Abstract
The Family Smoking Prevention and Tobacco Control Act gives the Food and Drug Administration power to regulate tobacco products. This commentary calls for immediate regulation of the carcinogenic tobacco-specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N’-nitrosonornicotine (NNN) in cigarette tobacco as a logical path to cancer prevention. NNK and NNN, powerful carcinogens in laboratory animals, have been evaluated as “carcinogenic to humans” by the International Agency for Research on Cancer. NNK and NNN are present in the tobacco of virtually all marketed cigarettes; levels in cigarette smoke are directly proportional to the amounts in tobacco. The NNK metabolite NNAL, itself a strong carcinogen, is present in the urine of smokers and non-smokers exposed to secondhand smoke. Some of the highest levels of NNK and NNN are found in U.S. products. It is well established that factors such as choice of tobacco blend, agricultural conditions, and processing methods influence levels of NNK and NNN in cigarette tobacco and cigarette smoke. Therefore, it is time to control these factors and produce cigarettes with 100 ppb or less each of NNK and NNN in tobacco, which would result in an approximate 15-20 fold reduction of these carcinogens in the mainstream smoke of popular cigarettes sold in the United States.
Keywords: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), N’-nitrosonornicotine (NNN), cigarette tobacco, FDA regulation
Introduction
The Family Smoking Prevention and Tobacco Control Act of 2009 gives the U.S. Food and Drug Administration power to regulate tobacco products, including the establishment of product standards (1). This commentary calls for immediate regulation to decrease levels of the carcinogenic tobacco specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N’-nitrosonornicotine (NNN)(Figure 1) in cigarette tobacco; this will lead to a corresponding reduction in their amounts in cigarette smoke. The need for regulation is based on the strong carcinogenicity of NNK and NNN in laboratory animals, their unreasonably high concentrations in cigarette tobacco, the well documented human exposure to their metabolites, and evidence of human carcinogenicity. NNK and NNN are also important contaminants of smokeless tobacco products which should be similarly regulated, but that aspect is not addressed here.
Figure 1.
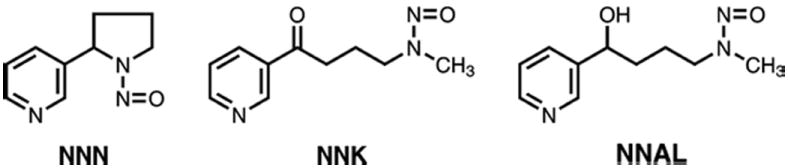
Structures of NNN, NNK, and NNAL
Nitrosamines are strong carcinogens
Following the seminal discovery by Magee and Barnes in 1956 of the powerful hepatocarcinogenicity in the rat of dimethylnitrosamine (2), several research groups in Germany and the U.S., led by Druckrey, Schmahl, Preussmann, Lijinsky, Mirvish and others performed multiple large carcinogenicity studies demonstrating conclusively that approximately 200 structurally varied nitrosamines were carcinogenic, inducing tumors at virtually every conceivable site in at least 30 different species ranging from mollusks to primates (3-6). While this immense and convincing body of research has receded into the shadows, away from the bright sunlight of the current fascination with cancer genomics, epigenetics and molecular networks, it does not alter the essential facts: some nitrosamines are extremely powerful genotoxic carcinogens. Examples can be found in the monumental dose-response studies on 4,080 rats of dimethylnitrosamine and diethylnitrosamine demonstrating a linear relationship between dose and carcinogenic activity down to levels of only 0.1 ppm administered in the drinking water with no sign of a threshold (7). Indeed, these findings caused considerable alarm and research extending through the second half of the twentieth century. Beginning in the 1970s, FDA held regular meetings to assess the threat of nitrosamines in the food supply, eventually leading to changes in processing that reduced levels of these carcinogens in commonly consumed foods and beverages, such as processed meats and beer, to their current levels, generally less than 10 ppb. Tobacco products were not considered at that time, but in 2009 FDA was empowered to regulate tobacco.
The tobacco-specific nitrosamines NNK and NNN are strong carcinogens
The discovery of nitrosamine carcinogenicity occurred soon after the first large epidemiologic studies showing that cigarette smoking caused lung cancer (8,9). Since tobacco contains the addictive compound nicotine as well as a number of structurally related “minor alkaloids” such as nornicotine, anabasine, and pseudooxynicotine among others, scientists were quick to make the logical suggestion that the corresponding “tobacco-specific nitrosamines” might be involved in tobacco carcinogenesis. Boyland was the first to demonstrate the carcinogenicity of NNN in mice (10). This was followed by our studies which showed that NNN was an esophageal carcinogen in rats(11) and that NNK induced lung tumors in mice and adenocarcinoma of the lung in addition to nasal cavity and liver tumors in rats (12,13). These studies were extended by our group and others demonstrating the carcinogenicity of NNK and NNN in rats, mice, hamsters, and mink; this research has been extensively reviewed (14,15). The powerful carcinogenicity of NNK and NNN is evident from these studies and is consistent with observations on many other nitrosamines as noted above. The ability of NNK to induce tumors of the lung, and in particular adenocarcinoma, is especially notable. These tumors are induced independent of the route of administration. Studies in F-344 rats, as an example, demonstrate the predominant induction of lung adenocarcinoma when NNK is given in the drinking water, by subcutaneous injection, by gavage, by oral swabbing, or by intravesicular administration; lung tumors are always induced preferentially over local tumors. The lowest total dose of NNK administered by multiple subcutaneous injections and shown to induce lung tumors in F-344 rats was 1.8 mg/kg, which was significant as part of a dose-response trend (16). Similarly, administration of NNK in the drinking water to F-344 rats, at a dose level of 0.5 ppm (total dose, 15 mg/kg) produced a significant incidence of lung tumors when considered as part of a dose-response trend (17).
Comparing rat and human doses of NNK demonstrates similarity. Based on the tobacco industry’s “total exposure study” study of 3585 smokers, mean excretion of the NNK metabolite NNAL in urine was 439 ng/24h, which is consistent with our data as well as data from the NHANES study (18-20). Assuming that this represents 30% of the NNK dose (21) and unpublished data) mean intake of NNK would be about 1460 ng/24h, or 16 mg in 30 years of smoking. (The 1460 ng figure is also consistent with the measured delivery of 73 ng NNK per cigarette in the mainstream smoke of popular cigarette brands; see also Figure 4) (22,23). Assuming a smoker’s body weight of 75 kg, the total dose of NNK in 30 years of smoking would be about 0.2 mg/kg, which is close to the 0.29 mg/kg human equivalent dose from the rat study, based on body surface area (24). Thus, there is little or no safety factor.
Figure 4.
