Abstract
Hemoglobin has been studied and well haracterized in red blood cells for over one hundred years. However, new work has indicated that the hemoglobin alpha subunit (Hbα) is also found within the blood vessel wall, where it appears to localize at the myoendothelial junction (MEJ) and plays a role in regulating nitric oxide (NO) signaling between endothelium and smooth muscle. This discovery has created a new paradigm for control of endothelial nitric oxide synthase activity, nitric oxide diffusion, and ultimately, control of vascular tone and blood pressure. This review will discuss the current knowledge of hemoglobin’s properties as a gas exchange molecule in the blood stream, and extrapolate the properties of Hbα biology to the MEJ signaling domain. Specifically, we propose that Hbα is present at the MEJ to regulate NO release and diffusion in a restricted physical space, which would have powerful implications for the regulation of blood flow in peripheral resistance arteries.
Keywords: hemoglobin, hemoglobin alpha, alpha thalassemia, myoendothelial junction, Cytochrome B5 reductase 3, nitric oxide
Introduction
For over one hundred years1, hemoglobin has been known as the gas exchange molecule found within red blood cells (RBC) that is responsible for delivering oxygen to tissues and subsequently removing carbon dioxide2. The structure of normal functional adult hemoglobin (HbA; Figure 1A) is composed of twin alpha and beta globin subunits (α2β2), each of which contains a heme, or iron ion, within a heterocyclic ring of four pyrrole (C4H4NH) molecules known as a porphyrin. The assembled tetramer can interact with the aforementioned gases, as well as carbon monoxide and NO. Today, this paradigm in RBC remains unchanged. However we3, and others4,5, have demonstrated that Hbα is expressed in the blood vessel wall but in contrast to other globins in the blood vessel wall3,6,7, Hbα localization and physiological effects are concentrated at myoendothelial junctions (MEJs) in the endothelial cells (EC) lining the lumen of blood vessels.
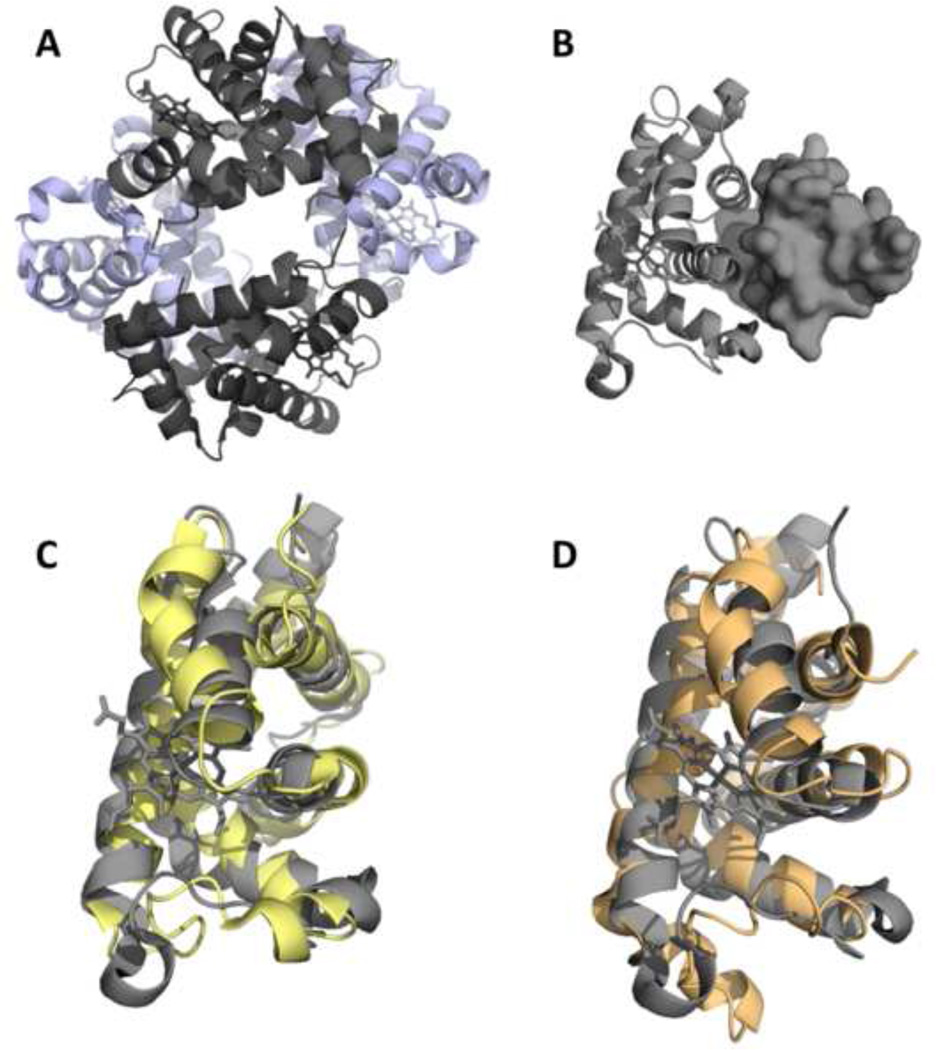
Figure 1. Structure of Hbα.
(A) HbA is a heterotetramer with two a subunits (gray) and two β subunits (purple). (B) Monomeric Hbα (rendered in cartoon) is stabilized by AHSP (surface representation). The overall backbone fold of Hbα bound to AHSP (C and D, gray) is similar to Hbα (C, yellow) and Hbβ (D, orange) of HbA.
