Abstract
We evaluated weight changes in obese patients at 6-months after they ended participation in a 12-month randomized controlled trial in which they received daily placebo, zonisamide 200 mg, or zonisamide 400 mg, in addition to lifestyle counseling. Of the originally randomized 225 patients, 218 completed month-12 when study interventions were discontinued. For the 154 patients who returned for 6-month follow-up off-treatment, weight changes between month-12 and month-18 for placebo (n=53), zonisamide 200 mg (n=49), and zonisamide 400 mg groups (n=52) were 0.5 kg (95% CI, −0.8 to 1.8; 0.7%), 1.5 kg (0.2 to 2.8; 1.6%; p=0.26 vs placebo) and 2.4 kg (1.1 to 3.7; 2.6%; p=0.04 vs placebo), respectively. Our results suggest that although zonisamide 400 mg daily for 12-months resulted in greater weight loss than with placebo, weight regain after discontinuation of interventions was greater in the zonisamide 400 mg group than placebo group.
Keywords: obesity, antiobesity drugs, zonisamide, weight loss, weight regain, weight maintenance
Introduction
In randomized controlled trials (RCTs) of pharmacological interventions for obesity, patients are seldom followed beyond the intervention period. In particular, there is sparse knowledge regarding weight changes after both pharmacological and ancillary lifestyle interventions are discontinued.
Zonisamide, an antiepileptic drug, was shown to enhance weight loss in obese adults in a short-term RCT [1]. Recently, Gadde et al reported results from a 12-month RCT in which daily treatment with zonisamide 400 mg was demonstrated to be more effective than placebo or zonisamide 200 mg [2]. In this paper, we report weight changes at 6-months after discontinuation of lifestyle counseling and double-blind zonisamide or placebo.
Methods
The study was conducted at Duke University Medical Center after institutional review board approval. This was an extension of a 12-month RCT (ClinicalTrials.gov Identifier: NCT00275834); eligibility criteria and details of the study design and conduct were described a previous publication [2]. The first phase was a randomized, double-blind, placebo-controlled trial in which a total 225 obese men and women without diabetes mellitus (mean body mass index [SD], 37.6 [4.9] kg/m2) were randomly assigned to treatment with daily placebo, zonisamide 200 mg, or zonisamide 400 mg for 12-months, when all study interventions and monthly assessments were discontinued. Patients were offered $25 for completing a 6-month follow-up visit at which they provided weight assessment and completed a brief questionnaire. All patients gave written informed consent first for the 12-month trial and later for the month-18 follow-up visit. Body weight, was measured on a calibrated electronic scale to the nearest 0.1 kg using standardized procedures.
The primary outcome was change in weight (weight at month-18 minus weight at month-12) by treatment (zonisamide 200 mg vs placebo, zonisamide 400 mg vs placebo). The resulting difference in weight change between treatment groups was regressed on a 3-level proxy variable (1, placebo; 2, zonisamide 200 mg; 3, zonisamide 400 mg) using analysis of covariance. If the ANCOVA omnibus test was significant, pairwise comparisons between treatments were subsequently tested with age, sex, race, weight at month-0 and weight at month-12 as covariates. Descriptive characteristics were computed with t- tests and analysis of variance for continuous variables and χ2 tests for categorical variables as appropriate. Pearson correlation test was used to assess relationship between change in weight from month-0 to month-12, and change in weight from month-12 to month-18.
Results
Of the 218 patients who completed the initial 12-month trial, 154 (placebo [n=53], zonisamide 200 mg [n=49], and zonisamide 400 mg [n=52]) returned to provide month-18 assessments. Of this sample of 154, 31 patients were not taking the study drug when they completed month-12. There were no significant differences in age, sex or race distribution, or weight at month-0 between the original cohort of 225 patients and the month-18 cohort of 154 patients (Table 1). We also compared these baseline characteristics between the 154 patients who completed month-18 and the 71 who did not; there were no differences (data not shown).
Table1.
Patient characteristics by original and follow-up cohorts, and follow-up treatment group
| Treatment groups of Follow-up Cohorts, 154 | |||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Original cohort n=225 |
Follow-Up cohort n=154 |
p- value |
Placebo n=53 |
Zonisamide 200 mg n=49 |
Zonisamide 400 mg n=52 |
p- value |
|
| Age, y | 43.4 (10.1) | 44.2 (10.8) | 0.46 | 45.3 (10.6) | 45.7 (10.9) | 41.5 (10.6) | 0.09 |
| Women, No (%) | 134 (59.6) | 93 (60.4) | 0.87 | 32 (60.4) | 30 (61.2) | 31 (59.6) | 0.99 |
| White, No (%) | 142 (63.1) | 97 (63.0) | 0.98 | 36 (67.9) | 30 (61.2) | 31 (59.6) | 0.88 |
| Weigh at 0-month, kg | 110.4 (18.4) | 108.9 (18.2) | 0.46 | 109.9 (18.8) | 109.4 (21.0) | 107.5 (14.8) | 0.78 |
Unless otherwise stated, the data are presented as mean (SD).
