Abstract
Genotoxins and other factors cause replication stress that activate the DNA damage response (DDR), comprising checkpoint and repair systems. The DDR suppresses cancer by promoting genome stability, and it regulates tumor resistance to chemo- and radiotherapy. Three members of the phosphatidylinositol 3-kinase-related kinase (PIKK) family, ATM, ATR, and DNA-PK, are important DDR proteins. A key PIKK target is replication protein A (RPA), which binds single-stranded DNA and functions in DNA replication, DNA repair, and checkpoint signaling. An early response to replication stress is ATR activation, which occurs when RPA accumulates on ssDNA. Activated ATR phosphorylates many targets, including the RPA32 subunit of RPA, leading to Chk1 activation and replication arrest. DNA-PK also phosphorylates RPA32 in response to replication stress, and we demonstrate that cells with DNA-PK defects, or lacking RPA32 Ser4/Ser8 targeted by DNA-PK, confer similar phenotypes, including defective replication checkpoint arrest, hyper-recombination, premature replication fork restart, failure to block late origin firing, and increased mitotic catastrophe. We present evidence that hyper-recombination in these mutants is ATM-dependent, but the other defects are ATM-independent. These results indicate that DNA-PK and ATR signaling through RPA32 plays a critical role in promoting genome stability and cell survival in response to replication stress.
1. Introduction
Cells respond to genotoxic stress by activating the DNA damage response (DDR), a network of damage sensor, signal transducer, and effector proteins that arrest the cell cycle and stimulate DNA repair. During S phase, replication forks stall at fragile sites, telomeres, DNA lesions, and when the replication machinery is disrupted by topoisomerase inhibitors or nucleotide pool depletion by hydroxyurea (HU) [1–5]. Prolonged fork stalling can result in fork collapse to one-ended double-strand breaks (DSBs) that promote genome instability and cancer. Collectively these events are termed “replication stress” and cells respond to replication stress by activating checkpoint and repair processes.
Replication checkpoints arrest the cell cycle, promote fork stabilization and repair, and prevent further encounters of replication forks with damage, thereby promoting cell survival and genome stability [6–8]. Key upstream checkpoint factors are replication protein A (RPA), a heterotrimeric single-stranded DNA (ssDNA) binding complex with critical roles in replication and DNA repair, and members of the phosphatidylinositol 3-kinase-related kinase (PIKK) family, ATR, ATM, and DNA-PK. Although early studies indicated that ATR and ATM respond to replication stress and replication-independent DSBs, respectively [9, 10], and DNA-PK functions in DSB repair by nonhomologous end-joining (NHEJ) [11], it is now clear that PIKKs have overlapping roles and display crosstalk in various DNA damage response pathways [12–23].
DSBs are also repaired by homologous recombination (HR), and HR proteins also play key roles in replication fork stabilization and restart [7, 8]. HR can result in accurate repair, but sometimes it leads to genome rearrangements including deletions, amplifications, and translocations through crossovers and strand-transfer reactions between non-allelic homologous sequences [24, 25]. Genome stability is maintained, in part, by crossover suppression [26–28]. Sister chromatid exchange (SCE) is mediated by HR and can be detected by cytogenetic methods [29, 30]. Most SCEs have no genetic consequence because sister chromatids typically have identical sequences. However, mammalian genomes comprise ~50% repeated sequences (e.g., Alu elements), and strand exchange can occur between linked repeats in equal or unequal fashion, with the latter resulting in genome rearrangement. While cytogenetic approaches cannot distinguish these outcomes, direct repeat HR substrates allow detection of unequal exchange events that produce a functional selectable marker, including gene conversion and repeat deletions, whereas equal exchange events are not detected (Fig. S1). Thus, all SCE events are detected cytogenetically, but HR substrates reveal additional information about HR accuracy.
RPA bound to ssDNA at stalled forks recruits and activates ATR through a Rad17-RFC, 9-1-1, MRN, and TopBP1-dependent pathway. ATR phosphorylates/activates Chk1 which signals downstream factors that stabilize and repair forks, arrest active forks, and stimulate dormant origin firing to complete replication adjacent to stalled forks [8, 31]. The RPA32 subunit of RPA is phosphorylated on Ser23 and Ser29 by CDK cyclically during the cell cycle, and in response to replication stress on Ser33 by ATR, and Ser4/Ser8, Ser12, and Thr21 by one or more PIKKs depending on the replication stress agent [13, 20, 32–36]. Certain replication stress-induced phosphorylation events in RPA32 are subject to “priming” by phosphorylation of other residues [13, 33]. DNA-PK phosphorylates RPA32 Ser4/Ser8, and defects in DNA-PK or RPA32 Ser4/Ser8 residues suppress replication stress-induced Chk1 activation, checkpoint arrest, and fork repair (revealed as persistent γ-H2AX foci) [33, 37]. Liaw et al. [37] showed that blocking RPA32 Ser4/Ser8 phosphorylation increases SCEs; as noted above, SCE analysis cannot distinguish accurate vs inaccurate HR. We previously showed that DNA-PK suppresses replication-associated (spontaneous) direct repeat HR (inaccurate HR) [38], suggesting that DNA-PK suppresses inaccurate HR, perhaps by modulating the interaction between p53 and RPA [39]. ATM/ATR-mediated phosphorylation of p53, and DNA-PKcs-mediated phosphorylation of RPA32, dissociates the p53-RPA complex which promote HR and cell cycle arrest [40].
In this study we define novel PIKK and RPA32 Ser4/Ser8 roles in replication stress responses. We demonstrate that DNA-PKcs phosphorylation of RPA32 Ser4/Ser8 regulates replication fork restart, new origin firing, HR, mitotic catastrophe, and cell survival in response to replication stress. These results indicate that in addition to its direct role in NHEJ, DNA-PKcs (along with ATM and ATR) helps maintain genome stability after replication stress by regulating checkpoint activation and suppressing inaccurate HR through RPA32 phosphorylation.
2. Material and methods
2.1. Cell lines
Human UM-SCC-38 oral squamous carcinoma cells expressing HA-tagged wild-type or Ser4 → Ala/Ser8 → Ala (S4A/S8A) mutant RPA32 were described previously [33, 41]. WT and S4A/S8A RPA32 are expressed at similar levels in the UM-SCC-38 derivatives (Fig. S2A). CHO V3 derivatives lacking DNA-PKcs (DNA-PKcs null) or complemented with wild-type (WT) or kinase-dead (KD; Lys3792 → Arg) and carrying neo direct repeat HR substrates were described previously [38, 42]. Human and CHO cells were cultured in DMEM or α-MEM, respectively, supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. ATR was downregulated in UM-SCC-38 cells as described [33].
