Abstract
The present investigation determined the molecular structure and the pharmacokinetic and pharmacodynamic profiles of oral unfractionated heparin containing oral absorption enhancer sodium N-[8-(2-hydroxybenzoyl) amino]caprylate, salcaprozate sodium (SNAC) and assessed the safety and tolerability of the orally dosed heparin solid dosage form versus other routes. Sixteen healthy men were included in this single-dose, 3-way crossover, randomized, open-label study. Disaccharide compositional analysis was performed using capillary high-performance liquid chromatography with electrospray ionization mass spectrometry detection. The pharmacodynamics of heparin were obtained from analysis of plasma anti–factor Xa, anti–factor IIa, activated partial thromboplastin time, and total tissue factor pathway inhibitor data. The molecular weight properties and the disaccharide composition of orally administered unfractionated heparin/SNAC and parenterally administered unfractionated heparin are identical and consistent with the starting pharmaceutical standard heparin. Furthermore, the anti–factor Xa/anti–factor IIa ratio achieved is of approximately 1:1. This is the first true pharmacokinetic study to measure the chemical compositions of heparin administered by different routes.
Keywords: Oral heparin, anticoagulants, pharmacokinetics, pharmacodynamics, solid dosage form, heparin composition
Heparin, a natural sulfated and highly acidic glycosaminoglycan, is a potent inhibitor of coagulation, primarily through formation of a complex with antithrombin (AT), resulting in indirect inhibition of factor Xa, factor IIa, and other AT-dependent coagulation factors in addition to other AT-independent pathways.1–3 Parenteral administration of heparin has been shown to prevent venous thrombosis and pulmonary embolism, as well as reduce the incidence of myocardial infarction and death in patients with unstable angina and stroke.1,4–8 Various low molecular weight heparins (LMWHs) have also established clinical efficacy in the prevention and treatment of thromboembolic disorders.3–5 Clinical benefit has been associated with heparin-induced prolongation of activated partial thromboplastin time (aPTT) 1.5- to 2.5-fold besides effects on anti–factor Xa and anti–factor IIa, as well as an increase in plasma levels of tissue factor pathway inhibitor (TFPI).1,2,4–8
Heparin is not orally absorbed, presumably because of its size and polyanionic charge, and hence is administered parenterally, either by continuous or intermittent infusion or by subcutaneous (SC) injection.9 However, a formulation that would result in absorption of heparin after oral administration would provide an attractive alternative to parenteral heparin. In that regard, several attempts to develop effective nonparenteral heparin formulations have been reported, but they have met with limited success.10–16 In contrast, synthesized delivery agents based on N-acylated α-amino acids17 and n-acylated non–α-amino acids18 demonstrated in animal models that they promote oral absorption of macromolecules. Sodium N-[8(-2-hydroxybenzoyl) amino] caprylate (SNAC)–mediated intestinal absorption of heparin occurs in a passive transcellular process.18–20 A significant anticoagulant effect was achieved with the oral liquid dosage form of heparin/SNAC in humans, but compliance was an issue because of the taste and its associated nausea and vomiting.21
In this study, a heparin/SNAC capsule formulation, unfractionated heparin (UFH)/SNAC soft gelatin capsules, was used to accomplish the oral delivery of UFH. Earlier studies demonstrated that after the oral administration of heparin/SNAC, anti–factor Xa/anti–factor IIa activities are achieved in a 1:1 ratio, as expected following administration of either intravenous (IV) or SC UFH. The present investigation was undertaken to confirm these earlier findings based on pharmacokinetic (PK) profiling in relation to pharmacodynamic (PD) profiles with the objectives of (1) obtaining the PK profile of heparin by characterizing the molecular structure of UFH in plasma after administration by the oral route, in the presence and absence of SNAC, and IV and SC routes in the absence of SNAC; (2) evaluating the PD profiles of UFH in plasma after administration by different routes, including the oral route in the presence and absence of SNAC; (3) characterizing the PK profile of the SNAC carrier in orally dosed heparin/SNAC capsules; and (4) assessing the safety and tolerance of orally dosed heparin/SNAC capsules compared to parenterally administered UFH.
MATERIALS AND METHODS
UFH/SNAC Soft Gelatin Oral Heparin Capsules
Treatment A: Subjects randomized to treatment A received 2 capsules of UFH/SNAC (heparin sodium, USP formulated with SNAC) soft gelatin capsules (Pharmaceutics International; Hunt Valley, Maryland) for a total dose of 75 000 U heparin sodium with 150 mL of water.
Treatment B: Subjects randomized to treatment B received 1 mL of heparin sodium USP (manufactured by Hyaluron for Emisphere) parenteral 10 000 U/mL via SC injection.
Treatment C: Subjects randomized to treatment C received 75 000 U heparin sodium milled powder for oral solution (manufactured by Emisphere Technologies) with 150 mL of water.
Treatment D: Subjects randomized to treatment D received 0.5 mL of heparin sodium USP (manufactured by Hyaluron for Emisphere) parenteral 10 000 U/mL solution via IV bolus.
Heparin
The main sugars occurring in heparin are (a) α-L-iduronic acid 2-sulfate, (b) 2-deoxy-2-sulfamino-α-D-glucose 6-sulfate, (c) β-D-glucuronic acid, (d) 2-acetamido-2-deoxy-α-D-glucose, and (e) α-L-iduronic acid. These sugars are present in decreasing amounts, usually in the order (b) > (a) > (d) > (c) > (e), and are joined by 1,4-glycosidic linkages forming polymers of varying sizes.
Functional Excipient (Noncompendial Excipient)—Salcaprozate Sodium (SNAC)
Chemical names: Sodium N-[8-(2-hydroxybenzoyl) amino]caprylate, salcaprozate sodium.
Method
This study was an open-label, active controlled, randomized, incomplete block design, 4-treatment, 3-way crossover design to compare single oral doses of UFH/SNAC soft gelatin capsules, oral heparin solution, SC heparin injection, and IV heparin injection. A total of 16 healthy adult male subjects were enrolled and completed the study. Each subject was randomly assigned to receive 3 of the 4 treatments so that a total of 12 subjects received each treatment. Four subjects received treatments A, B, and C; 4 subjects received treatments A, B, and D; 4 subjects received treatments A, C, and D; and 4 subjects received treatments B, C, and D. Each treatment was separated by a minimum 72-hour washout period. The study consisted of screening, 3 treatment periods, and an end-of-study assessment.
Screening
The screening visit took place within 14 days of the first dosing day. During screening, subjects were evaluated for study eligibility. Medical history, physical examination, electrocardiogram (ECG), vital signs, and clinical laboratory evaluations (including urine drug screen and urine alcohol test) were performed at screening. Subjects were screened for HIV, hepatitis B surface antigen (HBsAg), and hepatitis C antibody (HCAb). Upon meeting entry criteria for dosing, subjects entered the treatment phase.
Treatment Period
Subjects checked into the study unit on the evening before study drug administration in each of the 3 treatment periods. Subjects were administered the treatment the following morning, remained in the study unit for 8 to 12 hours, and were discharged after the last blood sample was taken and all safety parameters were within acceptable ranges. Urine drug screen and urine alcohol test were performed prior to dosing during each treatment period. While in the clinic, subjects began a 10-hour overnight fast starting at approximately 10 pm. Fasting blood samples were obtained prior to the first dose of study drug administration for baseline PK and PD analyses, clinical laboratory evaluations, and baseline heparin characterization. Subjects were administered each of their assigned treatments in the fasted state at approximately 8 am. Vital signs were taken approximately 30 minutes before study drug administration and 1, 2, 4, 6, and 8 hours after study drug administration. Blood samples were drawn for up to 8 hours after study drug administration to obtain the PK of SNAC (heparin/SNAC treatment only) and the PD of heparin, as well as up to 2.5 hours after study drug administration for heparin characterization. The doses selected for this trial were based on previous clinical experience.