Abbreviations: y, years; kg, kilograms
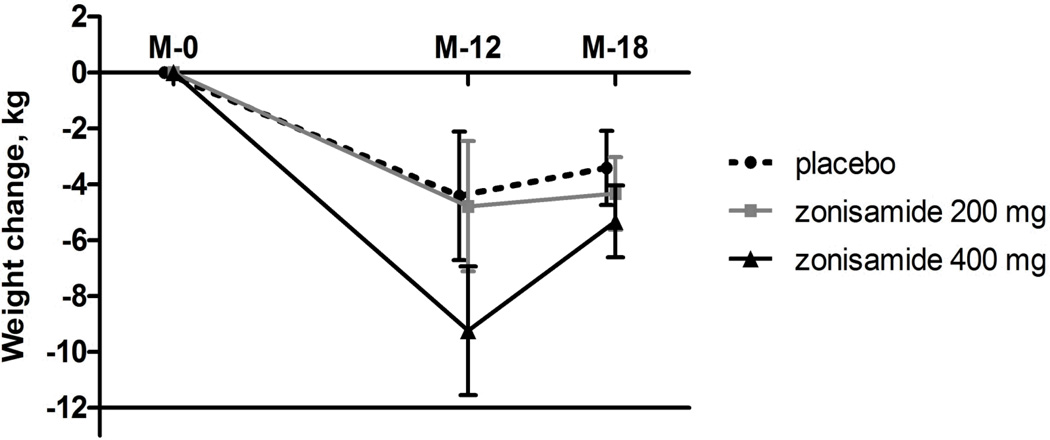
For the month-18 cohort of 154 patients, weight changes at month-12 were −4.4 kg (95% CI, −6.7 to −2.1 kg; least squares mean, −4.2%) for placebo, −4.8 kg (−7.1 to −2.4; −4.2%; p=0.82 vs placebo) for zonisamide 200 mg, and −9.2 kg [−11.5 to −6.9; −8.8%; p=0.003 vs placebo] for zonisamide 400 mg. Mean (least square) weights of the groups at month-12 were 104.9 kg (102.7 to 107.1) for placebo, 104.5 kg (102.3 to 106.7, p=0.96 vs placebo) for zonisamide 200 mg, and 100.1 kg (97.9 to 102.3, p=0.007 vs placebo) for zonisamide 400 mg.
Six-months after discontinuation of study interventions, mean weight change from month-12 for the entire cohort (n=154) was 1.4 kg (0.7 to 2.2) with all three groups gaining weight; change in the three groups were 0.5 kg (−0.8 to 1.8 kg; 0.7%) for placebo, 1.5 kg (0.2 to 2.8 kg; 1.6%; p=0.26 vs placebo) for zonisamide 200 mg, and 2.4 kg (1.1 to 3.7; 2.6%; p=0.04 vs placebo) for zonisamide 400 mg. The patterns of weight changes between month-0, month-12 and month-18 by treatment arm are depicted in Figure 1. Net weight changes (month-0 to month-18) for the treatment groups were: −3.4 kg (−4.7 to −2.1) for placebo, −4.3 kg (−5.6 to −3.0; p=0.26 vs placebo) for zonisamide 200 mg, and −5.3 kg (−6.6 to −4.0; p=0.035 vs placebo) for zonisamide 400 mg.
Figure 1. Weight changes from month-0 to month-12 on-treatment, and month-12 to month-18 off-treatment.
Data presented are least squares means (error bars, 95% confidence intervals) for the month-18 cohort of 154 patients (placebo [n=53], zonisamide 200 mg [n=49], zonisamide 400 mg [n=52]). p=0.035 vs placebo for zonisamide 400 mg for change from month-0 to month-18.
Weight changes for the subset of patients who completed month-12 on study drug (n=123) followed the same pattern observed in the total month-18 sample of 154 with the zonisamide 400 mg group regaining the most weight (2.9 kg [1.5 to 4.4]; p=0.008 vs placebo) whereas weight changes in the placebo (0.3 kg) and zonisamide 200 mg group (1.6 kg [0.1 to 3.1]; p=0.17) did not differ.
For the entire cohort of 154 patients, there was an inverse correlation between weight loss at month-12 and weight change at month-18 (r=.32; p<0.001), i.e. those who lost the most weight during the intervention period regained the most after treatment discontinuation.
Conclusions
The main finding of our 6-month follow-up investigation is that although zonisamide 400 mg led to greater weight loss than with placebo after 12 months, there was greater weight regain for the zonisamide 400 mg group than placebo group after treatment discontinuation.