2.2. Induction of replication stress, PIKK inhibition, and Western immunoblotting
Replication stress was induced by treating cells with etoposide, camptothecin (CPT), HU, or cis-platin at indicated concentrations/times, then growth medium was replaced and cells were harvested at various times after release from stress to analyze checkpoint arrest, RPA phosphorylation, DNA replication, cell survival, and homologous recombination as described below. In the case of etoposide, cells were treated for 2 h, and untreated cells are designated “−2 h.” To inhibit specific PIKKs, cells were pretreated with ATM inhibitors (ATMi) KU55933 (20 μM) or KU60019 (3 μM), DNA-PKi NU7026 (40 μM) or NU7441 (10 μM) (EMD Biosciences), or ATRi VE-821 (10 μM) (MedChem Express), before treatment with replication stress agents for specified periods, released from stress, and harvested for specific endpoints noted above. ATR was also inhibited by siRNA knockdown. Where tested, similar results were obtained with the two ATMi; the two DNA-PKi; and with siRNA knockdown of ATR and ATRi VE-821 (data not shown). UM-SCC-38 cell lysates were separated by SDS-PAGE, transferred to PVDF membranes, immunoblotted using RPA32 and β-actin primary antibodies (ThermoFisher Scientific) and horseradish peroxidase-conjugated secondary antibodies (GE Healthcare). RPA32 and β-actin signals were detected with a Typhoon 9410 (Molecular Dynamics) or Odyssey Infrared scanner (LI-COR), respectively.
2.3. Flow cytometry and immunofluorescence microscopy analysis
Cell cycle profiles were determined by flow cytometry after release from replication stress for indicated times and fixation in 70% ethanol overnight. Cells were incubated in 50 μg/ml propidium iodide (PI) and 100 μg/ml RNase A for 30 min and 10,000 cells per sample were analyzed on a BD FACSarray (BD Biosciences); data were analyzed using WinList™ (Verity Software House). Abnormal mitoses (i.e., anaphase bridges) were scored by immunofluorescence microscopy in CHO cells grown on 4-well chamber slides overnight and fixed in 100% ice-cold methanol for 10 min at −20°C. Cells were briefly permeabilized in 0.1% Triton X-100, washed and blocked in 1% milk in PBS for 30 min at room temperature. An anti-α tubulin antibody (Santa Cruz Biotechnology) was applied in blocking solution for 1 h at room temperature, then an appropriate Alexa Fluor-488 conjugated secondary antibody was incubated in blocking solution for 1 h at room temperature (Invitrogen). Cells were mounted in PermaFluor (Fisher) supplemented with 0.5 μg/ml DAPI (Roche). Images were acquired with a Zeiss Axiovert 200M microscope, randomized, and scored blindly. Human cells were fixed, and nuclei stained with DAPI, and imaged as described [33].
2.4. BrdU incorporation and DNA fiber analysis
BrdU incorporation was analyzed following cell growth in six-well culture dishes and treatment with 5 mM HU or PBS control in growth medium for 1 h 37°C. After 3 PBS washes, 100 μM BrdU was added in fresh growth medium. Samples were harvested at various times following HU release, fixed and processed with the FITC BrdU Flow Kit (BD Biosciences) according to manufacturer’s directions. To correlate BrdU incorporation with cell cycle phase, cells were co-stained with propidium iodide (PI). BrdU and PI fluorescence were analyzed with a MoFlo flow cytometer and Summit software (Dako). DNA fiber analysis was performed as described [43], with three determinations and >100 fibers scored per cell type per treatment.
2.5. Analysis of SCEs, direct repeat HR, and cell survival
Metaphase chromosomes were prepared using standard cytogenetic techniques and SCE analysis performed using Fluorescence Plus Giemsa (FPG) staining [44]. Briefly, near confluent cells were treated with 5 mM HU or mock treated for 16 h, then transferred to media containing 10 μM BrdU and incubated for two cell cycles to enable harlequin staining. Colcemid (Gibco) at 0.1 μg/ml was added 3.5–4.0 h before harvest to mitotically arrest cells. After trypsinization, cells were incubated in 75 mM KCl for 20–25 min, fixed in freshly prepared 3:1 methanol:acetic acid and dropped onto microscope slides. Slides were stained with Hoechst 33258 (0.5 μg/ml) for 15 min, exposed to 365 nm UV light for 30 min, incubated in 2xSSC at 60°C for 30 min, then stained in 5% Giemsa for 10–15 min. Brightfield images were captured and analyzed with an Olympus Bx41 microscope equipped with a Photometrics CoolSNAP ES2 camera and Metavue 7.1 software. Slides were randomized and scored blindly; SCEs were counted for each color switch from one chromatid to the sister. Thirty chromosome spreads were scored for each condition and significance was assessed using two-tailed t-tests. Replication stress induced neo direct repeat HR frequencies and cell survival were assayed as described [38] in cells treated for 16 h with 8 mM HU or 50 nM CPT.
3. Results
3.1. Replication stress-induced checkpoint arrest depends on ATR, DNA-PK, and RPA32 Ser4 and Ser8 phosphorylation, but not ATM
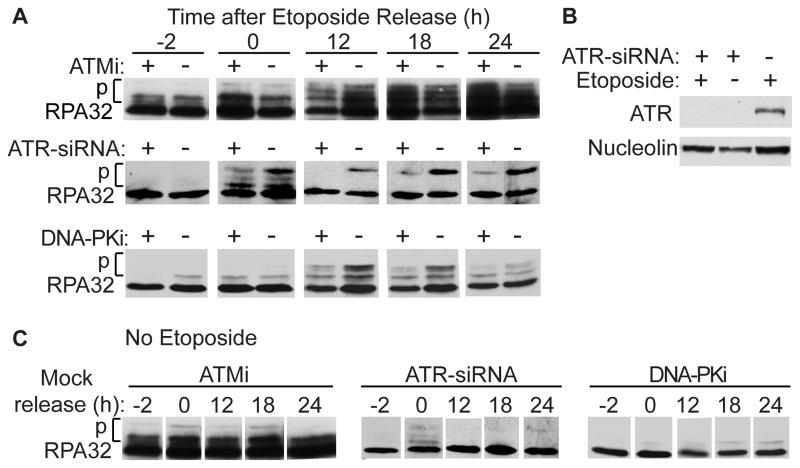
It has long been known that DNA-PKcs phosphorylates RPA in response to replication stress [32, 45], and recent studies indicate that DNA-PKcs has an important role in RPA32 Ser4/Ser8 phosphorylation [33, 37]. Phospho-Ser4/Ser8 are found only in the hyperphosphorylated form of RPA32 and are required for replication checkpoint arrest [46]. To further clarify PIKK roles in replication checkpoint arrest we used derivatives of UM-SCC-38 cells in which endogenous RPA32 was replaced with siRNA-resistant HA-tagged WT or S4A/S8A RPA32, previously shown to functionally interact with endogenous RPA14 and RPA70 [33]. We first examined WT RPA32 phosphorylation before and after a 2 h etoposide treatment in the presence or absence of PIKK inhibitors or siRNA knockdown of ATR. Here we show RPA32 phosphorylation via band shift on Western blots; we previously used phospho-specific antibodies to demonstrate that these shifts result from phosphorylation of Ser4, Ser8, Ser12, Thr21, and Ser33 by specific PIKKs and CDK [33] (Fig. S2B). In WT cells, RPA32 phosphorylation increased over a 24 h period following release from etoposide. RPA32 phosphorylation was reduced by ATMi 12 h after release from etoposide, but there was no effect after 18–24 h (Fig. 1A). In contrast, RPA32 phosphorylation was markedly reduced by ATR knockdown and DNA-PKi throughout the time course (Fig. 1A). ATR was depleted with equal efficiency in etoposide-treated and untreated cells (Fig. 1B), and PIKK inhibition or knockdown had no effect on RPA32 phosphorylation in the absence of etoposide (Fig. 1C). Thus, ATR and DNA-PK, but not ATM, are critical for replication stress-induced RPA32 phosphorylation, in agreement with prior studies of PIKK phosphorylation of specific RPA32 residues [13, 32, 33, 37].