Diagnosis and Main Criteria for Inclusion
All subjects enrolled in this study were judged by the investigator to be normal, healthy male volunteers who met all inclusion and none of the exclusion criteria.
Institutional Review Board
All pertinent study documents were reviewed by the MDS Pharma (Neptune, New Jersey) Services Institutional Review Board (IRB) prior to study initiation.
Inclusion Criteria
Subjects were in good health, as determined by no clinically significant findings in medical history, physical examination, ECGs, and clinical laboratory determinations. Body weight range was approximately 60 to 100 kg, and body mass index (BMI) was >18 and ≤32 kg/m2.
Exclusion Criteria
Subjects to whom any of the following applied were excluded from the study: hemoglobin <12 g/dL at screening or baseline; current use (within 14 days) of oral anticoagulants, platelet inhibitors, or anti-inflammatory drugs; history of gastrointestinal bleeding, deep vein thrombosis, or pulmonary embolism; history of coagulopathies and bleeding diatheses; absolute platelet count below 100 × 109/L; and any other clinically significant laboratory value at screening that, in the opinion of the investigator, suggested a condition that precluded the subject from entering this study.
Pharmacokinetics/Pharmacodynamics
To characterize the PK of SNAC after oral heparin/SNAC administration and the PD of heparin following the administration of heparin IV, SC, PO alone, and PO as heparin/SNAC, 21 blood samples were drawn from all of the subjects receiving treatments A, B, C, and D at the following time points: within 30 minutes predose; at 2, 5, 10, 15, 20, 25, 30, 35, 40, and 45 minutes postdose; and at 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, and 8 hours postdose. Blood was drawn into sodium citrate tubes for assessment of anti–factor Xa, anti–factor IIa, aPTT, and SNAC.
The PK study used 159 plasma samples from 16 subjects. Unfractionated heparin was recovered and purified from plasma samples using anion exchange spin columns, and UFH was quantified using a micro-carbazole assay. Molecular weight properties were determined using polyacrylamide gel electrophoresis (PAGE), and disaccharide composition was determined by capillary high-performance liquid chromatography (HPLC) using electrospray ionization mass spectrometry (ESI-MS) for detection.22 The PD study measured anti–factor Xa activity, anti–factor IIa activity, total plasma TFPI antigen, and aPTT activities in frozen plasma samples.21,23
Heparin anti–factor Xa and anti–factor IIa assays
The chromogenic assays for the determination of heparin activity in citrated plasma were performed on an ACL 8000 coagulation analyzer (Beckman Coulter; Fullerton, California). Neutralization of anti–factor Xa activity of heparin was determined using the Hemosil heparin kit (linearity: 0–0.8 U/mL; intraassay coefficient of variation [CV]: 8%; inter-assay CV: 9%), obtained from Instrumentation Laboratory Company (Lexington, Massachusetts). For the anti-IIa assay, the Actichrome heparin anti–factor IIa (American Diagnostica; Stamford, Connecticut) was adopted on the ACL 8000 coagulation analyzer (linearity: 0–0.6 U/mL; intra-assay CV: 9%; inter-assay CV: 7%).23
Total TFPI enzyme-linked immunosorbent assay (ELISA)
The AssayMax Human TFPI ELISA kit (AssayPro; Brooklyn, New York) was used for the quantitative analysis of total antigenic TFPI in citrated plasma (assay range: 0–10 ng/mL; intra-assay CV: 9%; inter-assay CV: 17%). The protocol was carried out as described by Mousa et al.24
SNAC bioanalytical assay
Following protein precipitation, samples were directly injected into an HPLC equipped with an Applied Biosystems MDS Sciex API 4000A liquid chromatography/tandem mass spectrometry (LC/MS/MS) system. For details on the analytical methodology, the method of validation, and the analytical within-study quality control procedures, see Brayden et al.19
Pharmacokinetic/Pharmacodynamics Methods
The PK parameters of SNAC and the PD parameters of heparin were analyzed by model-independent analysis of plasma concentrations and activities using SAS and WinNonlin Version 5.0. Plasma PK parameters determined for SNAC included Cmax, tmax, AUClast, AUC∞, t1/2, and Kel. Pharmacodynamic parameters were determined for the baseline-adjusted anti–factor Xa activity, anti–factor IIa activity, and aPTT. Pharmacodynamic parameters included Emax, tmax, and EAUClast. Bioavailability (F), EAUC∞, and t1/2 were also calculated for anti–factor Xa and anti–factor IIa.
Statistical Methods
Heparin PD and SNAC PK were listed with descriptive statistics (mean, standard deviation [SD], sample size [n], minimum, maximum, median, geometric mean, and standard error of the geometric mean) obtained from the SAS statistical package. Mean plasma concentrations versus time profiles of SNAC, baseline-adjusted anti–factor Xa activity, anti–factor IIa activity, and aPTT were presented graphically on a linear scale with SD.
Heparin PK Study
To characterize the molecular structure of heparin in plasma, 25-mL blood volume samples were collected from all subjects following treatments A, B, C, and D. Blood samples were collected at 5 and 35 minutes and at 2.5 hours. These time points were selected so that for any treatment, there would be a time point where heparin was expected to be at the maximum concentration depending on the route of administration. A predose sample (baseline) was also taken from all of the subjects. An extraction process followed by several analytical processes was used on the human plasma collected to characterize the heparin.
Recovery of heparin from plasma
The protocol for recovery of heparin from plasma using anion exchange spin columns was based on a previously developed protocol.22 A single sample for each treatment group was prepared by proportionally combining individual samples at each time point, and these proportionally combined samples were stored until analyzed.22
Uronic acid analysis of recovered heparin
The recovered sample solution was subjected to micro-carbazole assay to quantify the amount of UFH and endogenous glycosaminoglycan (GAG) in each sample.25
Polyacrylamide gel electrophoresis analysis
PAGE was applied to analyze the molecular weight of each sample according to a well-established protocol in our laboratory.26
Disaccharide analysis using LC/MS
Using an established protocol in our laboratory,27 preliminary studies showed that human plasma contains bikunin, a chondroitin sulfate (CS) proteoglycan that interferes with the analysis of heparin in plasma. Hence, CS (endogenous GAG) was subjected to exhaustive digestion with chondroitin lyases to eliminate its inference.26 In this quantitative heparin composition assay, unsaturated heparin disaccharide standards (Seikagaku; Tokyo, Japan) ΔUA-GlcNAc, ΔUA-GlcNS, ΔUA-GlcNAc6S, ΔUA2S-GlcNAc, ΔUAGlcNS6S, ΔUA2S-GlcNS, ΔUA2S-GlcNAc6S, and ΔUA2S-GlcNS6S (where ΔUA is Δ-deoxy-α-L–threo-hex-4-enopyranosyl uronic acid, GlcN is glucosamine, Ac is acetyl, and S is sulfo) were used to produce a standard LC/MS profile. ΔUA-GlcNS, ΔUA-GlcNAc6S, ΔUA2S-GlcNAc, ΔUA-GlcNS6S, ΔUA2S-GlcNS, ΔUA2S-GlcNAc6S, and ΔUA2S-GlcNS6S were used to prepare a standard curve for UFH analysis. Unsaturated chondroitin disaccharide standards ΔUA-GlcNAc, ΔUA2S-GlcNAc, ΔUA-GalNAc, ΔUA-GalNAc4S, ΔUA-GalNAc6S, ΔUA-GalNAc4S6S, and ΔUA2S-GalNAc4S6S (where GalN is galactosamine) were used to prepare a standard curve for CS analysis.27
Safety
Blood was collected at screening, baseline, and end of study for clinical laboratory tests. At check-in for treatment periods 2 and 3, a volume of 4 mL each was drawn to assess hemoglobin. Safety was evaluated through the monitoring of medical history findings, physical examination findings, concomitant medications, vital signs (including blood pressure, respiratory rate, heart rate, and temperature), laboratory tests (hematology, serum chemistry, and urinalysis values), ECGs, adverse events (AEs), and serious adverse events (SAEs).
