Dear Sir:
We thank Brannon et al for their thoughtful comments on our study (1), and greatly appreciate the opportunity to reply. In their correspondence, they incorrectly state that we defined vitamin D adequacy as 97.5% of the population achieving a 25-hydroxyvitamin D [25(OH)D] concentration of 20 ng/mL and raise concern about potential misapplication of the Recommended Dietary Allowance (RDA) for vitamin D intake in our analysis. They discuss that the RDA should not be used to assess vitamin D adequacy in populations or groups, because, by definition, the RDA-associated concentration of 20 ng/mL reflects a value that exceeds the needs of most individuals. They suggest that the correct approach is to estimate how much vitamin D is needed to ensure a low prevalence of plasma 25(OH)D below the estimated average requirement (EAR) of 16 ng/mL (2).
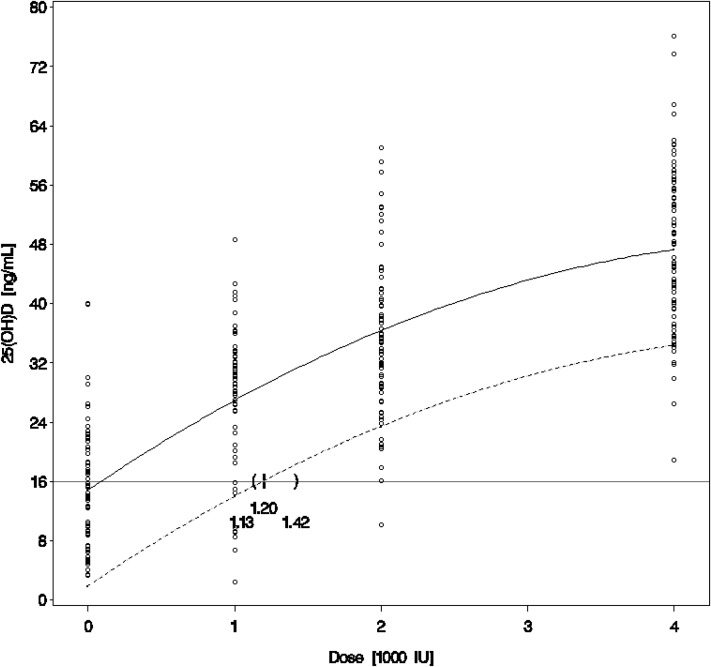
Although we agree with their statement on appropriate applications of the RDA, it is clear that Brannon et al misinterpreted the intent of our analysis. The EAR of 400 IU and RDA of 600 IU for adults up to age 70 y calculated by the Institute of Medicine (IOM) to be associated with plasma 25(OH)D concentrations of 16 and 20 ng/mL, respectively, are based on dose-response curves from studies in white populations. Given the lower baseline concentrations of 25(OH)D among African Americans, differences in lifestyle behaviors, and differences in germline genetic variation and vitamin D metabolism that have not yet been fully characterized, it is important to reexamine the vitamin D requirement associated with these target 25(OH)D concentrations by using dose-response curves that are actually constructed from studies in African Americans, rather than extrapolating from curves from whites. Until now, there have been very few studies evaluating a variety of vitamin D intakes in a large-enough cohort of African Americans with minimal UV-B radiation exposure to reliably construct such a dose-response curve. Our trial randomly assigned 328 community-based African Americans in Boston, MA, to receive placebo or 1000, 2000, or 4000 IU of vitamin D3/d for 3 mo during the winter, with plasma 25(OH)D concentrations assayed at baseline and at 3 and 6 mo. By using the resulting robust dose-response curve, we found a vitamin D requirement of 1640 IU/d to be the amount that corresponded to the RDA-associated 25(OH)D concentration of 20 ng/mL in African Americans. In fact, even when we consider the lower EAR-associated 25(OH)D concentration of 16 ng/mL as our target, as recommended by Brannon et al, the vitamin D intake required to reach that value in ≥97.5% of African Americans is still 1200 IU/d (95% CI: 1130, 1420 IU/d) (Figure 1).
FIGURE 1.
Plasma 25(OH)D concentrations at 3 mo and dose of vitamin D3 supplementation (n = 71 for placebo; n = 67 for 1000 IU/d; n = 76 for 2000 IU/d; n = 78 for 4000 IU/d). The solid line is a quadratic fit to the observed mean plasma 25(OH)D concentration. The dashed line falls below the mean line by 1.96 SDs of the distribution of the estimated within-subject mean concentration (obtained from the random patient effect in the mixed model) and represents the empirical Bayesian prediction interval to bound 97.5% of future subjects’ mean plasma 25(OH)D concentration. This prediction interval crosses the 16-ng/mL line at 1200 IU/d (95% CI: 1130, 1420 IU/d), indicating that an estimated dose of 1200 IU vitamin D3/d is required to achieve a mean plasma 25(OH)D ≥16 ng/mL in ≥97.5% of the study population. 25(OH)D, 25-hydroxyvitamin D.