Figure 1.
Etoposide-induced RPA32 phosphorylation depends on ATR and DNA-PK but not ATM. (A) UM-SCC-38 cells stably expressing WT RPA32 were treated for 3 h with KU55933 (ATMi), NU7026 (DNA-PKi), or 48 h with siRNA targeting ATR, then treated for 2 h with 20 μM etoposide. Perhaps because of drug uptake or other factors, more typical NU7026 concentrations (10–20 μM) were ineffective (data not shown). Cells were harvested before etoposide treatment, and 0–24 h after release. Inhibitors remained in the medium during etoposide treatment and recovery. Protein from whole-cell lysates were separated by SDS-PAGE and immunoblotted with antibodies to RPA32. (B) Western blot of ATR from cells treated with ATR-siRNA and/or etoposide; nucleolin was used as a loading control. (C) RPA32 was analyzed as in panel A except cells were not treated with etoposide.
We next monitored PIKK effects on G2/M arrest at intervals after etoposide release in cells expressing WT or S4A/S8A RPA32. In WT cells, etoposide causes cells to accumulate in S and G2/M phases, causing a steady decline in G1 cells. Checkpoint defects allow bypass of the G2/M checkpoint, increasing G1 cells at late (18–24 h) time points. We previously used a mitotic trap assay to show that increases in G1 cells represent G2/M bypass rather than failure to exit G1 [33]. WT cells treated with etoposide showed the expected reduction in G1 cells at late time points. ATMi had no effect on cell cycle distributions, but ATRi resulted in 2- to 3-fold increases in G1 cells 18–24 h after etoposide release, and similar increases were observed in DNA-PKi treated cells (Fig. 2A). RPA32 S4A/S8A mutations block etoposide-induced phosphorylation of these residues, as well as phosphorylation of Ser12 and Thr21, but not Ser33 which is an ATR target [33]. The S4A/S8 mutant showed a dramatic G2/M bypass defect 24 h after etoposide release, and PIKK inhibition did not result in further increases in G1 cells at late times (Fig. 2B). We observed similar G2/M bypass defects with ATRi and ATR knockdown, and with two DNA-PKis NU7026 and NU7441; no G2/M bypass defects were observed with two ATMis, KU55933 and KU60019 (Fig. 2 and data not shown). Together, these results indicate that ATR and DNA-PK play critical roles in maintaining etoposide-induced G2/M arrest. Since DNA-PKcs is the primary PIKK targeting RPA32 Ser4/Ser8 [33, 37], the results further indicate that G2/M arrest is largely mediated through phosphorylation of RPA32 Ser4/Ser8. At early times after etoposide release WT cells treated with ATMi showed reduced overall RPA32 phosphorylation (Fig. 1A) and reduced Ser4/Ser4 phosphorylation [33]. However, ATMi had no effect on overall cell cycle profiles at early or late times after release (Fig. 2A), suggesting that ATR and DNA-PK compensate for ATM defects. Thus, while ATM has an important role in IR-induced checkpoint arrest, it has little or no role in replication stress-induced checkpoint arrest.
Figure 2.
Etoposide-induced cell cycle arrest depends on RPA32 Ser4/Ser8 phosphorylation, ATR, and DNA-PK, but not ATM. UM-SCC-38 cells stably expressing WT or S4A/S8A RPA32 were treated with etoposide as described in Fig. 1. Cells were treated for 3 h with KU60019 (ATMi), VE-821 (ATRi) or NU7441 (DNA-PKi) prior to, during, and after etoposide treatment as indicated. Cells were harvested before etoposide treatment, and 0–24 h after release and cell cycle profiles of propidium iodide stained cells were determined by flow cytometry. (A) PIKK roles in etoposide-induced cell cycle arrest in cells expressing WT RPA32. For each treatment group, representative cell cycle profiles are presented above. G1 cells are the left-most peak of each profile; average percentages of G1 cells (±SEM) for 3 determinations are shown below. Dashed line indicates percent G1 cells 24 h after release. Statistics calculated using t tests are shown for etoposide treated vs etoposide + PIKK inhibitor treated cells at 18 and 24 h: * indicates P < 0.05 and ** indicates P < 0.02, and P values trending toward significance are shown. (B) Cell cycle profiles and percent G1 cells for etoposide-treated RPA32 S4A/S8A cells as in panel A. Statistics shown are for comparisons of WT vs S4A/S8A mutant cells in each treatment group at 24 hr; WT values (from panel A) are shown as hatched bars to illustrate these comparisons. * indicates P < 0.05 and ** indicates P < 0.02. At 24 h, there were no statistically significant differences in S4A/S8A cells treated vs not treated with any of the PIKK inhibitors.