RESULTS
Study Overall
Of the 16 subjects, 10 were black, 3 were white, 2 were Hispanic, and 1 was Asian. The mean age for all subjects was 36 years (range, 22–48), the mean weight was 81.2 kg (range, 69.7–93.5), and the mean height was 175 cm (range, 166–183). Mean BMI was 26.6 kg/m2 (range, 23.7–30.5). All subjects satisfied inclusion criteria.
SNAC Pharmacokinetics
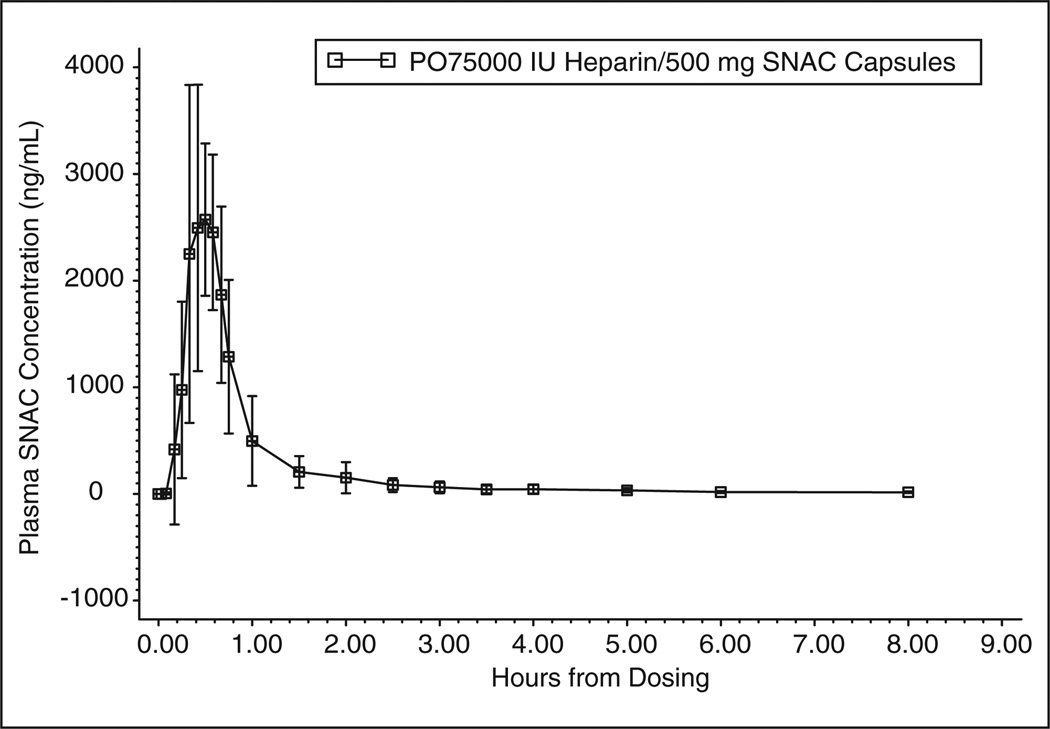
The pharmacokinetic profile of SNAC in plasma following treatment A is shown in Figure 1. Mean peak concentrations of 3316.0 ng/mL (± 1024 SD) were reached at 0.500 hours postdose (0.329 hours min, 0.980 hours max). The mean (± SD) values for AUClast and AUC∞ were 1905.6 (± 341.16) and 2017.8 (± 349.25) ng/mL·h, respectively. Mean (± SD) half-life values were 1.21 (± 0.360) hours with a Kel of 0.615 (± 0.177) hours.
Figure 1.
Sodium N-[8-(2-hydroxybenzoyl) amino] caprylate (SNAC) kinetics in plasma from subjects given treatment A (a single oral dose of unfractionated heparin [UFH]/SNAC). Data represent mean plasma levels of SNAC ± SD.
Pharmacokinetics: Heparin Measurements
Micro-carbazole analysis of recovered heparin
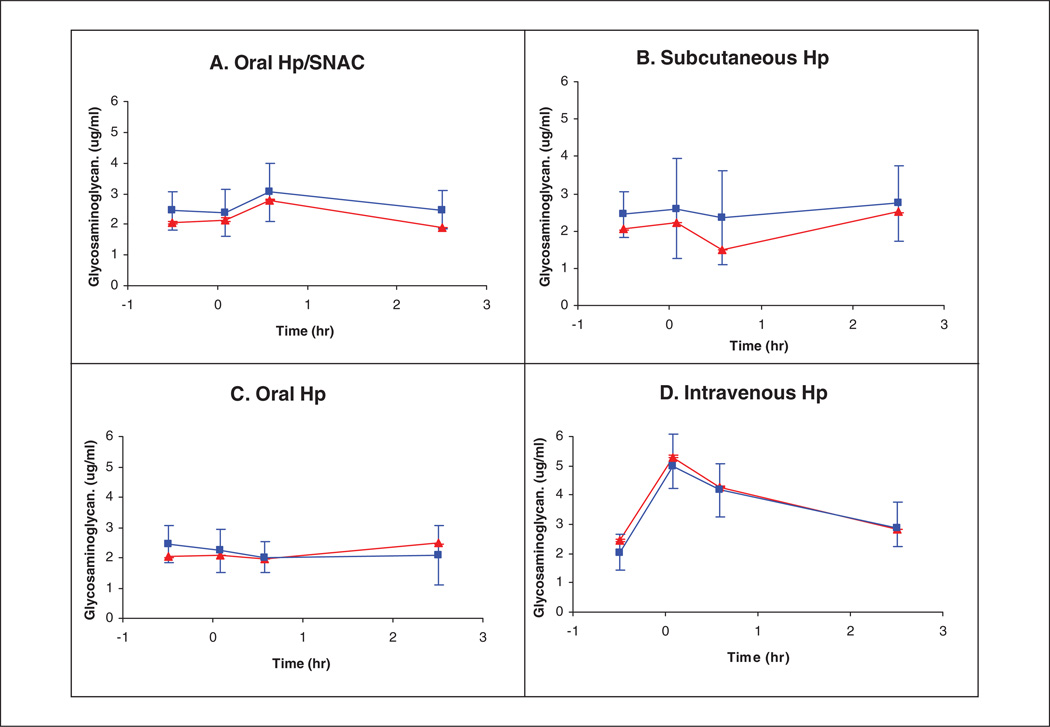
The UFH and endogenous bikunin GAG recovered from plasma samples using anion exchange spin columns were first subjected to micro-carbazole analysis. The results provide a clear PK profile of the 4 treatment groups (Figure 2). The curves corresponding to the averaged individual measurements show substantial error bars associated primarily with individual patient variability. The PK profile, determined on a single sample composed of proportionately recombined individual samples (shown in red), exhibited small error bars associated with analytical error. It is noteworthy that the GAG concentration did not drop below 2 µg/mL in any of the samples tested, as a result of the presence of the endogenous plasma CS associated with bikunin.26
Figure 2.