In 1999, Bray et al [3] reported a trial that randomized 1047 obese patients to placebo or 6 different doses of sibutramine ranging from 1 mg to 30 mg for 24-weeks. Weight loss with sibutramine ranged from 2.7% to 9.4% in a dose-ranging manner compared with 1.2% with placebo. Weights were assessed 6-weeks after drug discontinuation, and data were available for 399 (58%) of the 683 that completed 24-weeks on drug. It was stated that patients who lost the most weight regained the most. We estimated from the line-graph that the groups that discontinued sibutramine 20 mg, sibutramine 10 mg, and placebo regained approximately 3.1%, 2.0%, and 0.7%, respectively. Our study is similar to the sibutramine discontinuation study in that there were no weight maintenance interventions and no clinical monitoring after drug discontinuation. Results of the two studies are also similar in that patients who lost the most weight regained the most after discontinuation of treatment.
In addition to a relatively large sample size, the current study’s strengths include a fair distribution of participants by sex (40% men) and race (34% non-White). A limitation of our study is that only 154 (71%) of the 218 that completed month-12 returned for month-18 follow-up visit; however, this 71% return rate is still higher than that in other studies of this nature.
In summary, we found that obese patients assigned to daily treatment with zonisamide 400 mg achieved greater weight loss than those assigned to placebo at 12 months, but subsequent to discontinuation of the double-blind study drug along with diet and lifestyle counseling, regained more weight over the next 6 months, which may be attributable to greater degree of weight loss on treatment. As suggested by the sibutramine discontinuation study and the current study of zonisamide, weight regain after drug discontinuation appears to be faster than after discontinuation of lifestyle interventions [4,5]. Rapid physiological adaptation of energy regulation pathways after drug-induced suppression of hunger is lifted may explain faster weight regain following discontinuation of pharmacotherapy [6].
Acknowledgements
This study was supported by grant 5R01 DK67352 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to Dr. Gadde. The sponsor had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the official views of the NIDDK, the National Institutes of Health, or the Department of Health and Human Services.
K.M.G. obtained funding, had full access to all of the data in the study, and takes full responsibility for the integrity of the data. K.M.G. and G.A.B. contributed to study concept and design, and K.M.G. to data acquisition. J.H.S. contributed to data analysis, and J.H.S. and K.M.G. drafted the manuscript. All authors contributed to data interpretation and critical revision of the manuscript for important intellectual content.
We thank all of the patients who participated in this trial. We are grateful to Mariko F. Kopping, MS, RD, Gretchen M. Yonish, MPH, RD, and Catherine Gang, MPH, RD (Duke University Medical Center), for data collection and dietary counseling.
Dr Gadde reported receiving grants from Amylin, Eisai, Medical University of South Carolina, National Institute of Diabetes and Digestive and Kidney Diseases, and Vivus in the past 36 months. He has been awarded several patents in the name of his institution for use of zonisamide as monotherapy and in combination with other drugs for treatment of obesity as well as weight gain associated with psychotropic drugs; these patents have been licensed to Orexigen Therapeutics by his institution. Consequent to the licensing agreement, Dr Gadde owned equity (not currently) in Orexigen, which is developing zonisamide and bupropion combination therapy for obesity, based on his patents. However, to the best of Dr Gadde’s knowledge, no commercial entity has announced plans to develop zonisamide monotherapy for obesity or other applications claimed in his patents. Dr Bray reported that he received funding from National Institutes of Health; is an advisor to Medifast, Herbalife, and Global Direction in Medicine; and has received royalties for the Handbook of Obesity.
Abbreviations
- ZNS
zonisamide
- M-0
month-0
- M-12
month-12
- M-18
month-18
Footnotes
Parts of the study were presented in a poster format at the annual meeting of The Obesity Society in Atlanta on Nov 15, 2013.
Conflict of Interest
Drs Shin and Østbye have no conflicts to disclose.
References
- 1.Gadde KM, Franciscy DM, Wagner HR, II, Krishnan KR. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA. 2003;289:1820–1825. doi: 10.1001/jama.289.14.1820. [DOI] [PubMed] [Google Scholar]
- 2.Gadde KM, Kopping MF, Wagner HR, II, Yonish GM, Allison DB, Bray GA. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172:1557–1564. doi: 10.1001/2013.jamainternmed.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray GA, Blackburn GL, Ferguson JM, et al. Sibutramine produces dose-related weight loss. Obes Res. 1999;7:189–198. doi: 10.1002/j.1550-8528.1999.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 4.Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs diet-only interventions for weight loss: a meta-analysis. Obesity reviews. 2009;10:313–323. doi: 10.1111/j.1467-789X.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74:579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 6.Sumithran P, Proietto J. The defence of body weight: a physiological basis for weight regain after weight loss. Clin Sci. 2013;124:231–241. doi: 10.1042/CS20120223. [DOI] [PubMed] [Google Scholar]