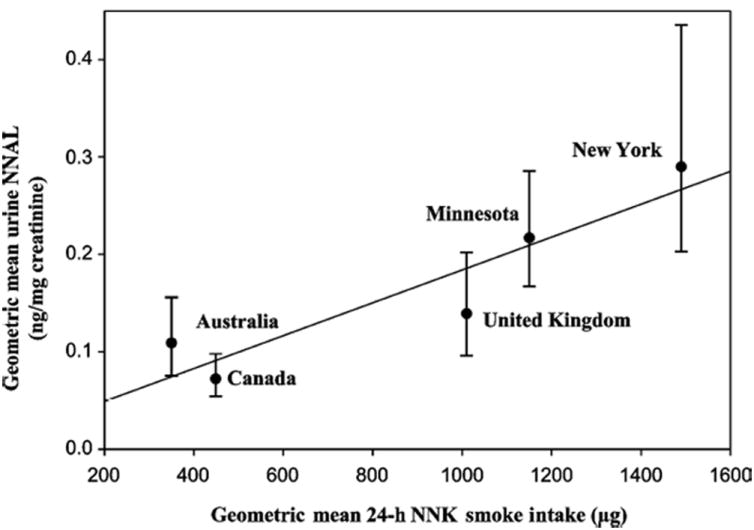
Geometric means of 24-h mouth-level exposure of NNK (ng) versus creatinine-corrected concentrations of urinary NNAL (ng/g creatinine) for the five study sites. Error bars are 95% confidence limits (43).
While less carcinogenic than NNK overall, NNN is remarkable for its ability to induce tumors of the oral cavity, esophagus, and nasal mucosa when administered to rats, tumors of the trachea and nasal cavity in Syrian golden hamsters, pulmonary tumors in mice, and malignant tumors of the nasal cavity, invading the forebrain, in mink (15). In a recent study, the enantiomers of NNN – (S)-NNN (the major form in tobacco) and (R)-NNN – were administered in the drinking water to F-344 rats at a dose of 14 ppm for 74 weeks. The results demonstrated that (S)-NNN is a powerful oral cavity carcinogen, inducing a total of 89 benign and malignant oral cavity tumors in a group of 20 rats, as well as a 100% incidence of esophageal tumors while (R)-NNN was somewhat less active (25). While extensive dose-response data are not available, a dose of racemic NNN of only 5 ppm, administered in the drinking water for 87 weeks, caused esophageal tumors in 71% of the treated rats (26).
It is notable that the induction of lung and oral cavity tumors is relatively rare and difficult in F-344 rats. In the National Toxicology Program, which tests compounds in F-344 rats at the maximum tolerated dose, only 38 of 574 tested compounds (6.6%) induced lung tumors and 4.6% induced oral cavity tumors (27).
NNK and NNN are present in virtually all cigarette brands (tobacco and smoke)
Prior to our initial study on NNN in tobacco, no organic carcinogen had been detected in any tobacco product and it was widely believed that the combustion process, leading to polycyclic aromatic hydrocarbons and other carcinogens, was the dominant factor in producing cigarette smoke carcinogens. Using gas chromatography and mass spectrometry, we positively identified NNN in cigarette tobacco, and quantified it at levels of 2.2 – 6.6 μg/g dry weight (parts per million) (28,29). This was remarkable not only because of the presence of this carcinogen in unburned tobacco, but even more so for its amounts which were far higher than those of carcinogenic nitrosamines in any other consumer product. Putting this in perspective, there was great concern at FDA and elsewhere regarding nitrosamines in cured meat and beer, but with process modification and improvement, the levels of these carcinogens were controlled and lowered to generally less than 10 parts per billion (30), about 1000 times less than in cigarette tobacco.
Following identification of NNN in tobacco, the formation of NNK was predicted based on a study of nicotine nitrosation (31), and ultimately NNK was detected in tobacco by high performance liquid chromatography with confirmation by mass spectrometry (13). Extensive research by the tobacco industry, government agencies, and the academic community followed, establishing highly reliable validated methods for the analysis of both tobacco and cigarette smoke for NNK and NNN as well as several other tobacco-specific nitrosamines not considered here because of their lower amounts or weaker carcinogenicity (14,23,32,33).
Major compendia of amounts of NNK and NNN in cigarette tobacco and smoke have been published (14,22,34). There is considerable variation in levels of these carcinogens in both tobacco and smoke, based on studies of brands marketed worldwide. In one compendium, amounts of NNK in cigarette tobacco ranged from undetectable to 10.7 μg and NNN from 0.045 – 58 μg/g dry weight in filler of cigarettes from various countries around the globe including every continent except Antarctica. Levels of NNK in mainstream smoke (ISO/FTC method) of commercial cigarettes sold internationally ranged from not detected to 1749 ng/cigarette while those of NNN were from 4-2830 ng/cigarette (34). In another collection of data from 401 cigarette samples, levels of NNK in mainstream smoke ranged from 8.7 – 868 ng/cigarette and NNN from 18 – 1760 ng/cigarette (22).
A remarkable study compared levels of NNK and NNN in the mainstream smoke of Marlboro cigarettes versus popular brands from different countries (35). Marlboro cigarettes purchased in various countries had significantly higher NNK plus NNN levels in mainstream smoke (FTC/ISO method) than did local-brand cigarettes from the same country. These results were consistent with an earlier study by the same group demonstrating higher NNK and NNN levels in the tobacco of Marlboro cigarettes compared to those from different countries(Figure 2) (36). The levels of NNK and NNN in the tobacco filler correlated with the levels in their mainstream smoke in this study (Figure 3).
Figure 2.
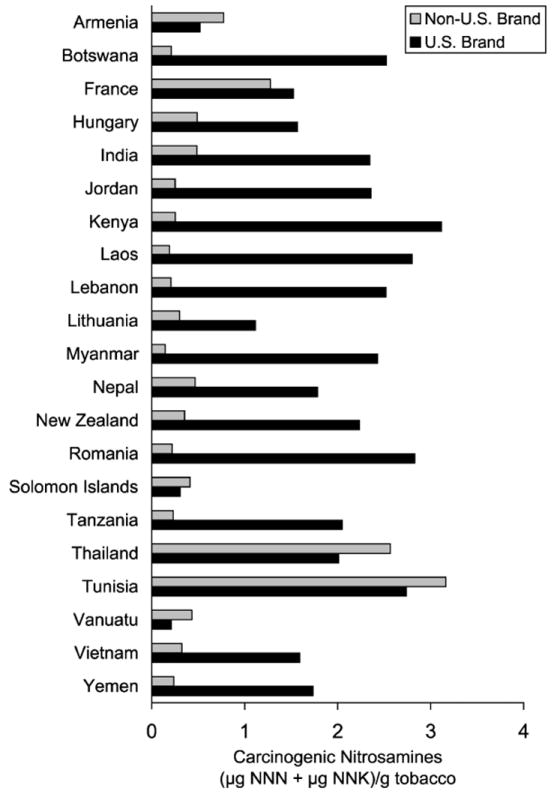
Levels of NNN plus NNK in Marlboro vs. local brands of cigarette tobacco from various countries (36).
Figure 3.
