dietary recall, are subject to both random and systematic measurement errors (55). The self-reported dietary information in the NHANES was collected by using the USDA's Automated Multiple-Pass Method. This 24-h recall methodology has been validated and is believed to provide reasonable estimates of dietary intakes at the group level (26, 27). Because of day-to-day variability in food intake of free-living individuals, a single 24-h recall can provide reasonable estimates of mean group intakes but is not suitable for examining nutrient intake distributions (56). Our use of a single recall to examine differences in covariate-adjusted regression–generated summary estimates of eating behaviors reported by respondents grouped into categories of sleep is therefore appropriate. Dietary misreporting, mostly underreporting, usually assessed by comparison of reported energy intakes to estimated energy requirements, is known to be prevalent in national surveys (30, 36). In our study, this will be especially problematic if the likelihood or the extent of energy underreporting differed across categories of hours of sleep duration. However, our results do not indicate such a differential. Also, our analysis was adjusted for several known correlates (eg, income, education, age, ethnicity, and BMI) of possible energy underreporting (57). The study focused on relative energy contribution of meals, snacks, macronutrients, and beverages to 24-h energy intake rather than to absolute energy intakes; this density approach, at least for protein, was reported to not relate to energy underreporting (58).
In conclusion, short-duration sleepers began eating earlier and ended their eating later in the day, but despite the longer eating period they did not report more eating events. Although eating behaviors that may potentially relate to positive energy balance (eg, relative contribution of main meals and snacks, beverages, and at or after 2000 h eating to 24-h energy intake) differed modestly among categories of sleep duration, total 24-h energy intake was not related with sleep duration.
Supplementary Material
Acknowledgments
We thank Lisa Licitra Kahle for expert programming support and David Check, National Cancer Institute, for graphic support.
The authors’ responsibilities were as follows—AKK: conceptualized the study question, designed the research, analyzed data, wrote the manuscript, and had primary responsibility for final content; and BIG: provided guidance on the study design and analytic strategy and reviewed the manuscript for important intellectual content. Neither of the authors declared a conflict of interest.
Footnotes
Abbreviations used: BEE, basal energy expenditure; MEC, mobile examination center; NCHS, National Center for Health Statistics.
REFERENCES
- 1.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008;31:619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Sleep duration among adults aged ≥20 years, by race/ethnicity—National Health and Nutrition Examination Survey, United States, 2007–2010. MMWR Morb Mortal Wkly Rep 2013;62:755 Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6236a9.htm?s_cid=mm6236a9_w (cited 20 October 2013). [Google Scholar]
- 4.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. Hyattsville, MD: National Center for Health Statistics, 2013. NCHS Data Brief 131. Available from: http://www.cdc.gov/nchs/data/databriefs/db131.pdf (cited 20 October 2013). [Google Scholar]
- 5.Shlisky JD, Hartman TJ, Kris-Etherton PM, Rogers CJ, Sharkey NA, Nickols-Richardson SM. Partial sleep deprivation and energy balance in adults: an emerging issue for consideration by dietetics practitioners. J Acad Nutr Diet 2012;112:1785–97. [DOI] [PubMed] [Google Scholar]
- 6.St-Onge MP. The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J Clin Sleep Med 2013;9:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaput JP. Sleep patterns, diet quality and energy balance. Physiol Behav (Epub ahead of print 17 September 2013). [DOI] [PubMed]
- 8.Tahara Y, Shibata S. Chronobiology and nutrition. Neuroscience 2013;253:78–88. [DOI] [PubMed] [Google Scholar]
- 9.Calvin AD, Carter RE, Adachi T, Macedo PG, Albuquerque FN, van der Walt C, Bukartyk J, Davison DE, Levine JA, Somers VK. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest 2013;144:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep 2013;36:981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP., Jr Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA 2013;110:5695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, Roy Choudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 2011;94:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 2010;91:1550–9. [DOI] [PubMed] [Google Scholar]
- 14.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009;89:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicks RA, McTighe S, Juarez M. Sleep duration and eating behaviors of college students. Percept Mot Skills 1986;62:25–6. [DOI] [PubMed] [Google Scholar]
- 16.Hulshof KF, Wedel M, Löwik MR, Kok FJ, Kistemaker C, Hermus RJ, ten Hoor F, Ockhuizen T. Clustering of dietary variables and other lifestyle factors (Dutch Nutritional Surveillance System). J Epidemiol Community Health 1992;46:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rontoyanni VG, Baic S, Cooper AR. Association between nocturnal sleep duration, body fatness, and dietary intake in Greek women. Nutrition 2007;23:773–7. [DOI] [PubMed] [Google Scholar]
- 18.Stamatakis KA, Brownson RC. Sleep duration and obesity-related risk factors in the rural Midwest. Prev Med 2008;46:439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep 2010;33:1201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, DeRoo LA, Sandler DP. Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutr 2011;14:889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaput JP, McNeil J, Després JP, Bouchard C, Tremblay A. Short sleep duration is associated with greater alcohol consumption in adults. Appetite 2012;59:650–5. [DOI] [PubMed] [Google Scholar]
- 22.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite 2013;64:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention; National Center for Health Statistics. About National Health and Nutrition Examination Survey. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; Available from: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm (cited 22 June 2013). [Google Scholar]
- 24.Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey: Questionnaires, Datasets, and Related documentation. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; Available from: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm (cited 22 June 2013). [Google Scholar]
- 25.Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey. Response rates and survey totals. Available from: http://www.cdc.gov/nchs/nhanes/response_rates_cps.htm (cited 15 October 2013).
