Abstract
Background
Attention-deficit /hyperactivity disorder (ADHD) is associated with substance use and substance use disorders (SUD). However, relatively little is known about the relationship between DSM-IV ADHD subtypes and substance use or DSM-IV abuse/dependence in epidemiological samples.
Methods
Data were obtained from the National Epidemiologic Survey of Alcohol and Related Conditions (NESARC, N=33,588). Respondents reported on ADHD symptoms (DSM-IV) for the period of time when they were 17 years or younger. Lifetime use and DSM-IV abuse/dependence of alcohol, nicotine, cannabis, cocaine, sedatives, stimulants and heroin/opiates were compared across those with ADHD symptoms but no diagnosis (ADHDsx; N=17,009), the Combined (ADHD-C; N=361), Predominantly Inattentive (ADHD-I; N=325), and the Predominantly Hyperactive-Impulsive (ADHD-HI; N=279) ADHD subtypes. Taking a more dimensional approach, inattentive and hyperactive-impulsive symptom counts and their associations with substance use and misuse were also examined.
Results
After adjustments for conduct disorder, major depressive disorder, any anxiety disorder and other socio-demographic covariates, substance use and SUD were associated with ADHDsx, ADHD-C, ADHD-I and ADHD-HI. Overall, substance use and SUD were more weakly associated with the ADHDsx group compared to the three ADHD diagnostic groups. Statistically significant differences were not evident across the three diagnostic groups. Hyperactive-impulsive symptoms were more consistently associated with substance use and SUD compared to inattentive symptoms.
Conclusions
ADHD subtypes are consistently associated with substance use and SUD. The relatively stronger association of hyperactive/impulsive symptoms with substance use and abuse/dependence is consistent with the extant literature noting impulsivity as a precursor of substance use and SUD.
Keywords: ADHD subtypes, substance use, substance use disorders
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD), comprising of inattentive, and/or hyperactive-impulsive symptoms, is known to affect approximately 4.4% adults in the United States (Kessler et al., 2006). Substance use and substance use disorders (SUD) are prominent amongst the numerous comorbid psychopathologies related to ADHD (Biederman, 2004; Sobanski, 2006). A diagnosis of ADHD is known to significantly increase the risk of substance use or SUD independent of other psychiatric comorbidity (Wilens et al. 1997).
Results from large cross-sectional population-based studies on ADHD and SUD have been mixed. Glantz et al., (2009) using the National Comorbidity Survey Replication (NCS-R) reported an association between a lifetime diagnosis of ADHD and SUD. Similarly, a recent study of the National Epidemiological Survey of Alcohol and Related Conditions (NESARC) found a modest association between ADHD and SUD after controlling for psychiatric comorbidity and socio-demographic variables (Bernardi et al., 2012). However, Kessler et al. (2012) using the NCS Adolescent sample reported that adolescent ADHD was associated with subsequent onset of substance related disorders but to a lesser degree than other childhood disorders (e.g. conduct disorder).
In two meta-analyses of this literature, Lee and colleagues (2011) reported that ADHD was prospectively associated with substance use disorders as well as nicotine and illicit drug use (including marijuana), but not alcohol use. Likewise, Charach and colleagues (2011) reported robust associations between ADHD, nicotine, and alcohol use disorders. However, a majority of the extant research on ADHD, substance use and SUD use a composite diagnosis of ADHD. Studies on ADHD subtypes utilizing clinical samples have found the ADHD-C (Combined) subtype to show high rates of substance use disorders (Sprafkin et al., 2007; Wilens et al., 2009).
However, the relationship between ADHD subtypes and substance involvement in the general population remains less well understood.
There is also growing evidence that symptoms of inattention and hyperactivity-impulsivity, even when not satisfying diagnostic thresholds, can influence the likelihood of co-occurring substance use/misuse. A study by Elkins et al (2007) found that hyperactive-impulsive symptoms were associated with initiation of substance use, onset of nicotine dependence, and cannabis use disorders while inattentive symptoms were not. Likewise, in a large Swedish registry, Chang, et al. (2012), found early onset substance use was associated with hyperactive-impulsive but not inattentive symptoms. In contrast, Pingault and colleagues (2013) found trajectories of inattention, but not hyperactivity, were associated with nicotine dependence in a longitudinal cohort of Canadian children. The use of such dimensional indices (e.g. inattentive or hyperactive-impulsive symptoms) is considered valuable even in clinical samples (Molina & Pelham, 2003) and warrant further study.
From the perspective of studying substance involvement, a significant shortcoming of the extant literature is the inability to distinguish the effects of ADHD on substance use versus SUD. For instance, is ADHD associated with starting to smoke, nicotine dependence or both? While there is some overlap in risk factors for initiation and dependence, others are distinct (Kendler et al., 1999; Neale et al., 2006). This distinction is helpful when examining subtypes as factors important to various stages of substance involvement; however, few studies have considered this approach.
Therefore, to contrast subtypes of ADHD, examine the role of non-diagnostic, dimensional (i.e. symptom count) indices of symptomatology, and to improve upon prior studies that have either collapsed across classes of drugs or not examined them in the context of prior substance use, this study leverages a large population based sample of 34,653 US adults (NESARC) to examine the relationship between ADHD subtypes and substance involvement using a categorical and dimensional approach. The main goals of this analysis were:
To examine the relationship between non-diagnostic but symptomatic ADHD as well as DSMIV ADHD subtypes, substance use, and DSM-IV defined SUD.
To examine the relationship between inattentive and hyperactive-impulsive symptom counts, regardless of diagnosis, substance use, and DSM-IV defined SUD.
To examine (a) and (b) for SUD but only in those who have a history of substance use/exposure.