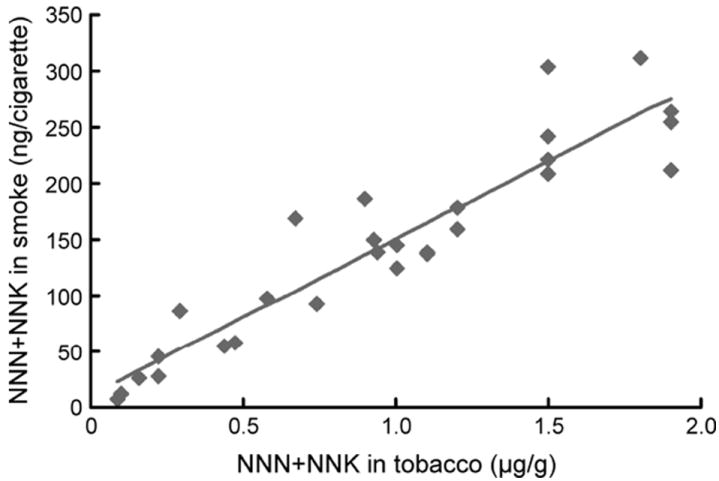
Correlation between amounts of NNN plus NNK in tobacco filler vs. mainstream cigarette smoke (35).
NNK and NNN in e-cigarettes, cigar and pipe tobacco
On April 24, 2014 the FDA announced its intention to regulate e-cigarettes, cigar tobacco and pipe tobacco. NNK and NNN have been detected in the replacement liquids and vapor of e-cigarettes, but the levels are generally considerably lower than in tobacco cigarettes (37,38). One study found that NNN concentrations were 380 times lower and those of NNK 40 times lower in the vapor of e-cigarettes than in the smoke of conventional cigarettes (38). The possibility of endogenous formation of NNN in e-cigarette users, as observed in some oral nicotine replacement product users (39) does not seem to have been investigated. Considerable levels of NNK and NNN have been detected in pipe tobacco and cigar tobacco as well as cigar smoke (14,34). NNAL has been detected in the urine of waterpipe smokers and children exposed to secondhand smoke from waterpipes (40-42). Thus, the regulation of these other types of products, while not the main topic of this commentary, is timely.
Factors Influencing Levels of NNK and NNN in Cigarette Tobacco and Smoke
Extensive studies have examined variables influencing levels of NNK and NNN in tobacco and smoke. A detailed review of these studies has been published (14). In general, NNK and NNN are either undetectable or present at relatively low levels in green tobacco. NNK and NNN are formed from tobacco alkaloids by reactions with nitrite during curing and processing. These reactions can be catalyzed by bacteria and other factors. Concentrations of NNK and NNN vary widely in cured tobacco, reflecting variations in tobacco variety (bright versus Burley), nitrate and nitrite concentrations, alkaloid levels, production year, climate, country of origin, agricultural practices including seed selection and fertilization, location in the tobacco plant, post-harvesting and curing methods, post-curing handling and storage conditions, and other factors. While cigarette design features can also influence levels of NNK and NNN in mainstream smoke, the major factor is clearly transfer from the tobacco filler.
Judicious use of favorable agricultural practices, selection of tobacco variety, formulation of the blend, choice of curing, handling, and storage processes as well as control of bacteria by pasteurization and other methods will lead to reductions of NNK and NNN in tobacco and mainstream smoke, as is clear from international comparisons (Figure 2) and a large amount of other data (14,22,43). The methods to reduce tobacco-specific nitrosamines are available and feasible, as demonstrated by the relatively low amounts of NNK and NNN in the smoke of some cigarettes (22) but manufacturers are reluctant to modify the blend or employ other methods because it could affect the organoleptic properties of the resulting smoke, or have other deleterious effects on marketing and profit.
NNK and NNN levels in American blended cigarettes are generally higher than those in other countries because American cigarettes are made from a blend of Burley tobacco (with high NNK and NNN) and bright tobacco, while cigarettes in many other countries including Canada, Australia, and England are made mainly from bright tobacco and therefore have lower levels of NNK and NNN.
Levels of NNK and NNN in the tobacco of popular products are still unreasonably high. This can be seen in Table 1 which summarizes NNK plus NNN levels in the filler of some popular U.S. cigarette brands marketed in 2010 (23). The levels range from 1.43 – 4.65 μg per gram wet weight. This can be compared to the levels of NNN of 2.2 and 6.6 μg per gram dry weight in the filler of 2 popular U.S. cigarette brands analyzed in our original study, published 40 years ago! There has been little change in 4 decades!
Table 1.
NNN + NNK (μg/g wet weight) in filler of some U.S. cigarettes marketed in 2010 (23).
| Altria Group, Inc. | RJ Reynolds | ||
|---|---|---|---|
| Marlboro Full Flavor | 2.78 | Camel Full Flavor | 1.96 |
| Marlboro Special Blend | 3.09 | Camel No. 9 | 2.28 |
| Marlboro Blend 27 | 3.06 | Camel Silver | 1.43 |
| Marlboro Blend 54 | 4.25 | Camel Crush | 1.77 |
| Marlboro Smooth Menthol | 4.65 | Winston FuII Flavor | 1.85 |
| Basic Full Flavor | 3.09 | Pall Mall Full Flavor | 1.91 |
In summary, there is no doubt: highly reliable methods for the reproducible determination of NNK and NNN in tobacco already exist and the major source of NNK and NNN in cigarette mainstream smoke is transfer from the tobacco. Thus, there is an urgent need to reduce levels of NNK and NNN in tobacco. To the extent that reduction in tobacco is accomplished, levels in cigarette smoke will follow.
All smokers have NNAL and its glucuronides in their urine
When NNK is introduced into virtually any biological system, from a red cell to an entire human, it is converted to varying extents to NNAL (Figure 1) in a reaction catalyzed by ubiquitous carbonyl reductase and related enzymes (15). The carcinogenicity of NNAL is similar to that of NNK (17,44). NNAL is further metabolized to its N- and O-glucuronides in laboratory animals and humans. Free NNAL and its glucuronides are excreted in urine. The sum of these compounds, called “total NNAL”, has been widely used as a biomarker of NNK exposure (45-48).
Several large studies have reported levels of total NNAL in the urine of smokers. The NHANES study of 1373 smokers found a geometric mean of 1.43 pmol/mL urine (20); the “Total Exposure Study” of 3585 smokers reported a weighted mean of 2.10 nmol/24h (18); our combined studies of 1088 smokers found a geometric mean of 1.12 pmol/mL (19); and a recent study of 2641 smokers reported a mean of 1.65 pmol/mL (45). In our experience in the analysis of thousands of urine samples for total NNAL, we have never encountered a negative sample from a smoker; this appears to be consistent with all other reports in the literature, and is also consistent with the fact that virtually all cigarette smoke contains NNK, and all humans convert NNK to NNAL to some extent.