Although the discovery of Hbα in the blood vessel wall is unique, there have been clues from human diseases of Hbα deletion, possibly independent of RBC function, that have already indicated its possible presence. The Hbα gene is located on chromosome 16 and has two identical but duplicated coding sequences, HBA-1 and HBA-2. Genetic deletion or loss-of-function mutations to the Hbα alleles are known as alpha-thalassemia, a condition which is defined by the severity of changes to red blood cell indices8,9. Patients with alpha+ thalassemia (silent carriers) will have deletion or loss of function mutations to one of the HBA-1 or HBA-2 alleles. However, compensation enables them to remain asymptomatic and many go undiagnosed. Deletion of two of the HBA-1 and HBA-2 alleles causes alpha0 thalassemia, which presents with moderate or severe RBC indices, depending on the deletion type or mutation9. In terms of the vascular phenotype, this frequently includes moderate hypotension. Deletion of three of the HBA-1 and HBA-2 alleles is known as HbH disease and characterized by hemolytic anemia, hepatosplenomegaly, and the formation of a tetramer of β chains in vivo. Due to severity of RBC indices, it remains unclear if the vascular phenotype remains consistent with alpha0 thalassemia or is hidden by development of other pathologies. Hydrops fetalis syndrome (HP Bart Syndrome) occurs with deletion of all of the Hbα alleles and results in death in utero10. The vascular phenotype of dilated cerebral arteries is observed via ultrasound,, and also used to assist in the diagnosis of the disease although difficult to delineate from the accompanied severe anemia (Table 1)11,15. Regardless, this would correlate with observations from patients with different degrees of Hgα deletion presenting with differences in capillary diameter.16 In each of these cases, a role for Hbα in RBC is challenging to fully explain the vascular phenotype presented. However, reduction or deletion of Hbα in EC of the resistance arteries, where it is hypothesized to regulate NO delivery to vascular smooth muscle cells (VSMC), could be a reason. Future research in this area could lead to important new insight into understanding the pathology as well as diagnosis related to these diseases.
Table 1.