Carbazole assay results. The heparin/glycosaminoglycan (GAG) concentrations in plasma were calculated from the data of the carbazole assay on recovered heparin/GAG from plasma (squares). The presenting data points are the average from the 12 subjects who received each treatment versus time. Error bars are the standard deviation (n = 16) for the first point (t = −0.5 h); n = 12 for the rest of the points (t = 0.08, 0.58, 2.5 h). Carbazole assay results on combined samples (30 µL each of recovered heparin/GAG samples was combined based on the same treatment, and the same time points are shown as triangles). The heparin/GAG concentrations in plasma were calculated from the data of carbazole assay. The presenting data points are the average from the duplicated assay versus time. Error bars that are smaller than the symbol are the deviation from duplicated carbazole assay.
Relative to intravenously administered heparin, treatment A (a single oral dose of UFH/SNAC) exhibited delayed absorption with a maximum concentration at 0.58 hours, followed by decreased plasma levels. Treatment B (a single subcutaneous dose of UFH) exhibited a delay in absorption for 0.58 hours with an increasing plasma concentration over time measured. Treatment C (a single oral dose of UFH) showed no absorption of UFH. Treatment D (a single intravenous bolus of UFH) showed UFH appearing in the plasma immediately after injection followed by its clearance.
Polyacrylamide gel electrophoresis analysis
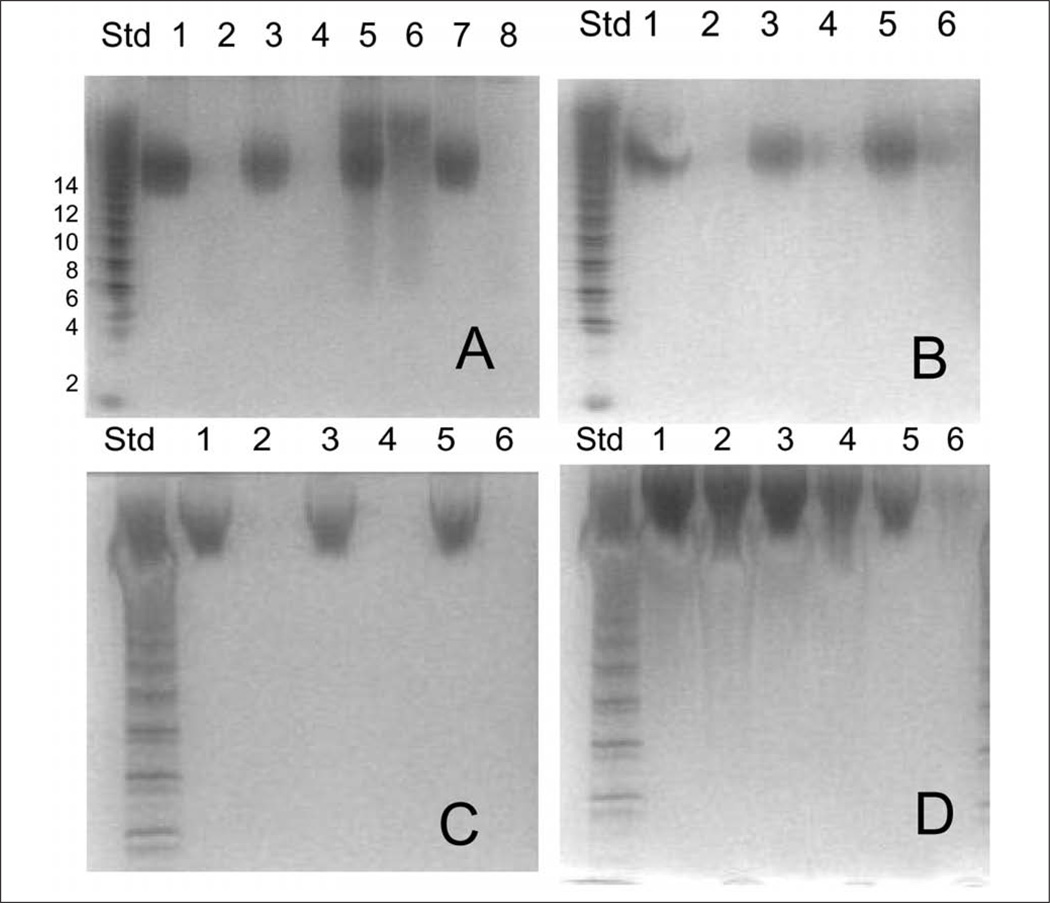
The GAG recovered from plasma was next analyzed by PAGE. Gels showing the analysis of proportionally combined individual samples are shown in Figure 3. The total recovered GAG content (shown in the oddnumbered lanes in each gel) represents a measure of endogenous CS (from bikunin) and exogenously administered UFH. Treatment of each time point with chondroitin lyases (shown in the even-numbered lanes) results in complete breakdown of CS to disaccharide products. These chondroitin disaccharides have insufficient sulfation to be stained; thus, only the UFH component in each time point was observed in the even-numbered lanes in panels A, B, and D. The qualitative results of PAGE analysis on individual samples correlate well with the quantitative results of the micro-carbazole analysis. Time points exhibiting concentrations >2 µg/mL in micro-carbazole analysis (Figure 2) show bands associated with UFH in the even-numbered lanes in gels A to D (Figure 3).
Figure 3.
Polyacrylamide gel electrophoresis (PAGE) analysis on the combined heparin/glycosaminoglycan (GAG) samples (with or without chondroitin lyase treatment) from different treatments. Gel A for treatment A: lane 1: −0.5 h, lane 2: −0.5 h + enzyme, lane 3: 0.08 h, lane 4: 0.08 h + enzyme, lane 5: 0.58 h, lane 6: 0.58 h + enzyme, lane 7: 2.5 h, lane 8: 2.5 h + enzyme. Gel B for treatment B: lane 1: 0.08 h, lane 2: 0.08 h + enzyme, lane 3: 0.58 h, lane 4: 0.58 h + enzyme, lane 5: 2.5 h, lane 6: 2.5 h + enzyme. Gel C for treatment C: lane 1: 0.08 h, lane 2: 0.08 h + enzyme, lane 3: 0.58 h, lane 4: 0.58 h + enzyme, lane 5: 2.5 h, lane 6: 2.5 h + enzyme. Gel D for treatment D: lane 1: −0.5 h, lane 2: −0.5 h + enzyme, lane 3: 0.08 h, lane 4: 0.08 h + enzyme, lane 5: 0.58 h, lane 6: 0.58 h + enzyme.
The individual recovered sample solutions treated with chondroitin lyases were also similarly analyzed by PAGE (not shown). On each gel, the pharmaceutical heparin administered and a mixture of standard heparin oligosaccharides were also analyzed. The heparin oligosaccharide standards (see Figure 3, standard lane) correspond to disaccharide to octadecasaccharide 18 of known molecular weights (MW). By plotting log MW as a function of migration distance, a standard curve was obtained and used to determine the average MW of the pharmaceutical UFH administered as well as the UFH recovered from each plasma sample (Table I). The results of the MW determination demonstrated that the pharmaceutical UFH used in this study had a MWavg 10000 ± 1100 (n = 24). All UFH recovered from plasma samples gave MWavg values identical to that of the pharmaceutical heparin administered (Table I), suggesting that neither preferential adsorption nor metabolism had taken place.
