Abstract
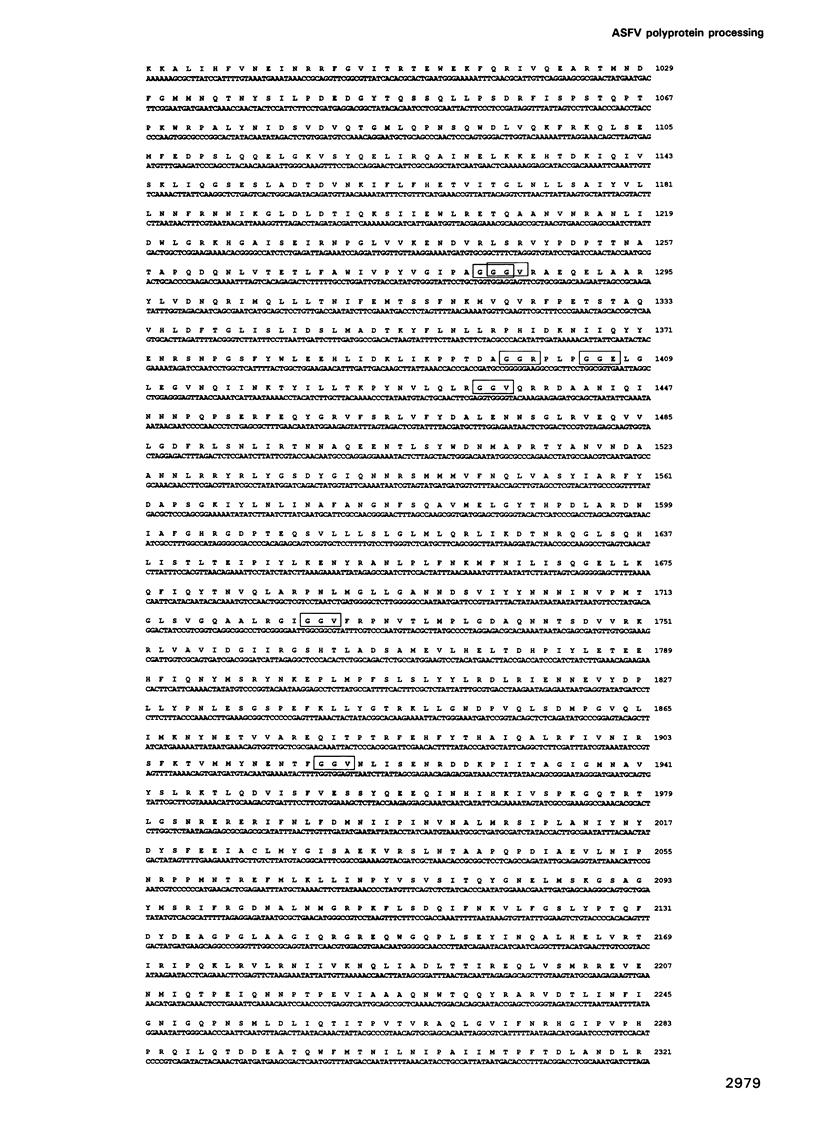
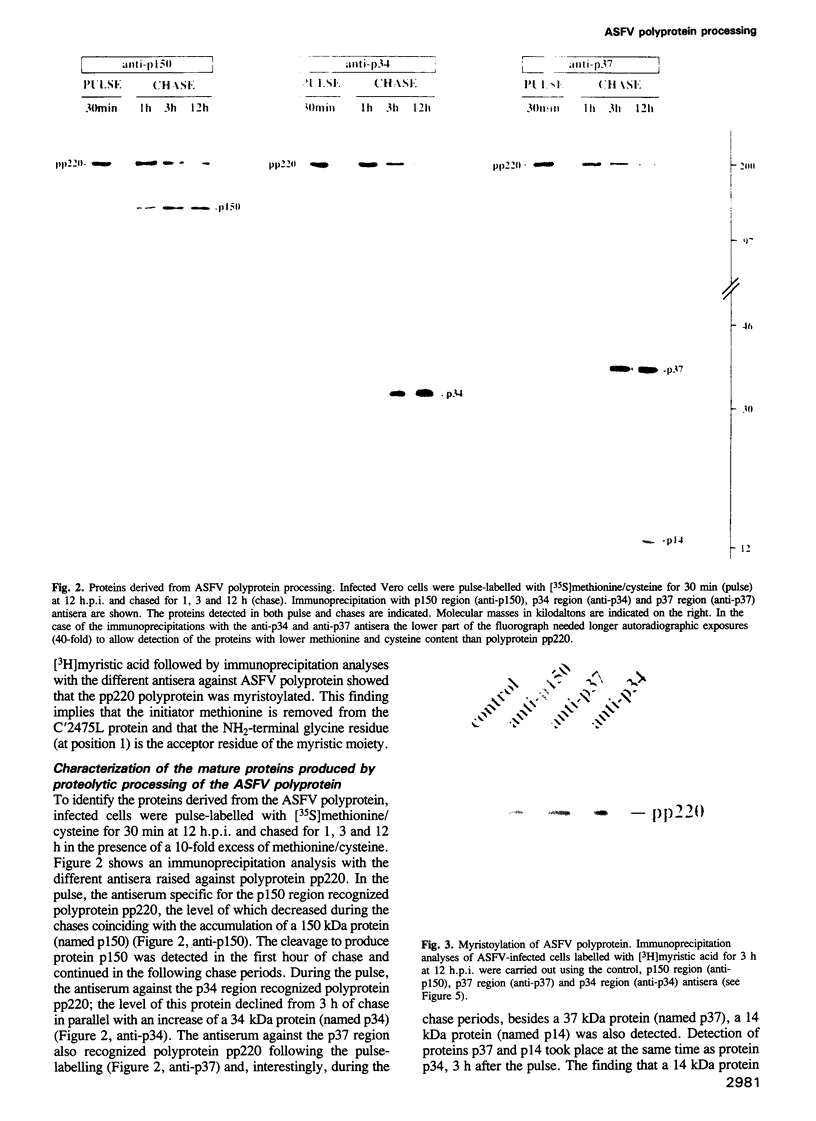
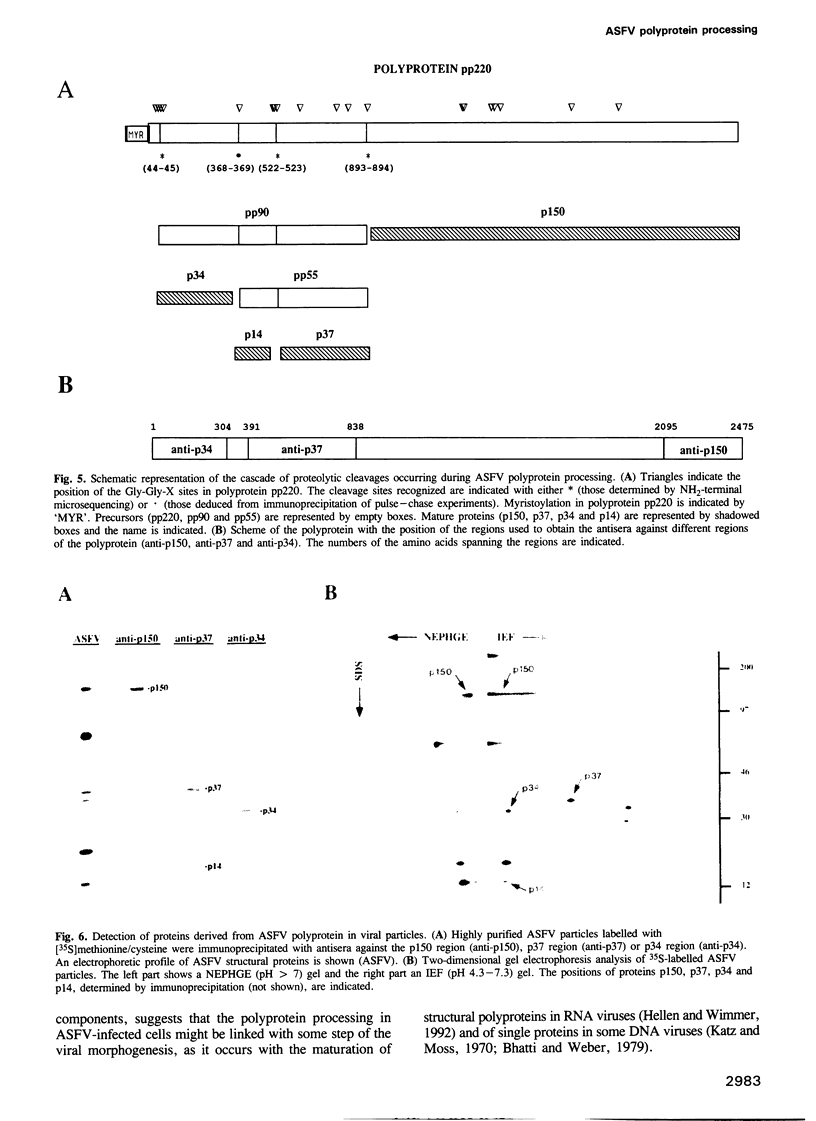
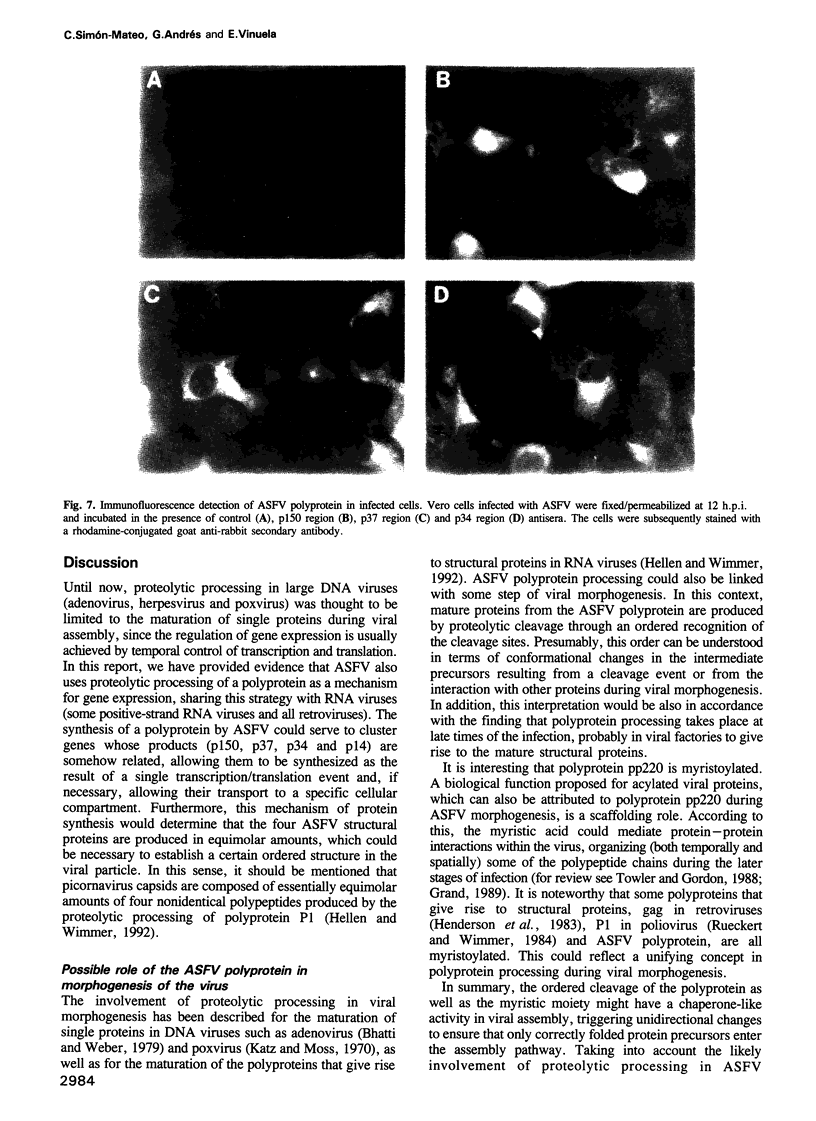
This report shows that African swine fever virus (ASFV)--a large DNA-containing virus--synthesizes a polyprotein to produce several of its structural proteins. By immunoprecipitation analysis, we have found that ASFV polyprotein is a 220 kDa myristoylated polypeptide (pp220) which, after proteolytic processing, gives rise to four major structural proteins: p150, p37, p34 and p14. Processing of the ASFV polyprotein takes place at the consensus sequence Gly-Gly-X and occurs through an ordered cascade of proteolytic cleavages. So far, polyprotein processing as a mechanism of gene expression had been found only in positive-strand RNA viruses and retroviruses. According to the results presented here, ASFV is the first example of a DNA virus that synthesizes a polyprotein as a strategy of gene expression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguado B., Viñuela E., Alcamí A. African swine fever virus fatty acid acylated proteins. Virology. 1991 Dec;185(2):942–945. doi: 10.1016/0042-6822(91)90578-y. [DOI] [PubMed] [Google Scholar]
- Akusjärvi G., Zabielski J., Perricaudet M., Pettersson U. The sequence of the 3' non-coding region of the hexon mRNA discloses a novel adenovirus gene. Nucleic Acids Res. 1981 Jan 10;9(1):1–17. doi: 10.1093/nar/9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcamí A., Angulo A., López-Otín C., Muñoz M., Freije J. M., Carrascosa A. L., Viñuela E. Amino acid sequence and structural properties of protein p12, an African swine fever virus attachment protein. J Virol. 