Chromosome 16 contains two identical coding sequences for hemoglobin alpha, hemoglobin alpha 2 and hemoglobin alpha 1 (shown here in reference to the zetaglobin gene, which is also located on chromosome 16). Deletions/non-functional mutations are depicted by the absence of a corresponding box. Genotype, red blood cell (RBC) indices, vascular phenotype with relation to blood pressure, and medical classification of the various alpha thalassemia’s are also listed8,10,44,74.
| Alpha Thalassemias | ||||||
|---|---|---|---|---|---|---|
| Chromosome 16 (both copies shown) | Genotype | RBC Indices |
Vascular Phenotype |
Classification | ||
| HBZ | m | HBA-1 | ||||
| ========|z|===========|α|=======|α|====== | αα/αα | Normal | Normal | Normal | ||
| ========|z|===========|α|=======|α|====== | ||||||
| ========|z|===========|α|=============== | α-/αα | Normal | Moderate hypotension with potential compensation | Alpha thalassemia minima, also known as heterozygo sity for alpha (+) thalassemia, silent carrier of alpha thalassemia, and alpha thalassemia-2 trait | ||
| ========|z|===========|α|=======|α|====== | ||||||
| ========|z|===========|α|=============== | α-/α- or --/αα | Minimal anemia, decreased MCV and MCH | Moderate hypotension | Alpha thalassemia minor, also known as alpha thalassemia-1 trait (Due to homozygosity for alpha (+) thalassemia (α-/α-) or heterozygo sity for alpha (0) thalassemia (--/αα)) | ||
| ========|z|===========|α|=============== | ||||||
| or | ||||||
| ========|z|============================ | ||||||
| ========|z|===========|α|=======|α|====== | ||||||
| ========|z|===========|α|=============== | α-/-- | Hemolytic anemia with the formation of P-chain tetramers | None documented | Hemoglobin H (HbH) disease | ||
| ========|z|============================ | ||||||
| ========|z|============================= | -/-- | Severe anemia due to formation of gamma-4 tetramers (hemoglobin Bart’s) | Hydrops fetalis with increased cerebral blood flow | Hydrops fetalis syndrome with hemoglobin Bart’s | ||
| ========|z|============================ | ||||||
Hemoglobin alpha, certae sedis
The first evidence of localization and function for Hbα in the blood vessel wall was seen in MEJ from resistance arteries in the systemic vasculature3. Resistance arteries are the small arteries that contribute the greatest amount to peripheral resistance and thus overall blood pressure regulation17,18. In resistance vasculature heterocellular communication is critical to the maintenance of vascular tone and blood pressure (for review, see 19,20). Crucial to heterocellular communication in resistance arteries are the presence of MEJs. The MEJ is the physical link between EC and the VSMC, characterized as a small protrusion of mostly EC (approximately 0.5 m wide and long) through the internal elastic lamina, linking with VSMC through gap junctions.12. Myoendothelial junctions are found throughout the vasculature; however, a gradient is observed such that the junctions are more prevalent as the diameter of the vascular tree decreases21. Thus, MEJs represent the closest physical location of EC to VSMC in the small resistance arteries. The MEJ is acknowledged as an important signaling microdomain (for review, see (Billaud et al., 2014)22) not only because of its spatially limited structure, but also because the MEJ serves as the gateway between EC signal transduction to the VSMC22. For instance, S-nitrosylation/denitrosylation of connexin 43 is controlled by specific proteins localized to the MEJ, including S-nitrosoglutatione reductase (GSNOR), endothelial nitric oxide synthase (eNOS), and IP3 receptor type 1 (IP3RI)23. Together, these proteins participate in localized signaling that promotes negative feedback on vasoconstriction induced by α1-adrenergic receptor activation.
Importantly, while eNOS is localized to intracellular regions including plasma membrane caveolae and the Golgi complex, it is also localized to the MEJ24,25. Endothelial cells exhibit a strict and varied control over the production of NO (reviewed in (Shaul, 2002)26) and the predominant enzyme responsible for the generation of NO in these cells is eNOS. This localization of eNOS at the MEJ presumably occurs because NO is a highly reactive gaseous free radical, enabling targeted release of NO at proximal sites to the overlying VSMCs, where it functions as potent vasodilator. Subsequent work demonstrated that eNOS and Hbα reside in close proximity to each other and potentially form a macromolecular complex at the MEJ3, where they provide a regulatory mechanism for NO-mediated vasodilation (Figure 2).