Table I.
Molecular Weight Analysis of Unfractionated Heparin Recovered From Plasma Samples
| Treatment Groups | ||||
|---|---|---|---|---|
| Time, h | A | B | C | D |
| −0.5 | ND | ND | ND | ND |
| 0.08 | ND | ND | 8.4a | 9.5 ± 0.7 (11) |
| 0.58 | 8.7 ± 0.8 (9.6)a | 6.3b (7.9) | ND | 9.5 ± 0.8 (11) |
| 2.5 | 9.3a | 7.8 ± 0.9 (7.9) | ND | 9.8 ± 1 (11) |
ND, not detected.
The number in parentheses corresponds to MWavg obtained from proportionally combined samples.
Only 1 subject shows a band corresponding to unfractionated heparin; no standard deviation was calculated.
Disaccharide analysis of UFH using HPLC-MS
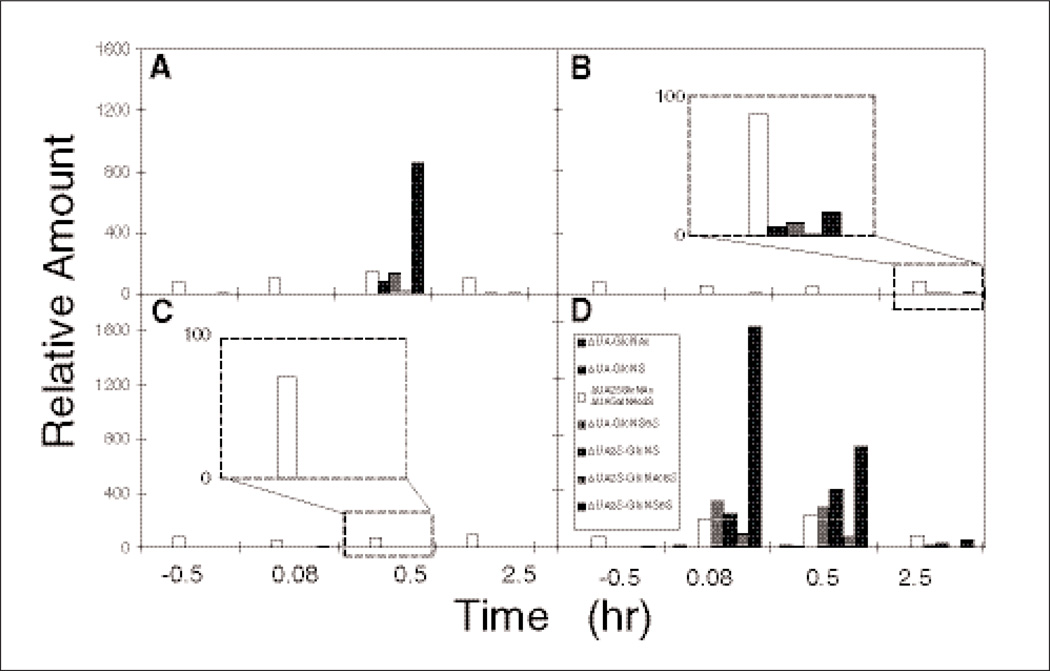
The disaccharide analysis of UFH recovered from proportionally combined samples is shown in Figure 4. Oral UFH/SNAC (Figure 4A) showed the appearance of UFH disaccharides at 0.58 hours. Subcutaneous UFH (Figure 4B) showed the appearance of low levels of UFH disaccharides at the 2.5-hour time point. Oral UFH (Figure 4C) showed only a small amount of disaccharide corresponding to incompletely removed ΔUAGalNAc4S, associated with endogenous CS from bikunin (this disaccharide coelutes with ΔUA2SGlc NAc), and the amount of this disaccharide remained unchanged throughout the time course of this study. These data are consistent with orally administered UFH having no bioavailability, as measured in the plasma compartment. Intravenous UFH (Figure 4D) showed a disaccharide composition at the 0.08-hour time point, with the amount of these disaccharides declining over time.
Figure 4.
Heparin disaccharide analysis (combined samples) using liquid chromatography/mass spectrometry. The different groups were as follows: treatment A = PO unfractionated heparin (UFH)/sodium N-[8-(2-hydroxybenzoyl) amino] caprylate (SNAC; heparin sodium, USP) soft gelatin capsules containing a total of 75 000 IU heparin, treatment B = SQ 10 000 IU heparin, treatment C = PO 75 000 IU heparin, and treatment D = intravenous 5000 IU heparin. The insets in panels B and C show a 16-fold magnification of the 2.5- and 0.5-hour time points in each panel, respectively. The black bar in panels A, B, and D corresponds to the major trisulfated disaccharide found in heparin, whereas the white bar corresponds to incompletely removed ΔUAGalNAc4S, associated with endogenous chondroitin sulfate coeluting with ΔUA2SGlcNAc.
The relative distribution of disaccharides observed in oral UFH/SNAC, intravenous UFH, and subcutaneous UFH was consistent with the composition of the UFH administered. The nearly constant amount of ΔUAGaINAc4S within each of the 4 treatment groups suggests that exogenously administered UFH has no effect on the plasma levels of endogenous GAG.
Heparin Pharmacodynamic
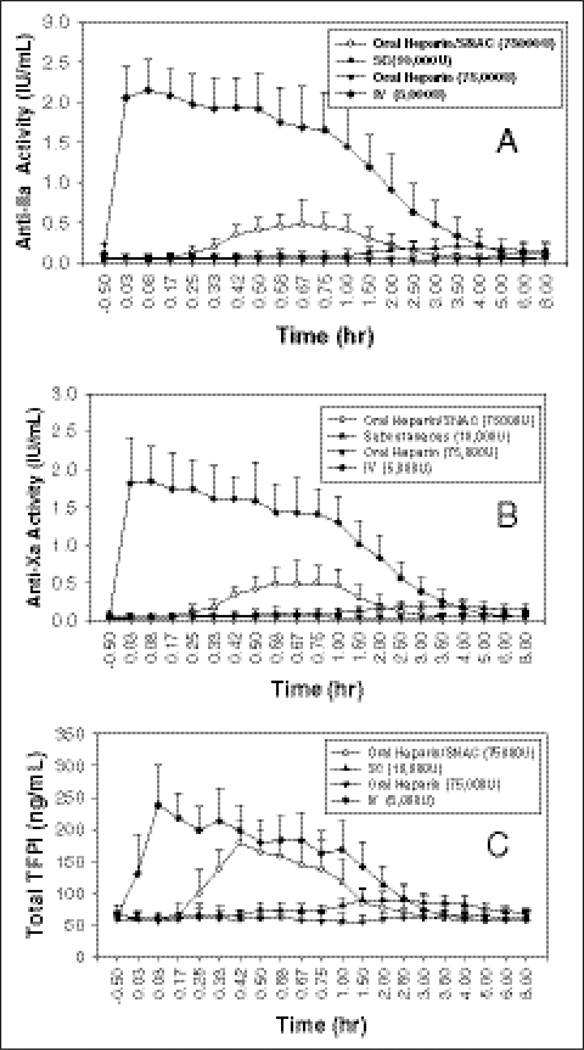
The PD profile of heparin as measured by anti–factor IIa and Xa activities versus time is shown in Figure 5. Although fewer blood samples were taken for the PK evaluation, the heparin PD profile well correlated with the heparin PK profile. The anti– factor IIa and Xa PD parameters of heparin are listed in Tables II and III. The administration of 5000 IU heparin IV resulted in the highest anti–factor IIa and anti–factor Xa activities, as determined by mean baseline adjusted Emax, EAUClast, and EAUC∞ values. Following oral administration of UFH/SNAC containing a total of 75 000 IU heparin, mean Emax was higher than following SC 10 000 IU heparin (0.494 U/mL vs 0.182 U/mL for anti–factor IIa and 0.547 U/mL vs 0.233 U/mL for anti–factor Xa). However, the EAUClast obtained following SC treatment B was comparable or slightly higher than that obtained following UFH/SNAC (0.8651 U·h/mL vs 0.7048 U·h/mL for anti–factor IIa and 1.101 U·h/mL vs 0.8236 U·h/mL for anti–factor Xa). Following a single dose of UFH/SNAC oral administration, the mean anti–factor Xa to anti–factor IIa activity ratio, based on the area under the anti–factor Xa and anti–factor IIa time profiles, approached 1/1.