3.2. DNA-PKcs suppresses inaccurate HR induced by replication stress
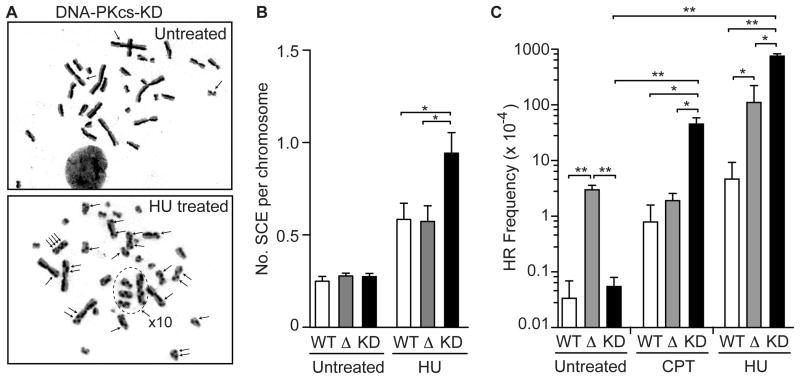
DNA-PKcs suppresses spontaneous HR [38], which initiates when replication forks encounter spontaneous DNA damage [47, 48]. Liaw et al. [37] showed that RPA32 S4A/S8A mutants yield 2-fold more UV-induced SCEs than WT, raising the possibility that DNA-PKcs phosphorylation of RPA32 Ser4/Ser8 suppresses replication stress-induced HR. To extend these findings, we measured HU-induced SCEs, and HU- and CPT-induced neo direct repeat HR in cells lacking DNA-PKcs or complemented with WT or kinase-dead (KD) DNA-PKcs. Although HU increased SCEs ~2-fold in DNA-PKcs null cells, this was not different than WT cells. In contrast, HU-induced SCEs were significantly higher in DNA-PKcs KD cells (~3-fold) than WT or DNA-PKcs null cells (Fig. 3A, B). These results support the proposal by Liaw et al. [37] that DNA-PKcs phosphorylation of RPA32 Ser4/Ser8 suppresses replication-stress induced SCEs. Because ATM is differentially expressed in DNA-PKcs null and KD cells [42, 49], these results also implicate ATM in the hyper-SCE phenotype of DNA-PKcs KD cells (see Discussion).
Figure 3.
DNA-PKcs suppresses replication stress-induced HR in an ATM-dependent manner. (A) Representative metaphase spreads of DNA-PKcs KD mutant cells treated with 5 mM HU or mock-treated. Arrows indicate SCEs. (B) Quantitation of SCEs in DNA-PKcs WT, null, and KD cells following HU or mock treatment. (C) Direct repeat HR frequencies in untreated DNA-PKcs WT, null, and KD cells, or treated with CPT or HU. Values are averages (±SEM) for 2–5 determinations; * and ** indicate P<0.05 or P<0.01, respectively (t tests).
Consistent with Lundin et al. [50], DNA-PKcs null cells showed mildly increased sensitivity to CPT and HU (Fig. S3). DNA-PKcs null and KD cells showed similar sensitivity to CPT, but DNA-PKcs null cells were significantly more sensitive to HU than DNA-PKcs KD cells, suggesting the reduced ATM levels in the DNA-PKcs null cells confers sensitivity to HU. Interestingly, replication stress-induced neo direct repeat HR (inaccurate HR; Fig. S1) was markedly increased in DNA-PKcs KD cells, but direct repeat HR was not significantly different between DNA-PKcs WT and null cells (Fig. 3C), similar to the SCE results. Note, however, in unstressed cells direct repeat HR, but not SCE, is significantly elevated in DNA-PKcs null cells compared to WT, yet neither SCE nor direct repeat HR is elevated in DNA-PKcs KD cells (Fig. 3B, C), suggesting differential roles for ATM in regulating HR accuracy under stress and non-stress conditions.
3.3. DNA-PKcs phosphorylation of RPA32 Ser4/Ser8 regulates replication fork restart and late-origin firing in response to replication stress
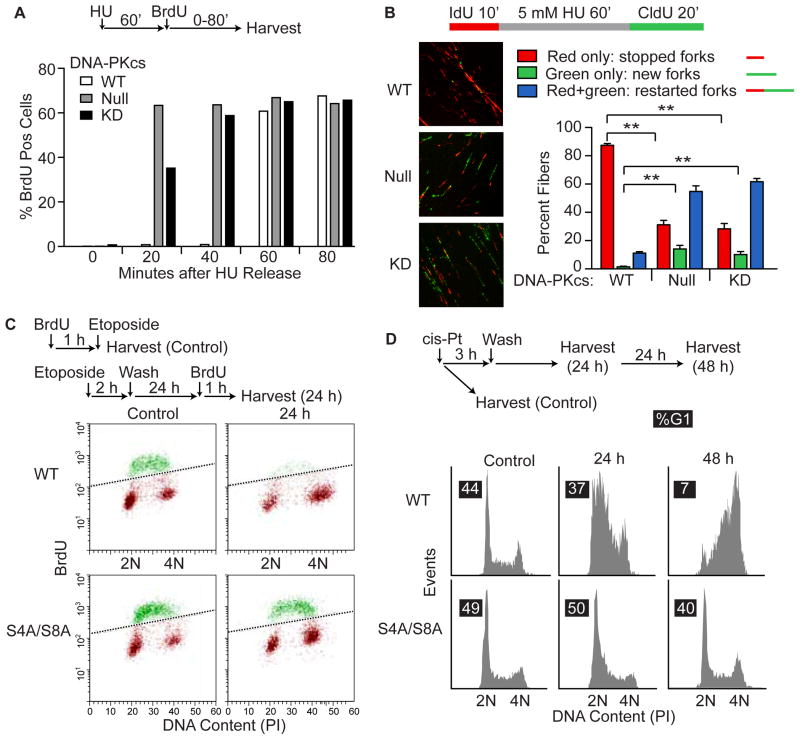
Replication stress-induced HR reflects unequal exchange occurring at stalled and collapsed replication forks. Replication stress also activates checkpoint responses that regulate fork restart and late origin firing [7]. Our recent study [33] and the data above indicate that DNA-PKcs phosphorylation of RPA32 Ser4/Ser8 plays an important role in replication stress-induced Chk1 activation and subsequent cell cycle arrest. We therefore investigated DNA-PKcs and RPA32 roles in replication recovery after release from stress induced by HU, etoposide, or cis-platin. Both DNA-PKcs null and KD cells incorporated BrdU within 20 min of release from HU, but replication recovery in WT cells was much slower, requiring >40 min (Fig. 4A). We then used DNA fiber analysis to determine whether the accelerated replication recovery of DNA-PKcs mutants reflected replication fork restart and/or late origin firing. In WT cells only ~10% of stalled forks restarted within 20 min of HU release, but this increased to ~70% in DNA-PKcs null and KD mutants (Fig. 4B). In addition, WT cells effectively blocked late origin firing (few new forks were observed 20 min after HU release), but this increased >15-fold in both DNA-PKcs mutants (Fig. 4B). Thus, DNA-PKcs slows replication fork restart and suppresses late origin firing during replication stress recovery.
Figure 4.