2. Methods
2.1 Sample
Data from the NESARC (waves 1 and 2) were used in these analyses (see Grant et al. 2005 for detailed information on data collection and adjustments). Interviews were conducted by the US Bureau of Census on behalf of the National Institute of Alcohol Abuse and Alcoholism (NIAAA) on non-institutionalized US citizens and non-citizens who were 18 years and older. Wave 1 included 43,093 participants, and data were collected on 34, 653 of these participants approximately 3 years later at Wave 2. Psychiatric diagnoses were assessed using the Alcohol Use Disorder and Associated Disabilities Schedule (AUDADIS-IV). The reliability and validity of AUDADIS-IV has been previously established (Grant et al., 2003; Ruan et al., 2008). Data related to the lifetime presence of Conduct Disorder (CD) was obtained from wave 1, while all other measures were obtained from wave 2. CD related questions were not included in wave 2 since all participants were older than 18 years. Of these, 33,588 had symptom data and were used in analyses.
2.2 Measures
2.2.1 ADHD subtypes and symptom counts
During wave 2, participants were queried about ADHD symptom criteria based on DSMIV (that currently also apply to DSM-5; American Psychiatric Association, 1994; 2013). Twenty questions operationalized the 18 ADHD criterion A symptoms. Each item referred to when the respondent was 17 years or younger (e.g. before age 18, frequently lose things, like assignments or books). The overall DSM-IV ADHD diagnosis has good test-retest reliability with a kappa=0.63-0.71 (Ruan et al. 2008). ADHD subtypes were categorized using the DSM-IV symptom count classification, with the requirement that the requisite number of symptoms persist for a period of 6 months or longer (i.e. Criterion A). DSM-IV B (some symptoms present before age 7), C (impairment in at least two settings), D (interference with developmentally appropriate functioning) and E (exclusions) criteria were not utilized.
Combined subtype (ADHD-C): The presence of six or more inattentive symptoms and six or more hyperactive-impulsive symptoms.
Predominantly Inattentive subtype (ADHD-I): The presence of six or more inattentive symptoms and five or fewer hyperactive-impulsive symptoms.
Predominantly Hyperactive-Impulsive subtype (ADHD-HI): The presence of six or more hyperactive-impulsive symptoms and five or fewer inattentive symptoms.
Individuals not meeting diagnostic criteria were separated into two groups: those that experienced ADHD symptoms (ADHDsx) and those with no symptoms. Dimensional indices of inattentiveness and hyperactivity-impulsivity were created by summing the 9 inattentive and 9 hyperactive-impulsive symptoms.
2.2.2 Substance use and SUD
Responses related to lifetime alcohol, nicotine, cannabis, cocaine, sedative, stimulant, and opiate (including heroin) use and DSM-IV diagnoses of abuse and dependence were utilized. Diagnoses were not extended to DSM-5 as data on craving was unavailable.
Substance use
Substance use was variously defined. Lifetime alcohol use was defined as ever having drunk 12 or more drinks within one year. This higher threshold was used to account for the ubiquitous nature of ever consuming one alcoholic beverage in U.S. populations. Consistent with the CDC definition, lifetime cigarette smoking was defined as having smoked 100 or more cigarettes during the lifetime (Centers for Disease Control, 2007). The use of cannabis, cocaine, sedatives, stimulants, and heroin/opiates even once was considered as lifetime use of the substance as is the norm in the epidemiological literature (e.g. Compton et al., 2013).
Substance use disorders (SUD)
Substance use disorders (SUD) were defined for alcohol, nicotine, cannabis, cocaine, sedatives, stimulants, and heroin/opiates using DSM-IV criteria as the presence of either a lifetime abuse or dependence diagnosis. Abuse was non-hierarchically diagnosed, irrespective of a dependence diagnosis, as the endorsement of one of four criteria. A diagnosis of dependence required the clustering of 3 or more of the 7 dependence criteria within a single 12 month period.
2.2.3 Covariates
Socio-demographic covariates included sex, age (dummy-coded as the lower three quartiles), Caucasian ancestry, and poverty level (defined using census data). Research also shows that conduct disorder (CD), major depressive disorder (MDD), and anxiety disorders (Bernardi et al. 2012, Fischer et al. 2002) are associated with both ADHD and SUD. Also, CD has been found to mediate ADHD and substance involvement (Fergusson et al. 1997; Lynskey & Fergusson, 1995). Therefore, lifetime DSM-IV diagnoses of CD, MDD, and anxiety disorders were included as covariates in all analyses.
2.4 Data analysis
Descriptive statistics and cross tabulations were computed in SAS v9 (SAS institute, 1999) while logistic regressions were conducted in STATA v9.2 (Stata Corp, 2003). All regression analyses included the complex survey design characteristics of the NESARC.
In all analyses, the binary substance use or SUD measure was the dependent variable. Hence, logistic regression was conducted to examine the associations between substance use and SUD measures (adjusting for study design and covariates described above), the ADHD subtypes (ADHD-C, ADHD-I, and ADHD-HI) and the ADHDsx group. Those who did not report any ADHD symptoms served as the reference group. Post-hoc Wald chi-square tests were used to examine whether odds-ratios across the ADHDsx and the three diagnostic subtypes statistically differed from each other. In other words, while the odds-ratios for each group were scaled to refer to those individuals who had no ADHD symptoms, each individual odds-ratio was also compared to the odds-ratios for the other groups via post hoc tests. A similar analysis was conducted using the inattentive and hyperactive-impulsive symptom counts. Counts were standardized to a mean of 0 and a standard deviation of 1.0 such that odds-ratios reflected change for every unit increase or decrease.
Across all analyses SUD were examined in two ways. First, SUD was coded in the full dataset such that those who had never used the substance were coded as 0 for a SUD diagnosis. Second, analyses were done on a conditional definition of SUD for which those who had never used the substance were coded as missing (and excluded from the analyses). The latter analytic strategy allowed us to investigate whether the association between ADHD and SUD persisted after accounting for the association between ADHD subtypes/symptom counts and substance use.