Figure 5.
Pharmacodynamics of oral heparin versus injectable heparin. Mean and standard deviation of (A) anti–factor Xa and (B) anti–factor IIa activities following administration of oral heparin/sodium N-[8-(2-hydroxybenzoyl) amino] caprylate (SNAC; 75 000 IU), subcutaneous (SC) heparin (10 000 IU), oral heparin (75 000 IU), and intravenous (IV) heparin (5000 IU) to healthy volunteers; n = 12 per group. (C) Mean and standard deviation of plasma total tissue factor pathway inhibitor (TFPI) following administration of oral heparin/SNAC (75 000 IU), SC heparin (10 000 IU), oral heparin (75 000 IU), and IV heparin (5000 IU) to healthy volunteers; n = 12 per group.
Table II.
Summary of the Pharmacodynamic Parameters of Baseline-Adjusted Anti–Factor Xa Activity
| Pharmacodynamic Parameters | Treatment A | Treatment B | Treatment C | Treatment D |
|---|---|---|---|---|
| Emax, U/mL | 0.547 ± 0.261 | 0.233 ± 0.077 | 0.043 ± 0.055 | 1.914 ± 0.464 |
| (12) | (12) | (12) | (12) | |
| tmax, h | 0.666 (0.411, 1.02) | 3.99 (2.50, 7.99) | 0.455 (0.0328, 4.00) | 0.0841 (0.0311, 0.424) |
| (12) | (12) | (6) | (12) | |
| EAUClast, U·h/mL | 0.8236 ± 0.4592 | 1.101 ± 0.5655 | 0.08277 ± 0.2390 | 3.203 ± 0.9855 |
| (12) | (12) | (12) | (12) | |
| EAUC∞, U·h/mL | 1.048 ± 0.4143 | — | — | 3.345 ± 0.9573 |
| (8) | — | — | (12) | |
| t1/2, h | 0.936 ± 0.349 (8) | — | — | 0.703 ± 0.212 (12) |
| F | 0.0219 ± 0.0129 | — | — | — |
| (6) |
Data presented as mean ± SD (n), except for tmax, which is presented as median (minimum, maximum). — = value missing or not reportable. Treatment A = PO unfractionated heparin (UFH)/sodium N-[8(-2-hydroxybenzoyl) amino] caprylate (SNAC) (heparin sodium, USP) soft gelatin capsules containing a total of 75 000 IU heparin. Treatment B = subcutaneous 10 000 IU heparin. Treatment C = PO 75 000 IU heparin. Treatment D = intravenous 5000 IU heparin.
Table III.
Summary of the Pharmacodynamic Parameters of Baseline-Adjusted Anti–Factor IIa Activity
| Pharmacodynamic Parameters | Treatment A | Treatment B | Treatment C | Treatment D |
|---|---|---|---|---|
| Emax, U/mL | 0.494 ± 0.288 | 0.182 ± 0.127 | 0.047 ± 0.065 | 2.156 ± 0.235 |
| (12) | (12) | (12) | (12) | |
| tmax, h | 0.679 (0.498, 1.02) | 3.74 (0.617, 7.99) | 0.592 (0.0828, 4.00) | 0.0883 (0.0311, 0.584) |
| (12) | (10) | (5) | (12) | |
| EAUClast, U·h/mL | 0.7048 ± 0.4773 | 0.8651 ± 0.7034 | 0.1868 ± 0.3392 | 3.666 ± 1.156 |
| (12) | (12) | (12) | (12) | |
| EAUC∞, U·h/mL | 0.7512 ± 0.3514 | — | — | 3.832 ± 1.191 |
| (9) | — | — | (12) | |
| t1/2, h | 0.635 ± 0.291 | — | — | 0.868 ± 0.386 |
| (9) | (12) | |||
| F | 0.0133 ± 0.00811 | — | — | — |
Data presented as mean ± SD (n), except for tmax, which is presented as median (minimum, maximum). — = value missing or not reportable. Treatment A = PO unfractionated heparin (UFH)/sodium N-[8(-2-hydroxybenzoyl) amino] caprylate (SNAC) (heparin sodium, USP) soft gelatin capsules containing a total of 75 000 IU heparin. Treatment B = subcutaneous 10 000 IU heparin. Treatment C = PO 75 000 IU heparin. Treatment D = intravenous 5000 IU heparin.
When heparin was administered PO alone in treatment C (PO 75 000 IU heparin), negligible PD response was observed, whereas when SNAC was coadministered with heparin (UFH/SNAC, treatment A), a significant increase in anti–factor Xa, anti–factor IIa, TFPI, and aPTT activities was observed.
As shown in Figure 6, overall, the aPTT versus time profile correlated well with the antifactors activity profile discussed earlier. Treatment D (IV 5000 IU heparin) resulted in the highest aPTT as determined by mean Emax and EAUClast. Following the oral administration of UFH/SNAC (treatment A), mean Emax values were higher than following heparin SC administration (treatment B) (42 vs 26 seconds). However, the mean EAUClast of treatment B was higher than treatment A (62.26 vs 43.22 s·h). The oral administration of heparin alone (treatment C) showed the lowest aPTT values, as described by the mean EAUClast (15.68 s·h). The mean aPTT values following the oral administration of heparin alone were skewed by a few atypical high aPTT values measured in 3 subjects of this treatment group. As a result, the mean Emax values after this treatment were similar to those observed following the SC administration of heparin and may not be a reliable indicator of mean aPTT activity.
Figure 6.
Activated partial thromboplastin time (aPTT) over time as a function of the different heparin regimens. Data represent mean ± SD.
Adverse Events
All subjects completed the study. No serious AEs occurred. A total of 4 treatment-emergent adverse events (TEAEs) were reported by 2 (19%) subjects in treatment A, and 1 (8%) of the 12 subjects reported TEAEs following treatment D. All TEAEs were mild in severity. The investigator considered 2 of the 4 TEAEs (chest pain and dyspepsia) to be possibly related to study treatment and the 2 remaining TEAEs to be unlikely related to study treatment.
No clinically significant treatment-related trends were observed in the clinical laboratory (serum chemistry, hematology, and urinalysis), vital sign, ECG, or physical examination assessments with respect to subject safety.