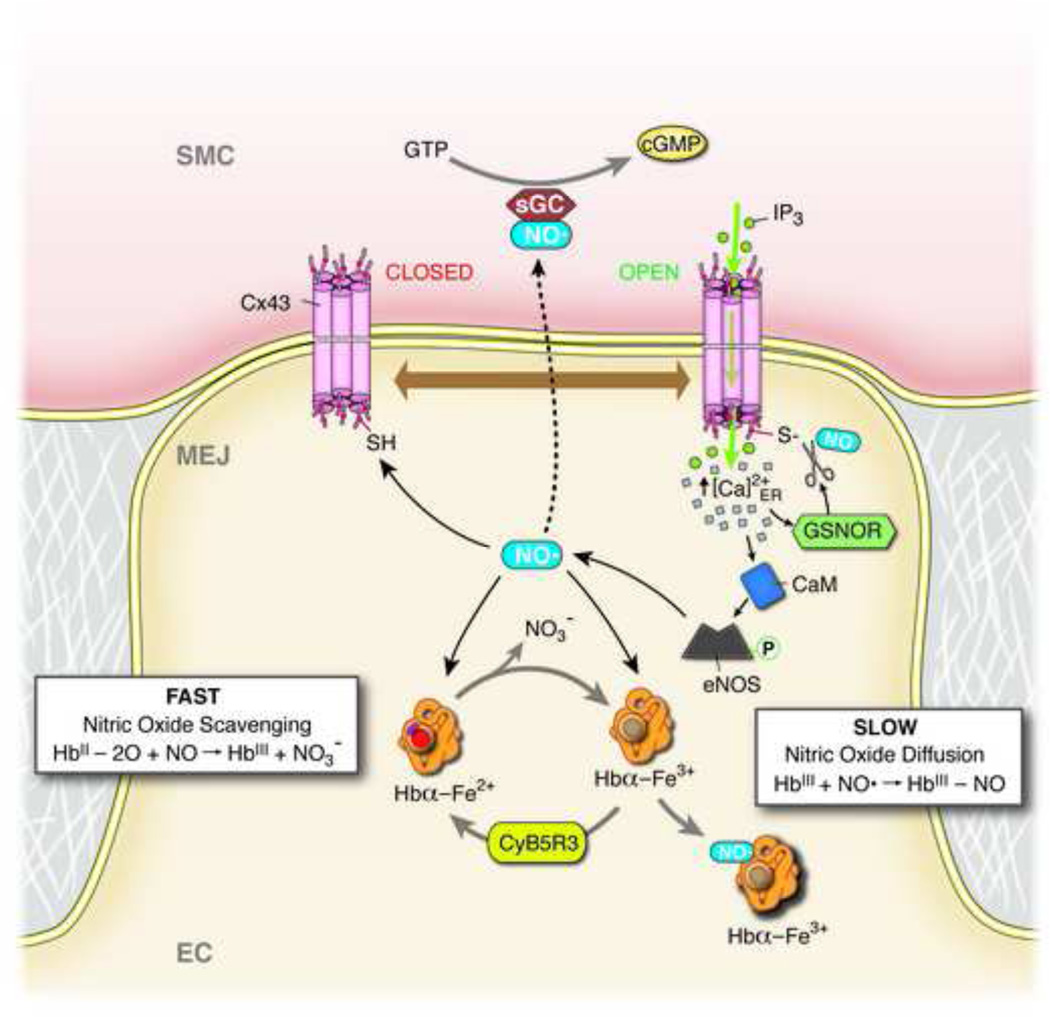
Figure 2. Schematic of NO regulation at the MEJ.