3. Results
3.1 Prevalence of ADHD subtypes/subgroups
The majority of the sample (N=32,623) did not satisfy the DSM-IV symptom criterion (Criterion A) to be classified into one of the three ADHD subtypes, however, 50.6% reported some symptoms (N=17,009). The next largest was the ADHD-C group (N= 361) followed by the ADHD-I group (N=325). The ADHD-HI group was the smallest (N=279). Socio demographic characteristics of the sample are included in Table 1. The range of inattentive (mean=0.90, weighted SD=144.4) and hyperactive-impulsive (mean=1.28, weighted SD=152.3) symptoms counts are shown in Figure 1.
Table 1.
Socio Demographic information of participants (N=33,588) in the DSM-IV ADHD subtypes/subgroups and individuals with ADHD symptoms but not meeting diagnostic thresholds (ADHDsx) and individuals with no ADHD symptoms.
| No symptoms | ADHDsx | ADHD -Combined | ADHD- Inattentive | ADHD-Hyperactive-Impulsive | |
|---|---|---|---|---|---|
| N | 15,614 | 17,009 | 361 | 325 | 279 |
| Mean Age (yrs, SD) | 49.2 | 47.8 | 37.5 | 41.5 | 42.5 |
| Females (%) | 55.9 | 49.4 | 35.38 | 48.31 | 39.11 |
| Caucasian (%) | 69.2 | 72.0 | 77.39 | 78.62 | 78.30 |
| Hispanic (%) | 12.6 | 10.8 | 9.3 | 7.3 | 9.9 |
| Married (%) | 65.7 | 62.6 | 53.1 | 58.1 | 58.8 |
| Completed High School (%) | 86.7 | 85.5 | 78.8 | 84.2 | 87.4 |
| Cohort 1 (%) 18-35 years |
25.1 | 28.5 | 50.14 | 40.90 | 37.86 |
| Cohort 2 (%) 36-47 years |
25.4 | 24.7 | 26.23 | 21.25 | 26.98 |
| Cohort 3(%) 48-61 years |
25.4 | 24.1 | 19.42 | 30.95 | 25.02 |
| Conduct Disorder (%) | 2.5 | 5.8 | 21.40 | 16.04 | 16.82 |
| Lifetime Major Depression (%) | 16.3 | 25.0 | 55.26 | 54.98 | 39.50 |
| Any lifetime Anxiety Disorder* (%) | 19.4 | 29.0 | 60.5 | 53.2 | 44.2 |
| Sought treatment (%)# | - | - | 44.9 | 40.7 | 32.8 |
| Poverty (%) | 11.7 | 12.2 | 19.61 | 16.11 | 13.77 |
Includes a lifetime DSM-IV diagnosis of generalized anxiety disorder, panic disorder, agoraphobia, social anxiety disorder or specific phobias.
Ever saw a counselor/therapist/doctor for help or prescribed medicine; only those with a diagnosis were queried about treatment.
Figure 1.
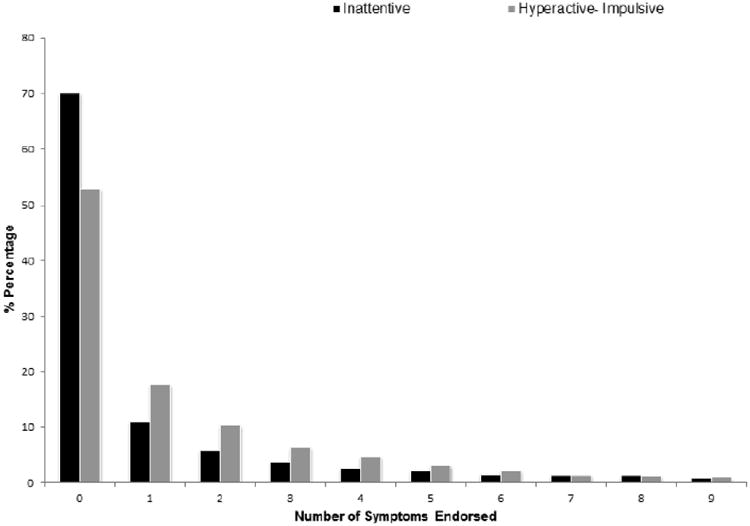
Distribution of 9 inattentive and 9 hyperactive-impulsive symptoms endorsed by a sample of 33,588 adults aged 18 years and older.
3.2 ADHD subtypes/subgroups, substance use, and SUD
Figure 2 shows the prevalence of substance use and SUD (unconditional and conditional on use) across the 5 comparison groups (i.e. no ADHDsx, ADHDsx, ADHD-C, ADHD-I, ADHD-HI). The corresponding odds-ratios (upon adjustment for covariates) depicting the association between individuals with ADHD symptoms who do not meet diagnostic thresholds (ADHDsx), the three ADHD subtypes (ADHD-C, ADHD-I and ADHD-HI) and substance use and SUD are summarized in Table 2.
Figure 2.
