Abstract
The dystrophin-associated glycoprotein complex (DGC) is a collection of glycoproteins that are essential for the normal function of striated muscle and many other tissues. Recent genetic studies have implicated the components of this complex in over a dozen forms of muscular dystrophy. Furthermore, disruption of the DGC has been implicated in many forms of acquired disease. This review aims to summarize the current state of knowledge regarding the processing and assembly of dystrophin associated proteins with a focus primarily on the dystroglycan heterodimer and the sarcoglycan complex. These proteins form the transmembrane portion of the DGC and undergo a complex multi-step processing with proteolytic cleavage, differential assembly, and both N- and O-glycosylation. The enzymes responsible for this processing and a model describing the sequence and subcellular localization of these events are discussed.
Introduction
Dystrophin and its associated proteins are critical for the normal function of both skeletal and cardiac muscle, as is evident from the severity of the disease resulting from their absence (Bushby et al., 2010a, 2010b; Norwood et al., 2007). The most common of these diseases is Duchenne muscular dystrophy (DMD), which was first described in the 19th century (Clarke and Gowers, 1874; Duchenne, 1867; Ross, 1883). Our understanding of this disease process was greatly enhanced with the identification of dystrophin as the protein whose loss resulted in DMD (E P Hoffman et al., 1987a). This discovery facilitated the characterization of an entire complex of proteins, collectively called dystrophin-associated glycoprotein complex (DGC; Figure 1). These proteins interact with dystrophin to mediate cellular interactions with the extracellular matrix important in membrane stabilization, force transmission, and synapse formation (Ervasti and Campbell, 1993, 1991; Ervasti et al., 1990; Yoshida and Ozawa, 1990). Mutations within many of these genes or in genes involved in post-translational modifications of DGC proteins have been demonstrated to cause multiple forms of recessive muscular dystrophy (Table 1).
Figure 1.
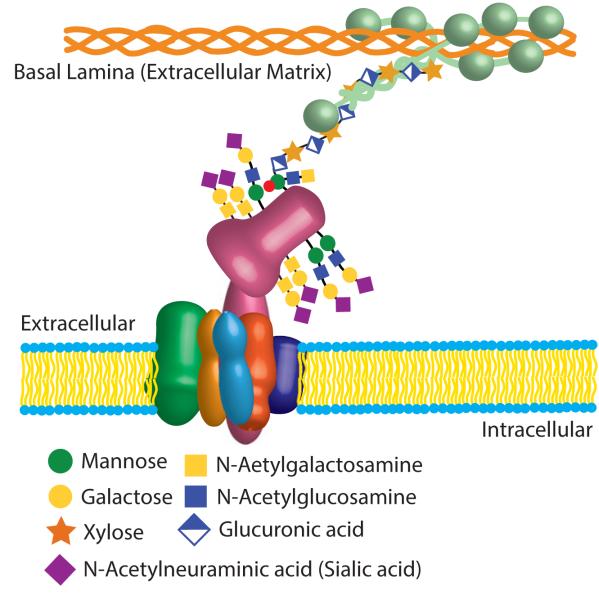
A schematic cartoon of the DGC in the sarcolemmal membrane. The sarcoglycan complex (SGC) consists of α-SG (green), β-SG (orange), γ-SG (gold), σ-SG (light blue), and sarcospan (purple). This protein complex associates with β-dystroglycan (DG; pink) which binds to α-DG. α-DG is heavily glycosylated and functions as a receptor for laminin (light green), which in turn binds to collagen (orange). All of the components of the DGC shown here are also N-glycosylated; these features are not shown, but details can be found in the text.
Table 1.
| Gene | OMIM ID | Disease | Reference |
|---|---|---|---|
| Dystrophin | 300377 | DMD, BMD, XLCM | (E. P Hoffman et al., 1987; Towbin et al., 1993) |
| γ-Sarcoglycan | 608896 | LGMD-2C | (Noguchi et al., 1995) |
| α-Sarcoglycan | 600119 | LGMD-2D | (Matsumura et al., 1992b) |
| β-Sarcoglycan | 600900 | LGMD-2E | (Bonnemann et al., 1995) |
| δ-Sarcoglycan | 601411 | LGMD-2C | (Nigro et al., 1996a) |
| Protein O-mannosyltransferase-1 (POMT-1) | 607423 | MDDG-A1; LGMD-2K | (Beltran-Valero de Bernabe et al., 2002) |
| Protein O-mannosyltransferase-2 (POMT-2) | 607439 | MDDG-A2; LGMD-2N | (Willer et al., 2002) |
| Protein O-mannose β-1,2-N- Acetylglucosaminyltransferase (POMGNT1) |
606822 | MDDG-A3; LGMD-2O | (Yoshida et al., 2001) |
| Fukutin | 607440 | MDDG-A4; LGMD-2M | (Kobayashi et al., 1998) |
| Fukutin-related protein (FKRP) | 606596 | MDDG-A5; LGMD-2I | (Brockington et al., 2001a) |
| Acetylglucosaminyltransferase-like protein (LARGE) |
603590 | MDDG-A6 | (van Reeuwijk et al., 2007) |
| Isoprenoid synthase domain-containing protein (ISPD) |
614631 | MDDG-A7 | (Roscioli et al., 2012; Willer et al., 2012) |
| Glycosyltransferase-like domain-containing protein 2 (GTDC2) |
614828 | MDDG-A8 | (Manzini et al., 2012) |
| Dystrophin-associated glycoprotein 1 (DAG1); Dystroglycan |
128239 | MDDG-C9; LGMD-2P | (Hara et al., 2011) |
| Transmembrane protein 5 (TMEM5) | 605862 | MDDG-A10 | (Jae et al., 2013) |
| β-1,3-N-Acetylgalactosaminyltransferase 2 (B3GALNT2) |
610194 | MDDG-A11 | (Stevens et al., 2013) |
| Protein kinase-like protein (SGK196) | 615247 | MDDG-A12 | (Jae et al., 2013) |
| b-1,3-N-Acetylglucosaminyltransferase 1 | 605517 | MDDG-A13 | (Buysse et al., 2013) |
| GDP-Mannose pyrophosphorylase B (GMPPB) | 615320 | MDDG-A14 | (Carss et al., 2013) |
| Dolichyl-phosphate Mannosyltransferase 2 (DPM2) |
603564 | CDG1U | (Barone et al., 2012) |
| Dolichyl-phosphate Mannosyltransferase 3 (DPM3) |
605951 | CDG1O | (Lefeber et al., 2009) |
| Dolichol kinase (DOLK) | 610746 | CDG1M | (Lefeber et al., 2011) |
LGMD-Limb girdle muscular dystrophy; MDDG-Muscular dystrophy-dystroglycanopathy; CDG-Congenital disorder of glycosylation
Along with these genetic diseases, it has become evident that dystrophin and its related proteins are important in the pathophysiology of many acquired diseases. Patients with genetic disruptions of dystrophin clearly demonstrate that the loss of dystrophin is sufficient to cause significant cardiac disease. The marked reduction of dystrophin in patients with heart failure (Vatta et al., 2004, 2002) and viral myocarditis (Lee et al., 2000; Lim et al., 2013; Xiong et al., 2002) implicate dystrophin in the pathophysiology of these common forms of heart disease. The loss of dystrophin in the heart has been seen with pulmonary hypertension (Daicho et al., 2009) and in aging (Townsend et al., 2011). Reductions in dystrophin and the DGC have also been implicated in cancer cachexia (Acharyya et al., 2005) and disuse atrophy (Chockalingam et al., 2002). Given the importance of the DGC to the pathogenesis of many diseases, this review will focus on recent developments in our understanding of the assembly and function of these proteins.
DGC Components
Dystroglycan
Dystroglycan (DG) is a heterodimer that consists of a transmembrane β-subunit and a large, heavily glycosylated α-subunit (Figure 1). These proteins were first identified as part of the complex that tightly associates with dystrophin (Ervasti et al., 1990). The α-and β-subunits of DG are proteolytic cleavage products of a single polypeptide (Ibraghimov-Beskrovnaya et al., 1992). The C-terminal domain of β-DG faces the cytosol, where it interacts with dystrophin or utrophin (Jung et al., 1995; Matsumura et al., 1992a). This interaction is thought to localize DG and associated proteins to a defined subcellular region. On the extracellular surface α-DG, anchored to β-DG, functions as a receptor for extracellular matrix components such as laminin, neurexin, and agrin (Ervasti and Campbell, 1993; Michele et al., 2002). DG is expressed widely throughout the body, with the highest levels present in striated muscle and the brain (Ibraghimov-Beskrovnaya et al., 1993). The main function of DG is centered on its ability to bind to various elements of the extracellular matrix, a process that is critically dependent on post-translational glycosylation (Michele et al., 2002).
Dystroglycan Processing
The dystroglycan gene (DAG1) expression is regulated by a combination of chromatin remodeling and transcription factors binding to SP1 and E-Box sites in the promoter region (Rettino et al., 2009). This promoter is relatively active in myoblasts and displays increased activity during differentiation (Noguchi et al., 1999). Following the excision of two large introns the mature mRNA is translated and the protein is inserted into the ER membrane (Ibraghimov-Beskrovnaya et al., 1993, 1992). Dystroglycan is cleaved into a large extracellular domain (α-DG) and a smaller transmembrane protein (β-DG). While both α- and β-DG are glycoproteins, the glycosylation of α-DG is a particularly complex process involving many glycosyltransferases and demonstrating distinct tissue (Ervasti et al., 1997) and developmental regulation (Goddeeris et al., 2013; Leschziner et al., 2000). A tremendous amount of work has been done to understand the processing of DG. Over half a dozen genetic diseases have been linked to alterations in this pathway. The following section will focus on describing a model of DG glycosylation that attempts to incorporate findings ranging from patients to cell lines.
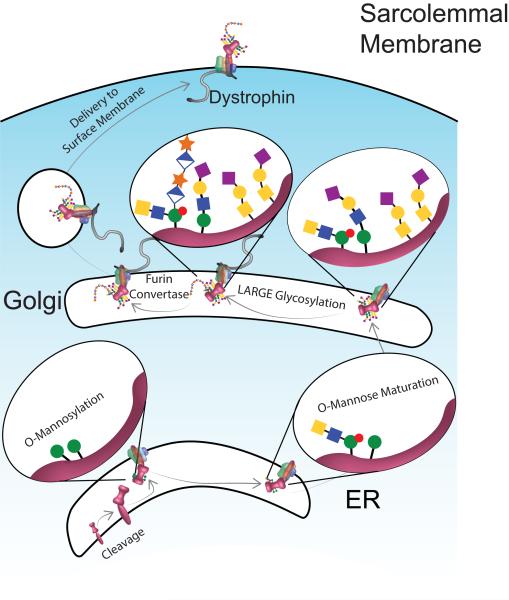
The cleavage of dystroglycan into two polypeptides is critical for proper post-translational processing (Esapa et al., 2003; Jayasinha et al., 2003). Both α- and β-DG are N-glycosylated; although these modifications are limited in size and number (Ervasti and Campbell, 1991; Ibraghimov-Beskrovnaya et al., 1992), they are critically important for subsequent processing (Esapa et al., 2003). α-DG has two globular domains flanking a central mucin domain that is characterized by a high concentration of serine, threonine, and proline residues and is heavily glycosylated (Ibraghimov-Beskrovnaya et al., 1992). In addition to proteolysis and N-glycosylation, nearly half of the O-glycosylation of α-DG begins with the addition of mannose (Stalnaker et al., 2010). Mannose-1-phosphate is converted to GDP-mannose by guanosine diphosphate mannose pyrophosphorylase B (GMPPB; Carss et al., 2013). GDP-mannose serves as a substrate for dolichol-phosphate-mannose (Dol-P-Man) synthase, an enzyme consisting of three subunits, DPM1-3 (Lefeber et al., 2009). Dol-P-Man is the substrate utilized by a variety of mannosyltransferases, including those involved in the glycosylation of α-DG. The function of the enzyme isoprenoid synthase domain (ISPD) has not been clearly defined, however, its homologous enzymes in plants and bacteria suggest it may have a role in synthesizing a new nucleotide sugar (Roscioli et al., 2012; Willer et al., 2012). The protein O-mannosyltransferase (POMT)-1 and POMT-2 transfer mannose to the α-DG polypeptide (Beltran-Valero de Bernabe et al., 2002; Manya et al., 2004; Willer et al., 2002). A subset of these mannose moieties are modified by POMGnT2, which adds an N-Acetyl glucosamine in a β1-4 configuration to the mannose, followed by the addition of an N-acetyl glacatosamine by B3GALnT2 (Yoshida-Moriguchi et al., 2013). The kinase SGK196 is responsible for the phosphorylation the sixth carbon of mannose in this trisaccharide (Yoshida-Moriguchi et al., 2013). Failure of any of these processes completely prevents the subsequent ability of α-DG to bind its extracellular matrix ligands (Beltran-Valero de Bernabe et al., 2002; van Reeuwijk et al., 2005). The addition of O-linked mannose has been documented throughout the mucin domain, but it is concentrated in the N-terminal half of this domain (Kanagawa et al., 2004; Stalnaker et al., 2010). As DG leaves the ER and transits to the Golgi, it has been cleaved into two subunits, N-glycosylation has been initiated, and O-linked Mannose moieties have been attached and partially modified (Figure 2).