A similar approach to determine the vitamin D intake corresponding to the RDA-associated 25(OH)D concentration was undertaken by Gallagher et al (3), who randomly assigned 163 postmenopausal white women with 25(OH)D ≤20 ng/mL to 7 doses of vitamin D3 compared with placebo for 12 mo. By using the dose-response curve that resulted from this study in whites, the authors were able to confirm the IOM RDA of 600–800 IU/d, supporting the appropriateness of the methodology. No concerns have been published about misuse of the RDA in that study.
Brannon et al also state that we did not account for “background” intake of dietary vitamin D. However, all subjects in our study were administered dietary and lifestyle questionnaires, and Table 1 in our article clearly shows that in this representative cohort of African Americans, the contribution of vitamin D from dietary sources was extremely low at <200 IU/d at baseline (1). Moreover, plasma 25(OH)D concentrations reflect contributions from all sources of vitamin D, including diet; therefore, our determination of the vitamin D3 dose associated with specific target 25(OH)D concentrations can be interpreted as the amount of vitamin D3 needed in addition to underlying dietary intake.
Finally, Brannon et al raise concerns about vitamin D toxicity at doses near or above the Tolerable Upper Intake Level; however, we did not see any evidence of clinically significant hypercalcemia in our African American cohort, including those treated with 4000 IU vitamin D3/d for 3 mo (1). They also claim that high doses of vitamin D supplementation may pose special concerns for African Americans; they cite a study from the NHANES cohort that reported a reverse J-shaped relation between 25(OH)D concentrations and all-cause mortality, with an adjusted RR of 2.4 for blacks with serum 25(OH)D ≥48 ng/mL compared with ∼30–40 ng/mL, whereas the adjusted RR was 1.6 for the same comparison in whites (4). However, Brannon et al fail to point out that blacks comprised only 10% of the population in that study, for which the abstract explicitly states, “the study was too small to evaluate the association in non-Hispanic black . . . adults.” This statement is supported by a very telling, extremely wide 95% CI surrounding the RR of 2.4 for blacks (95% CI: 0.8, 7.0), which was pointedly omitted from the body of their letter. In addition, Brannon et al also did not mention that the RRs for death were adjusted for only age, sex, race-ethnicity, and season, and that all significant associations between serum 25(OH)D ≥48 ng/mL and increased mortality disappeared when the model was adjusted for additional critical confounding variables, such as BMI, physical activity, and various comorbidities and socioeconomic factors. Last, the suggestion that African Americans are adapted to low circulating 25(OH)D and may therefore be particularly susceptible to vitamin D toxicity seems unlikely in light of observations that African hunter-gatherers in Tanzania with year-round UV-B exposure have mean circulating 25(OH)D concentrations of 46 ng/mL (5).
In conclusion, the intent of our RDA analysis was not to define vitamin D adequacy in a population of African Americans but rather to provide a more accurate estimation of the vitamin D intake that is associated with the IOM-determined 25(OH)D target concentration of 20 ng/mL by using dose-response curves constructed from a rigorous, randomized clinical trial of vitamin D supplementation in African Americans. We believe these dose estimates of vitamin D from race-specific dose-response curves provide a better assessment of the relation between vitamin D supplementation and change in 25(OH)D concentrations in African Americans than does extrapolation from data obtained from white populations.
Acknowledgments
BWH has received support from DiaSorin SpA for serving as an academic consultant. No other relevant financial disclosures or conflicts of interest were reported by the authors for themselves or their spouses, partners, or children.
REFERENCES
- 1.Ng K, Scott JB, Drake BF, Chan AT, Hollis BW, Chandler PD, Bennett GG, Giovannucci EL, Gonzalez-Suarez E, Meyerhardt JA, et al. Dose response to vitamin D supplementation in African Americans: results of a 4-arm, randomized, placebo-controlled trial. Am J Clin Nutr 2014;99:587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press, 2011. [PubMed] [Google Scholar]
- 3.Gallagher JC, Sai A, Templin T, 2nd, Smith L. Dose response to vitamin d supplementation in postmenopausal women: a randomized trial. Ann Intern Med 2012;156:425–37. [DOI] [PubMed] [Google Scholar]
- 4.Sempos CT, Durazo-Arvizu RA, Dawson-Hughes B, Yetley EA, Looker AC, Schleicher RL, Cao G, Burt V, Kramer H, Bailey RL, et al. Is there a reverse J-shaped association between 25-hydroxyvitamin D and all-cause mortality? Results from the U.S. nationally representative NHANES. J Clin Endocrinol Metab 2013;98:3001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luxwolda MF, Kuipers RS, Kema IP, Janneke Dijck-Brouwer DA, Muskiet FA. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr 2012;108(9):1557–61. [DOI] [PubMed] [Google Scholar]