Ashley et al demonstrated a relationship between mouth-level exposure to NNK, determined by analyzing cigarette butts, versus urinary concentrations of total NNAL among 126 daily smokers in four countries with products having differing NNK levels (43). The four countries were Australia and Canada, with relatively low NNK levels in the smoke of cigarettes made mainly from bright tobacco, the United Kingdom with intermediate levels, and the U.S. with the highest amounts. The highest mouth level exposures to NNK and NNN were in the U.S. and the lowest were in Australia and Canada, with intermediate levels found in the United Kingdom. After adjustment for covariates, there was a significant relationship between 24-hour mouth level NNK exposure and creatinine-normalized urinary total NNAL (Figure 4). These results clearly demonstrate and confirm the expected dependence of urinary total NNAL on NNK levels in cigarette smoke. Overall, it is clear that NNK levels in tobacco translate to carcinogen dose, determined by urinary total NNAL in smokers.
Non-smokers exposed to secondhand tobacco smoke have the lung carcinogen NNAL and its glucuronides in their urine
The effects of NNK in cigarette smoke reaches beyond active smokers. Urinary NNAL, itself a potent lung carcinogen, is an important biomarker to explore the effects of secondhand smoke exposure. Its detection in urine signals exposure to NNK, and that exposure can arise only from tobacco products since NNK is a tobacco-specific compound, not found in the diet or general environment (unless tobacco smoke or its residues are present). Exposure to secondhand smoke is an accepted cause of lung cancer in non-smokers (34). The detection of NNAL in the urine of non-smokers creates a plausible biochemical link between exposure and outcome. It is the only tobacco carcinogen biomarker consistently elevated in non-smokers exposed to secondhand tobacco smoke. Following our original studies on NNAL in the urine of men exposed to secondhand smoke (48), we and others have demonstrated its presence in the urine of non-smokers exposed to secondhand smoke, essentially throughout life – from the fetus onward (19,49-52). We have not detected NNAL in the urine of non-smokers who were not exposed to secondhand smoke. The NHANES study of 6599 persons confirmed these results; NNAL was detected in the urine of 44% of the non-smoking participants (53). Furthermore, levels in young children exposed to their parents’ cigarette smoke are higher than those seen in exposed adult non-smokers, probably because young children are frequently in proximity to their smoking parents (53,54). The relatively high levels of NNAL in the urine of young children may have dreadful consequences in the future.
Smokers have NNN and its glucuronides in their urine
NNN is extensively metabolized in laboratory animals, with only a small percentage excreted in the urine. This unchanged NNN as well as its N-glucuronide can be detected in the urine of smokers. Reported values range from about 0.02 – 0.14 pmol/mg creatinine, in the relatively small number of published studies (39,55-59). The lower values of total NNN compared to total NNAL in spite of the higher levels of NNN than NNK in most products most likely result from the relatively high metabolic conversion of NNK to NNAL, while a similar pathway does not exist for NNN.
NNK could be the cause of increasing adenocarcinoma of the lung in U.S. smokers
During the past several decades, adenocarcinoma of the lung has increased in the United States, both in terms of absolute incidence rates and as a fraction of all lung cancers (1,60-63). Adenocarcinoma comprised about 35% of all lung cancers in females in the 1890 birth cohort, whereas in the 1955 birth cohort it comprised 70%; similar increases were seen in males. This increase has been observed among smokers, but not among non-smokers, indicating that it is associated with a change in cigarette design, smoking patterns, or related factors. Two explanations have been widely proposed in the literature for this change (1,61-63). The first concerns changes in cigarette design, including the introduction of filters, the advent of lower tar cigarettes that also delivered less nicotine, and the introduction of other design modifications such as filter ventilation resulting in greater depth of inhalation by smokers and concomitant delivery of smoke constituents to the periphery of the lung (61,64). This explanation is plausible and widely quoted; however, actual physical evidence for penetration of smoke constituents to the lung periphery is lacking.
The second explanation revolves around NNK, which robustly induces adenocarcinoma of the lung in animal models, as noted above. One study noted an increase in levels of NNK in the mainstream smoke (FTC/ISO method) of a leading U.S. non-filter cigarette between 1978, when measurements of this carcinogen first became available, and 1992 while levels of benzo[a]pyrene (BaP) in the mainstream smoke of the same cigarette decreased from 1959-1992 (62). There is debate about the trend in levels of NNK in mainstream smoke since 1992; one publication from the tobacco industry notes a decrease during the short recent interval studied (22). There is no doubt however that levels of BaP, and most likely other polycyclic aromatic hydrocarbons, have decreased from the 1950s to the present. Levels from 1959-1992 were typically 20-40 ng per cigarette while in a 2004 study of more than 40 Philip Morris commercial brands from various international market regions, the average level of BaP was about 7 ng per cigarette while the amount of NNK in the mainstream smoke (FTC/ISO method) of these same cigarettes was 53.5 ng per cigarette (62,65). Therefore, the ratio of NNK to BaP increased from about 2:1 in 1978 to 7.4:1 in 2004 (65). BaP and polycyclic aromatic hydrocarbons tend to induce tumors locally and are plausibly involved in causing squamous cell carcinoma of the lung (66). The increased ratio of NNK to BaP in cigarette mainstream smoke is therefore consistent with the increasing ratio of adenocarcinoma to squamous cell carcinoma of the lung.
Further evidence supporting a role for NNK in the changing histology of lung cancer derives from comparison of rates in the U.S. and Australia. Mouth level exposure to NNK is greater in the U.S. than in Australia (1150-1490 vs. 350 ng/24h), while Canada (449 ng/24h) and the U.K. (1010 ng/24 h) have intermediate levels. As mentioned above, total NNAL levels in the urine of smokers correlated with mouth level exposures to NNK (Figure 4). It has been noted that the increases in adenocarcinoma in Australia, England and Scotland, and Canada have not been as great as in the U.S. (61).
It is plausible that the increased levels of adenocarcinoma are attributable to both higher levels of NNK in cigarette tobacco and to cigarette design changes which lead smokers to smoke more intensely, thereby increasing the areas of the lung exposed to carcinogens. Thus, the U.S. Surgeon General has recently concluded that “there is suggestive evidence that ventilated filters and increased levels of tobacco-specific nitrosamines have played a role” in the increasing incidence of adenocarcinoma of the lung among smokers in the U.S. (1).
Urinary total NNAL and total NNN are related to cancer in smokers; results of prospective epidemiologic studies
The first study to assess the relationship between total NNAL and lung cancer was the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, sponsored by the U.S. National Cancer Institute (67). Levels of total NNAL were compared in serum samples from 100 lung cancer cases and 100 controls, all current smokers, selected from approximately 25,000 smokers in the overall study. A statistically significant association between total NNAL (odds ratio 1.57 per unit standard deviation increase) and lung cancer risk was observed, after correction for number of years of smoking and number of cigarettes smoked per day; a similar significant result was obtained for adenocarcinoma.