1992 Jun;66(6):3860–3868. doi: 10.1128/jvi.66.6.3860-3868.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés G., Simón-Mateo C., Viñuela E. Characterization of two African swine fever virus 220-kDa proteins: a precursor of the major structural protein p150 and an oligomer of phosphoprotein p32. Virology. 1993 May;194(1):284–293. doi: 10.1006/viro.1993.1259. [DOI] [PubMed] [Google Scholar]
- Bhatti A. R., Weber J. Protease of adenovirus type 2. Subcellular localization. J Biol Chem. 1979 Dec 25;254(24):12265–12268. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Carrascosa A. L., del Val M., Santarén J. F., Viñuela E. Purification and properties of African swine fever virus. J Virol. 1985 May;54(2):337–344. doi: 10.1128/jvi.54.2.337-344.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Lewis M., Govindarajan S., Shapiro M., Moskal T., Purcell R. H. cDNA clone of hepatitis A virus encoding a virulent virus: induction of viral hepatitis by direct nucleic acid transfection of marmosets. J Virol. 1992 Nov;66(11):6649–6654. doi: 10.1128/jvi.66.11.6649-6654.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Carrascosa A. L., Moreno M. A., Viñuela E. Titration of African swine fever (ASF) virus. J Gen Virol. 1976 Sep;32(3):471–477. doi: 10.1099/0022-1317-32-3-471. [DOI] [PubMed] [Google Scholar]
- González A., Talavera A., Almendral J. M., Viñuela E. Hairpin loop structure of African swine fever virus DNA. Nucleic Acids Res. 1986 Sep 11;14(17):6835–6844. doi: 10.1093/nar/14.17.