DNA-PKcs and RPA32 regulate replication recovery after stress. (A) WT, DNA-PKcs null and KD cells were treated for 60 min with 5 mM HU, HU was removed, 10 μM BrdU was added, and BrdU incorporation was detected with immunofluorescence/flow cytometry. (B) DNA fiber analysis procedure is outlined above and representative images are shown. The plot includes data based on >250 fibers scored for each of WT, DNA-PKcs null and KD cells. Values are averages (±SEM) for 4–6 images. ** indicates P<0.01 (t tests). (C) BrdU incorporation in WT and RPA32 S4A/S8A cells procedures are outlined above for controls and replication recovery after etoposide. Histograms of flow cytometry data scoring BrdU incorporation and DNA content by PI staining. Points above diagonal line (green) represent BrdU positive cells. Both cell lines are replication competent prior to etoposide (control). (D) RPA32 S4A/S8A abrogates S/G2 arrest after release from cis-platin-induced replication stress. Procedure is outlined above, and cell cycle profiles of PI-stained cells were determined by flow cytometry. Percent G1 cells for each condition are shown in black boxes.
We next measured BrdU incorporation in WT and RPA32 S4A/S8A mutant cells after release from etoposide, and correlated this endpoint with DNA content in propidium iodide stained cells. WT cells showed little BrdU incorporation within 24 h of etoposide release, whereas BrdU incorporation was robust in both etoposide treated S4A/S8A mutant cells and untreated controls (Fig. 4C). Thus, the RPA32 S4A/S8A mutant is defective in replication stress checkpoint arrest, similar to DNA-PKcs defective cells. We also measured cell cycle profiles of WT and S4A/S8A mutant cells 24 and 48 h after release from a 2 h cis-platin treatment and again, the S4A/S8A mutant failed to arrest in S/G2 (Fig. 4D). Together these results indicate that DNA-PKcs phosphorylation of RPA32 S4/S8 phosphorylation is critical for replication checkpoint arrest in response to replication stress induced by several agents.
3.4. DNA-PKcs and RPA32 S4/S8 phosphorylation suppresses replication stress-induced mitotic catastrophe
DNA-PKcs defects and RPA32 S4A/S8A mutations confer Chk1 activation and checkpoint arrest defects (refs. [33, 37] and this study). In addition, RPA32 S4A/S8A mutants progress into M phase in the presence of DSBs marked by γ-H2AX [33]. To determine the consequences of M phase progression with unrepaired replication stress-induced DNA damage, we treated WT, DNA-PKcs mutant, and RPA32 S4A/S8A mutant cells with HU or etoposide and then scored cells stained with anti-tubulin antibodies and/or DAPI using fluorescence microscopy. Abnormal cells, including giant cells, cells with abnormal nuclear morphology, and anaphase bridges reflecting failed cytokinesis, were induced by HU at significantly increased frequencies in DNA-PKcs null and KD cells relative to WT (Fig. 5A, B). Anaphase bridges were also apparent in RPA32 S4A/S8A mutants treated with etoposide (Fig. 5C). These data indicate that DNA-PKcs and RPA32 mutants suffer mitotic problems when they fail to arrest in S/G2 in response to stress and progress to M phase with damage.
Figure 5.
DNA-PKcs – RPA32 signaling suppresses mitotic catastrophe after replication stress. (A) Representative images of WT, DNA-PKcs null and KD cells after 16 h treatment with 10 mM HU and staining with DAPI (blue) and anti-tubulin antibodies (green). A higher HU concentration was used in this experiment to increase sensitivity to detect abnormal cells. Arrows indicate anaphase bridges (failed cytokinesis) and giant cells. (B) Average percentage (±SEM) of abnormal cells for 4 determinations. (C) Images of mitotic RPA32 S4A/S8A and WT cells stained with DAPI following etoposide or mock treatment. WT cells treated with etoposide are not shown because they arrest before M phase. In etoposide-treated cells, arrows indicate anaphase bridges (left and center) and nuclear blebs (left and right).
4. Discussion
RPA32 phosphorylation is a hallmark of the replication stress response, and Ser4/Ser8 phosphorylation marks the hyperphosphorylated form required for replication checkpoint arrest [46, 51–53]. We recently showed that in vitro, ATR phosphorylates RPA32 Ser33, but not Ser4/Ser8 or Thr21; ATM phosphorylates Ser4/Ser8 and Thr21, but not Ser33; and DNA-PKcs phosphorylates all of these residues [33] (Fig. S2B). ATR also specifically targets Ser33 in vivo [54]. ATR indirectly regulates Ser4/Ser8 phosphorylation through priming by phospho-Ser33 [13, 34]. Given that both ATM and DNA-PKcs phosphorylate Ser4/Ser8 in vitro, and inhibition of either kinase markedly reduces Ser4/Ser8 phosphorylation in response to etoposide [33], it was plausible that all three PIKKs are involved in Ser4/Ser8 phosphorylation in vivo, and thus all three were expected to regulate replication checkpoint arrest. However, while inhibition of ATR and DNA-PKcs severely compromised replication checkpoint arrest, inhibition of ATM had no effect (Fig. 2). The idea that ATR and DNA-PKcs, but not ATM, regulate replication checkpoint arrest appears to contradict the prior observation that ATM inhibition blocks etoposide-induced Ser4/Ser8 phosphorylation [33]. This is likely a timing issue as Ser4/Ser8 phosphorylation was previously monitored immediately after a 2 h etoposide treatment, while arrest in the current study was monitored 12–24 h after etoposide release (Fig. 2). Thus, although ATM inhibition suppresses Ser4/Ser8 phosphorylation at early times, RPA32 becomes hyperphosphorylated within 12 h (Fig. 1A), suggesting that ATR and DNA-PKcs eventually compensate for the ATM defect and enforce checkpoint arrest (Fig. 2A).
Comparisons of checkpoint defects with ATR or DNA-PKcs inhibition and the RPA32 S4A/S8A mutant shed additional light on the relative importance of PIKKs in checkpoint signaling. Checkpoint arrest is fully abrogated in the S4A/S8A mutant, and this severe defect is mimicked by DNA-PKcs inhibition (Fig. 2). Given the dominant role of DNA-PKcs in Ser4/Ser8 phosphorylation [33, 37], these genetic results strongly support the idea that DNA-PKcs phosphorylation of RPA32 Ser4/Ser8 is critical for replication checkpoint arrest. ATR inhibition and ATR knockdown conferred a similarly strong arrest defect as DNA-PK inhibition (Fig. 2 and data not shown). This probably reflects the importance of ATR phosphorylation of Ser33 and the strong priming effects on phospho-Ser33 on Ser4/Ser8 phosphorylation [33]. ATR is frequently described as the master regulator of the replication checkpoint. The current study and our recent study [33] indicate that at least for etoposide-induced replication stress, DNA-PKcs and ATR are equally important for enforcing the replication checkpoint through RPA32 Ser4/Ser8 phosphorylation.
Chk1 phosphorylation in response to etoposide, CPT, and HU is reduced several-fold in cells lacking DNA-PKcs, or expressing kinase dead DNA-PKcs or RPA32 S4A/S8A [33]. Although the residual Chk1 phosphorylation is insufficient to enforce arrest (Fig. 2), it may trigger other responses. In unstressed S phase cells, the RPA-ATR-Chk1 pathway is partially active, regulating origin firing and the number of active replicons, and enabling rapid responses when stress exceeds a threshold [55, 56]. This allows for graded stress responses in lieu of, or in addition to arrest, including stabilization of stalled forks, induction of DNA repair, suppression of late origin firing, and activation of dormant origins adjacent to irreparably damaged forks.