Figure 2.
A model of the processing of dystroglycan (DG; pink). The DAG polypeptide is cleaved into α- and β-DG. While shown in the ER, the nature of the protease and its precise subcellular location are unknown. O-linked mannosylation (green circles) of α-DG occurs within the ER. During O-mannose maturation, mannose moieties on which the LARGE-glycan will be added are phosphorylated (red circle) and N-acetylglucosamine (filled blue square) and N-acetylgalactosamine (filled yellow square) are added. Following transit to the Golgi, additional O-glycosylation occurs with many structures capped with galactose (yellow circle) and sialic acid (purple diamond). Next a polymer of glucuronic acid (half-filled diamond) and xylose (orange star) is added by the glycosyltransferase LARGE. Following the cleavage of the N-terminal globular domain of α-DG by a furin convertase the complex is exported to the sarcolemmal membrane. The number of sites and structures of the glycosylation moieties has been simplified for clarity (see text for more details). Note the subcellular location where dystrophin first interacts with the DGC is not clear, while evident at the membrane so data suggests that dystrophin has a role in exporting the DGC from the Golgi (see text for details).
Within the Golgi, DG interacts with many glycosyltransferases and other regulatory proteins. Protein O-mannose beta-1,2-N-acetylglucosaminyltransferase (POMGnT1) is present within the Golgi and attaches an N-acetylglucosamine to the O-linked mannose moieties (Yoshida et al., 2001). Also in the Golgi, POMGnT1 forms a complex with fukutin, a protein whose disruption causes Fukuyama-type congenital muscular dystrophy (Kobayashi et al., 1998; Xiong et al., 2006). The function of fukutin is currently unknown; but interestingly, it shares homology with a yeast protein that regulates mannose phosphorylation (Aravind and Koonin, 1999). Furthermore, phosphorylated mannose is a poor substrate for POMGnT1 and thus prevents the addition of N-acetylglucosamine (Mo et al., 2011). These data raise the interesting hypothesis that a complex containing POMGnT1, fukutin, and α-DG is responsible for preparing α-DG to be a suitable substrate for subsequent steps. Nearly all of the mannose moieties are expanded in the Golgi through the addition of N-acetylglucosamine by POMGnT1 (Yoshida et al., 2001; Zhang et al., 2002). Most of these receive an additional galactose and a bit more than half of these receive a terminal sialic acid moiety (Stalnaker et al., 2010). This later motif has been extensively characterized in isolated mature α-DG (Chiba et al., 1997; Sasaki et al., 1998).
The ability to bind laminin is an essential function of DG and is completely dependent on the presence of a unique glycan (Michele et al., 2002). This glycan is added by a glycosyltranserase called LARGE (Like-acetylglucoseaminyltransferase). The LARGE-glycan consists of a long polymer of 3-xylose-α-1,3-glucuronic acid (Inamori et al., 2012) that is attached to the α-DG backbone at a phosphorylated mannose group (Yoshida-Moriguchi et al., 2010). Most of the evidence supports a model whereby the LARGE-glycan is added to only the O-linked mannose group attached to Thr379 of α-DG (Kanagawa et al., 2004; Stalnaker et al., 2011b; Yoshida-Moriguchi et al., 2010), although other sites may also contribute. Increased expression of LARGE results in increased LARGE-glycan deposition (Gumerson et al., 2013; Yu et al., 2013; Zhang and Hu, 2012). However, with overexpression there is nonspecific addition of LARGE-glycan to non-DG proteins (Zhang and Hu, 2012).
Fukutin-related protein (FKRP) is a homodimer that also colocalizes with fukutin in the Golgi (Esapa et al., 2002). Mutations within FKRP result in muscular dystrophy-dystroglycanopathy type 5, a combined clinical descriptor encompassing both congenital muscular dystrophy type 1 (MDC1C) and the milder limb-girdle muscular dystrophy 2I (LGMD2I; Table 1; Alhamidi et al., 2011; Brockington et al., 2001a, 2001b). The effect of these mutations on α-DG glycosylation is variable with some patients displaying very little LARGE-glycan, while others have only slight reductions (Brockington et al., 2001a, 2001b). Similarly, mouse models of FKRP mutations also have variable phenotypes ranging from no LARGE-glycan to nearly normal levels (Blaeser et al., 2013). In samples from patients with FKRP mutations there is an increased gel mobility of the LARGE-glycan immunoreactivity (Blaeser et al., 2013; Brockington et al., 2001a). The gel migration of the α-DG core protein in FKRP mutant mice is not altered by aqueous hydrofluoric acid treatment, which cleaves phosphodiester bonds and releases the LARGE-glycan. Therefore, the lack of an effect indicates that there is less LARGE-glycan and suggests that FKRP may function to increase the efficiency of LARGE-glycan addition (Kuga et al., 2012). Interestingly, despite this proposed role in the maturation of α-DG, FKRP accompanies the entire DGC to the surface membrane (Beedle et al., 2007). Other studies demonstrate that the N-terminal domain of α-DG is essential for the addition of the LARGE-glycan, but is cleaved off by a furin convertase prior to final transport to the surface membrane (Kanagawa et al., 2004).
As noted above, the LARGE-glycan accounts for only a very small portion of the significant level of O-glycans present on α-DG. The remainder of these moieties are either the POMGnT1-dependent mannose-initiated or O-linked N-acetylgalactosamine initiated mucin groups (Stalnaker et al., 2011a, 2010). The latter of these glycans is initiated in the Golgi by the actions of polypeptide N-acetylgalactosaminyltransferase (Homa et al., 1993; Mo et al., 2011; Röttger et al., 1998; White et al., 1995). Like the mannosylation in the ER, N-acetylgalactosaminylation occurs stochastically on specific Ser/Thr residues throughout the central mucin domain of α-DG, although they appear to occur more frequently in the C-terminal half of the domain (Stalnaker et al., 2010). The importance of these tetrasaccharides and trisaccharides for LARGE function is evident by the dramatic decrease in LARGE-glycan addition in patients with mutations in POMGnT1 or the inhibition of ppGalNAcT, the enzymes that initiate these glycans (Esapa et al., 2003; Michele et al., 2002). The mechanism by which these changes limit addition of the LARGE-glycan are not clear, but likely result from the significant structural differences imparted by O-glycosylation on α-DG.
The majority of these O-glycans terminate with a sialic acid moiety (Stalnaker et al., 2010). Despite its abundance, removal of the terminal sialic acid has no significant effect on α-DG laminin binding activity, although it does prevent the binding of several lectins (Combs and Ervasti, 2005). Other studies demonstrate that overexpression of LARGE in cells with defective sialic acid metabolism results in normal levels of LARGE-glycan addition (Patnaik and Stanley, 2005). However patients with disruption of sialic acid synthesis display a variable phenotype; most appear to retain the LARGE-glycan and laminin binding (Broccolini et al., 2005; Saito et al., 2004), although some patients display a loss of the LARGE-glycan (Huizing et al., 2004).
There are clear differences in the glycosylation of DG in different tissues (Ervasti et al., 1997), but the underlying mechanism remains unclear. Recent studies in mice lacking LARGE indicate that tissue-dependent differences in the gel migration of the α-DG core protein remain (Goddeeris et al., 2013), suggesting that O-glycans, other than LARGE-glycan, contribute to these differences. Consistent with this model is the increased gel migration of the α-DG protein core with the loss of POMGnT1 function, which transfers N-acetylglucosamine to mannose (Willer et al., 2012). Differences in tissue expression of other α-DG processing enzymes have also been described and may contribute to these tissue-specific forms of α-DG (Homa et al., 1993; Margeta et al., 2009; White et al., 1995).
The importance of the LARGE-glycan for the binding of extracellular matrix proteins is well established (Barresi et al., 2004; Michele et al., 2002). The function of the other glycosylation sites remains less clear. One possible function is that these modifications improve the interaction between α- and β-DG (Zhang and Hu, 2012) or perhaps to protect β-DG from the action of extracellular proteases (Hnia et al., 2006). Another intriguing possibility is that these additional O-glycan structures provide mechanical support of the LARGE-glycan domain. Full O-glycosylation of the mucin domain would be expected to greatly increase the stiffness of the region (Jentoft, 1990). This stiffness may be important for extending the LARGE-glycan containing N-terminus of α-DG out toward the extracellular matrix components it interacts with.
Sarcoglycan Complex
The sarcoglycan complex (SGC) is a group of four tightly associated proteins that have been implicated in causing several forms of recessive limb-girdle muscular dystrophies (LGMD). This section will introduce each of the sarcoglycans (SG) and then will discuss what is known regarding the assembly of this complex.
α-Sarcoglycan
α-Sarcoglycan was the first member of the SGC to be identified as the gene responsible for causing LGMD2D (Matsumura et al., 1992b). The gene encoding α-SG is located on human chromosome 17 and consists of 10 exons (Roberds et al., 1994). The protein has two putative N-glycosylation sites and a single transmembrane domain with a relatively short intracellular C-terminal domain (Roberds et al., 1993). Most disease causing mutations are found in the extracellular domain (Bonnemann et al., 1995; McNally et al., 1994; Piccolo et al., 1995). α-SG contains a divalent cation dependent ecto-ATPase activity within its extracelluar domain that has been demonstrated in both myoblasts and HEK-293 cells (Betto et al., 1999; Sandona et al., 2004). The physiological importance of this activity is currently unknown.
ε-Sarcoglycan
Muscular dystrophy resulting from mutations in α-SG have milder cardiac disease than mutations caused by other members of the SGC (Carrie et al., 1997). This is explained, in part, by the presence of ε-SG, an α-SG homologue (Ettinger et al., 1997; McNally et al., 1998). ε-SG is widely expressed in non-skeletal muscle tissues, including the heart (Ettinger et al., 1997; Imamura et al., 2005; McNally et al., 1998; Straub et al., 1999). The ε-SG gene structure is similar to that of α-SG (McNally et al., 1998) and in the absence of α-SG, ε-SG can support the SGC (Liu and Engvall, 1999). ε-SG is expressed early in development in striated muscle and is found in smooth muscle tissues in adults (Imamura et al., 2005; Straub et al., 1999). ε-SG levels decrease in skeletal muscle shortly after birth (Liu and Engvall, 1999; Straub et al., 1999). Ablation of both α- and ε-SG results in the loss of the cardiac SGC and a phenotype much more severe than that present in either knockout individually (Lancioni et al., 2011).