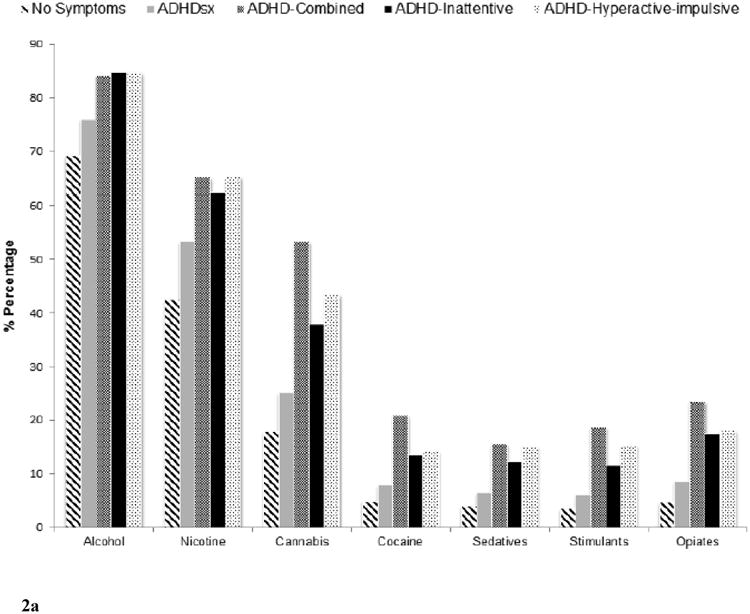
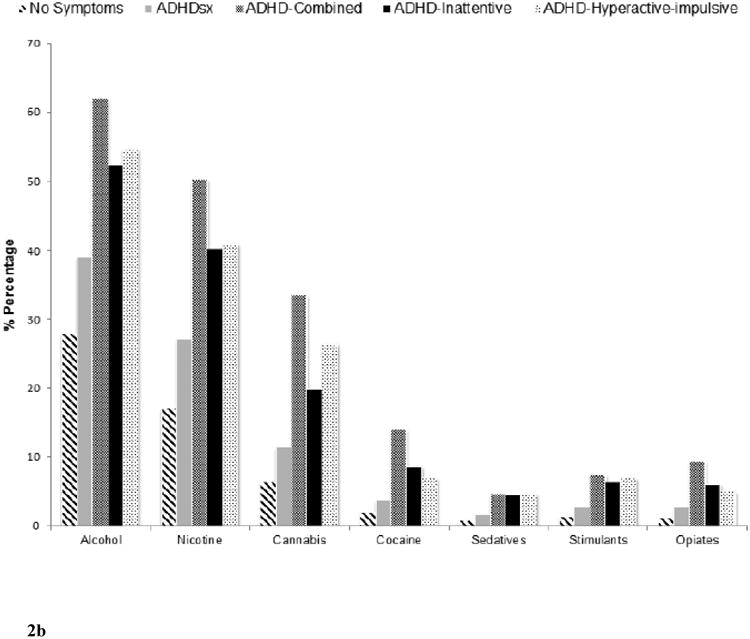
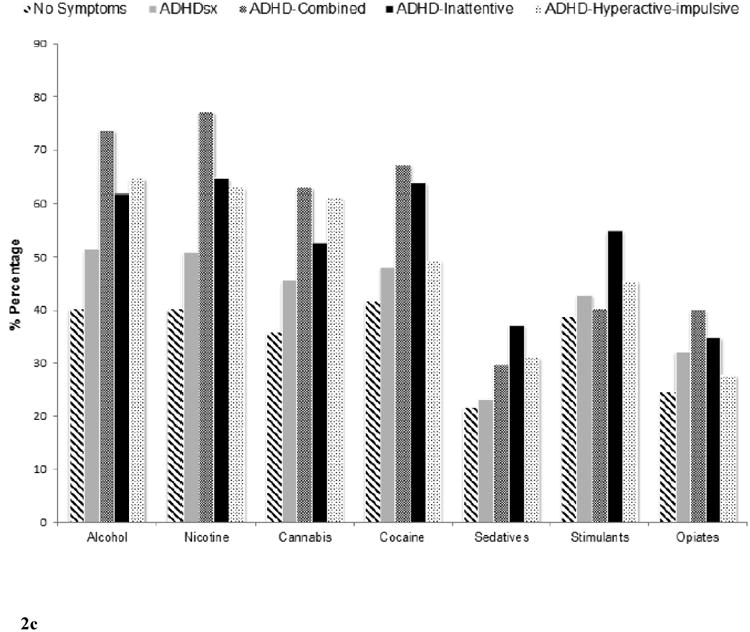
Prevalence of alcohol, nicotine, cannabis, cocaine, sedative, stimulant, and opiate use (Figure 2a), use disorders (Figure 2b) and use disorders conditional on use (Figure 2c) across those with no ADHD symptoms (N=15,614), ADHD symptoms without meeting criteria for a diagnosis (ADHDsx; N=17,009), ADHD Combined (ADHD-C; N=361), ADHD predominantly inattentive (ADHD-I; N=325), and ADHD predominantly hyperactive-impulsive (ADHD-HI; N=279) comparison groups.
Table 2.
Adjusted Odds Ratios with their 95% confidence limits reflecting associations between substance use, abuse/dependence and ADHD subtypes/subgroups and individuals with ADHD symptoms but not meeting diagnostic thresholds (ADHDsx) in a sample of 33,588 adults aged 18 years and older after controlling for age, sex, poverty, ethnicity, and a lifetime history of conduct disorder, major depression and any anxiety disorder.