When ATR or Chk1 are not activated properly, stalled forks are not stabilized and they frequently collapse to DSBs [33, 57, 58] causing genome instability and lethality. Liaw et al. [37] demonstrated that UV-induced SCEs are elevated in RPA32 S4A/S8A mutants, and here we extend this by showing that DNA-PKcs KD mutant cells show increased replication stress-induced SCEs, and HU- and CPT-induced direct repeat HR, with the latter resulting from inaccurate HR (unequal exchange; Fig. S1). Interestingly, replication stress did not elevate SCEs in DNA-PKcs null cells over WT, and direct repeat HR was either not increased (CPT) or moderately increased (HU), whereas marked increases were seen in DNA-PKcs KD cells (Fig. 3). This is probably due to low levels of ATM in DNA-PKcs null and knockdown cells [42, 49]. Thus, ATM appears to promote replication stress-induced HR, as is the case with DSB-induced HR [42, 59–62]. In this model, the failure to phosphorylate RPA32 Ser4/Ser8 in response to replication stress enhances HR in an ATM-dependent manner. This model is consistent with the elevated SCEs in S4A/S8A cells [37] as mutant RPA32 is not expected to affect ATM levels. p53 interacts with RPA70 and this inhibits HR [39]. Serrano et al. [40] recently showed that PIKK phosphorylation of p53 and RPA dissociates the p53-RPA complex, promoting HR and cell cycle arrest, and that an RPA32 mutant lacking 7 hyperphosphorylation sites prevents p53-RPA dissociation, and suppresses HR, consistent with results by Shi et al. [63]. These results contrast with the hyper-HR observed in DNA-PKcs KD and RPA32 Ser4A/Ser8A mutants [37] (Fig. 3). We propose that residual phosphorylation at other residues in the S4A/S8A mutant is sufficient to promote p53-RPA dissociation, and that this, coupled with replication of damaged templates due to the replication arrest defect, accounts for the hyper-HR phenotype.
Activated Chk1 regulates restart of stalled and collapsed replication forks, and late origin firing. DNA-PKcs null, KD, and RPA32 S4A/S8A mutants all suppress Chk1 activation [33], all show accelerated replication recovery, and both DNA-PKcs mutants fail to suppress late origin firing after release from stress (Fig. 4B). Because ATM levels differ markedly in DNA-PKcs null vs KD cells [42], these results indicate that unlike HR, replication recovery and suppression of late origin firing are ATM independent. These results further show that premature replication recovery is not sufficient to enhance replication stress-induced HR. Defects in DNA-PKcs – RPA32 signaling result in more persistent DSB damage (γ-H2AX) after release from replication stress [33]. Because HR is an important mechanism for restarting collapsed forks [8], one possibility is that persistent damage underlies the elevated HR in DNA-PKcs KD and S4A/S8A mutants. However, DNA-PKcs null cells (with low ATM) display persistent γ-H2AX, as do DNA-PKcs KD and S4A/S8A mutants [33, 37], yet HR is not elevated above WT in DNA-PKcs null mutants. Thus, neither premature replication recovery nor persistent DSBs are sufficient to elevate HR when ATM levels are low. ATM is activated by DSBs [64], suggesting it may function in HR-mediated restart of collapsed forks. RPA32 S4A/S8 cells are checkpoint defective, they show persistent DSB damage upon release from replication stress, and they progress into M phase with damage [33], accounting for the mitotic problems observed in S4A/S8A and DNA-PKcs mutants (Fig. 5). Mitotic problems were observed at similar frequencies in both DNA-PKcs null and KD mutants. Thus, increased ATM-dependent HR does not prevent mitotic catastrophe when the checkpoint is defective.
In conclusion, DNA-PKcs is the major PIKK that phosphorylates RPA32 Ser4/Ser8 in response to replication stress, and is thus critical for maturation of hyperphosphorylated RPA32 required for checkpoint arrest. DNA-PKcs and RPA32 S4A/S8A mutants share many similar replication stress phenotypes including defective Chk1 activation and G2/M arrest; persistent DSB damage; premature replication recovery and failure to prevent late origin firing after release from stress; ATM-dependent hyper-HR; and increased mitotic catastrophe and lethality. A model illustrating PIKK-RPA-Chk1 network regulation of HR and mitotic catastrophe is shown in Fig. S4. Inhibition of DNA-PKcs-RPA32 signaling may prove valuable as an adjunct to cancer chemotherapy. ATM inhibitors may also prove useful as adjunct therapy to further enhance tumor cell killing while suppressing HR-mediated genome instability which could drive progression of rare surviving tumor cells.
Supplementary Material
Highlights.
We demonstrate a critical role for DNA-PK in cell responses to replication stress.
DNA-PK is the primary kinase targeting RPA32 Ser4/Ser8.
RPA32 phosphorylation regulates replication arrest, recombination, late origin firing, and mitotic catastrophe.
Replication stress induced recombination is ATM-dependent.