β-Sarcoglycan
β-SG is a 43 kDa protein, the mutation of which has been linked to LGMD2E (Bonnemann et al., 1995; Lim et al., 1995). The β-SG gene is found on chromosome 4 and consists of 6 exons (Bonnemann et al., 1995; Duclos et al., 1998; Lim et al., 1995). The protein contains a single transmembrane domain and a short intracellular N-terminal domain. The longer extracellular domain contains three N-glycosylation sites and is the location of most of the disease causing mutations (Bonnemann et al., 1998; Lim et al., 1995). Loss of β-SG results in the complete absence of the SGC without significant changes in transcript levels (Araishi et al., 1999). Importantly, laminin, dystrophin, and β-DG are normally localized (Araishi et al., 1999; Duclos et al., 1998); although expression of fully glycosylated α-DG appears to be reduced (Araishi et al., 1999).
γ-Sarcoglycan
γ-SG is a 35 kDa protein also with a single transmembrane domain and an extracellular C-terminal domain, which contains a single N-glycosylation domain and a series of conserved cysteines (McNally et al., 1996; Noguchi et al., 1995). Mutations in γ-SG cause LGMD2C, with most of the mutations occurring in the extracellular domain (McNally et al., 1996; Torelli et al., 2005). Loss of γ-SG results in the absence of the other three SGC components (Hack et al., 1998; McNally et al., 1996) σ-Sarcoglycan σ-SG is also a 35 kDa protein that shares significant homology with γ-SG. Like γ-SG, σ-SG consists of a single transmembrane domain with a small intracellular N-terminus and a larger extracellular C-terminal domain, with a single N-glycosylation site and the cluster of cysteine residues present in several other members of the SGC (Nigro et al., 1996b). Mutations in σ-SG result in LGMD2F (Nigro et al., 1996a; Passos-Bueno et al., 1996) and the loss of the entire SGC (Hack et al., 2000; Straub et al., 1998). Interestingly, similar to the absence of β-SG, the loss of σ-SG results in the disproportional loss of the LARGE-glycan expression relative to β-DG (Sakamoto et al., 1997; Straub et al., 1998).
ζ-Sarcoglycan
ζ-SG is homologous to gamma- and delta-SG with 55-57% amino acid identity and 74-75% similarity. It is present in the SGC in both skeletal and cardiac muscle, especially in the absence of γ- or σ-SG (Wheeler et al., 2002). ζ-SG also contains the conserved cluster of cysteine residues that are found in β-,γ-, and σ-SG.
Sarcospan
Sarcospan (SSPN) is a 25kDa protein with 4 transmembrane domains with both N- and C-termini on the intracellular face that is localized to cardiac and skeletal muscle sarcolemma. Primary expression is in striated muscle, although lower levels of expression are present in the lung, brain, and testes. SSPN is markedly down-regulated in DMD patients and in dystrophin deficient mdx mice, including the heart where the DGC is largely intact, suggesting a unique dependence on dystrophin for stabilization (Crosbie et al., 1999, 1997). SSPN is also expressed at the neuromuscular junction, where it is stabilized by either dystrophin or utrophin (Crosbie et al., 1999). SSPN expression is also dependent on the presence of an intact SGC (Araishi et al., 1999; Coral-Vazquez et al., 1999; Crosbie et al., 2000, 1999). Loss of SSPN does not result in a significant myopathy, although molecular changes include a reduction in dystrophin binding to the DGC and increased susceptibility to lengthening contractions (Lebakken et al., 2000; Marshall et al., 2012a). While high levels of overexpression are detrimental, lower levels of human SSPN expression increase the presence of the DGC and utrophin in the sarcolemma of mdx skeletal muscle resulting in improved muscle function (Peter et al., 2008, 2007). SSPN is capable of forming higher order oligomers (Marshall and Watson, 2013; Miller et al., 2007), which in other structurally related proteins results in the formation of a lattice network. It has been proposed that SSPN functions, in part, to hold the components of the DGC in tight proximity (Marshall and Watson, 2013).
Assembly of the SGC
The individual components of the SGC are inserted into the membrane of the ER where the process of N-glycosylation begins. Blockade of ER export demonstrates that the initial addition of mannose and subsequent addition of sialic acid and N-acetylglucosamine all occur within the ER (Noguchi et al., 2000). The N-glycan structures on the SGs are critically important for subsequent trafficking to the membrane and association with the DG heterodimer (J. Chen et al., 2006; Noguchi et al., 2000; Shi et al., 2004). The timing and location of the interaction of SSPN with the reminder of the complex is not clear, although there is evidence that SSPN is present in the ER/Golgi compartment and that transgenic expression of SSPN alters the glycosylation of α-DG in the Golgi (Marshall et al., 2012b). Current evidence suggests that the SGC forms around a complex of β- and σ-SG (Chan et al., 1998; Noguchi et al., 2000) and is capable of binding to co-expressed β-DG/dystrophin complex (J. Chen et al., 2006). When expressed in HEK cells the β-/σ-SG dimer is sufficient to traffic to the membrane (Chan et al., 1998; J. Chen et al., 2006; Draviam et al., 2006a; Noguchi et al., 2000). The next significant complex is the β-/γ-/σ-SG trimer; each of these proteins contains a conserved cluster of cysteine residues that participate in intramolecular disulfide bond formation that is important for trafficking of the complex (Chan et al., 1998; J. Chen et al., 2006). The final form of the SGC is completed with the addition of α-SG (Noguchi et al., 2000). α-SG, which binds to the γ-SG subunit, is capable of being transported to the plasma membrane independent of other parts of the SGC, however it is not stable at the cell surface without the rest of the complex (J. Chen et al., 2006; Draviam et al., 2006b; Noguchi et al., 2000; Shi et al., 2004). Many of the disease-causing mutations in the SGC affect either the stability of the proteins or involve mutations that disrupt the formation of these intermediate complexes (J. Chen et al., 2006; Draviam et al., 2006a; Holt and Campbell, 1998; Shi et al., 2004). The SGC is largely assembled and glycosylated within the ER and is tightly associated with the partially processed DG heterodimer as it transits to the Golgi (Noguchi et al., 2000). Furthermore, the addition of the LARGE-glycan, assessed by IIH6 immunoreactivity, to α-DG is critically dependent on the presence of an intact SGC (Hack et al., 2000; Straub et al., 1998). While the SGC will form without DG, it is unstable and continued expression is dependent on the presence of an intact DG (Cote et al., 2002; Holt and Campbell, 1998; Kanagawa et al., 2004; Michele et al., 2009)
Dystrophin and Utrophin
Dystrophin is the protein product of the DMD gene that is responsible for Duchenne muscular dystrophy (E P Hoffman et al., 1987a). The dystrophin protein consists of four major domains, an N-terminal actin binding domain, a central rod domain with 24 spectrin-like repeats, a cysteine-rich β-DG binding domain, and a carboxy-terminal domain (E P Hoffman et al., 1987a, 1987b; Koenig and Kunkel, 1990). Utrophin is a homologous protein with a similar domain structure (Matsumura et al., 1992a; Tinsley et al., 1992). Furthermore, overexpression of utrophin has been shown to largely rescue the phenotype of the mdx mouse (Tinsley et al., 1998) and utrophin expression correlates with a milder phenotype in DMD patients (Kleopa et al., 2006). Much has been written regarding the function of dystrophin and utrophin, but for the purposes of this review, only their ability to bind to the components of the DGC will be explored. The proteins of the DGC were first identified by their absence in skeletal muscle from patients with DMD (Ervasti et al., 1990; Ohlendieck and Campbell, 1991). In skeletal muscle, utrophin is normally localized to the neuromuscular junction where it binds to β-DG and the SGC to aid in the formation of the synaptic structure (Matsumura et al., 1992a; Tinsley et al., 1992). In cardiac tissue, utrophin also has a different subcellular localization pattern compared to dystrophin, suggesting it has a unique function in this tissue as well (Pons et al., 1994). In the absence of dystrophin, utrophin can largely replace dystrophin as evidenced by the severity of the dystrophy present in mice lacking both utrophin and dystrophin (Deconinck et al., 1997; Grady et al., 1997) and the ability of utrophin overexpression to significantly improve the phenotype of dystrophin-deficient mice (Rafael et al., 1998). In skeletal muscle, the absence of dystrophin results in dramatic decreases in the amount of DGC proteins that are present (Ervasti et al., 1990; Ohlendieck and Campbell, 1991). In smooth muscle, much of the DGC remains intact without dystrophin, but is absent in the dystrophin/utrophin double knockout (Straub et al., 1999), indicating that utrophin is largely able to compensate for the absence of dystrophin. Interestingly, the DGC is largely intact in the dystrophin-deficient myocardium with or without utrophin (Bies et al., 1997; Matsumura et al., 1992a; Sharpe et al., 2013; Townsend et al., 2007).
The roles of dystrophin and utrophin in the processing and stabilizing of the DGC are not clear. Both dystrophin and utrophin messages are expressed very early in muscle differentiation, but dystrophin protein does not accumulate until later in the differentiation process (Noguchi et al., 2000, 1999; Tanaka and Ozawa, 1990; Tomé et al., 1994). During early differentiation, utrophin is prominent at the surface of the myotube while dystrophin expression is largely cytoplasmic in nature as are both β-DG and the sarcoglycans (Noguchi et al., 2000). This data suggests that in developing skeletal muscle, dystrophin interacts with the DGC components prior to insertion into the surface membrane. This raises the possibility that dystrophin may modulate the processing of the DGC as it moves through the cellular export pathways. Less is known about the maturation of the DGC in cardiac tissues, but recent studies suggest that dystrophin and utrophin may have a role in the glycosylation of α-DG in this tissue (Sharpe et al., 2013). This study provides evidence that the addition/maturation of non-LARGE-glycan O-glycosylation is dependent on the presence of dystrophin or utrophin (Sharpe et al., 2013). It is not clear if these changes in glycosylation result from the pathology present in these mice or if they provide support for a model where dystrophin or utrophin directly affect the processing and assembly of the DGC in the Golgi. In skeletal muscle, the absence of dystrophin results in the accumulation of the DGC components in the ER/Golgi compartment (Marshall et al., 2012b). In both skeletal and cardiac muscle increases in dystrophin levels result in increased levels of the DGC (Cox et al., 1993; Townsend et al., 2007). This data suggests that dystrophin is the rate-limiting step in the accumulation of the DGC at the surface membrane; the more dystrophin binding sites, the more DGC is stabilized and saved from the proteasome (Bonuccelli et al., 2003). There is significant evidence indicating that the DGC is present in distinct domains within the surface membranes (Byers et al., 1991; Klietsch et al., 1993; Ohlendieck et al., 1991; Watkins et al., 1987), however, the functional importance of these distinct domains is not clear.
Other Peripheral Components of the DGC
In addition to the membrane-bound components of the DGC, several soluble proteins are found tightly associated with dystrophin and the rest of the DGC. The most abundant of these are syntrophin (Ahn et al., 1994; Ohlendieck and Campbell, 1991) and dystrobrevin (Dwyer and Froehner, 1995). In the DGC, syntrophin’s PDZ domain facilitates the localization of a variety of binding partners ranging from nNOS (Brenman et al., 1995) and α1D-adrenergic receptors (Z. Chen et al., 2006) to ion channels (Gavillet et al., 2006; Vandebrouck et al., 2007) and aquaporin (Adams et al., 2001). The function of dystrobrevin is less clear. Its structure suggests that it too functions to bind proteins together, but the targets of these interactions are largely cytoskeletal in nature (Albrecht and Froehner, 2004; Benson et al., 2001; Mizuno et al., 2001; Newey et al., 2001). Dystrobrevin’s interaction with both dystrophin and the sarcoglycan complex also suggests a potential role reinforcing dystrophin binding to the membrane (Bunnell et al., 2008; Sadoulet-Puccio et al., 1997; Yoshida et al., 2000). Both syntrophin and dystrobrevin have multiple isoforms and splice variants that display unique subcellular localization and binding interactions, however, the importance of these individual isoforms is less clearly understood. In addition to syntrophin and dystrobrevin, there are a variety of other proteins that have been shown to interact with dystrophin and the DGC, including ankyrin-B and –G (Ayalon et al., 2008), microtubules (Prins et al., 2009), cavin-1, CRYAB, and AHNAK1 (Johnson et al., 2012). Little is known regarding the timing and localization of the association of these soluble proteins with the DGC core proteins.