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand R. J. Acylation of viral and eukaryotic proteins. Biochem J. 1989 Mar 15;258(3):625–638. doi: 10.1042/bj2580625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen C. U., Wimmer E. The role of proteolytic processing in the morphogenesis of virus particles. Experientia. 1992 Feb 15;48(2):201–215. doi: 10.1007/BF01923512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch J., Barbour A. G. Linear plasmids of Borrelia burgdorferi have a telomeric structure and sequence similar to those of a eukaryotic virus. J Bacteriol. 1991 Nov;173(22):7233–7239. doi: 10.1128/jb.173.22.7233-7239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Moss B. Formation of a vaccinia virus structural polypeptide from a higher molecular weight precursor: inhibition by rifampicin. Proc Natl Acad Sci U S A. 1970 Jul;66(3):677–684. doi: 10.1073/pnas.66.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P. M., Davison A. J., Lowe R. S., Bennett C. D., Ellis R. W. Identification and structure of the gene encoding gpII, a major glycoprotein of varicella-zoster virus. Virology. 1986 Jul 15;152(1):181–191. doi: 10.1016/0042-6822(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Kuznar J., Salas M. L., Viñuela E. DNA-dependent RNA polymerase in African swine fever virus. Virology. 1980 Feb;101(1):169–175. doi: 10.1016/0042-6822(80)90493-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ley V., Almendral J. M., Carbonero P., Beloso A., Viñuela E., Talavera A. Molecular cloning of African swine fever virus DNA. Virology. 1984 Mar;133(2):249–257. doi: 10.1016/0042-6822(84)90392-1. [DOI] [PubMed] [Google Scholar]