The next study assessed the relationship between total NNAL and lung cancer in two prospective cohorts of Chinese cigarette smokers, from Singapore and Shanghai (68). Urinary levels of total NNAL were significantly associated with the risk of lung cancer in a dose-dependent manner. Relative to the lowest tertile, risks associated with the second and third tertiles of total NNAL were 1.43 and 2.11 after adjustment for self-reported smoking history and urinary cotinine.
The third study was a more extensive investigation of male smokers from Shanghai (69). The cohort consisted of 18,244 men enrolled in the 1980s. For the NNAL study, 476 lung cancer cases and 476 matched controls were selected. Again, total NNAL in urine was significantly associated with lung cancer among current smokers, with an odds ratio of 1.98 in the third versus the first tertile, after adjustment for cigarettes smoked per day, number of years of smoking, urinary total cotinine, and urinary phenanthrene tetraol, a biomarker of polycyclic aromatic hydrocarbon uptake.
Collectively, these results provide convincing evidence that total NNAL is a risk biomarker for lung cancer, above and beyond the commonly used parameters of number of years of smoking and cigarettes per day (70,71). These observations are fully consistent with the powerful lung carcinogenicity of NNK. Total NNAL is a much better indicator of NNK exposure than is cigarettes per day or number of years of smoking because it is a direct measure of lung carcinogen uptake.
The relationship between urinary total NNN and esophageal cancer was also examined prospectively in the Shanghai cohort study (55). In 77 individuals who presented with esophageal cancer and 223 individually matched controls, odds ratios for esophageal cancer in the second and third tertiles of total NNN were 3.99 and 17.0, respectively, compared with the first tertile after adjustment for urinary total NNAL and total cotinine as well as smoking intensity and duration. NNN was not associated with lung cancer in this study nor was NNAL associated with esophageal cancer (72). These results show a noteworthy association of NNN with esophageal cancer and demonstrate coherence with carcinogenicity studies of NNN and NNK in rats, in which NNN induces predominantly esophageal and oral cavity cancer, but not lung cancer while NNK induced predominantly lung cancer but not esophageal cancer. Thus, NNN appears to be a risk biomarker for esophageal cancer in smokers.
The goal of these studies is to develop a predictive algorithm that would allow one to identify smokers particularly susceptible to cancer. While that goal has not yet been achieved, the results do indicate that total NNAL and total NNN will be part of the formula, perhaps combined with biomarkers of relevant cellular pathways that may enhance DNA damage. One limitation of these studies is that they were mainly carried out in Asian smokers, who generally smoke different types of cigarettes (usually with lower levels of NNK and NNN) than smokers from the U.S. There are known ethnic differences in susceptibility to lung cancer among smokers, but the underlying reasons have not been fully elucidated (73).
Recommendation
Given that there are already cigarettes with levels of NNK and NNN as low as about 10 ng per cigarette of each in mainstream smoke (22,65), and that the transfer rate from tobacco to mainstream smoke is about 12 - 14% (23,35), a reasonable and achievable standard would be 100 ppb of each in the tobacco filler. The result would be cigarettes with levels of NNK and NNN in mainstream smoke that are approximately 15-20 fold lower than in current popular U.S. brands.
This proposed regulation is somewhat different from that recommended by the World Health Organization under the Framework Convention on Tobacco Control (74). Their recommendation was that levels of NNK and NNN in mainstream smoke of international brands should not exceed 72 and 114 ng/mg nicotine, respectively. These are the median values for NNK and NNN in the data set from a worldwide sample of cigarette brands (65). The intent of this regulation is that levels of the mandated constituents would gradually decrease over time as the brands with levels higher than the mean would be eliminated. This is a more conservative approach than that proposed here.
Possible results of regulation
The cancers most closely associated with exposure to NNK and NNN – lung, oral cavity, and esophagus – will kill an average of more than 500 people per day in the U.S. alone in 2014 (75). Complete avoidance of all tobacco products is the most effective path to significantly decreasing the massive death toll from these diseases. But there are still 42.1 million smokers in the U.S., 18.1% of the adult population (76). We urgently need product standards to help protect those individuals who are addicted to nicotine and will continue to use tobacco products.
The data presented here provide a compelling argument for establishing standards for NNK and NNN in tobacco. It is recognized however that NNK and NNN are only two of the more than 70 established carcinogens in cigarette smoke, listed among others by FDA as “harmful and potentially harmful constituents” (HPHC) of tobacco products (77). Ideally, FDA would require that all HPHC be significantly reduced or eliminated from tobacco products, but that may not be feasible any time soon. FDA regulation of the tobacco-specific nitrosamines NNK and NNN is a logical starting point for constituent regulation, for all the reasons discussed in this commentary. There are no positive attributes of NNK and NNN; only negative. And the methods for controlling their levels in tobacco are well established. But still there are some complex issues which need to be considered. Entire monographs have been written about the potential pitfalls associated with modifications of tobacco products (78-81). A full discussion of these issues is beyond the scope of this commentary, but a number of points deserve mention.
First, would FDA-mandated decreases in levels of NNK and NNN be accompanied by increases in amounts of other toxic and carcinogenic constituents? For example, there are ample data in the literature indicating an inverse relationship between tobacco-specific nitrosamine concentrations and those of carcinogenic polycyclic aromatic hydrocarbons (PAH) in cigarette smoke. Thus, decreases in tobacco nitrate resulting in decreased NNK and NNN concentrations in smoke could lead to an increase in levels of PAH (82). In establishing regulations for NNK and NNN in cigarette tobacco, as suggested here, FDA should require that levels of other HPHC do not significantly increase. With respect to PAH concentrations, there are already data in the literature indicating that this is feasible (65).
Second, if mainstream smoke levels of NNK and NNN were 10 ng each and all other HPHC remained at their current levels, what would be the effect on cancer incidence in smokers, and how long would it take to observe a change? All data summarized here strongly indicate that a decrease in lung, oral cavity, and esophageal cancer incidence among long-term smokers of these modified cigarettes will occur, but predicting the extent or the timing of that decrease would be speculative because there are too many other variables.
Third, post-marketing surveillance, or pharmaco-vigilance, is a critical aspect of the introduction of any new drug or tobacco product (83). What would be the public’s perception of this new product? Would the modified product encourage continued use of cigarettes among smokers who would have otherwise quit? Would it lead to initiation of use among those who thought smoking is now safe? The Family Smoking Prevention and Tobacco Control Act addresses post-marketing surveillance, requiring, for “modified risk tobacco products”, a determination of their impact on consumer perception, behavior, and health (1,83). Much has been written about the ways in which “potentially reduced exposure products” or “modified risk tobacco products” are presented to the public and the potential consequences of misleading statements (78,79). The history of light cigarettes, widely and incorrectly accepted as less harmful, is a well-documented example (84).
Finally, a sequence of studies has been suggested by the Institute of Medicine to evaluate “modified risk tobacco products” (79). Among these, lower exposures as determined by constituent analysis and biomarkers, as discussed here in the preceding sections, represent a necessary first step. Randomized controlled trials and observational epidemiologic studies would play an important role in definitively establishing the benefits of cigarettes with lower levels of NNK and NNN in their smoke.