Conclusion
The DGC is essential to the normal functioning of striated muscle and many other tissues. We have significantly improved our understanding of the role these molecules play in muscle function. The data reviewed here indicate that the transmembrane core DGC proteins associate early within the protein export pathways (Figure 2; Noguchi et al., 2000). This tight association is essential for the normal processing and function of the mature DGC. The addition of dystrophin/utrophin appears to be required for these complexes to exit the Golgi, but the role of other components of the DGC remains unclear. Despite the detail by which this process has been defined many questions about the role of α-DG glycosylation remain, including: how does it define ligand selectivity, does it modify the structural features of the core protein, or dose it possess some other function? Recent studies have demonstrated that α-DG is present in significant excess relative to dystrophin, suggesting that most of this protein is not normally associated with dystrophin (Johnson et al., 2013); the function of such a complex remains unclear. Further complexity arises from the diversity of DGC structures within cells (surface vs. NMJ) and between different cell types (skeletal vs. cardiac muscle) and the functional significance of these differences will likely provide insight into role of the DGC in a variety of tissues. Diseases of the DGC are largely incurable in part because of a poor mechanistic understanding of the pathophysiology resulting from the loss of these proteins. A more complete understanding of the mechanism of disease would allow the identification of specific pathways that may be amenable to the development of specific therapeutics. Furthermore, there is increasing evidence that the proteins in the DGC may have a significant role in the pathophysiology of more common acquired diseases ranging from cancer cachexia to heart failure (Acharyya et al., 2005; Fearon et al., 2012; Lim et al., 2013; Toyo-Oka et al., 2004; Vatta et al., 2002). Further studies into the physiology of the DGC will be important for the development of new therapies for both rare genetic disorders and more common diseases where the DGC has been implicated.
Acknowledgments
Grants: NIH K08HL102066 NIH R01HL114832
REFERENCES
- Acharyya S, Butchbach MER, Sahenk Z, Wang H, Saji M, Carathers M, Ringel MD, Skipworth RJE, Fearon KCH, Hollingsworth MA, Muscarella P, Burghes AHM, Rafael-Fortney JA, Guttridge DC. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 2005;8:421–432. doi: 10.1016/j.ccr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Adams ME, Mueller HA, Froehner SC. In vivo requirement of the alpha-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J Cell Biol. 2001;155:113–122. doi: 10.1083/jcb.200106158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn AH, Yoshida M, Anderson MS, Feener CA, Selig S, Hagiwara Y, Ozawa E, Kunkel LM. Cloning of human basic A1, a distinct 59-kDa dystrophin-associated protein encoded on chromosome 8q23-24. Proc Natl Acad Sci U S A. 1994;91:4446–4450. doi: 10.1073/pnas.91.10.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DE, Froehner SC. DAMAGE, a novel alpha-dystrobrevin-associated MAGE protein in dystrophin complexes. J Biol Chem. 2004;279:7014–7023. doi: 10.1074/jbc.M312205200. [DOI] [PubMed] [Google Scholar]
- Alhamidi M, Kjeldsen Buvang E, Fagerheim T, Brox V, Lindal S, Van Ghelue M, Nilssen O. Fukutin-related protein resides in the Golgi cisternae of skeletal muscle fibres and forms disulfide-linked homodimers via an N-terminal interaction. PLoS One. 2011;6:e22968. doi: 10.1371/journal.pone.0022968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araishi K, Sasaoka T, Imamura M, Noguchi S, Hama H, Wakabayashi E, Yoshida M, Hori T, Ozawa E. Loss of the sarcoglycan complex and sarcospan leads to muscular dystrophy in beta-sarcoglycan-deficient mice. Hum. Mol. Genet. 1999;8:1589–1598. doi: 10.1093/hmg/8.9.1589. [DOI] [PubMed] [Google Scholar]
- Aravind L, Koonin EV. The fukutin protein family--predicted enzymes modifying cell-surface molecules. Curr. Biol. 1999;9:R836–7. doi: 10.1016/s0960-9822(00)80039-1. [DOI] [PubMed] [Google Scholar]
- Ayalon G, Davis JQ, Scotland PB, Bennett V. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell. 2008;135:1189–1200. doi: 10.1016/j.cell.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Barone R, Aiello C, Race V, Morava E, Foulquier F, Riemersma M, Passarelli C, Concolino D, Carella M, Santorelli F, Vleugels W, Mercuri E, Garozzo D, Sturiale L, Messina S, Jaeken J, Fiumara A, Wevers R. a, Bertini E, Matthijs G, Lefeber DJ. DPM2-CDG: a muscular dystrophy-dystroglycanopathy syndrome with severe epilepsy. Ann. Neurol. 2012;72:550–8. doi: 10.1002/ana.23632. [DOI] [PubMed] [Google Scholar]
- Barresi R, Michele DE, Kanagawa M, Harper HA, Dovico SA, Satz JS, Moore SA, Zhang W, Schachter H, Dumanski JP, Cohn RD, Nishino I, Campbell KP. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med. 2004;10:696–703. doi: 10.1038/nm1059. [DOI] [PubMed] [Google Scholar]
- Beedle AM, Nienaber PM, Campbell KP. Fukutin-related protein associates with the sarcolemmal dystrophin-glycoprotein complex. J Biol Chem. 2007;282:16713–16717. doi: 10.1074/jbc.C700061200. [DOI] [PubMed] [Google Scholar]
- Beltran-Valero de Bernabe D, Currier S, Steinbrecher A, Celli J, van Beusekom E, van der Zwaag B, Kayserili H, Merlini L, Chitayat D, Dobyns WB, Cormand B, Lehesjoki AE, Cruces J, Voit T, Walsh CA, van Bokhoven H, Brunner HG. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet. 2002;71:1033–1043. doi: 10.1086/342975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem. 2001;276:24232–24241. doi: 10.1074/jbc.M010418200. [DOI] [PubMed] [Google Scholar]
- Betto R, Senter L, Ceoldo S, Tarricone E, Biral D, Salviati G. Ecto-ATPase activity of alpha-sarcoglycan (adhalin) J Biol Chem. 1999;274:7907–7912. doi: 10.1074/jbc.274.12.7907. [DOI] [PubMed] [Google Scholar]
- Bies RD, Maeda M, Roberds SL, Holder E, Bohlmeyer T, Young JB, Campbell KP. A 5′ dystrophin duplication mutation causes membrane deficiency of alpha-dystroglycan in a family with X-linked cardiomyopathy. J Mol Cell Cardiol. 1997;29:3175–3188. doi: 10.1006/jmcc.1997.0568. [DOI] [PubMed] [Google Scholar]
- Blaeser A, Keramaris E, Chan YM, Sparks S, Cowley D, Xiao X, Lu QL. Mouse models of fukutin-related protein mutations show a wide range of disease phenotypes. Hum. Genet. 2013;132:923–934. doi: 10.1007/s00439-013-1302-7. [DOI] [PubMed] [Google Scholar]
- Bonnemann CG, Modi R, Noguchi S, Mizuno Y, Yoshida M, Gussoni E, McNally EM, Duggan DJ, Angelini C, Hoffman EP. Beta-sarcoglycan (A3b) mutations cause autosomal recessive muscular dystrophy with loss of the sarcoglycan complex. Nat Genet. 1995;11:266–273. doi: 10.1038/ng1195-266. [DOI] [PubMed] [Google Scholar]
- Bonnemann CG, Wong J, Ben Hamida C, Hamida MB, Hentati F, Kunkel LM. LGMD 2E in Tunisia is caused by a homozygous missense mutation in beta-sarcoglycan exon 3. Neuromuscul Disord. 1998;8:193–197. doi: 10.1016/s0960-8966(98)00014-5. [DOI] [PubMed] [Google Scholar]
- Bonuccelli G, Sotgia F, Schubert W, Park DS, Frank PG, Woodman SE, Insabato L, Cammer M, Minetti C, Lisanti MP. Proteasome inhibitor (MG-132) treatment of mdx mice rescues the expression and membrane localization of dystrophin and dystrophin-associated proteins. Am. J. Pathol. 2003;163:1663–1675. doi: 10.1016/S0002-9440(10)63523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- Broccolini A, Gliubizzi C, Pavoni E, Gidaro T, Morosetti R, Sciandra F, Giardina B, Tonali P, Ricci E, Brancaccio A, Mirabella M. alpha-Dystroglycan does not play a major pathogenic role in autosomal recessive hereditary inclusion-body myopathy. Neuromuscul. Disord. 2005;15:177–84. doi: 10.1016/j.nmd.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Brockington M, Blake DJ, Prandini P, Brown SC, Torelli S, Benson MA, Ponting CP, Estournet B, Romero NB, Mercuri E, Voit T, Sewry CA, Guicheney P, Muntoni F. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am J Hum Genet. 2001a;69:1198–1209. doi: 10.1086/324412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington M, Yuva Y, Prandini P, Brown SC, Torelli S, Benson M. a, Herrmann R, Anderson LV, Bashir R, Burgunder JM, Fallet S, Romero N, Fardeau M, Straub V, Storey G, Pollitt C, Richard I, Sewry C. a, Bushby K, Voit T, Blake DJ, Muntoni F. Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum. Mol. Genet. 2001b;10:2851–9. doi: 10.1093/hmg/10.25.2851. [DOI] [PubMed] [Google Scholar]
- Bunnell TM, Jaeger MA, Fitzsimons DP, Prins KW, Ervasti JM. Destabilization of the dystrophin-glycoprotein complex without functional deficits in alpha-dystrobrevin null muscle. PLoS One. 2008;3:e2604. doi: 10.1371/journal.pone.0002604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010a;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010b;9:177–189. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- Buysse K, Riemersma M, Powell G, van Reeuwijk J, Chitayat D, Roscioli T, Kamsteeg E-J, van den Elzen C, van Beusekom E, Blaser S, Babul-Hirji R, Halliday W, Wright GJ, Stemple DL, Lin Y-Y, Lefeber DJ, van Bokhoven H. Missense mutations in β-1,3-N-acetylglucosaminyltransferase 1 (B3GNT1) cause Walker-Warburg syndrome. Hum. Mol. Genet. 2013;22:1746–54. doi: 10.1093/hmg/ddt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers TJ, Kunkel LM, Watkins SC. The subcellular distribution of dystrophin in mouse skeletal, cardiac, and smooth muscle. J. Cell Biol. 1991;115:411–421. doi: 10.1083/jcb.115.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie A, Piccolo F, Leturcq F, de Toma C, Azibi K, Beldjord C, Vallat JM, Merlini L, Voit T, Sewry C, Urtizberea JA, Romero N, Tome FM, Fardeau M, Sunada Y, Campbell KP, Kaplan JC, Jeanpierre M. Mutational diversity and hot spots in the alpha-sarcoglycan gene in autosomal recessive muscular dystrophy (LGMD2D) J Med Genet. 1997;34:470–475. doi: 10.1136/jmg.34.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carss KJ, Stevens E, Foley a R., Cirak S, Riemersma M, Torelli S, Hoischen A, Willer T, van Scherpenzeel M, Moore S. a, Messina S, Bertini E, Bönnemann CG, Abdenur JE, Grosmann CM, Kesari A, Punetha J, Quinlivan R, Waddell LB, Young HK, Wraige E, Yau S, Brodd L, Feng L, Sewry C, MacArthur DG, North KN, Hoffman E, Stemple DL, Hurles ME, van Bokhoven H, Campbell KP, Lefeber DJ, Lin Y-Y, Muntoni F. Mutations in GDP-mannose pyrophosphorylase B cause congenital and limb-girdle muscular dystrophies associated with hypoglycosylation of α-dystroglycan. Am. J. Hum. Genet. 2013;93:29–41. doi: 10.1016/j.ajhg.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YM, Bönnemann CG, Lidov HG, Kunkel LM. Molecular organization of sarcoglycan complex in mouse myotubes in culture. J. Cell Biol. 1998;143:2033–44. doi: 10.1083/jcb.143.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Shi W, Zhang Y, Sokol R, Cai H, Lun M, Moore BF, Farber MJ, Stepanchick JS, Bönnemann CG, Chan YM. Identification of functional domains in sarcoglycans essential for their interaction and plasma membrane targeting. Exp. Cell Res. 2006;312:1610–25. doi: 10.1016/j.yexcr.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hague C, Hall RA, Minneman KP. Syntrophins regulate alpha1D-adrenergic receptors through a PDZ domain-mediated interaction. J Biol Chem. 2006;281:12414–12420. doi: 10.1074/jbc.M508651200. [DOI] [PubMed] [Google Scholar]
- Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A, Endo T. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve alpha-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of alpha-dystroglycan with laminin. J. Biol. Chem. 1997;272:2156–2162. doi: 10.1074/jbc.272.4.2156. [DOI] [PubMed] [Google Scholar]
- Chockalingam PS, Cholera R, Oak SA, Zheng Y, Jarrett HW, Thomason DB. Dystrophin-glycoprotein complex and Ras and Rho GTPase signaling are altered in muscle atrophy. Am J Physiol Cell Physiol. 2002;283:C500–11. doi: 10.1152/ajpcell.00529.2001. [DOI] [PubMed] [Google Scholar]
- Clarke JL, Gowers WR. On a Case of Pseudo-hypertrophic Muscular Paralysis. Med. Chir. Trans. 1874;57:247–260. 5. doi: 10.1177/095952877405700121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs AC, Ervasti JM. Enhanced laminin binding by alpha-dystroglycan after enzymatic deglycosylation. Biochem J. 2005;390:303–309. doi: 10.1042/BJ20050375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coral-Vazquez R, Cohn RD, Moore SA, Hill JA, Weiss RM, Davisson RL, Straub V, Barresi R, Bansal D, Hrstka RF, Williamson R, Campbell KP. Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell. 1999;98:465–474. doi: 10.1016/s0092-8674(00)81975-3. [DOI] [PubMed] [Google Scholar]
- Cote PD, Moukhles H, Carbonetto S. Dystroglycan is not required for localization of dystrophin, syntrophin, and neuronal nitric-oxide synthase at the sarcolemma but regulates integrin alpha 7B expression and caveolin-3 distribution. J. Biol. Chem. 2002;277:4672–4679. doi: 10.1074/jbc.M106879200. [DOI] [PubMed] [Google Scholar]
- Cox GA, Cole NM, Matsumura K, Phelps SF, Hauschka SD, Campbell KP, Faulkner JA, Chamberlain JS. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature. 1993;364:725–729. doi: 10.1038/364725a0. [DOI] [PubMed] [Google Scholar]
- Crosbie RH, Heighway J, Venzke DP, Lee JC, Campbell KP. Sarcospan, the 25-kDa transmembrane component of the dystrophin-glycoprotein complex. J Biol Chem. 1997;272:31221–31224. doi: 10.1074/jbc.272.50.31221. [DOI] [PubMed] [Google Scholar]
- Crosbie RH, Lebakken CS, Holt KH, Venzke DP, Straub V, Lee JC, Grady RM, Chamberlain JS, Sanes JR, Campbell KP. Membrane targeting and stabilization of sarcospan is mediated by the sarcoglycan subcomplex. J. Cell Biol. 1999;145:153–165. doi: 10.1083/jcb.145.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosbie RH, Lim LE, Moore SA, Hirano M, Hays a P., Maybaum SW, Collin H, Dovico SA, Stolle C. a, Fardeau M, Tomé FM, Campbell KP. Molecular and genetic characterization of sarcospan: insights into sarcoglycansarcospan interactions. Hum. Mol. Genet. 2000;9:2019–27. doi: 10.1093/hmg/9.13.2019. [DOI] [PubMed] [Google Scholar]
- Daicho T, Daisho Y, Kojima S, Takano S, Tejima Y, Marunouchi T, Takagi N, Takeo S, Tanonaka K. Alterations in dystrophin-related glycoproteins in development of right ventricular failure in rats. J. Pharmacol. Sci. 2009;111:405–15. doi: 10.1254/jphs.09208fp. [DOI] [PubMed] [Google Scholar]
- Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- Draviam R. a, Shand SH, Watkins SC. The beta-delta-core of sarcoglycan is essential for deposition at the plasma membrane. Muscle Nerve. 2006a;34:691–701. doi: 10.1002/mus.20640. [DOI] [PubMed] [Google Scholar]
- Draviam R. a, Wang B, Shand SH, Xiao X, Watkins SC. Alpha-sarcoglycan is recycled from the plasma membrane in the absence of sarcoglycan complex assembly. Traffic. 2006b;7:793–810. doi: 10.1111/j.1600-0854.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- Duchenne GB. The Pathology of Paralysis with Muscular Degeneration (Paralysie Myosclerotique), or Paralysis with Apparent Hypertrophy. Brit Med J. 1867;2:541–542. doi: 10.1136/bmj.2.363.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos F, Broux O, Bourg N, Straub V, Feldman GL, Sunada Y, Lim LE, Piccolo F, Cutshall S, Gary F, Quetier F, Kaplan JC, Jackson CE, Beckmann JS, Campbell KP. Beta-sarcoglycan: genomic analysis and identification of a novel missense mutation in the LGMD2E Amish isolate. Neuromuscul Disord. 1998;8:30–38. doi: 10.1016/s0960-8966(97)00135-1. [DOI] [PubMed] [Google Scholar]
- Dwyer TM, Froehner SC. Direct binding of Torpedo syntrophin to dystrophin and the 87 kDa dystrophin homologue. FEBS Lett. 1995;375:91–94. doi: 10.1016/0014-5793(95)01176-f. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Burwell AL, Geissler AL. Tissue-specific heterogeneity in alpha-dystroglycan sialoglycosylation. Skeletal muscle alpha-dystroglycan is a latent receptor for Vicia villosa agglutinin b4 masked by sialic acid modification. J Biol Chem. 1997;272:22315–22321. doi: 10.1074/jbc.272.35.22315. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophinglycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990;345:315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Esapa CT, Benson MA, Schroder JE, Martin-Rendon E, Brockington M, Brown SC, Muntoni F, Kroger S, Blake DJ. Functional requirements for fukutin-related protein in the Golgi apparatus. Hum. Mol. Genet. 2002;11:3319–3331. doi: 10.1093/hmg/11.26.3319. [DOI] [PubMed] [Google Scholar]
- Esapa CT, Bentham GRBB, Schröder JE, Kröger S, Blake DJ. The effects of post-translational processing on dystroglycan synthesis and trafficking. FEBS Lett. 2003;555:209–216. doi: 10.1016/s0014-5793(03)01230-4. [DOI] [PubMed] [Google Scholar]
- Ettinger a. J., Feng G, Sanes JR. epsilon -Sarcoglycan, a Broadly Expressed Homologue of the Gene Mutated in Limb-Girdle Muscular Dystrophy 2D. J. Biol. Chem. 1997;272:32534–32538. doi: 10.1074/jbc.272.51.32534. [DOI] [PubMed] [Google Scholar]
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Gavillet B, Rougier JS, Domenighetti AA, Behar R, Boixel C, Ruchat P, Lehr HA, Pedrazzini T, Abriel H. Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ Res. 2006;99:407–414. doi: 10.1161/01.RES.0000237466.13252.5e. [DOI] [PubMed] [Google Scholar]
- Goddeeris MM, Wu B, Venzke D, Yoshida-Moriguchi T, Saito F, Matsumura K, Moore S. a, Campbell KP. LARGE glycans on dystroglycan function as a tunable matrix scaffold to prevent dystrophy. Nature. 2013;503:136–140. doi: 10.1038/nature12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90:729–738. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- Gumerson JD, Davis CS, Kabaeva ZT, Hayes JM, Brooks SV, Michele DE. Muscle-specific expression of LARGE restores neuromuscular transmission deficits in dystrophic LARGE(myd) mice. Hum. Mol. Genet. 2013;22:757–768. doi: 10.1093/hmg/dds483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack AA, Lam MY, Cordier L, Shoturma DI, Ly CT, Hadhazy MA, Hadhazy MR, Sweeney HL, McNally EM. Differential requirement for individual sarcoglycans and dystrophin in the assembly and function of the dystrophinglycoprotein complex. J Cell Sci. 2000;113(Pt 1):2535–2544. doi: 10.1242/jcs.113.14.2535. [DOI] [PubMed] [Google Scholar]
- Hack AA, Ly CT, Jiang F, Clendenin CJ, Sigrist KS, Wollmann RL, McNally EM. Gamma-sarcoglycan deficiency leads to muscle membrane defects and apoptosis independent of dystrophin. J Cell Biol. 1998;142:1279–1287. doi: 10.1083/jcb.142.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Balci-Hayta B, Yoshida-Moriguchi T, Kanagawa M, Beltran-Valero de Bernabe D, Gundesli H, Willer T, Satz JS, Crawford RW, Burden SJ, Kunz S, Oldstone MB, Accardi A, Talim B, Muntoni F, Topaloglu H, Dincer P, Campbell KP. A dystroglycan mutation associated with limb-girdle muscular dystrophy. N Engl J Med. 2011;364:939–946. doi: 10.1056/NEJMoa1006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnia K, Hugon G, Masmoudi A, Mercier J, Rivier F, Mornet D. Effect of beta-dystroglycan processing on utrophin/Dp116 anchorage in normal and mdx mouse Schwann cell membrane. Neuroscience. 2006;141:607–620. doi: 10.1016/j.neuroscience.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr., Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987a;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Knudson CM, Campbell KP, Kunkel LM. Subcellular fractionation of dystrophin to the triads of skeletal muscle. Nature. 1987b;330:754–758. doi: 10.1038/330754a0. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Monaco AP, Feener CC, Kunkel LM. Conservation of the Duchenne muscular dystrophy gene in mice and humans. Science (80-. ) 1987;238:347–350. doi: 10.1126/science.3659917. [DOI] [PubMed] [Google Scholar]
- Holt KH, Campbell KP. Assembly of the Sarcoglycan Complex. INSIGHTS FOR MUSCULAR DYSTROPHY. J. Biol. Chem. 1998;273:34667–34670. doi: 10.1074/jbc.273.52.34667. [DOI] [PubMed] [Google Scholar]
- Homa FL, Hollander T, Lehman DJ, Thomsen DR, Elhammer AP. Isolation and expression of a cDNA clone encoding a bovine UDPGalNAc:polypeptide N-acetylgalactosaminyltransferase. J. Biol. Chem. 1993;268:12609–16. [PubMed] [Google Scholar]