- Liu F. Y., Roizman B. The herpes simplex virus 1 gene encoding a protease also contains within its coding domain the gene encoding the more abundant substrate. J Virol. 1991 Oct;65(10):5149–5156. doi: 10.1128/jvi.65.10.5149-5156.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C., Simón-Mateo C., Martínez L., Viñuela E. Gly-Gly-X, a novel consensus sequence for the proteolytic processing of viral and cellular proteins. J Biol Chem. 1989 Jun 5;264(16):9107–9110. [PubMed] [Google Scholar]
- López-Otín C., Simón C., Méndez E., Viñuela E. Mapping and sequence of the gene encoding protein p37, a major structural protein of African swine fever virus. Virus Genes. 1988 Jun;1(3):291–303. doi: 10.1007/BF00572708. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Morse M. A., Marriott A. C., Nuttall P. A. The glycoprotein of Thogoto virus (a tick-borne orthomyxo-like virus) is related to the baculovirus glycoprotein GP64. Virology. 1992 Feb;186(2):640–646. doi: 10.1016/0042-6822(92)90030-s. [DOI] [PubMed] [Google Scholar]
- Nunes J. F., Vigário J. D., Terrinha A. M. Ultrastructural study of African swine fever virus replication in cultures of swine bone marrow cells. Arch Virol. 1975;49(1):59–66. doi: 10.1007/BF02175596. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Rueckert R. R., Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984 Jun;50(3):957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Salas M. L., Rey-Campos J., Almendral J. M., Talavera A., Viñuela E. Transcription and translation maps of African swine fever virus. Virology. 1986 Jul 15;152(1):228–240. doi: 10.1016/0042-6822(86)90387-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarén J. F., Viñuela E. African swine fever virus-induced polypeptides in Vero cells. Virus Res. 1986 Sep;5(4):391–405. doi: 10.1016/0168-1702(86)90031-6. [DOI] [PubMed] [Google Scholar]