Acknowledgments
We thank Christopher P. Allen and Karoline Manthey for helpful comments. This work was supported by grants from the National Institutes of Health (R01 GM084020 to J.A.N., P20 RR018759-08 to G.G.O), the American Cancer Society (RSG-10-031-01-CCG to G.G.O.), and the Nebraska Department of Human and Health Services (G.G.O.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barlow JH, Faryabi RB, Callen E, Wong N, Malhowski A, Chen HT, Gutierrez-Cruz G, Sun HW, McKinnon P, Wright G, Casellas R, Robbiani DF, Staudt L, Fernandez-Capetillo O, Nussenzweig A. Identification of early replicating fragile sites that contribute to genome instability. Cell. 2013;152:620–632. doi: 10.1016/j.cell.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen Z, Nickoloff JA. Mammalian homologous recombination repair and cancer intervention. In: Wei Q, Li L, Chen DJ, editors. DNA Repair, Genetic Instability, and Cancer. World Scientific Publishing Co; Singapore: 2007. pp. 119–156. [Google Scholar]
- 3.Bosco EE, Mayhew CN, Hennigan RF, Sage J, Jacks T, Knudsen ES. RB signaling prevents replication-dependent DNA double-strand breaks following genotoxic insult. Nucleic Acids Res. 2004;32:25–34. doi: 10.1093/nar/gkg919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limoli CL, Giedzinski E, Bonner WM, Cleaver JE. UV-induced replication arrest in the xeroderma pigmentosum variant leads to DNA double-strand breaks, γ-H2AX formation, and Mre11 relocalization. Proc Natl Acad Sci USA. 2002;99:233–238. doi: 10.1073/pnas.231611798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mankouri HW, Huttner D, Hickson ID. How unfinished business from S-phase affects mitosis and beyond. EMBO J. 2013;32:2661–2671. doi: 10.1038/emboj.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warmerdam DO, Kanaar R. Dealing with DNA damage: relationships between checkpoint and repair pathways. Mutat Res. 2010;704:2–11. doi: 10.1016/j.mrrev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Allen C, Ashley AK, Hromas R, Nickoloff JA. More forks on the road to replication stress recovery. J Mol Cell Biol. 2011;3:4–12. doi: 10.1093/jmcb/mjq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budzowska M, Kanaar R. Mechanisms of dealing with DNA damage-induced replication problems. Cell Biochem Biophys. 2009;53:17–31. doi: 10.1007/s12013-008-9039-y. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- 10.Paulsen RD, Cimprich KA. The ATR pathway: fine-tuning the fork. DNA Repair. 2007;6:953–966. doi: 10.1016/j.dnarep.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 13.Anantha RW, Vassin VM, Borowiec JA. Sequential and synergistic modification of human RPA stimulates chromosomal DNA repair. J Biol Chem. 2007;282:35910–35923. doi: 10.1074/jbc.M704645200. [DOI] [PubMed] [Google Scholar]
- 14.Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 15.Hurley PJ, Bunz F. ATM and ATR: components of an integrated circuit. Cell Cycle. 2007;6:414–417. doi: 10.4161/cc.6.4.3886. [DOI] [PubMed] [Google Scholar]
- 16.Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 18.Myers JS, Cortez D. Rapid activation of ATR by ionizing radiation requires ATM and Mre11. J Biol Chem. 2006;281:9346–9350. doi: 10.1074/jbc.M513265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stiff T, O’Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Guan J, Perrault AR, Wang Y, Iliakis G. Replication protein A2 phosphorylation after DNA damage by the coordinated action of ataxia telangiectasia-mutated and DNA-dependent protein kinase. Cancer Res. 2001;61:8554–8563. [PubMed] [Google Scholar]
- 21.Yajima H, Lee K-J, Chen BPC. ATR-dependent DNA-PKcs phosphorylation in response to UV-induced replication stress. Mol Cell Biol. 2006;26:7520–7528. doi: 10.1128/MCB.00048-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo HY, Kumagai A, Shevchenko A, Dunphy WG. Ataxia-telangiectasia mutated (ATM)-dependent activation of ATR occurs through phosphorylation of TopBP1 by ATM. J Biol Chem. 2007;282:17501–17506. doi: 10.1074/jbc.M701770200. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Paull TT. DNA-dependent protein kinase regulates DNA end resection in concert with Mre11-Rad50-Nbs1 (MRN) and ataxia telangiectasia-mutated (ATM) J Biol Chem. 2013;288:37112–37125. doi: 10.1074/jbc.M113.514398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickoloff JA. Recombination: mechanisms and roles in tumorigenesis. In: Bertino JR, editor. Encyclopedia of Cancer. 2. Elsevier Science (USA); San Diego: 2002. pp. 49–59. [Google Scholar]
- 25.Hastings PJ, Lupski JR, Rosenberg SM, Ira G. Mechanisms of change in gene copy number. Nat Rev Genet. 2009;10:551–564. doi: 10.1038/nrg2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstock DM, Richardson CA, Elliott B, Jasin M. Modeling oncogenic translocations: distinct roles for double-strand break repair pathways in translocation formation in mammalian cells. DNA Repair. 2006;5:1065–1074. doi: 10.1016/j.dnarep.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Moynahan ME, Jasin M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat Rev Mol Cell Biol. 2010;11:196–207. doi: 10.1038/nrm2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, Hickson ID. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 29.Sonoda E, Sasaki MS, Morrison C, Yamaguchi-Iwai Y, Takata M, Takata S. Sister chromatid exchanges are mediated by homologous recombination in vertebrate cells. Mol Cell Biol. 1999;19:5166–5169. doi: 10.1128/mcb.19.7.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson DM, 3rd, Thompson LH. Molecular mechanisms of sister-chromatid exchange. Mutat Res. 2007;616:11–23. doi: 10.1016/j.mrfmmm.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Doksani Y, Bermejo R, Fiorani S, Haber JE, Foiani M. Replicon dynamics, dormant origin firing, and terminal fork integrity after double-strand break formation. Cell. 2009;137:247–258. doi: 10.1016/j.cell.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Block WD, Yu Y, Lees-Miller SP. Phosphatidyl inositol 3-kinase-like serine/threonine protein kinases (PIKKs) are required for DNA damage-induced phosphorylation of the 32 kDa subunit of replication protein A at threonine 21. Nucleic Acids Res. 2004;32:997–1005. doi: 10.1093/nar/gkh265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S, Opiyo SO, Manthey K, Glanzer JG, Ashley AK, Troksa K, Shrivastav M, Nickoloff JA, Oakley GG. Distinct roles for DNA-PK, ATM, and ATR in RPA phosphorylation and checkpoint activation in response to replication stress. Nucleic Acids Res. 2012;40:10780–10794. doi: 10.1093/nar/gks849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson E, Nievera CJ, Klimovich V, Fanning E, Wu X. RPA2 is a direct downstream target for ATR to regulate the S-phase checkpoint. J Biol Chem. 2006;281:39517–39533. doi: 10.1074/jbc.M605121200. [DOI] [PubMed] [Google Scholar]
- 35.Anantha RW, Borowiec JA. Mitotic crisis: the unmasking of a novel role for RPA. Cell Cycle. 2009;8:357–361. doi: 10.4161/cc.8.3.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zernik-Kobak M, Vasunia K, Connelly M, Anderson CW, Dixon K. Sites of UV-induced phosphorylation of the p34 subunit of replication protein A from HeLa cells. J Biol Chem. 1997;272:23896–23904. doi: 10.1074/jbc.272.38.23896. [DOI] [PubMed] [Google Scholar]