- Huizing M, Rakocevic G, Sparks SE, Mamali I, Shatunov A, Goldfarb L, Krasnewich D, Gahl W. a, Dalakas MC. Hypoglycosylation of alpha-dystroglycan in patients with hereditary IBM due to GNE mutations. Mol. Genet. Metab. 2004;81:196–202. doi: 10.1016/j.ymgme.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Milatovich A, Ozcelik T, Yang B, Koepnick K, Francke U, Campbell KP. Human dystroglycan: skeletal muscle cDNA, genomic structure, origin of tissue specific isoforms and chromosomal localization. Hum Mol Genet. 1993;2:1651–1657. doi: 10.1093/hmg/2.10.1651. [DOI] [PubMed] [Google Scholar]
- Imamura M, Mochizuki Y, Engvall E, Takeda S. Epsilon-sarcoglycan compensates for lack of alpha-sarcoglycan in a mouse model of limb-girdle muscular dystrophy. Hum. Mol. Genet. 2005;14:775–83. doi: 10.1093/hmg/ddi072. [DOI] [PubMed] [Google Scholar]
- Inamori K, Yoshida-Moriguchi T, Hara Y, Anderson ME, Yu L, Campbell KP. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science (80-. ) 2012;335:93–96. doi: 10.1126/science.1214115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jae LT, Raaben M, Riemersma M, van Beusekom E, Blomen V. a, Velds A, Kerkhoven RM, Carette JE, Topaloglu H, Meinecke P, Wessels MW, Lefeber DJ, Whelan SP, van Bokhoven H, Brummelkamp TR. Deciphering the glycosylome of dystroglycanopathies using haploid screens for lassa virus entry. Science. 2013;340:479–83. doi: 10.1126/science.1233675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasinha V, Nguyen HH, Xia B, Kammesheidt A, Hoyte K, Martin PT. Inhibition of dystroglycan cleavage causes muscular dystrophy in transgenic mice. Neuromuscul. Disord. 2003;13:365–375. doi: 10.1016/s0960-8966(03)00040-3. [DOI] [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem. Sci. 1990;15:291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Johnson EK, Li B, Yoon JH, Flanigan KM, Martin PT, Ervasti J, Montanaro F. Identification of new dystroglycan complexes in skeletal muscle. PLoS One. 2013;8:e73224. doi: 10.1371/journal.pone.0073224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EK, Zhang L, Adams ME, Phillips A, Freitas MA, Froehner SC, Green-Church KB, Montanaro F. Proteomic analysis reveals new cardiac-specific dystrophin-associated proteins. PLoS One. 2012;7:e43515. doi: 10.1371/journal.pone.0043515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Yang B, Meyer J, Chamberlain JS, Campbell KP. Identification and characterization of the dystrophin anchoring site on beta-dystroglycan. J Biol Chem. 1995;270:27305–27310. doi: 10.1074/jbc.270.45.27305. [DOI] [PubMed] [Google Scholar]
- Kanagawa M, Saito F, Kunz S, Yoshida-Moriguchi T, Barresi R, Kobayashi YM, Muschler J, Dumanski JP, Michele DE, Oldstone MB, Campbell KP. Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell. 2004;117:953–964. doi: 10.1016/j.cell.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Kleopa KA, Drousiotou A, Mavrikiou E, Ormiston A, Kyriakides T. Naturally occurring utrophin correlates with disease severity in Duchenne muscular dystrophy. Hum Mol Genet. 2006;15:1623–1628. doi: 10.1093/hmg/ddl083. [DOI] [PubMed] [Google Scholar]
- Klietsch R, Ervasti JM, Arnold W, Campbell KP, Jorgensen AO. Dystrophin-glycoprotein complex and laminin colocalize to the sarcolemma and transverse tubules of cardiac muscle. Circ Res. 1993;72:349–360. doi: 10.1161/01.res.72.2.349. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Nakahori Y, Miyake M, Matsumura K, Kondo-Iida E, Nomura Y, Segawa M, Yoshioka M, Saito K, Osawa M, Hamano K, Sakakihara Y, Nonaka I, Nakagome Y, Kanazawa I, Nakamura Y, Tokunaga K, Toda T. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998;394:388–392. doi: 10.1038/28653. [DOI] [PubMed] [Google Scholar]
- Koenig M, Kunkel LM. Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J Biol Chem. 1990;265:4560–4566. [PubMed] [Google Scholar]
- Kuga A, Kanagawa M, Sudo A, Chan YM, Tajiri M, Manya H, Kikkawa Y, Nomizu M, Kobayashi K, Endo T, Lu QL, Wada Y, Toda T. Absence of post-phosphoryl modification in dystroglycanopathy mouse models and wild-type tissues expressing non-laminin binding form of alpha-dystroglycan. J Biol Chem. 2012;287:9560–9567. doi: 10.1074/jbc.M111.271767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancioni A, Rotundo IL, Kobayashi YM, D’Orsi L, Aurino S, Nigro G, Piluso G, Acampora D, Cacciottolo M, Campbell KP, Nigro V. Combined deficiency of alpha and epsilon sarcoglycan disrupts the cardiac dystrophin complex. Hum. Mol. Genet. 2011;20:4644–4654. doi: 10.1093/hmg/ddr398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebakken CS, Venzke DP, Hrstka RF, Consolino CM, Faulkner JA, Williamson RA, Campbell KP. Sarcospan-deficient mice maintain normal muscle function. Mol Cell Biol. 2000;20:1669–1677. doi: 10.1128/mcb.20.5.1669-1677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GH, Badorff C, Knowlton KU. Dissociation of sarcoglycans and the dystrophin carboxyl terminus from the sarcolemma in enteroviral cardiomyopathy. Circ Res. 2000;87:489–495. doi: 10.1161/01.res.87.6.489. [DOI] [PubMed] [Google Scholar]
- Lefeber DJ, de Brouwer APM, Morava E, Riemersma M, Schuurs-Hoeijmakers JHM, Absmanner B, Verrijp K, van den Akker WMR, Huijben K, Steenbergen G, van Reeuwijk J, Jozwiak A, Zucker N, Lorber A, Lammens M, Knopf C, van Bokhoven H, Grünewald S, Lehle L, Kapusta L, Mandel H, Wevers R. a. Autosomal recessive dilated cardiomyopathy due to DOLK mutations results from abnormal dystroglycan O-mannosylation. PLoS Genet. 2011;7:e1002427. doi: 10.1371/journal.pgen.1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefeber DJ, Schönberger J, Morava E, Guillard M, Huyben KM, Verrijp K, Grafakou O, Evangeliou A, Preijers FW, Manta P, Yildiz J, Grünewald S, Spilioti M, van den Elzen C, Klein D, Hess D, Ashida H, Hofsteenge J, Maeda Y, van den Heuvel L, Lammens M, Lehle L, Wevers R. a. Deficiency of Dol-P-Man synthase subunit DPM3 bridges the congenital disorders of glycosylation with the dystroglycanopathies. Am. J. Hum. Genet. 2009;85:76–86. doi: 10.1016/j.ajhg.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschziner A, Moukhles H, Lindenbaum M, Gee SH, Butterworth J, Campbell KP, Carbonetto S. Neural regulation of alpha-dystroglycan biosynthesis and glycosylation in skeletal muscle. J Neurochem. 2000;74:70–80. doi: 10.1046/j.1471-4159.2000.0740070.x. [DOI] [PubMed] [Google Scholar]
- Lim B, Peter AK, Xiong D, Narezkina A, Yung A, Dalton ND, Hwang K-K, Yajima T, Chen J, Knowlton KU. Inhibition of Coxsackievirus-associated dystrophin cleavage prevents cardiomyopathy. J. Clin. Invest. 2013;123:5146–51. doi: 10.1172/JCI66271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LE, Duclos F, Broux O, Bourg N, Sunada Y, Allamand V, Meyer J, Richard I, Moomaw C, Slaughter C, et al. Beta-sarcoglycan: characterization and role in limb-girdle muscular dystrophy linked to 4q12. Nat Genet. 1995;11:257–265. doi: 10.1038/ng1195-257. [DOI] [PubMed] [Google Scholar]
- Liu LA, Engvall E. Sarcoglycan isoforms in skeletal muscle. J Biol Chem. 1999;274:38171–38176. doi: 10.1074/jbc.274.53.38171. [DOI] [PubMed] [Google Scholar]
- Manya H, Chiba A, Yoshida A, Wang X, Chiba Y, Jigami Y, Margolis RU, Endo T. Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity. Proc. Natl. Acad. Sci. U. S. A. 2004;101:500–5. doi: 10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini MC, Tambunan DE, Hill RS, Yu TW, Maynard TM, Heinzen EL, Shianna K, Stevens CR, Partlow JN, Barry BJ, Rodriguez J, Gupta V. a, Al-Qudah A-K, Eyaid WM, Friedman JM, Salih M. a, Clark R, Moroni I, Mora M, Beggs AH, Gabriel SB, Walsh C. a. Exome sequencing and functional validation in zebrafish identify GTDC2 mutations as a cause of Walker-Warburg syndrome. Am. J. Hum. Genet. 2012;91:541–7. doi: 10.1016/j.ajhg.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta M, Connolly AM, Winder TL, Pestronk A, Moore SA. Cardiac pathology exceeds skeletal muscle pathology in two cases of limb-girdle muscular dystrophy type 2I. Muscle Nerve. 2009;40:883–889. doi: 10.1002/mus.21432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JL, Chou E, Oh J, Kwok A, Burkin DJ, Crosbie-Watson RH. Dystrophin and utrophin expression require sarcospan: loss of alpha7 integrin exacerbates a newly discovered muscle phenotype in sarcospan-null mice. Hum. Mol. Genet. 2012a doi: 10.1093/hmg/dds271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JL, Holmberg J, Chou E, Ocampo AC, Oh J, Lee J, Peter AK, Martin PT, Crosbie-Watson RH. Sarcospan-dependent Akt activation is required for utrophin expression and muscle regeneration. J. Cell Biol. 2012b;197:1009–1027. doi: 10.1083/jcb.201110032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JL, Watson RH. Sarcospan: a small protein with large potential for Duchenne muscular dystrophy. Skelet. Muscle. 2013;3:1. doi: 10.1186/2044-5040-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Ervasti JM, Ohlendieck K, Kahl SD, Campbell KP. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature. 1992a;360:588–591. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Tome FM, Collin H, Azibi K, Chaouch M, Kaplan JC, Fardeau M, Campbell KP. Deficiency of the 50K dystrophin-associated glycoprotein in severe childhood autosomal recessive muscular dystrophy. Nature. 1992b;359:320–322. doi: 10.1038/359320a0. [DOI] [PubMed] [Google Scholar]
- McNally EM, Duggan D, Gorospe JR, Bonnemann CG, Fanin M, Pegoraro E, Lidov HG, Noguchi S, Ozawa E, Finkel RS, Cruse RP, Angelini C, Kunkel LM, Hoffman EP. Mutations that disrupt the carboxyl-terminus of gamma-sarcoglycan cause muscular dystrophy. Hum Mol Genet. 1996;5:1841–1847. doi: 10.1093/hmg/5.11.1841. [DOI] [PubMed] [Google Scholar]
- McNally EM, Ly CT, Kunkel LM. Human epsilon-sarcoglycan is highly related to alpha-sarcoglycan (adhalin), the limb girdle muscular dystrophy 2D gene. FEBS Lett. 1998;422:27–32. doi: 10.1016/s0014-5793(97)01593-7. [DOI] [PubMed] [Google Scholar]