- Sanz A., García-Barreno B., Nogal M. L., Viñuela E., Enjuanes L. Monoclonal antibodies specific for African swine fever virus proteins. J Virol. 1985 Apr;54(1):199–206. doi: 10.1128/jvi.54.1.199-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirako Y., Strauss J. H. Cleavage between nsP1 and nsP2 initiates the processing pathway of Sindbis virus nonstructural polyprotein P123. Virology. 1990 Jul;177(1):54–64. doi: 10.1016/0042-6822(90)90459-5. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Almendral J. M., Talavera A., Viñuela E. Terminal and internal inverted repetitions in African swine fever virus DNA. Virology. 1984 Mar;133(2):271–275. doi: 10.1016/0042-6822(84)90394-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Vanslyke J. K., Whitehead S. S., Wilson E. M., Hruby D. E. The multistep proteolytic maturation pathway utilized by vaccinia virus P4a protein: a degenerate conserved cleavage motif within core proteins. Virology. 1991 Aug;183(2):467–478. doi: 10.1016/0042-6822(91)90976-i. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. The N-end rule. Cell. 1992 May 29;69(5):725–735. doi: 10.1016/0092-8674(92)90285-k. [DOI] [PubMed] [Google Scholar]
- Wellink J., van Kammen A. Proteases involved in the processing of viral polyproteins. Brief review. Arch Virol. 1988;98(1-2):1–26. doi: 10.1007/BF01321002. [DOI] [PubMed] [Google Scholar]
- del Val M., Viñuela E. Glycosylated components induced in African swine fever (ASF) virus-infected Vero cells. Virus Res. 1987 Jun;7(4):297–308. doi: 10.1016/0168-1702(87)90044-x. [DOI] [PubMed] [Google Scholar]