Although there are some unknown factors associated with the suggested regulation of NNK and NNN in tobacco, it is crucial to do everything in our power, without further delay, to protect millions of addicted smokers from unnecessary exposure to powerful tobacco-specific carcinogens. Carcinogens of this strength in any other consumer product designed for human consumption would be banned immediately.
Acknowledgments
Grant support: This work was supported by grant CA-81301 (S. S. Hecht) from the U.S. National Cancer Institutute.
Footnotes
Conflict of Interest Statement: I have acted as a paid consultant for Tobacco-Free Kids, addressing the issues covered in this commentary. I have no other financial arrangements related to this work.
Reference List
- 1.United States Department of Health and Human Services. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The Health Consequences of Smoking: 50 Years of Progress. [Google Scholar]
- 2.Magee PN, Barnes JM. The production of malignant primary hepatic tumors in the rat by feeding dimethylnitrosamine. Br J Cancer. 1956;10:114–22. doi: 10.1038/bjc.1956.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Druckrey H, Preussmann R, Ivankovic S, Schmähl D. Organotrope Carcinogen Wirkungen bei 65 verschiedenen N-Nitrosoverbindungen an BD-ratten. Z Krebsforsch Klin Onkol. 1967;69:103–201. [PubMed] [Google Scholar]
- 4.Preussmann R, Stewart BW. N-nitroso carcinogens. In: Searle C, editor. Chemical Carcinogens. Second Edition. Vol. 2. Washington, D.C.: American Chemical Society; 1981. pp. 643–828. ACS Monograph 182. [Google Scholar]
- 5.Lijinsky W. Cambridge Monographs on Cancer Research. Cambridge, UK: Cambridge University Press; 1992. Chemistry and Biology of N-nitroso compounds; pp. 251–403. [Google Scholar]
- 6.Bogovski P, Bogovski S. Animal species in which N-nitroso compounds induce cancer. Int J Cancer. 1981;27:471–74. doi: 10.1002/ijc.2910270408. [DOI] [PubMed] [Google Scholar]
- 7.Peto R, Gray R, Brantom P, Grasso P. Effects on 4080 rats of chronic ingestion of N-nitrosodiethylamine or N-nitrosodimethylamine: a detailed dose-response study. Cancer Res. 1991;51:6415–51. [PubMed] [Google Scholar]
- 8.Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma. A study of six hundred and eighty-four proved cases. J Amer Med Assoc. 1950;143:329–36. doi: 10.1001/jama.1950.02910390001001. [DOI] [PubMed] [Google Scholar]
- 9.Doll R, Hill AB. Smoking and carcinoma of the lung. A preliminary report. Br Med J. 1950;ii:739–48. doi: 10.1136/bmj.2.4682.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyland E, Roe FJC, Gorrod JW. Induction of pulmonary tumours in mice by nitrosonornicotine, a possible constituent of tobacco smoke. Nature. 1964;202:1126. doi: 10.1038/2021126a0. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann D, Raineri R, Hecht SS, Maronpot R, Wynder EL. Effects of N’-nitrosonornicotine and N’-nitrosoanabasine in rats. J Natl Cancer Inst. 1975;55:977–81. doi: 10.1093/jnci/55.4.977. [DOI] [PubMed] [Google Scholar]
- 12.Hecht SS, Chen CB, Ohmori T, Hoffmann D. Comparative carcinogenicity in F344 rats of the tobacco specific nitrosamines, N’-nitrosonornicotine and 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1980;40:298–302. [PubMed] [Google Scholar]
- 13.Hecht SS, Chen CB, Hirota N, Ornaf RM, Tso TC, Hoffmann D. Tobacco specific nitrosamines: formation from nicotine in vitro and during tobacco curing and carcinogenicity in strain A mice. J Natl Cancer Inst. 1978;60:819–24. doi: 10.1093/jnci/60.4.819. [DOI] [PubMed] [Google Scholar]
- 14.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 89. Lyon, FR: IARC; 2007. Smokeless tobacco and tobacco-specific nitrosamines; pp. 421–583. [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 16.Belinsky SA, Foley JF, White CM, Anderson MW, Maronpot RR. Dose-response relationship between O6-methylguanine formation in Clara cells and induction of pulmonary neoplasia in the rat by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1990;50:3772–80. [PubMed] [Google Scholar]
- 17.Rivenson A, Hoffmann D, Prokopczyk B, Amin S, Hecht SS. Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Areca-derived N-nitrosamines. Cancer Res. 1988;48:6912–17. [PubMed] [Google Scholar]
- 18.Roethig HJ, Munjal S, Feng S, Liang Q, Sarkar M, Walk RA, et al. Population estimates for biomarkers of exposure to cigarette smoke in adult U.S. cigarette smokers. Nicotine Tob Res. 2009;11:1216–25. doi: 10.1093/ntr/ntp126. [DOI] [PubMed] [Google Scholar]
- 19.Vogel RI, Carmella SG, Stepanov I, Hatsukami DK, Hecht SS. The ratio of a urinary tobacco-specific lung carcinogen metabolite to cotinine is significantly higher in passive than in active smokers. Biomarkers. 2011;16:491–97. doi: 10.3109/1354750X.2011.598565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia Y, Bernert JT, Jain RB, Ashley DL, Pirkle JL. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in smokers in the United States: NHANES 2007-2008. Biomarkers. 2011;16:112–19. doi: 10.3109/1354750X.2010.533288. [DOI] [PubMed] [Google Scholar]
- 21.Stepanov I, Upadhyaya P, Feuer R, Jensen J, Hatsukami DK, Hecht SS. Extensive metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers. Cancer Epidemiol Biomarkers Prev. 2008;17:1764–73. doi: 10.1158/1055-9965.EPI-07-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appleton S, Olegario RM, Lipowicz PJ. TSNA levels in machine-generated mainstream cigarette smoke: 35 years of data. Regul Toxicol Pharmacol. 2013;66:197–207. doi: 10.1016/j.yrtph.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Stepanov I, Knezevich A, Zhang L, Watson CH, Hatsukami DK, Hecht SS. Carcinogenic tobacco-specific N-nitrosamines in US cigarettes: three decades of remarkable neglect by the tobacco industry. Tob Control. 2012;21:44–48. doi: 10.1136/tc.2010.042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 25.Balbo S, James-Yi S, Johnson CS, O’Sullivan G, Stepanov I, Wang M, et al. (S)-N’-Nitrosonornicotine, a constituent of smokeless tobacco, is a powerful oral cavity carcinogen in rats. Carcinogenesis. 2013;34:2178–83. doi: 10.1093/carcin/bgt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoner GD, Adams C, Kresty LA, Hecht SS, Murphy SE, Morse MA. Inhibition of N’-nitrosonornicotine-induced esophageal tumorigenesis by 3-phenylpropyl isothiocyanate. Carcinogenesis. 1998;19:2139–43. doi: 10.1093/carcin/19.12.2139. [DOI] [PubMed] [Google Scholar]
- 27.National Institute of Environmental Health Sciences and National Institutes of Health. [3/12/2-14];National Toxicology Program. 2014 http://ntp.niehs.nih.gov.