- McNally EM, Yoshida M, Mizuno Y, Ozawa E, Kunkel LM. Human adhalin is alternatively spliced and the gene is located on chromosome 17q21. Proc. Natl. Acad. Sci. U. S. A. 1994;91:9690–4. doi: 10.1073/pnas.91.21.9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Michele DE, Kabaeva Z, Davis SL, Weiss RM, Campbell KP. Dystroglycan matrix receptor function in cardiac myocytes is important for limiting activity-induced myocardial damage. Circ Res. 2009;105:984–993. doi: 10.1161/CIRCRESAHA.109.199489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Wang EL, Nassar KL, Peter AK, Crosbie RH. Structural and functional analysis of the sarcoglycan-sarcospan subcomplex. Exp. Cell Res. 2007;313:639–51. doi: 10.1016/j.yexcr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Thompson TG, Guyon JR, Lidov HG, Brosius M, Imamura M, Ozawa E, Watkins SC, Kunkel LM. Desmuslin, an intermediate filament protein that interacts with alpha -dystrobrevin and desmin. Proc Natl Acad Sci U S A. 2001;98:6156–6161. doi: 10.1073/pnas.111153298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo KF, Fang T, Stalnaker SH, Kirby PS, Liu M, Wells L, Pierce M, Live DH, Boons GJ. Synthetic, structural, and biosynthetic studies of an unusual phospho-glycopeptide derived from alpha-dystroglycan. J. Am. Chem. Soc. 2011;133:14418–14430. doi: 10.1021/ja205473q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey SE, Howman EV, Ponting CP, Benson MA, Nawrotzki R, Loh NY, Davies KE, Blake DJ. Syncoilin, a novel member of the intermediate filament superfamily that interacts with alpha-dystrobrevin in skeletal muscle. J Biol Chem. 2001;276:6645–6655. doi: 10.1074/jbc.M008305200. [DOI] [PubMed] [Google Scholar]
- Nigro V, de Sa Moreira E, Piluso G, Vainzof M, Belsito A, Politano L, Puca AA, Passos-Bueno MR, Zatz M. Autosomal recessive limb-girdle muscular dystrophy, LGMD2F, is caused by a mutation in the delta-sarcoglycan gene. Nat Genet. 1996a;14:195–198. doi: 10.1038/ng1096-195. [DOI] [PubMed] [Google Scholar]
- Nigro V, Piluso G, Belsito A, Politano L, Puca AA, Papparella S, Rossi E, Viglietto G, Esposito MG, Abbondanza C, Medici N, Molinari AM, Nigro G, Puca GA. Identification of a novel sarcoglycan gene at 5q33 encoding a sarcolemmal 35 kDa glycoprotein. Hum Mol Genet. 1996b;5:1179–1186. doi: 10.1093/hmg/5.8.1179. [DOI] [PubMed] [Google Scholar]
- Noguchi S, McNally EM, Ben Othmane K, Hagiwara Y, Mizuno Y, Yoshida M, Yamamoto H, Bonnemann CG, Gussoni E, Denton PH, Kyriakides T, Middleton L, Hentati F, Ben Hamida M, Nonaka I, Vance JM, Kunkel LM, Ozawa E. Mutations in the dystrophin-associated protein gamma-sarcoglycan in chromosome 13 muscular dystrophy. Science (80-. ) 1995;270:819–822. doi: 10.1126/science.270.5237.819. [DOI] [PubMed] [Google Scholar]
- Noguchi S, Wakabayashi E, Imamura M, Yoshida M, Ozawa E. Developmental expression of sarcoglycan gene products in cultured myocytes. Biochem. Biophys. Res. Commun. 1999;262:88–93. doi: 10.1006/bbrc.1999.1163. [DOI] [PubMed] [Google Scholar]
- Noguchi S, Wakabayashi E, Imamura M, Yoshida M, Ozawa E. Formation of sarcoglycan complex with differentiation in cultured myocytes. Eur J Biochem. 2000;267:640–8. doi: 10.1046/j.1432-1327.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- Norwood F, de Visser M, Eymard B, Lochmuller H, Bushby K. EFNS guideline on diagnosis and management of limb girdle muscular dystrophies. Eur J Neurol. 2007;14:1305–1312. doi: 10.1111/j.1468-1331.2007.01979.x. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K, Campbell KP. Dystrophin-associated proteins are greatly reduced in skeletal muscle from mdx mice. J Cell Biol. 1991;115:1685–1694. doi: 10.1083/jcb.115.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K, Ervasti JM, Matsumura K, Kahl SD, Leveille CJ, Campbell KP. Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron. 1991;7:499–508. doi: 10.1016/0896-6273(91)90301-f. [DOI] [PubMed] [Google Scholar]
- Passos-Bueno MR, Moreira ES, Vainzof M, Marie SK, Zatz M. Linkage analysis in autosomal recessive limb-girdle muscular dystrophy (AR LGMD) maps a sixth form to 5q33-34 (LGMD2F) and indicates that there is at least one more subtype of AR LGMD. Hum Mol Genet. 1996;5:815–820. doi: 10.1093/hmg/5.6.815. [DOI] [PubMed] [Google Scholar]
- Patnaik SK, Stanley P. Mouse large can modify complex N- and mucin O-glycans on alpha-dystroglycan to induce laminin binding. J. Biol. Chem. 2005;280:20851–20859. doi: 10.1074/jbc.M500069200. [DOI] [PubMed] [Google Scholar]
- Peter AK, Marshall JL, Crosbie RH. Sarcospan reduces dystrophic pathology: stabilization of the utrophin-glycoprotein complex. J. Cell Biol. 2008;183:419–427. doi: 10.1083/jcb.200808027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter AK, Miller G, Crosbie RH. Disrupted mechanical stability of the dystrophin-glycoprotein complex causes severe muscular dystrophy in sarcospan transgenic mice. J Cell Sci. 2007;120:996–1008. doi: 10.1242/jcs.03360. [DOI] [PubMed] [Google Scholar]
- Piccolo F, Roberds SL, Jeanpierre M, Leturcq F, Azibi K, Beldjord C, Carrie A, Recan D, Chaouch M, Reghis A, et al. Primary adhalinopathy: a common cause of autosomal recessive muscular dystrophy of variable severity. Nat Genet. 1995;10:243–245. doi: 10.1038/ng0695-243. [DOI] [PubMed] [Google Scholar]
- Pons F, Robert A, Fabbrizio E, Hugon G, Califano JC, Fehrentz JA, Martinez J, Mornet D. Utrophin localization in normal and dystrophin-deficient heart. Circulation. 1994;90:369–374. doi: 10.1161/01.cir.90.1.369. [DOI] [PubMed] [Google Scholar]
- Prins KW, Humston JL, Mehta A, Tate V, Ralston E, Ervasti JM. Dystrophin is a microtubule-associated protein. J Cell Biol. 2009;186:363–369. doi: 10.1083/jcb.200905048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafael JA, Tinsley JM, Potter AC, Deconinck AE, Davies KE. Skeletal muscle-specific expression of a utrophin transgene rescues utrophin-dystrophin deficient mice. Nat Genet. 1998;19:79–82. doi: 10.1038/ng0598-79. [DOI] [PubMed] [Google Scholar]
- Rettino A, Rafanelli F, Genovese G, Goracci M, Cifarelli RA, Cittadini A, Sgambato A. Identification of Sp1 and GC-boxes as transcriptional regulators of mouse Dag1 gene promoter. Am. J. Physiol. Cell Physiol. 2009;297:C1113–23. doi: 10.1152/ajpcell.00189.2009. [DOI] [PubMed] [Google Scholar]
- Roberds SL, Anderson RD, Ibraghimov-Beskrovnaya O, Campbell KP. Primary structure and muscle-specific expression of the 50-kDa dystrophin-associated glycoprotein (adhalin) J Biol Chem. 1993;268:23739–23742. [PubMed] [Google Scholar]
- Roberds SL, Leturcq F, Allamand V, Piccolo F, Jeanpierre M, Anderson RD, Lim LE, Lee JC, Tome FM, Romero NB, et al. Missense mutations in the adhalin gene linked to autosomal recessive muscular dystrophy. Cell. 1994;78:625–633. doi: 10.1016/0092-8674(94)90527-4. [DOI] [PubMed] [Google Scholar]
- Roscioli T, Kamsteeg E-J, Buysse K, Maystadt I, van Reeuwijk J, van den Elzen C, van Beusekom E, Riemersma M, Pfundt R, Vissers LELM, Schraders M, Altunoglu U, Buckley MF, Brunner HG, Grisart B, Zhou H, Veltman J, Gilissen C, Mancini GMS, Delrée P, Willemsen M. a, Ramadža DP, Chitayat D, Bennett C, Sheridan E, Peeters E. a J., Tan-Sindhunata GMB, de Die-Smulders CE, Devriendt K, Kayserili H, El-Hashash OAE-F, Stemple DL, Lefeber DJ, Lin Y-Y, van Bokhoven H. Mutations in ISPD cause Walker-Warburg syndrome and defective glycosylation of α-dystroglycan. Nat. Genet. 2012;44:581–5. doi: 10.1038/ng.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. On a Case of Pseudo-Hypertrophic Paralysis. Br. Med. J. 1883;1:200–202. doi: 10.1136/bmj.1.1153.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röttger S, White J, Wandall HH, Olivo JC, Stark a, Bennett EP, Whitehouse C, Berger EG, Clausen H, Nilsson T. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J. Cell Sci. 1998;111(Pt 1):45–60. doi: 10.1242/jcs.111.1.45. [DOI] [PubMed] [Google Scholar]
- Sadoulet-Puccio HM, Rajala M, Kunkel LM. Dystrobrevin and dystrophin: an interaction through coiled-coil motifs. Proc Natl Acad Sci U S A. 1997;94:12413–12418. doi: 10.1073/pnas.94.23.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito F, Tomimitsu H, Arai K, Nakai S, Kanda T, Shimizu T, Mizusawa H, Matsumura K. A Japanese patient with distal myopathy with rimmed vacuoles: missense mutations in the epimerase domain of the UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) gene accompanied by hyposialylation of skeletal muscle glycoproteins. Neuromuscul. Disord. 2004;14:158–161. doi: 10.1016/j.nmd.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Ono K, Abe M, Jasmin G, Eki T, Murakami Y, Masaki T, Toyooka T, Hanaoka F. Both hypertrophic and dilated cardiomyopathies are caused by mutation of the same gene, delta-sarcoglycan, in hamster: an animal model of disrupted dystrophin-associated glycoprotein complex. Proc Natl Acad Sci U S A. 1997;94:13873–13878. doi: 10.1073/pnas.94.25.13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandona D, Gastaldello S, Martinello T, Betto R. Characterization of the ATP-hydrolysing activity of alpha-sarcoglycan. Biochem J. 2004;381:105–112. doi: 10.1042/BJ20031644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Yamada H, Matsumura K, Shimizu T, Kobata A, Endo T. Detection of O-mannosyl glycans in rabbit skeletal muscle alpha-dystroglycan. Biochim. Biophys. Acta. 1998;1425:599–606. doi: 10.1016/s0304-4165(98)00114-7. [DOI] [PubMed] [Google Scholar]
- Sharpe KM, Premsukh MD, Townsend D. Alterations of dystrophin-associated glycoproteins in the heart lacking dystrophin or dystrophin and utrophin. J. Muscle Res. Cell Motil. 2013 doi: 10.1007/s10974-013-9362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Chen Z, Schottenfeld J, Stahl RC, Kunkel LM, Chan Y-MM. Specific assembly pathway of sarcoglycans is dependent on beta- and delta-sarcoglycan. Muscle Nerve. 2004;29:409–419. doi: 10.1002/mus.10566. [DOI] [PubMed] [Google Scholar]