| ADHDsx (N=17,009) | ADHD Combined (N=361) | ADHD Inattentive (N=325) | ADHD Hyperactive-Impulsive (N=279) | |
|---|---|---|---|---|
| Alcohol Use | 1.25*a(1.17-1.33) | 1.25a(0.84-1.88) | 1.58*a(1.10-2.26) | 1.49a (0.92-2.44) |
| Nicotine Use | 1.38*a(1.30-1.47) | 1.63*a(1.19-2.22) | 1.66*a(1.24-2.23) | 1.87*a(1.35-2.59) |
| Cannabis Use | 1.29*a (1.20-1.38) | 2.14*b (1.58-2.90) | 1.33ac (1.00-1.78) | 1.87*bc(1.33-2.62) |
| Cocaine Use | 1.30*a(1.16-1.47) | 1.88*a(1.27-2.77) | 1.35a(0.85-2.13) | 1.49a(0.95-2.31) |
| Sedative Use | 1.30*a (1.15-1.48) | 1.54*ab(1.02-2.32) | 1.41ab(0.90-2.22) | 2.07*b(1.36-3.15) |
| Stimulant Use | 1.40*a(1.24-1.60) | 2.26*b(1.49-3.40) | 1.50ab (0.97-2.31) | 2.33*b(1.53-3.58) |
| Opiate Use | 1.50*a(1.33-1.69) | 2.19*b(1.55-3.10) | 1.88*ab(1.30-2.72) | 2.18*b(1.50-3.16) |
| Alcohol Use Disorder | 1.39*a(1.31-1.48) | 1.76*a(1.28-2.42) | 1.51*a(1.09-2.07) | 1.65*a(1.16-2.35) |
| Nicotine Dependence | 1.51*a (1.40-1.62) | 2.10*b(1.57-2.81) | 1.65*ab(1.25-2.18) | 1.94*ab(1.40-2.68) |
| Cannabis Use Disorder | 1.45*a(1.31-1.61) | 2.41*b(1.72-3.37) | 1.49ac (1.00-2.22) | 2.43*bc(1.68-3.51) |
| Cocaine Use Disorder | 1.39*a(1.17-1.66) | 2.45*b(1.57-3.82) | 1.80*ab(1.05-3.06) | 1.50ab(0.87-2.54) |
| Sedative Use Disorder | 1.18a (0.90-1.53) | 1.32a(0.68-2.55) | 1.58a (0.76-3.29) | 2.13*a(1.03-4.38) |
| Stimulant Use Disorder | 1.43*a(1.17-1.75) | 1.66a(0.98-2.80) | 1.76a (0.98-3.14) | 2.23*a(1.28-3.88) |
| Opiate Use Disorder | 1.71*a(1.35-2.15) | 2.27*a(1.40-3.67) | 1.77a(0.95-3.31) | 1.71a(0.90-3.22) |
| In Lifetime Users | ||||
| Alcohol Use Disorder (N=24,759) | 1.35*a(1.26-1.44) | 1.96*b(1.37-2.81) | 1.41ab(0.98-2.02) | 1.64*ab(1.13-2.38) |
| Nicotine Dependence (N=16,297) | 1.33*a(1.22-1.46) | 2.23*b(1.53-3.28) | 1.34a(0.95-1.87) | 1.61*ab(1.08-2.39) |
| Cannabis Use Disorder (N=7,438) | 1.26*a(1.11-1.43) | 1.86*b(1.30-2.66) | 1.28ab(0.79-2.08) | 1.95*b(1.31-2.92) |
| Cocaine Use Disorder (N=2,284) | 1.17a(0.94-1.46) | 2.04*a(1.14-3.66) | 1.99*a(1.07-3.70) | 1.07a(0.54-2.10) |
| Sedative Use Disorder (N=1,809) | 0.97a(0.72-1.31) | 0.93a(0.43-2.02) | 1.34a(0.51-3.51) | 1.10a(0.47-2.57) |
| Stimulant Use Disorder (N=1,622) | 1.09a(0.84-1.41) | 0.86a(0.46-1.61) | 1.38a(0.65-2.89) | 1.10a(0.50-2.43) |
| Opiate Use Disorder (N=2,276) | 1.20a(0.91-1.58) | 1.27a(0.71-2.26) | 0.94a(0.44-2.00) | 0.77a(0.37-1.60) |
Indicates significant at p < 0.05 when compared with the reference group of 15,614 individuals with no symptoms.
=Odds Ratios with identical superscripts could be statistically equated to each other at p > 0.05 in post-hoc between group comparisons. For instance, for nicotine use, the odds-ratio for ADHD Combined is 1.63 while the odds-ratio for ADHD Inattentive is 1.66. These are both statistically significant (as indicated by *) and show that, relative to those with no ADHD symptoms, those in these two groups are at higher likelihood for using nicotine. The “a” superscript, in addition, indicates that while these odds-ratios are statistically significant (i.e. greater than 1.0), they are not statistically different from each other (i.e. 1.63 can be statistically equated to 1.66) in posthoc tests.
Compared to those who did not report any ADHD symptoms (the reference group), the ADHDsx group showed elevated rates of use across all of the substances with odds ratios (ORs) ranging from 1.25-1.50. The ADHD-C group also showed elevated levels of use for all substances (except alcohol) (ORs 1.54-2.26). Those in the ADHD-HI group had significantly higher levels of nicotine, cannabis, sedative, stimulant, and opiate use (ORs 1.87-2.33), while those in the ADHD-I group had elevated levels of alcohol (OR=1.58), nicotine (OR=1.66) and opiate use (OR=1.88).
Relative to the reference group, a lifetime history of SUD was significantly more common in the ADHDsx group (except for sedatives- ORs 1.39-1.71) and the ADHD-C subtype (alcohol, nicotine, cannabis, cocaine, and opiates- ORs 1.76-2.45). The ADHD-HI group had elevated rates of alcohol, nicotine, cannabis, sedative, and stimulant use disorders (ORs 1.65-2.43), while the ADHD-I group showed high rates of nicotine dependence, alcohol, and cocaine use disorders (ORs 1.51-1.80).
Overall, the ADHDsx group showed relatively weaker associations with substance use and SUD across all substances (except opiate use disorder) compared to the three ADHD subgroups. (ORs 1.25-1.50 vs. 1.25-2.33 for substance use and ORs 1.18-1.51 vs. 1.32-2.45 for SUD), although only a few of these across-group differences were statistically significant. Importantly, even though associations were not always significant for the ADHD-I or ADHD-HI group, the magnitude of odds-ratios suggested that this may be due to the smaller size of these groups, and hence, lower power.
Subsetting on those with a lifetime history of use (i.e. SUD conditional on substance use) revealed elevated rates of alcohol, nicotine, cannabis, and cocaine use disorders in the ADHD-C subtype (ORs 1.86-2.23). The ADHD-HI subtype had elevated rates of alcohol, nicotine, and cannabis use disorders (ORs 1.61-1.95), while the ADHD-I subtype showed elevated associations with cocaine use disorder (OR=1.99). The ADHDsx group too showed elevated levels of alcohol, nicotine, and cannabis use disorders (ORs 1.26-1.35). However, the magnitude of association for the three disorders was weaker in the ADHDsx group compared to the ADHDC group. No across group differences were apparent except for nicotine dependence which was more weakly associated with the ADHD-I compared to the ADHD-C group.