- 28.Hoffmann D, Hecht SS, Ornaf M, Wynder WL. N’-nitrosonornicotine in tobacco. Science. 1974;186:265–67. doi: 10.1126/science.186.4160.265. [DOI] [PubMed] [Google Scholar]
- 29.Hecht SS, Ornaf RM, Hoffmann D. N’-Nitrosonornicotine in tobacco: Analysis of possible contributing factors and biologic implications. J Natl Cancer Inst. 1974;54:1237–44. doi: 10.1093/jnci/54.5.1237. [DOI] [PubMed] [Google Scholar]
- 30.National Academy of Sciences - National Research Council Academy of Life Sciences. The Health Effects of Nitrate, Nitrite and N-Nitroso Compounds. Washington D.C.: National Academy Press; 1981. [Google Scholar]
- 31.Hecht SS, Chen CB, Ornaf RM, Jacobs E, Adams JD, Hoffmann D. Reaction of nicotine and sodium nitrite: Formation of nitrosamines and fragmentation of the pyrrolidine ring. J Org Chem. 1978;43:72–76. doi: 10.1021/jo00395a017. [DOI] [PubMed] [Google Scholar]
- 32.Morgan WT, Reece JB, Risner CH, Bennett CB, Midgett CH, Johnson KS, et al. A collaborative study for the determination of tobacco specific nitrosamines in tobacco. Beitrage zur Tabakforsch Intl. 2004;21:192–203. [Google Scholar]
- 33.Wu W, Ashley DL, Watson CH. Simultaneous determination of five tobacco-specific nitrosamines in mainstream cigarette smoke by isotope dilution liquid chromatography/electrospray ionization tandem mass spectrometry. Anal Chem. 2003;75:4827–32. doi: 10.1021/ac030135y. [DOI] [PubMed] [Google Scholar]
- 34.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. Lyon, FR: IARC; 2004. Tobacco Smoke and Involuntary Smoking; pp. 33–1413. [PMC free article] [PubMed] [Google Scholar]
- 35.Wu W, Zhang L, Jain RB, Ashley DL, Watson CH. Determination of carcinogenic tobacco-specific nitrosamines in mainstream smoke from U.S.-brand and non-U.S.-brand cigarettes from 14 countries. Nicotine Tob Res. 2005;7:443–51. doi: 10.1080/14622200500125898. [DOI] [PubMed] [Google Scholar]
- 36.Ashley DL, Beeson MD, Johnson DR, McCraw JM, Richter P, Pirkle JL, et al. Tobacco-specific nitrosamines in tobacco from U.S. brand and non-U.S. brand cigarettes. Nicotine Tob Res. 2003;5:323–31. doi: 10.1080/1462220031000095311. [DOI] [PubMed] [Google Scholar]
- 37.Kim HJ, Shin HS. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2013;1291:48–55. doi: 10.1016/j.chroma.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 38.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2013 doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stepanov I, Carmella SG, Briggs A, Hertsgaard L, Lindgren B, Hatsukami DK, et al. Presence of the carcinogen N’-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Res. 2009;69:8236–40. doi: 10.1158/0008-5472.CAN-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al Ali R, Rastam S, Ibrahim I, Bazzi A, Fayad S, Shihadeh AL, et al. A comparative study of systemic carcinogen exposure in waterpipe smokers, cigarette smokers and non-smokers. Tob Control. 2013 doi: 10.1136/tobaccocontrol-2013-051206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radwan G, Hecht SS, Carmella SG, Loffredo CA. Tobacco-specific nitrosamine exposures in smokers and nonsmokers exposed to cigarette or waterpipe tobacco smoke. Nicotine Tob Res. 2013;15:130–38. doi: 10.1093/ntr/nts099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kassem NO, Daffa RM, Liles S, Jackson SR, Kassem NO, Younis MA, et al. Children’s exposure to secondhand and thirdhand smoke carcinogens and toxicants in homes of hookah smokers. Nicotine Tob Res 2014. 2014 Mar 3; doi: 10.1093/ntr/ntu016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashley DL, O’Connor RJ, Bernert JT, Watson CH, Polzin GM, Jain RB, et al. Effect of differing levels of tobacco-specific nitrosamines in cigarette smoke on the levels of biomarkers in smokers. Cancer Epidemiol Biomarkers Prev. 2010;19:1389–98. doi: 10.1158/1055-9965.EPI-10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Upadhyaya P, Kenney PMJ, Hochalter JB, Wang M, Hecht SS. Tumorigenicity and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) enantiomers and metabolites in the A/J mouse. Carcinogenesis. 1999;20:1577–82. doi: 10.1093/carcin/20.8.1577. [DOI] [PubMed] [Google Scholar]
- 45.Carmella SG, Ming X, Olvera N, Brookmeyer C, Yoder A, Hecht SS. High throughput liquid and gas chromatography-tandem mass spectrometry assays for tobacco-specific nitrosamine and polycyclic aromatic hydrocarbon metabolites associated with lung cancer in smokers. Chem Res Toxicol. 2013;26:1209–17. doi: 10.1021/tx400121n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carmella SG, Akerkar S, Hecht SS. Metabolites of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers’ urine. Cancer Res. 1993;53:721–24. [PubMed] [Google Scholar]
- 47.Shah KA, Karnes HT. A review of the analysis of tobacco-specific nitrosamines in biological matrices. Crit Rev Toxicol. 2010;40:305–27. doi: 10.3109/10408440903394435. [DOI] [PubMed] [Google Scholar]
- 48.Hecht SS, Carmella SG, Murphy SE, Akerkar S, Brunnemann KD, Hoffmann D. A tobacco-specific lung carcinogen in the urine of men exposed to cigarette smoke. N Engl J Med. 1993;329:1543–46. doi: 10.1056/NEJM199311183292105. [DOI] [PubMed] [Google Scholar]
- 49.Lackmann GM, Salzberger U, Tollner U, Chen M, Carmella SG, Hecht SS. Metabolites of a tobacco-specific carcinogen in the urine of newborns. J Natl Cancer Inst. 1999;91:459–65. doi: 10.1093/jnci/91.5.459. [DOI] [PubMed] [Google Scholar]
- 50.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–22. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 51.Milunsky A, Carmella SG, Ye M, Hecht SS. A tobacco-specific carcinogen in the fetus. Prenat Diagn. 2000;20:307–10. doi: 10.1002/(sici)1097-0223(200004)20:4<307::aid-pd797>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 52.Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, et al. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob Res. 2011;13:202–08. doi: 10.1093/ntr/ntq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernert JT, Pirkle JL, Xia Y, Jain RB, Ashley DL, Sampson EJ. Urine concentrations of a tobacco-specific nitrosamine carcinogen in the U.S. population from secondhand smoke exposure. Cancer Epidemiol Biomarkers Prev. 2010;19:2969–77. doi: 10.1158/1055-9965.EPI-10-0711. [DOI] [PubMed] [Google Scholar]