- 37.Liaw H, Lee D, Myung K. DNA-PK-dependent RPA2 hyperphosphorylation facilitates DNA repair and suppresses sister chromatid exchange. PLoS One. 2011;6:e21424. doi: 10.1371/journal.pone.0021424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen C, Kurimasa A, Brenneman MA, Chen DJ, Nickoloff JA. DNA-dependent protein kinase suppresses double-strand break-induced and spontaneous homologous recombination. Proc Natl Acad Sci USA. 2002;99:3758–3763. doi: 10.1073/pnas.052545899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romanova LY, Willers H, Blagosklonny MV, Powell SN. The interaction of p53 with replication protein A mediates suppression of homologous recombination. Oncogene. 2004;23:9025–9033. doi: 10.1038/sj.onc.1207982. [DOI] [PubMed] [Google Scholar]
- 40.Serrano MA, Li Z, Dangeti M, Musich PR, Patrick S, Roginskaya M, Cartwright B, Zou Y. DNA-PK, ATM and ATR collaboratively regulate p53-RPA interaction to facilitate homologous recombination DNA repair. Oncogene. 2013;32:2452–2462. doi: 10.1038/onc.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manthey KC, Opiyo S, Glanzer JG, Dimitrova D, Elliott J, Oakley GG. NBS1 mediates ATR-dependent RPA hyperphosphorylation following replication-fork stall and collapse. J Cell Sci. 2007;120:4221–4229. doi: 10.1242/jcs.004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shrivastav M, Miller CA, De Haro LP, Durant ST, Chen BP, Chen DJ, Nickoloff JA. DNA-PKcs and ATM co-regulate DNA double-strand break repair. DNA Repair. 2009;8:920–929. doi: 10.1016/j.dnarep.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Haro LP, Wray J, Williamson EA, Durant ST, Corwin L, Gentry AC, Osheroff N, Lee SH, Hromas R, Nickoloff JA. Metnase promotes restart and repair of stalled and collapsed replication forks. Nucleic Acids Res. 2010;38:5681–5691. doi: 10.1093/nar/gkq339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagelstrom RT, Askin KF, Williams AJ, Ramaiah L, Desaintes C, Goodwin EH, Ullrich RL, Bailey SM. DNA-PKcs and ATM influence generation of ionizing radiation-induced bystander signals. Oncogene. 2008;27:6761–6769. doi: 10.1038/onc.2008.276. [DOI] [PubMed] [Google Scholar]
- 45.Shao RG, Cao CX, Zhang H, Kohn KW, Wold MS, Pommier Y. Replication-mediated DNA damage by camptothecin induces phosphorylation of RPA by DNA-dependent protein kinase and dissociates RPA:DNA-PK complexes. EMBO J. 1999;18:1397–1406. doi: 10.1093/emboj/18.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oakley GG, Patrick SM. Replication protein A: directing traffic at the intersection of replication and repair. Front Biosci. 2010;15:883–900. doi: 10.2741/3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnaudeau C, Tenorio Miranda E, Jenssen D, Helleday T. Inhibition of DNA synthesis is a potent mechanism by which cytostatic drugs induce homologous recombination in mammalian cells. Mutat Res. 2000;461:221–228. doi: 10.1016/s0921-8777(00)00052-5. [DOI] [PubMed] [Google Scholar]
- 48.Arnaudeau C, Lundin C, Helleday T. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J Mol Biol. 2001;307:1235–1245. doi: 10.1006/jmbi.2001.4564. [DOI] [PubMed] [Google Scholar]
- 49.Peng Y, Woods RG, Beamish H, Ye R, Lees-Miller SP, Lavin MF, Bedford JS. Deficiency in the catalytic subunit of DNA-dependent protein kinase causes down-regulation of ATM. Cancer Res. 2005;65:1670–1677. doi: 10.1158/0008-5472.CAN-04-3451. [DOI] [PubMed] [Google Scholar]
- 50.Lundin C, Erixon K, Arnaudeau C, Schultz N, Jenssen D, Meuth M, Helleday T. Different roles for nonhomologous end joining and homologous recombination following replication arrest in mammalian cells. Mol Cell Biol. 2002;22:5869–5878. doi: 10.1128/MCB.22.16.5869-5878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oakley GG, Loberg LI, Yao J, Risinger MA, Yunker RL, Zernik-Kobak M, Khanna KK, Lavin MF, Carty MP, Dixon K. UV-induced hyperphosphorylation of replication protein a depends on DNA replication and expression of ATM protein. Mol Biol Cell. 2001;12:1199–1213. doi: 10.1091/mbc.12.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cruet-Hennequart S, Glynn MT, Murillo LS, Coyne S, Carty MP. Enhanced DNA-PK-mediated RPA2 hyperphosphorylation in DNA polymerase η-deficient human cells treated with cisplatin and oxaliplatin. DNA Repair. 2008;7:582–596. doi: 10.1016/j.dnarep.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 53.Wu X, Yang Z, Liu Y, Zou Y. Preferential localization of hyperphosphorylated replication protein A to double-strand break repair and checkpoint complexes upon DNA damage. Biochem J. 2005;391:473–480. doi: 10.1042/BJ20050379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vassin VM, Anantha RW, Sokolova E, Kanner S, Borowiec JA. Human RPA phosphorylation by ATR stimulates DNA synthesis and prevents ssDNA accumulation during DNA-replication stress. J Cell Sci. 2009;122:4070–4080. doi: 10.1242/jcs.053702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shechter D, Costanzo V, Gautier J. ATR and ATM regulate the timing of DNA replication origin firing. Nat Cell Biol. 2004;6:648–655. doi: 10.1038/ncb1145. [DOI] [PubMed] [Google Scholar]
- 56.Shechter D, Gautier J. ATM and ATR check in on origins: a dynamic model for origin selection and activation. Cell Cycle. 2005;4:235–238. [PubMed] [Google Scholar]
- 57.Brown EJ, Baltimore D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 2003;17:615–628. doi: 10.1101/gad.1067403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, Helleday T, Sehested M, Lukas J, Bartek J. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol. 2005;25:3553–3562. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuhne M, Riballo E, Rief N, Rothkamm K, Jeggo PA, Lobrich M. A double-strand break repair defect in ATM-deficient cells contributes to radiosensitivity. Cancer Res. 2004;64:500–508. doi: 10.1158/0008-5472.can-03-2384. [DOI] [PubMed] [Google Scholar]
- 60.Morrison C, Sonoda E, Takao N, Shinohara A, Yamamoto K, Takeda S. The controlling role of ATM in homologous recombinational repair of DNA damage. EMBO J. 2000;19:463–471. doi: 10.1093/emboj/19.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo C-M, Tang W, Mekeel KL, DeFrank JS, Anne PR, Powell SN. High frequency and error-prone DNA recombination in ataxia telangiectasia cell lines. J Biol Chem. 1996;271:4497–4503. doi: 10.1074/jbc.271.8.4497. [DOI] [PubMed] [Google Scholar]
- 62.Xie A, Puget N, Shim I, Odate S, Jarzyna I, Bassing CH, Alt FW, Scully R. Control of sister chromatid recombination by histone H2AX. Mol Cell. 2004;16:1017–1025. doi: 10.1016/j.molcel.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi W, Feng Z, Zhang J, Gonzalez-Suarez I, Vanderwaal RP, Wu X, Powell SN, Roti Roti JL, Gonzalo S, Zhang J. The role of RPA2 phosphorylation in homologous recombination in response to replication arrest. Carcinogenesis. 2010;31:994–1002. doi: 10.1093/carcin/bgq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.