3.3 ADHD symptom counts, substance use, and SUD
The odds-ratios (upon adjustment for covariates) depicting the associations between ADHD symptom counts (inattentive vs. hyperactive-impulsive) and alcohol, nicotine, cannabis, cocaine, sedative, stimulant, and opiate use and use disorders are summarized in Table 3. Hyperactive-impulsive symptoms were associated with the use of all substances (ORs 1.05-1.10) while inattentive symptoms were also associated with substance use (except alcohol and sedatives) (ORs 1.02-1.06) compared to the reference group (individuals with no symptoms). Hyperactive-impulsive symptoms showed stronger associations with a lifetime history of SUD across all substances (ORs 1.08-1.11) compared to inattentive symptoms (only associated with cannabis use disorder, OR=1.05). For SUD conditional on lifetime use, hyperactive-impulsive symptoms were associated with SUD for alcohol, nicotine and cannabis (ORs 1.05-1.1.08), while inattentive symptoms were associated with cannabis use disorder (OR=1.04).
Table 3.
Adjusted Odds Ratios with their 95% confidence limits reflecting associations between substance use, abuse/dependence and ADHD symptom counts (inattentive vs. hyperactive-impulsive) in a sample of 33,588 adults aged 18 years and older after controlling for age, sex, poverty, ethnicity, and a lifetime history of conduct disorder major depression, and any anxiety disorder.
| ADHD Inattentive Symptoms | ADHD Hyperactive-Impulsive Symptoms | |
|---|---|---|
| Alcohol Use | 0.99 (0.97-1.02) | 1.07* (1.05-1.10) |
| Nicotine Use | 1.02* (1.00-1.04) | 1.10* (1.08-1.12) |
| Cannabis Use | 1.04*a (1.02-1.06) | 1.06*a (1.04-1.08) |
| Cocaine Use | 1.03*a (1.00-1.07) | 1.05*a (1.02-1.09) |
| Sedative Use | 1.02 a (1.00-1.06) | 1.05*a (1.02-1.08) |
| Stimulant Use | 1.06*a (1.02-1.10) | 1.06*a (1.03-1.10) |
| Opiate Use | 1.05*a (1.02-1.08) | 1.07*a (1.04-1.11) |
| Alcohol Use Disorder | 1.01 (0.99-1.03) | 1.08*(1.06-1.11) |
| Nicotine Dependence | 1.02 (1.00-1.04) | 1.11*(1.09-1.13) |
| Cannabis Use Disorder | 1.05*a(1.02-1.08) | 1.08*a(1.05-1.10) |
| Cocaine Use Disorder | 1.03a (1.00-1.07) | 1.08*a(1.03-1.12) |
| Sedative Use Disorder | 0.99a (0.93-1.06) | 1.09*a(1.02-1.16) |
| Stimulant Use Disorder | 1.02a (0.97-1.07) | 1.08*a(1.03-1.13) |
| Opiate Use Disorder | 1.03a (0.99-1.08) | 1.09*a(1.05-1.15) |
| In Lifetime Users | ||
| Alcohol Use Disorder (N=24,759) | 1.02(1.00-1.04) | 1.08*(1.05-1.10) |
| Nicotine Dependence (N=16,297) | 1.02 (1.00-1.04) | 1.08*(1.06-1.10) |
| Cannabis Use Disorder (N=7,438) | 1.04*a(1.02-1.08) | 1.05*a(1.01-1.08) |
| Cocaine Use Disorder (N=2,284) | 1.01a (0.96-1.07) | 1.05a (1.00-1.11) |
| Sedative Use Disorder (N=1,809) | 0.98 a (0.92-1.05) | 1.04 a (0.97-1.12) |
| Stimulant Use Disorder (N=1,622) | 0.95a (0.89-1.01) | 1.05a (0.99-1.12) |
| Opiate Use Disorder (N=2,276) | 0.99a (0.94-1.05) | 1.04a (0.99-1.11) |
Indicates significant at p < 0.05 when compared with the reference group of 15,614 individuals with no symptoms
=Odds Ratios with identical superscripts could be statistically equated to each other at p > 0.05 in post-hoc between symptom count (inattentive vs. hyperactive-impulsive) comparisons.
Overall, for substance use, SUD, and SUD conditional on lifetime use, the associations between inattentive and hyperactive-impulsive symptom counts were similar across all substances with the exception of alcohol use/use disorder and nicotine use/dependence which were more weakly associated with inattentive symptoms.
4. Discussion
This study examines ADHD subtypes, dimensional measures of inattention and hyperactivity-impulsivity (i.e. symptoms counts) and their associations with substance use and SUD in a large representative sample. Importantly, the large sample size helped to examine the association between ADHD and individual SUD, including individual illicit drugs, while accounting for prior associations with substance use. Overall, the prevalence of substance use and SUD in the NESARC was representative of the U.S. population with the possible exception of one study that notes lower rates of illicit drug use and use disorders in this sample (Grucza et al. 2007). Likewise, the prevalence of ADHD is highly comparable to those reported by Kessler and colleagues (2006) in the NCS-R (4.4%). As was done in NCS-R, sub-setting on those aged 18-44 years resulted in a nearly identical prevalence of 4.2%.
Our results are consistent with the extant literature and two meta-analyses on ADHD and substance involvement (Charach, et al., 2011; Lee, et al., 2011).We found associations between nicotine use and nicotine dependence across the three ADHD subtypes. Similar to the meta-analyses, we also found associations between ADHD and alcohol use disorders but no influence of ADHD on drinking onset (except for the ADHD-I subtype). Lee and colleagues (2011) have also reported associations between ADHD and cannabis use, albeit with a high degree of across-study heterogeneity, as well as with cannabis abuse/dependence and other illicit drug use disorders. Our findings reveal similar associations with cannabis and other illicit drugs. However, the associations were restricted to the ADHD-C and ADHD-HI groups. In addition, our study made an important observation – those reporting symptoms of ADHD but not meeting diagnostic criteria (ADHDsx) were also more likely to report comorbid substance use and SUD. As these individuals are often neglected from an etiological and treatment perspective, our analyses draw attention to the high rates of comorbid problems in them.