- Stalnaker SH, Aoki K, Lim JM, Porterfield M, Liu M, Satz JS, Buskirk S, Xiong Y, Zhang P, Campbell KP, Hu H, Live D, Tiemeyer M, Wells L. Glycomic analyses of mouse models of congenital muscular dystrophy. J Biol Chem. 2011a;286:21180–21190. doi: 10.1074/jbc.M110.203281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker SH, Hashmi S, Lim JM, Aoki K, Porterfield M, Gutierrez-Sanchez G, Wheeler J, Ervasti JM, Bergmann C, Tiemeyer M, Wells L. Site mapping and characterization of O-glycan structures on alpha-dystroglycan isolated from rabbit skeletal muscle. J Biol Chem. 2010;285:24882–24891. doi: 10.1074/jbc.M110.126474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker SH, Stuart R, Wells L. Mammalian O-mannosylation: unsolved questions of structure/function. Curr. Opin. Struct. Biol. 2011b;21:603–609. doi: 10.1016/j.sbi.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens E, Carss KJ, Cirak S, Foley AR, Torelli S, Willer T, Tambunan DE, Yau S, Brodd L, Sewry CA, Feng L, Haliloglu G, Orhan D, Dobyns WB, Enns GM, Manning M, Krause A, Salih MA, Walsh CA, Hurles M, Campbell KP, Manzini MC, Stemple D, Lin YY, Muntoni F. Mutations in B3GALNT2 Cause Congenital Muscular Dystrophy and Hypoglycosylation of alpha-Dystroglycan. Am J Hum Genet. 2013;92:354–365. doi: 10.1016/j.ajhg.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub V, Duclos F, Venzke DP, Lee JC, Cutshall S, Leveille CJ, Campbell KP. Molecular pathogenesis of muscle degeneration in the delta-sarcoglycan-deficient hamster. Am J Pathol. 1998;153:1623–1630. doi: 10.1016/s0002-9440(10)65751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub V, Ettinger AJ, Durbeej M, Venzke DP, Cutshall S, Sanes JR, Campbell KP. epsilon-sarcoglycan replaces alpha-sarcoglycan in smooth muscle to form a unique dystrophin-glycoprotein complex. J Biol Chem. 1999;274:27989–27996. doi: 10.1074/jbc.274.39.27989. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Ozawa E. Expression of dystrophin mRNA and the protein in the developing rat heart. Biochem Biophys Res Commun. 1990;172:824–829. doi: 10.1016/0006-291x(90)90749-d. [DOI] [PubMed] [Google Scholar]
- Tinsley J, Deconinck N, Fisher R, Kahn D, Phelps S, Gillis JM, Davies K. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med. 1998;4:1441–1444. doi: 10.1038/4033. [DOI] [PubMed] [Google Scholar]
- Tinsley JM, Blake DJ, Roche A, Fairbrother U, Riss J, Byth BC, Knight AE, Kendrick-Jones J, Suthers GK, Love DR, et al. Primary structure of dystrophin-related protein. Nature. 1992;360:591–593. doi: 10.1038/360591a0. [DOI] [PubMed] [Google Scholar]
- Tomé FM, Matsumura K, Chevallay M, Campbell KP, Fardeau M. Expression of dystrophin-associated glycoproteins during human fetal muscle development: a preliminary immunocytochemical study. Neuromuscul. Disord. 1994;4:343–8. doi: 10.1016/0960-8966(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Torelli S, Brown SC, Brockington M, Dolatshad NF, Jimenez C, Skordis L, Feng LH, Merlini L, Jones DH, Romero N, Wewer U, Voit T, Sewry CA, Noguchi S, Nishino I, Muntoni F. Sub-cellular localisation of fukutin related protein in different cell lines and in the muscle of patients with MDC1C and LGMD2I. Neuromuscul. Disord. 2005;15:836–843. doi: 10.1016/j.nmd.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Towbin JA, Hejtmancik JF, Brink P, Gelb B, Zhu XM, Chamberlain JS, McCabe ER, Swift M. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation. 1993;87:1854–1865. doi: 10.1161/01.cir.87.6.1854. [DOI] [PubMed] [Google Scholar]
- Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS, Metzger JM. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther. 2007;15:1086–1092. doi: 10.1038/sj.mt.6300144. [DOI] [PubMed] [Google Scholar]
- Townsend D, Daly M, Chamberlain JS, Metzger JM. Age-dependent Dystrophin Loss and Genetic Reconstitution Establish a Molecular Link Between Dystrophin and Heart Performance During Aging. Mol Ther. 2011;19:1821–1825. doi: 10.1038/mt.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyo-Oka T, Kawada T, Nakata J, Xie H, Urabe M, Masui F, Ebisawa T, Tezuka A, Iwasawa K, Nakajima T, Uehara Y, Kumagai H, Kostin S, Schaper J, Nakazawa M, Ozawa K. Translocation and cleavage of myocardial dystrophin as a common pathway to advanced heart failure: a scheme for the progression of cardiac dysfunction. Proc Natl Acad Sci U S A. 2004;101:7381–7385. doi: 10.1073/pnas.0401944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeuwijk J, Grewal PK, Salih M. a M., Beltrán-Valero de Bernabé D, McLaughlan JM, Michielse CB, Herrmann R, Hewitt JE, Steinbrecher A, Seidahmed MZ, Shaheed MM, Abomelha A, Brunner HG, van Bokhoven H, Voit T. Intragenic deletion in the LARGE gene causes Walker-Warburg syndrome. Hum. Genet. 2007;121:685–90. doi: 10.1007/s00439-007-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeuwijk J, Janssen M, van den Elzen C, Beltran-Valero de Bernabé D, Sabatelli P, Merlini L, Boon M, Scheffer H, Brockington M, Muntoni F, Huynen M. a, Verrips A, Walsh C. a, Barth PG, Brunner HG, van Bokhoven H. POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J. Med. Genet. 2005;42:907–12. doi: 10.1136/jmg.2005.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebrouck A, Sabourin J, Rivet J, Balghi H, Sebille S, Kitzis A, Raymond G, Cognard C, Bourmeyster N, Constantin B. Regulation of capacitative calcium entries by alpha1-syntrophin: association of TRPC1 with dystrophin complex and the PDZ domain of alpha1-syntrophin. Faseb J. 2007;21:608–617. doi: 10.1096/fj.06-6683com. [DOI] [PubMed] [Google Scholar]
- Vatta M, Stetson SJ, Jimenez S, Entman ML, Noon GP, Bowles NE, Towbin JA, Torre-Amione G. Molecular normalization of dystrophin in the failing left and right ventricle of patients treated with either pulsatile or continuous flow-type ventricular assist devices. J Am Coll Cardiol. 2004;43:811–817. doi: 10.1016/j.jacc.2003.09.052. [DOI] [PubMed] [Google Scholar]
- Vatta M, Stetson SJ, Perez-Verdia A, Entman ML, Noon GP, Torre-Amione G, Bowles NE, Towbin JA. Molecular remodelling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet. 2002;359:936–941. doi: 10.1016/S0140-6736(02)08026-1. [DOI] [PubMed] [Google Scholar]
- Watkins SC, Samuel JL, Marotte F, Bertier-Savalle B, Rappaport L. Microtubules and desmin filaments during onset of heart hypertrophy in rat: a double immunoelectron microscope study. Circ Res. 1987;60:327–336. doi: 10.1161/01.res.60.3.327. [DOI] [PubMed] [Google Scholar]
- Wheeler MT, Zarnegar S, McNally EM. Zeta-sarcoglycan, a novel component of the sarcoglycan complex, is reduced in muscular dystrophy. Hum Mol Genet. 2002;11:2147–2154. doi: 10.1093/hmg/11.18.2147. [DOI] [PubMed] [Google Scholar]
- White T, Bennett EP, Takio K, Sørensen T, Bonding N, Clausen H. Purification and cDNA Cloning of a Human UDP-N-acetyl-alpha- D-galactosamine:polypeptide N-Acetylgalactosaminyltransferase. J. Biol. Chem. 1995;270:24156–24165. doi: 10.1074/jbc.270.41.24156. [DOI] [PubMed] [Google Scholar]
- Willer T, Amselgruber W, Deutzmann R, Strahl S. Characterization of POMT2, a novel member of the PMT protein O-mannosyltransferase family specifically localized to the acrosome of mammalian spermatids. Glycobiology. 2002;12:771–783. doi: 10.1093/glycob/cwf086. [DOI] [PubMed] [Google Scholar]
- Willer T, Lee H, Lommel M, Yoshida-Moriguchi T, de Bernabe DBV, Venzke D, Cirak S, Schachter H, Vajsar J, Voit T, Muntoni F, Loder AS, Dobyns WB, Winder TL, Strahl S, Mathews KD, Nelson SF, Moore S. a, Campbell KP. ISPD loss-of-function mutations disrupt dystroglycan O-mannosylation and cause Walker-Warburg syndrome. Nat. Genet. 2012;44:575–80. doi: 10.1038/ng.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D, Lee GH, Badorff C, Dorner A, Lee S, Wolf P, Knowlton KU. Dystrophin deficiency markedly increases enterovirus-induced cardiomyopathy: a genetic predisposition to viral heart disease. Nat Med. 2002;8:872–877. doi: 10.1038/nm737. [DOI] [PubMed] [Google Scholar]
- Xiong H, Kobayashi K, Tachikawa M, Manya H, Takeda S, Chiyonobu T, Fujikake N, Wang F, Nishimoto A, Morris GE, Nagai Y, Kanagawa M, Endo T, Toda T. Molecular interaction between fukutin and POMGnT1 in the glycosylation pathway of alpha-dystroglycan. Biochem. Biophys. Res. Commun. 2006;350:935–41. doi: 10.1016/j.bbrc.2006.09.129. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, Herrmann R, Straub V, Talim B, Voit T, Topaloglu H, Toda T, Endo T. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell. 2001;1:717–724. doi: 10.1016/s1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Hama H, Ishikawa-Sakurai M, Imamura M, Mizuno Y, Araishi K, Wakabayashi-Takai E, Noguchi S, Sasaoka T, Ozawa E. Biochemical evidence for association of dystrobrevin with the sarcoglycan-sarcospan complex as a basis for understanding sarcoglycanopathy. Hum Mol Genet. 2000;9:1033–1040. doi: 10.1093/hmg/9.7.1033. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Ozawa E. Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem. 1990;108:748–752. doi: 10.1093/oxfordjournals.jbchem.a123276. [DOI] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Lipshitz HD, Stevenson VA, Theurkauf WE, Kirschner M, Maric C, Kriegstein HJ, Hogness DS, Walter JC, Bradley RH, Gao Y, Coue M, Shevchenko A, Dunphy WG, Kumano M, Kubota Y, Hashimoto Y, Takisawa H, Newport JW, Kiehle CP, Schubiger G, Dasso M, Sible JC, Lewellyn AL, Maller JL, Mackenzie A, Donaldson A, Zegerman P, Pestell RQ, Scherson T, Li JM, Elemento O, Tavazoie S, Wieschaus EF, Mccleland ML, Shermoen AW, Willer T, Anderson ME, Venzke D, Whyte T, Muntoni F, Lee H, Nelson SF, Yu L, Campbell KP. SGK196 Is a Glycosylation-Specific O-Mannose Kinase Required for Dystroglycan Function. Science (80-. ) 2013;341:896–9. doi: 10.1126/science.1239951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP. O-mannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science (80-. ) 2010;327:88–92. doi: 10.1126/science.1180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, He Y, Wang K, Zhang P, Zhang S, Hu H. Adeno-Associated Viral-Mediated LARGE Gene Therapy Rescues the Muscular Dystrophic Phenotype in Mouse Models of Dystroglycanopathy. Hum. Gene Ther. 2013;24:317–330. doi: 10.1089/hum.2012.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Hu H. Differential glycosylation of alpha-dystroglycan and proteins other than alpha-dystroglycan by like-glycosyltransferase. Glycobiology. 2012;22:235–247. doi: 10.1093/glycob/cwr131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Betel D, Schachter H. Cloning and expression of a novel UDPGlcNAc:alpha-D-mannoside beta1,2-N-acetylglucosaminyltransferase homologous to UDP-GlcNAc:alpha-3-D-mannoside beta1,2-N-acetylglucosaminyltransferase I. Biochem. J. 2002;361:153–62. doi: 10.1042/0264-6021:3610153. [DOI] [PMC free article] [PubMed] [Google Scholar]