DISCUSSION
The primary objective of the current study was to demonstrate that heparin administered orally as UFH/SNAC soft gelatin capsules had the same molecular structure in plasma as the heparin administered IV and SC. Blood samples were collected at time points at which heparin in the dosing arms was at the expected maximum. In addition, samples from a control treatment of oral liquid heparin were analyzed. A crossover design was selected to reduce the intersubject variability and to allow maximum use of the limited sample size. The PD profile of heparin following the studied route of administrations was also characterized, and for this purpose, blood samples were collected up to 8 hours postdose. A 72-hour washout period eliminated pharmacological treatment interactions. The PK and PD parameters reported here are those widely used for the description of concentration or activity versus time data using model-independent analysis. The safety measures were also well-established standard procedures for monitoring a broad range of potential safety issues and possible AEs.
In summary, the MW properties and the disaccharide composition of orally administered heparin/SNAC and parenterally administered heparin are identical and consistent with the starting standard heparin. The results of the carbazole assay showed different patterns of heparin in plasma for each administration route. Relative to IV heparin, treatment A (a single oral dose of heparin/SNAC) showed absorption with maximum concentration at 0.58 hours, followed by decreased plasma heparin concentrations. Treatment B (a single SC dose of heparin) showed sustained plasma concentrations over the time measured. Treatment C (a single oral dose of heparin) showed no absorption based on plasma concentration. Treatment D (a single IV bolus of heparin) showed a maximum heparin concentration immediately after injection followed by clearance.
In the present investigation, combining UFH with the carrier sodium N-(8[2-hydroxybenzoyl] amino) caprylate, or SNAC, has markedly increased the gastrointestinal absorption of this drug, as evident from the present PK/PD study. This study represents the first true PK determination for UFH. Previous PK studies were actually PD studies because they relied on anti-Xa or anti-IIa activities as a surrogate marker for heparin. Intravenous UFH, subcutaneous UFH, and oral UFH/SNAC all showed bioavailability as determined by the presence of chemical UFH in the plasma compartment. Oral UFH administered in the absence of SNAC showed no measurable bioavailability. The UFH observed in the plasma, in each of the 3 study arms showing bioavailability, was identical in MW properties and disaccharide composition to the pharmaceutical UFH administered. No endogenous UFH or heparin sulfate was observed in the plasma. However, a substantial quantity (~2 µg/mL plasma) of CS, derived from the trypsin inhibitor, bikunin, was found in all plasma samples.
The PD parameters confirm the results of the PK study, clearly demonstrating the oral bioavailability of UFH formulated with SNAC. Furthermore, the anti–factor Xa/anti–factor IIa ratio was identical in the oral UFH/SNAC, IV UFH, and SC UFH arms, with a value of 1.0.
This report describes the first controlled evidence for the oral absorption of the heparin solid dosage form in humans. Initial observations were obtained after drug delivery by gavage under double-blind conditions and involved 4 indexes of anticoagulant effect.21 The results indicate that SNAC does promote oral absorption of heparin in humans. The evidence that links the anticoagulant effect of heparin to the prevention of thrombosis holds most strongly for aPTT. The changes in aPTT observed after the single oral dose of heparin administered by gavage were modest. However, TFPI may also reflect the effects of heparin in vivo.28 The maximal effects on TFPI that we observed after oral heparin were an increase of 2.5- to 3-fold. Such increases are within the range of those reported after IV dosing of 7500 U heparin (1.5- to 6.5-fold) and similar to that observed after 5000 anti–factor Xa units of an LMWH.28 However, interindividual differences in the anticoagulant response to conventional heparins are well recognized.28,29 The peak TFPI concentration we observed was at 1 hour after dosing. This is similar to the response reported by Mousa et al24 in healthy male and female human volunteers. Peak values for anti–factor IIa and Xa values were also observed 1 hour after dosing. Thus, the TFPI and anti–factor IIa and Xa concentrations suggest that the timing of the aPTT samples in the first study may have led to an underestimation of the maximum response to oral heparin on this index of anticoagulant effect. Similarly, plasma levels of heparin were not estimated by protamine titration.30 Such an approach may minimize variability in aPTT responses attributable to differences in test reagents.31
This study establishes the feasibility of oral heparin delivery in humans. Both UFH and LMWHs have been demonstrated to be efficacious in the prevention of thrombotic venous and arterial disease.1–7,32 Furthermore, non-anticoagulant properties of heparin may also reduce cardiovascular risk.33 Future studies will focus on different variables such as meals, other commonly used drugs, renal function, and age on the PK/PD of this oral solid dosage of heparin.
Acknowledgments
The authors would like to acknowledge MDS Pharma Services, Neptune, New Jersey; Avantix, New Castle, Delaware; Joseph Fotso, Lewis B. Bender, and Ehud Arbit of Emisphere for his technical support; and Pearl Weisinger for editorial work.
Financial disclosure: This study was supported by research funding from Emisphere to S. A. Mousa and R. J. Linhardt. S. A. Mousa and R. J. Linhardt are consultants to Emisphere, Inc; E. Arbit, M. C. Castelli, K. Friedman, and M. M. Gldberg have stock ownership in Emisphere, Inc; other authors are staff researchers at Pharmaceutical Research Institute and Rensselaer Polytechnic Institute and thus have no financial involvement.
Footnotes
S. A. Mousa designed the research, analyzed data, and wrote the article; F. Zhang, A. Aljada, S. Chaturvedi, M. Takieddin, H. Zhang, and L. Chi performed research; E. Arbit and M. M. Goldberg designed the research; M. C. Castelli and K. Friedman designed the research and analyzed data; and R. J. Linhardt designed research, performed research, and analyzed data.