When considering the dimensional indices, hyperactive-impulsive symptoms showed stronger associations with alcohol and nicotine use and use disorders. Inattentive symptoms were not associated with cocaine, sedative, stimulant, and opiate use disorders while hyperactive-impulsive symptoms were (when not conditional on prior exposure). These results are consistent with two prior studies (Elkins et al., 2007; Chang et al., 2012). Also, substance use shares some of its variance with a general predisposition to disinhibited behaviors, including impulsivity (Hicks et al., 2011; Iacono et al., 2008) and it is possible that this shared predisposition is indexed by symptoms of hyperactivity-impulsivity. This is further evidenced by our analysis of substance use disorders conditional on use. In those analyses, the role of hyperactive-impulsive symptoms (and of the ADHD-HI subtype) appear attenuated. This is likely because while this general disinhibition facilitates onset of use, the progression from use to use disorders is possibly influenced by additional factors.
Differences between our study and the extant clinical literature can be attributed to several factors. Most clinical studies include measures of current ADHD symptoms, or combined assessments of childhood and current symptoms. This is different from the retrospective self-reports used in the current analysis, which may be limited by recall bias. In the current study, we utilized self-report items related to symptoms of inattention and hyperactivity-impulsivity. Subjects recalled the presence or absence of these symptoms prior to age 18. However, the average age of respondents in NESARC (at wave 2) was 49.1 years (range 20-90 years). Further, we noted a decrease in prevalence of ADHD subtypes as a function of interview age, suggesting that younger individuals may have preferentially recalled symptomatology, either due to a current diagnosis as well as decreased participation by older individuals with a current diagnosis (Murphy & Barkley, 1996). Such biases may also be subtype specific, particularly impacting ADHD-HI (Ramtekkar et al., 2010; Willcutt et al., 2012). To account for age-related recall-bias, we conducted sensitivity analyses restricting the sample to those aged 35 and younger (i.e. bottom quartile of wave 2 data). Odds-ratios from this subset of data were well within the confidence limits of the estimates reported in Tables 2 and 3.
Considering the epidemiological nature of this study, it is possible that individuals with a diagnosis of ADHD differed in severity of illness from those in clinical studies. Even though we did not utilize items related to social, academic and occupational impairment to further refine the diagnostic definition, 35% of those diagnosed with any subtype of ADHD reported impairment in interpersonal, social as well as academic/occupational domains. Furthermore, while it is typical to confine the diagnosis of ADHD subtypes to symptoms occurring in the past 6 months, we took a lifetime approach. All of these may have attenuated the severity of the diagnosis. However, studies have suggested that five or more symptoms are empirically optimal in identifying some degree of impairment in children and adults (Lahey et al., 2005).
Individuals with ADHD tend to under-report their symptoms possibly due to a lack of insight into their limitations. Parent and informant reports have been found to be more diagnostically sensitive even in young adults with ADHD (Barkley et al., 2002; Sibley et al., 2012). This too might have influenced the attenuated severity of the ADHD diagnosis as we did not have informant reports. However, Barkley et al. (2002) report the recollection of childhood ADHD symptoms in young adults to correlate moderately with parent reports collected during childhood, supporting the reliability of retrospective self reports to some extent.
Two additional caveats of our study are worth noting – ours is a cross-sectional study, thus, we cannot make any causal inferences about the nature of the relationship between ADHD subtypes, symptoms, and substance involvement. Second, ADHD treatment may have influenced our findings. However, only those with a diagnosis of ADHD were queried about treatment (Table 1). Thus, informative data were not available across all groups to make adequate adjustments for treatment effects.
5. Conclusion
This study adds to the literature by showing that ADHD symptoms (inattentive and hyperactive impulsive) and DSM-IV ADHD subtypes are robustly related to substance use and SUD across a range of substances. Importantly our findings suggest that the presence of ADHD symptoms, even when not satisfying diagnostic criteria, is strongly associated with substance use and SUD. This is an important factor for consideration when tailoring intervention. Furthermore, the current study is the first to explore the associations between ADHD and SUD based on a history of substance use/exposure, indicating that the relationship between ADHD and substance involvement extends well beyond experimentation to maladaptive use and use disorders. While these important observations reinforce the need to study ADHD in the context of SUD and vice versa, longitudinal studies are needed to further disentangle the causal role of these behaviors on each other and their etiologic connections.
Highlights (De Alwis et al).
Data from 33,588 adults from the NESARC are used.
ADHD, regardless of subtype, is associated with substance use and use disorders.
ADHD symptoms without diagnosis are associated with substance involvement.
Hyperactive-impulsive symptoms more consistently correlate with substance involvement.
Acknowledgments
NA
Role of funding source: This research was funded by the National Institute of Drug Abuse (NIDA) grants T32DA007313 (DDA), K02DA32573, DA25886, DA23668 (AA) and National Institute of Mental Health (NIMH) grant MH-080287(AMR). AMR also receives grant funds from the McDonnell Center for Systems Neuroscience and the McDonnell Center for Cellular and Molecular Neurobiology. The content in this paper is the responsibility of the authors and does not represent the official views of the funding agencies.
Footnotes
Contributors: DDA and AA conceived the hypothesis, conducted the analyses, wrote the first draft and revised all versions of the manuscript. MTL and AMR provided feedback on phenotype coding, analysis and critically revised the manuscript.