Negative feedback following α1-adrenergic receptor induced vasoconstriction at the myoendothelial junction (MEJ) of an endothelial cell (EC). α1-adrenergic receptor agonists cause an increase in smooth muscle cell (VSMC) cytosolic inositol 1,4,5-trisphosphate (IP3), which travels down its concentration gradient through open (i.e. S-nitrosylated) connexin 43 (Cx43) channels. IP3 binds to localized IPs-receptor 1 on the endoplasmic reticulum (not shown), which causes localized calcium (Ca2+) release and an increase in cytosolic Ca2+ concentration at the MEJ. This increase in cytosolic Ca2+ facilitates activation of S-nitrosoglutathione reductase (GSNOR), which denitrosylates and closes Cx43 channels, making them impermeable to IP3. The increase in cytosolic Ca2+ also activates calmodulin (CaM) through binding-induced conformational changes. The Ca2+/CaM complex binds to endothelial nitric oxide synthase (eNOS), facilitating its phosphorylation, activation, and production of nitric oxide (NO). Some of this newly-produced NO will diffuse into VSMC, where it binds to and activates soluble guanylyl cyclase (sGC), facilitating the conversion of GTP to cGMP, which ultimately leads to a reduction in constriction. The newly-produced NO may also facilitate opening of Cx43 channels through S-nitrosylation. Finally, excess NO may be scavenged by free hemoglobin alpha (Hbα) at the MEJ through reaction with oxyhemoglobin alpha (fast), or NO may be chelated through reaction with methemoglobin alpha (slow). Cytochrome B5 reductase (CyB5R3) converts methemoglobin alpha to Hbα, which readily binds oxygen3,6,21–23.
Three general outcomes await NO once it is produced in EC: it can 1) diffuse into the blood stream where it is rapidly scavenged, first by cell free hemoglobin in the plasma and even further by hemoglobin in RBC27, 2) diffuse to neighboring VSMCs and cause vasorelaxation, 3) be scavenged by any number of molecules, such as reactive oxygen species (ROS) (for review, see Martinez et al., 200928). In the blood stream, most NO diffuses across its concentration gradient into RBC, where it is either scavenged or stored. NO scavenging proceeds via oxidation by oxyhemoglobin, which produces methemoglobin and nitrate. This is the favored reaction; however, NO can also bind directly to deoxygenated hemoglobin in a simple addition reaction to form iron-nitrosyl-hemoglobin (Hb-NO)29. Furthermore, it has been proposed that nitric oxide reacts in an oxygen-sensitive mechanism with the conserved Cys93 residue of the hemoglobin β-chain30; however, these findings have been intensely debated31–33. Finally, data has been suggested that eNOS is present in RBC34, which possibly contributes to vasoprotectivity35–37. Once in the VSMC, NO activates soluble guanylyl cyclase (sGC) by binding to its heme moiety, allowing a several hundred-fold increase in the catalysation of GTP to cGMP38 and causing vasodilation through several mechanisms39. Scavenging of NO occurs in pathologies that result in, produce, or maintain elevated oxidant stress, especially when accompanied with an inability to be counter balanced by antioxidants. Increased ROS can scavenge NO directly or alter pathways that mediate NO production. The multiple fates of NO in the vasculature is further complicated by its very short half-life (<5 seconds)40. Conceivably, in the EC at the MEJ, any one of the above mechanisms may be in play41.