REFERENCES
- 1.Hirsh J, Warkentin TE, Raschke R, Granger C, Ohman EM, Dalen JE. Heparin and low-molecular-weight-heparin: mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest. 1998;114:489S–510S. doi: 10.1378/chest.114.5_supplement.489s. [DOI] [PubMed] [Google Scholar]
- 2.Jaques LB. Heparins: anionic polyelectrolyte drugs. Pharmacol Rev. 1979;31:99–166. [PubMed] [Google Scholar]
- 3.Jin L, Abrahams JP, Skinner R, Petitou M, Pike RN, Carrell RW. The anticoagulant activation of antithrombin by heparin. Proc Natl Acad Sci USA. 1997;94:14683–14688. doi: 10.1073/pnas.94.26.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hull R, Raskob G, Pineo G, et al. A comparison of subcutaneous low-molecular-weight heparin with warfarin sodium for prophylaxis against deep-vein thrombosis after hip or knee implantation. N Engl J Med. 1993;329:1370–1376. doi: 10.1056/NEJM199311043291902. [DOI] [PubMed] [Google Scholar]
- 5.Koopman MM, Prandoni P, Piovella F, et al. for the Tasman Study Group. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. N Engl J Med. 1996;334:682–687. doi: 10.1056/NEJM199603143341102. [DOI] [PubMed] [Google Scholar]
- 6.Klein W, Buchwald A, Hillis SE, et al. for the FRIC Investigators. Comparison of low-weight heparin with unfractionated heparin acutely and with placebo for 6 weeks in the management of unstable coronary artery disease: Fragmin in Unstable Coronary Artery Disease Study (FRIC) Circulation. 1997;96:61–68. doi: 10.1161/01.cir.96.1.61. [DOI] [PubMed] [Google Scholar]
- 7.FRISC Study Group. Low-molecular weight heparin during instability in coronary artery disease. Lancet. 1996;347:561–568. [PubMed] [Google Scholar]
- 8.Kay R, Wong KS, Yu YL, et al. Low-molecular-weight heparin for the treatment of acute ischemic stroke. N Engl J Med. 1995;333:1588–1593. doi: 10.1056/NEJM199512143332402. [DOI] [PubMed] [Google Scholar]
- 9.DalPozzo A, Acquasaliente M, Geron MR. New heparin complexes active by intestinal absorption: I. Multiple ion pairs with basic organic compounds. Thromb Res. 1989;56:119–124. doi: 10.1016/0049-3848(89)90014-5. [DOI] [PubMed] [Google Scholar]
- 10.Tidball CS, Lipman RI. Enhancement of jejunal absorption of heparinoid sodium ethylenediamine-tetraacetate in the dog. Proc Soc Exp Biol Med. 1962;111:713–715. doi: 10.3181/00379727-111-27900. [DOI] [PubMed] [Google Scholar]
- 11.Sue TK, Jaques LB, Yuen E. Effects of acidity, cations, and alcoholic fractionation on absorption of heparin from the gastrointestinal tract. Can J Physiol Pharmacol. 1976;54:613–618. doi: 10.1139/y76-084. [DOI] [PubMed] [Google Scholar]
- 12.Engel RH, Rigg GJ. Intestinal absorption of heparin facilitated by sulphate or sulfonated surfactant. Proc Soc Exp Bio Med. 1969;13:706–710. doi: 10.1002/jps.2600580612. [DOI] [PubMed] [Google Scholar]
- 13.Guarani S, Ferrari W. Olive oil-provoked, bile-dependent absorption of heparin from the gastrointestinal tract in rats. Pharm Res Commun. 1985;17:685–694. doi: 10.1016/0031-6989(85)90086-4. [DOI] [PubMed] [Google Scholar]
- 14.Uno M, Nagasaki T, Hiroshi I, Sakuragawa N. Oral administration of liposomally-entrapped heparin to beagle dogs. Chem Pharm Bull. 1982;30:2245–2247. doi: 10.1248/cpb.30.2245. [DOI] [PubMed] [Google Scholar]
- 15.Artmann C, Roding J, Ghyczy M, Pratzel HG. Liposomes from soya phospholipids as percutaneous drug carriers. Arzneimittelforschung. 1990;40:1365–1368. [PubMed] [Google Scholar]
- 16.Jacques LB, Hiebert LM, Wice SM. Evidence from endothelium of gastric absorption of heparin and of dextran sulfates 8000. J Lab Clin Med. 1991;117:122–130. [PubMed] [Google Scholar]
- 17.Leone-Bay A, Santiago N, Achan D, et al. N-acylated α-amino acids as novel oral delivery agents for proteins. J Med Chem. 1995;38:4263–4269. doi: 10.1021/jm00021a015. [DOI] [PubMed] [Google Scholar]
- 18.Leone-Bay A, Ho K-K, Agarwal R, et al. 4-[4-(2 hydroxybenzoyl) amino] phenyl butyric acid as a novel delivery agent for recombinant human growth hormone. J Med Chem. 1996;39:2571–2576. doi: 10.1021/jm960038f. [DOI] [PubMed] [Google Scholar]
- 19.Brayden D, Creed E, O’Connell A, Leipold H, Agarwal R, Leone-Bay A. Heparin absorption across the intestin: effects of sodium N-[8-(2-hydroxybenzoyl)amino]caprylate in rat in situ intestinal installations and in caco-2 monolayers. Pharm Res. 1997;14:1772–1779. doi: 10.1023/a:1012192115828. [DOI] [PubMed] [Google Scholar]
- 20.Rivera T, Leone-Bay A, Paton D, Leipold H, Baughman R. Oral delivery of heparin in combination with sodium N-[8-(2-hydroxybenzoyl) amino]caprylate: pharmacology considerations. Pharm Res. 1997;14:1830–1834. doi: 10.1023/a:1012160703533. [DOI] [PubMed] [Google Scholar]
- 21.Baughman RA, Kapoor SC, Agarwal RK, Kisicki J, Catella-Lawson F, FitzGerald GA. Oral delivery of anticoagulant doses of heparin: a randomized, double-blind, controlled study in humans. Circulation. 1998;98:1610–1615. doi: 10.1161/01.cir.98.16.1610. [DOI] [PubMed] [Google Scholar]
- 22.Zhang F, Sun P, Muñoz E, et al. Microscale isolation and analysis of heparin from plasma using an anion-exchange spin column. Anal Biochem. 2006;353:284–286. doi: 10.1016/j.ab.2006.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mousa SA, Linhardt R, Francis JL, Amirkhosravi A. Anti-metastatic effect of a non-anticoagulant low-molecular-weight heparin versus the standard low-molecular-weight heparin, enoxaparin. Thromb Haemost. 2006;96:816–821. doi: 10.1160/th06-05-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mousa SA, Bozarth J, Barrett JS. Pharmacodynamic properties of the low molecular weight heparin, tinzaparin: effect of molecular weight distribution on plasma tissue factor pathway inhibitor in healthy human subjects. J Clin Pharmacol. 2003;43:727–734. [PubMed] [Google Scholar]
- 25.Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 26.Edens RE, Al-Hakim A, Weiler JM, Rethwisch DG, Fareed J, Linhardt RJ. Gradient polyacrylamide gel electrophoresis for determination of molecular weights of heparin preparations and low-molecular-weight heparin derivatives. J Pharm Sci. 1992;81:823–827. doi: 10.1002/jps.2600810821. [DOI] [PubMed] [Google Scholar]
- 27.Thanawiroon C, Rice KG, Toida T, Linhardt RJ. Liquid chromatography/mass spectrometry sequencing approach for highly sulfated heparin-derived oligosaccharides. J Biol Chem. 2004;279:2608–2615. doi: 10.1074/jbc.M304772200. [DOI] [PubMed] [Google Scholar]
- 28.Novotny WF, Brown SG, Miletich JP, Rader DJ, Broze GJ., Jr Plasma antigen levels of the lipoprotein-associated coagulation inhibitor in patient samples. Blood. 1991;78:387–393. [PubMed] [Google Scholar]
- 29.Mousa SA, Kaiser B. Tissue factor pathway inhibitor in thrombosis and beyond: role of heparin. Drugs Future. 2004;29:751–762. doi: 10.1385/1-59259-658-4:133. [DOI] [PubMed] [Google Scholar]
- 30.Hull RD, Raskob GE, Hirsh J, et al. Continuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1986;315:1109–1114. doi: 10.1056/NEJM198610303151801. [DOI] [PubMed] [Google Scholar]
- 31.Brill-Edwards P, Ginsberg JS, Johnston M, Hirsh J. Establishing a therapeutic range for heparin therapy. Ann Intern Med. 1993;119:104–109. doi: 10.7326/0003-4819-119-2-199307150-00002. [DOI] [PubMed] [Google Scholar]
- 32.Cohen M, Demers C, Gurfinkel EP, et al. for the Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group. A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. N Engl J Med. 1997;337:447–452. doi: 10.1056/NEJM199708143370702. [DOI] [PubMed] [Google Scholar]
- 33.Lane DA, Adams L. Non-anti-coagulant uses of heparin. N Engl J Med. 1993;329:129–130. doi: 10.1056/NEJM199307083290212. [DOI] [PubMed] [Google Scholar]