Conflict of interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Washington, DC: Author; 2013. [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111:279–289. [PubMed] [Google Scholar]
- Bernardi S, Faraone SV, Cortese S, Kerridge BT, Pallanti S, Wang S, Blanco C. The lifetime impact of attention deficit hyperactivity disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Psychol Med. 2012;42:875–887. doi: 10.1017/S003329171100153X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J. Impact of comorbidity in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2004;65(Suppl 3):3–7. [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Cigarette smoking among adults: United States, 2006. Morbidity and Mortality Weekly Report. 2007;56:1157–1161. [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, Larsson H. The effects of childhood ADHD symptoms on early-onset substance use: a Swedish twin study. J Abnorm Child Psychol. 2012;40:425–435. doi: 10.1007/s10802-011-9575-6. [DOI] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011;50:9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Compton WM, Dawson D A, Conway KP, Brodsky M, Grant BF. Transitions in illicit drug use status over 3 years: a prospective analysis of a general population sample. American Journal of Psychiatry. 2013;170:660–670. doi: 10.1176/appi.ajp.2012.12060737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT, Horwood LJ. Attentional difficulties in middle childhood and psychosocial outcomes in young adulthood. J Child Psychol Psychiatry. 1997;38:633–644. doi: 10.1111/j.1469-7610.1997.tb01690.x. [DOI] [PubMed] [Google Scholar]
- Fischer M, Barkley RA, Smallish L, Fletcher K. Young Adult Follow-Up of Hyperactive Children: Self-Reported Psychiatric Disorders, Comorbidity, and the Role of Childhood Conduct Problems and Teen CD. Journal of Abnormal Child Psychology. 2002;30:463–475. doi: 10.1023/a:1019864813776. [DOI] [PubMed] [Google Scholar]
- Glantz MD, Anthony JC, Berglund PA, Degenhardt L, Dierker L, Kalaydjian A, et al. Kessler RC. Mental disorders as risk factors for later substance dependence: estimates of optimal prevention and treatment benefits. Psychol Med. 2009;39:1365–1377. doi: 10.1017/S0033291708004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADISIV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend. 2003;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Blanco C, Stinson FS, Chou PS, Goldstein RB, Dawson DA, Smith S, Saha TD, Huang B. The epidemiology of social anxiety disorder in the United States: results from the national epidemiologic survey of alcohol and related conditions. Journal of Clinical Psychiatry. 2005;66:1351–1361. doi: 10.4088/jcp.v66n1102. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Abbachi AM, Przybeck TR, Gfroerer JC. Discrepancies in estimates of prevalence and correlates of substance use and disorders between two national surveys. Addiction. 2007;102:623–629. doi: 10.1111/j.1360-0443.2007.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Schalet BD, Malone SM, Iacono WG, McGue M. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behav Genet. 2011;41:459–475. doi: 10.1007/s10519-010-9417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, McLaughlin KA, Green JG, Lakoma MD, Petukhova M, et al. Merikangas KR. Lifetime co-morbidity of DSM-IV disorders in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A) Psychol Med. 2012;42:1997–2010. doi: 10.1017/S0033291712000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSMIV Subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Fergusson DM. Childhood conduct problems, attention deficit behaviors, and adolescent alcohol, tobacco, and illicit drug use. J Abnorm Child Psychol. 1995;23:281–302. doi: 10.1007/BF01447558. [DOI] [PubMed] [Google Scholar]
- Molina BS, Pelham WE., Jr Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Murphy KR, Barkley RA. Prevelance of DSM-IV symtpoms of ADHD in adult licensed drivers: implications for clinical diagnosis. J Attention Dis. 1996;1:147–161. [Google Scholar]
- Neale MC, Harvey E, Maes HH, Sullivan PF, Kendler KS. Extensions to the modeling of initiation and progression: applications to substance use and abuse. Behav Genet. 2006;36:507–524. doi: 10.1007/s10519-006-9063-x. [DOI] [PubMed] [Google Scholar]
- Pingault JB, Cote SM, Galera C, Genolini C, Falissard B, Vitaro F, Tremblay RE. Childhood trajectories of inattention, hyperactivity and oppositional behaviors and prediction of substance abuse/dependence: a 15-year longitudinal population-based study. Mol Psychiatry. 2013;18:806–812. doi: 10.1038/mp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J Am Acad Child Adolesc Psychiatry. 2010;49:217–228. [PMC free article] [PubMed] [Google Scholar]
- Ruan WJ, Goldstein RB, Chou SP, Smith SM, Saha TD, Pickering RP, et al. Grant BF. The alcohol use disorder and associated disabilities interview schedule-IV (AUDADIS-IV): reliability of new psychiatric diagnostic modules and risk factors in a general population sample. Drug Alcohol Depend. 2008;92:27–36. doi: 10.1016/j.drugalcdep.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS institute. SAS User Guide, Version 8.2. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- Sibley MH, Pelham WE, Molina BS, Gnagy EM, Waxmonsky JG, Waschbusch DA, et al. Kuriyan B. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80:1052–1061. doi: 10.1037/a0029098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobanski E. Psychiatric comorbidity in adults with attention-deficit/hyperactivity disorder (ADHD) Eur Arch Psychiatry Clin Neurosci. 2006;256 Suppl 1:i26–31. doi: 10.1007/s00406-006-1004-4. [DOI] [PubMed] [Google Scholar]
- Sprafkin J, Gadow KD, Weiss MD, Schneider J, Nolan EE. Psychiatric comorbidity in ADHD symptom subtypes in clinic and community adults. J Atten Disord. 2007;11:114–124. doi: 10.1177/1087054707299402. [DOI] [PubMed] [Google Scholar]
- STATA Corp. STATA, Statistical Software. College Station, TX: STATA Corp; 2003. [Google Scholar]
- Wilens TE, Biederman J, Faraone SV, Martelon M, Westerberg D, Spencer TJ. Presenting ADHD symptoms, subtypes, and comorbid disorders in clinically referred adults with ADHD. J Clin Psychiatry. 2009;70:1557–1562. doi: 10.4088/JCP.08m04785pur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Mick E, Faraone SV, Spencer T. Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. J Nerv Ment Dis. 1997;185:475–482. doi: 10.1097/00005053-199708000-00001. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, et al. Lahey BB. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 2012;121:991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]