- 26.Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J Nutr 2006;136:2594–9. [DOI] [PubMed] [Google Scholar]
- 27.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr 2008;88:324–32. [DOI] [PubMed] [Google Scholar]
- 28.Kant AK, Schatzkin A, Graubard BI, Ballard-Barbash R. Frequency of eating occasions and weight change in the NHANES I Epidemiologic Follow-up Study. Int J Obes Relat Metab Disord 1995;19:468–74. [PubMed] [Google Scholar]
- 29.Kant AK, Schatzkin A, Ballard-Barbash R. Evening eating and subsequent long-term weight change in a national cohort. Int J Obes Relat Metab Disord 1997;21:407–12. [DOI] [PubMed] [Google Scholar]
- 30.Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971–1975 to NHANES 1999–2002. Am J Clin Nutr 2006;84:1215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kant AK, Graubard BI. Family income and education were related with 30-year time trends in dietary and meal behaviors of American children and adolescents. J Nutr 2013;143:690–700 (Published erratum appears in J Nutr 2013;143:1348.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kant AK, Graubard BI, Mattes RD. Association of food form with self-reported 24-h energy intake and meal patterns in US adults: NHANES 2003–2008. Am J Clin Nutr 2012;96:1369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics 1999;55:652–9. [DOI] [PubMed] [Google Scholar]
- 34.Korn EL, Graubard BI. Analysis of health surveys. New York, NY: John Wiley and Sons, 1999. [Google Scholar]
- 35.Centers for Disease Control and Prevention; National Center for Health Statistics. The National Health and Nutrition Examination Survey analytic and reporting guidelines. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; Available from: http://www.cdc.gov/nchs/nhanes/analytic_guidelines.htm (cited 15 October 2013). [Google Scholar]
- 36.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr 2003;133(suppl 3):895S–920S. [DOI] [PubMed] [Google Scholar]
- 37.Kant AK. Nature of dietary reporting by adults in the third National Health and Nutrition Examination survey, 1988–1994. J Am Coll Nutr 2002;21:315–27. [DOI] [PubMed] [Google Scholar]
- 38. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: National Academies Press, 2005ndash2006.
- 39.Casazza K, Fontaine KR, Astrup A, Birch LL, Brown AW, Bohan Brown MM, Durant N, Dutton G, Foster EM, Heymsfield SB, et al. Myths, presumptions, and facts about obesity. N Engl J Med 2013;368:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sebastian RS, Wilkinson Enns C, Goldman JD. Snacking patterns of U.S. adults: what we eat in America, NHANES 2007-2008. June 2011. Food Surveys Research Group Dietary Data Brief 4. Available from: http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/DBrief/4_adult_snacking_0708.pdf (cited 20 October 2013).
- 41.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19:1374–81. [DOI] [PubMed] [Google Scholar]
- 42.Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37:604–11 (Published erratum appears in Int J Obes (Lond) 2013;37:624.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan EL, Daniels AJ, Koegler FH, Cameron JL. Evidence in female rhesus monkeys (Macaca mulatta) that nighttime caloric intake is not associated with weight gain. Obes Res 2005;13:2072–80. [DOI] [PubMed] [Google Scholar]
- 44.Baron KG, Reid KJ, Horn LV, Zee PC. Contribution of evening macronutrient intake to total caloric intake and body mass index. Appetite 2013;60:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi Z, McEvoy M, Luu J, Attia J. Dietary fat and sleep duration in Chinese men and women. Int J Obes (Lond) 2008;32:1835–40. [DOI] [PubMed] [Google Scholar]
- 46.Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med 2010;11:180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc 2005;105:110–3 (Published erratum appears in J Am Diet Assoc 2008;108:727.) [DOI] [PubMed] [Google Scholar]
- 48.Roehrs T, Roth T. Caffeine: sleep and daytime sleepiness. Sleep Med Rev 2008;12:153–62. [DOI] [PubMed] [Google Scholar]
- 49.Rolls BJ, Fedoroff IC, Guthrie JF. Gender differences in eating behavior and body weight regulation. Health Psychol 1991;10:133–42. [DOI] [PubMed] [Google Scholar]
- 50.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr 2006;83:1297–305. [DOI] [PubMed] [Google Scholar]
- 51.Provencher V, Drapeau V, Tremblay A, Després JP, Lemieux S. Eating behaviors and indexes of body composition in men and women from the Québec family study. Obes Res 2003;11:783–92. [DOI] [PubMed] [Google Scholar]
- 52.Woods SC, Gotoh K, Clegg DJ. Gender differences in the control of energy homeostasis. Exp Biol Med (Maywood) 2003;228:1175–80. [DOI] [PubMed] [Google Scholar]
- 53.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology 2008;19:838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva GE, Goodwin JL, Sherrill DL, Arnold JL, Bootzin RR, Smith T, Walsleben JA, Baldwin CM, Quan SF. Relationship between reported and measured sleep times: the sleep heart health study (SHHS). J Clin Sleep Med 2007;3:622–30. [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson FE, Subar AF. Dietary assessment methodology. Available from: http://appliedresearch.cancer.gov/diet/adi/thompson_subar_dietary_assessment_methodology.pdf (cited 22 October 2013).
- 56.Institute of Medicine. Dietary Reference Intakes: applications in dietary assessment. Washington, DC: National Academies Press, 2000. [PubMed] [Google Scholar]
- 57.Macdiarmid J, Blundell J. Assessing dietary intake: who, what, and why of under-reporting. Nutr Res Rev 1998;11:231–53. [DOI] [PubMed] [Google Scholar]
- 58.Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S, Ballard-Barbash R, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol 2003;158:1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.