- 54.Thomas JL, Guo H, Carmella SG, Balbo S, Han S, Davis A, et al. Metabolites of a tobacco-specific lung carcinogen in children exposed to secondhand or thirdhand tobacco smoke in their homes. Cancer Epidemiol Biomarkers Prev. 2011;20:1213–21. doi: 10.1158/1055-9965.EPI-10-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I. Urinary levels of the tobacco-specific carcinogen N’-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis. 2011;32:1366–71. doi: 10.1093/carcin/bgr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stepanov I, Hecht SS. Tobacco-specific nitrosamines and their N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol Biomarkers Prev. 2005;14:885–91. doi: 10.1158/1055-9965.EPI-04-0753. [DOI] [PubMed] [Google Scholar]
- 57.Kavvadias D, Scherer G, Cheung F, Errington G, Shepperd J, McEwan M. Determination of tobacco-specific N-nitrosamines in urine of smokers and non-smokers. Biomarkers. 2009;14:547–53. doi: 10.3109/13547500903242883. [DOI] [PubMed] [Google Scholar]
- 58.Urban M, Scherer G, Kavvadias D, Hagedorn HW, Feng S, Serafin R, et al. Quantitation of N’-nitrosonornicotine (NNN) in smokers’ urine by liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 2009;33:260–65. doi: 10.1093/jat/33.5.260. [DOI] [PubMed] [Google Scholar]
- 59.Xia B, Xia Y, Wong J, Nicodemus KJ, Xu M, Lee J, et al. Quantitative analysis of five tobacco-specific N-nitrosamines in urine by liquid chromatography-atmospheric pressure ionization tandem mass spectrometry. Biomed Chromatogr 2013. 2013 Oct 11; doi: 10.1002/bmc.3031. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60.Devesa SS, Shaw G, Blot WJ. Changing patterns of lung cancer incidence by histological type. Cancer Epidemiol Biomarkers Prev. 1991;1:29–34. [PubMed] [Google Scholar]
- 61.Burns DM, Anderson CM, Gray N. Do changes in cigarette design influence the rise in adenocarcinoma of the lung? Cancer Causes Control. 2011;22:13–22. doi: 10.1007/s10552-010-9660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoffmann D, Rivenson A, Murphy SE, Chung FL, Amin S, Hecht SS. Cigarette smoking and adenocarcinoma of the lung: the relevance of nicotine-derived N-nitrosamines. Journal of Smoking-Related Disorders. 1993;4:165–89. [Google Scholar]
- 63.Thun MJ, Lopez AD, Hartge P. Smoking-related mortality in the United States. N Engl J Med. 2013;368:1753. doi: 10.1056/NEJMc1302783. [DOI] [PubMed] [Google Scholar]
- 64.Hoffmann D, Hoffmann I. The changing cigarette, 1950-1995. J Toxicol Environ Health. 1997;50:307–64. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 65.Counts ME, Hsu FS, Laffoon SW, Dwyer RW, Cox RH. Mainstream smoke constituent yields and predicting relationships from a worldwide market sample of cigarette brands: ISO smoking conditions. Regul Toxicol Pharmacol. 2004;39:111–34. doi: 10.1016/j.yrtph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 66.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 92. Lyon, FR: IARC; 2010. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; pp. 35–818. [PMC free article] [PubMed] [Google Scholar]
- 67.Church TR, Anderson KE, Caporaso NE, Geisser MS, Le C, Zhang Y, et al. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers Prev. 2009;18:260–66. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuan JM, Koh WP, Murphy SE, Fan Y, Wang R, Carmella SG, et al. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 2009;69:2990–95. doi: 10.1158/0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan J-M, Gao Y-T, Murphy SE, Carmella SG, Wang R, Zhong Y, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 2011;71:6749–57. doi: 10.1158/0008-5472.CAN-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuan JM, Butler LM, Stepanov I, Hecht SS. Urinary tobacco smoke-constituent biomarkers for assessing risk of lung cancer. Cancer Res. 2014;74:401–11. doi: 10.1158/0008-5472.CAN-13-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hecht SS, Murphy SE, Stepanov I, Nelson HH, Yuan J-M. Tobacco smoke biomarkers and cancer risk among male smokers in the Shanghai Cohort Study. Cancer Lett. 2012;334:34–38. doi: 10.1016/j.canlet.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stepanov I, Sebero E, Wang R, Gao YT, Hecht SS, Yuan JM. Tobacco-specific N-nitrosamine exposures and cancer risk in the Shanghai Cohort Study: remarkable coherence with rat tumor sites. Int J Cancer. 2014;134:2278–83. doi: 10.1002/ijc.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354:333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 74.Burns DM, Dybing E, Gray N, Hecht S, Anderson C, Sanner T, et al. Mandated lowering of toxicants in cigarette smoke: a description of the World Health Organization TobReg proposal. Tob Control. 2008;17:132–41. doi: 10.1136/tc.2007.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 76.Agaku IT, King BA, Dube SR. Current cigarette smoking among adults - United States, 2005-2012. MMWR Morb Mortal Wkly Rep. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- 77.U.S. Food and Drug Administration. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List. 2012;77:20034–37. Fed Regist. [Google Scholar]
- 78.Stratton K, Shetty P, Wallace R, Bondurant S. Institute of Medicine. Washington, DC: National Academy Press; 2001. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. [PubMed] [Google Scholar]
- 79.Institute of Medicine. Scientific Standards for Studies on Modified Risk Tobacco Products. Washington, D.C.: National Academy Press; 2012. [Google Scholar]
- 80.CEBP Focus: Tobacco Research. Cancer Epidemiol Biomarkers Prev. 2009;18:3139–3348. [Google Scholar]
- 81.Leischow S, Zeller M. Research opportunities to inform tobacco product regulation in the United States. Nicotine Tob Res. 2012;14:1–74. [Google Scholar]
- 82.Ding YS, Zhang L, Jain RB, Jain N, Wang RY, Ashley DL, et al. Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol Biomarkers Prev. 2008;17:3366–71. doi: 10.1158/1055-9965.EPI-08-0320. [DOI] [PubMed] [Google Scholar]
- 83.O’Connor RJ. Postmarketing surveillance for “modified-risk” tobacco products. Nicotine Tob Res. 2012;14:29–42. doi: 10.1093/ntr/ntq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.National Cancer Institute. Smoking and Tobacco Control Monograph no 13. Bethesda, MD: U.S. Dept of Health and Human Services, National Institutes of Health, National Cancer Institute; 2001. Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine. NIH Pub. No. 99-4645. [Google Scholar]