An important regulator in the binding of NO (and oxygen) to hemoglobin is the oxidation state of the heme iron: ferrous (Fe2+), or ferric (Fe3+). The ferric state of hemoglobin is also frequently known as methemoglobin (MHb)42,43. However, the HbA in the bloodstream contains less than 1% MHb due to the fact that it possesses a higher affinity to oxygen and excess MHb can result in tissue hypoxia and even death44. The oxidation state of iron also controls the sensitivity of Hbα to NO; the Fe2+ state rapidly scavenges NO and the resulting production of nitrate and methemoglobin decreases NO bioavailability, whereas the heme moiety in the Fe3+ state reacts slowly and transiently with NO, allowing for increased diffusion of NO into smooth muscle45–47. This reaction would presumably hold true for the Hbα found in the MEJ. In RBC, a methemoglobin reductase, cytochrome b5 reductase 3 (CytB5R3, also known as diaphorase 1), is present to recycle the Fe3+ state and prevent the accumulation of MHb. When CytB5R3 is absent due to genetic defect or ingestion of oxidizing toxins the resulting methemoglobinemia48(famously characterized as a bluish tint to the skin) causes decreased tissue oxygenation, hypoxia, and cyanosis49. Not surprisingly, the enzyme CytB5R3 found in RBC is also found in EC and at the MEJ [although not as strictly localized as Hbα). However, in contrast to the RBC where the Fe2+ oxidation state predominates, the MEJ heme moieties in resistance arteries exist in both states (Fe3+ approximately 58%). Knockdown of CytB5R3 reveals altered reactivity in ex vivo vessels to adrenergic and endothelial dependent NO stimulus, revealing a permissive effect of CytB5R3 on NO bioavailability3. Interestingly, the CytB5R3 inhibitor and anti-thyroid drug propylthiouracil (PTU) also presents with a reduction in blood pressure in rats50. This could be explained by PTU preventing the CytB5R3 enzyme from reducing the Fe3+ heme moieties in Hbα the MEJ, thus maintaining the slower NO scavenging reaction and allowing for increased NO diffusion into the VSMC. The evidence increasingly indicates that Hbα could play an important role in regulating NO bioavailability through control of the heme oxidation state.
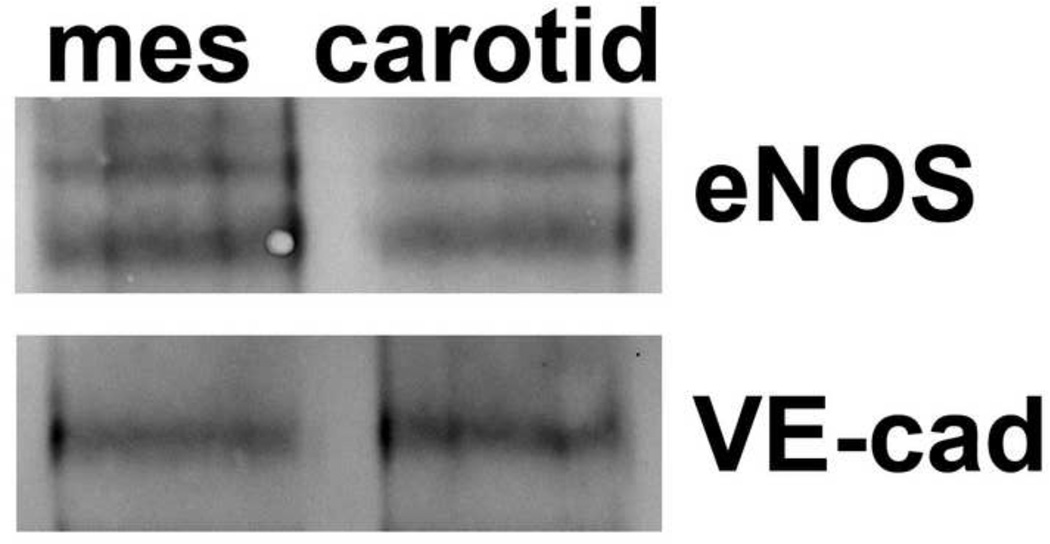
Lastly, it’s possible that Hbα is found in resistance arteries to chelate excessive NO. This rationale is based on the fact that conduit arteries have been previously characterized as largely dependent on NO for vasodilator activity, whereas smaller resistance vessels rely on the action of endothelium-derived hyperpolarizing factor (EDHF)22,51. However, there is no observable difference in the total protein expression of eNOS in carotid and third order mesenteric arteries (i.e., resistance arteries; Figure 3). Thus, Hbα at the MEJ in resistance arteries may be chelating the NO generated by eNOS, allowing for EDHF to remain dominant for increased endothelial dependent vasodilation, and maintaining strict control over NO for other cellular functions (e.g., negative feedback after vasoconstriction). It remains unclear how Hbα scavenges the excessive NO, and whether this action results in a permanent loss of NO or simply a reservoir of NO that can be released upon appropriate stimulus. It is also possible that the amount of eNOS between resistance arteries and conduit arteries is inconsequential because the eNOS that is present in resistance arteries is uncoupled as compared to conduit arteries, negating eNOS generation (e.g.,52,53). It is unclear why such a potent enzyme would still be present if this were the case, however the possibility exists and it is clear more work needs to be done in this regard.
Figure 3. Expression of eNOS between resistance and conduit arteries is equal.
Representative western blot analysis of endothelial nitric oxide synthase (eNOS) protein level in third-order mesenteric arteries compared to carotid. Samples were equalized according to the amount of VE-cadherin, an endothelial cell marker.
The importance of NO scavenging by Hbα at the MEJ is predominantly derived from its location. Nitric oxide scavenging by RBC hemoglobin requires diffusion through the EC monolayer, the glycocalyx, the plasma, and then interacts with cell free hemoglobin or must cross the RBC membrane before it reaches HbA. Based on these assumptions, it is tempting to speculate that the likelihood of locally produced NO at the MEJ interacting with Hbα is greatly increased due to it’s proximity to the NO source. However, NO produced on the luminal side of the endothelium would favor scavenging by RBC hemoglobin. This implies that although the relative abundance of Hb in RBC may be greater, it’s importance for the regulation of diffusion of NO into VSMC may be reduced, although this may change with deviation from basal conditions or pathological states.
Why hemoglobin α in the endothelium?
One of the interesting questions that arises from the discovery of Hbα at MEJs of EC is, why Hbα, and not Hbβ? Wouldn’t both subunits be better than one for microcirculation where MEJs are prevalent? As of yet there is no clear answer to this question.
Expression of both hemoglobin subunits has beef reported in many cell types beyond erythrocytes54–59, which has sparked hypotheses of functions in addition to oxygen transport and delivery. The oxygen transport by Hb is specific to vertebrates with globins in other walks of life having a variety of functions such as NO dioxygenase and peroxidase activity60. Individually, the Hb subunits have damaging effects; thus, the assembly of HbA is regulated. Only Hbα has a chaperone, alpha hemoglobin stabilizing protein [AHSP], which maintains Hbα solubility and reduces reactive oxygen species production by stabilizing the Fe3+ state61. AHSP binds to Hbα in proximity to the Hbβ binding site [Fig. 1B] and is displaced during the assembly of Hb. Therefore, Hbα subunits may be considered the less toxic of the two subunits for additional physiological roles due to the added protection of AHSP. The Hbα and Hbβ subunits have very similar structures [Figure 1C–D) yet very different amino acid sequences (43% identity]. There are some structural rearrangements of Hbα upon binding AHSP; however, the overall structure is very similar to both Hbα and Hbβ in the heterotetramer. Both subunits similarly bind haem; thus, in terms of oxygen binding and reactivity the subunits are similar. However, the sequence differences between the subunits could certainly provide differences in signaling. For instance, Hbα molecular interactions that regulate NO signaling at the MEJ3 may be mediated by distinct amino acid regions of Hbα that are different than Hbβ such that Hbβ would not elicit the same signaling cascade. More work on this fundamental question will certainly be required.
Vasculature effects of genetic hemoglobin deletion
In mice, similar to humans, deletion of all of the Hbα alleles is lethal, making genetic studies on animals in vivo difficult. However, there are other animal models found in nature where Hbα and β have been reported to be deleted, yet the animals still prove viable, with very interesting effects on the vasculature62,63. These animals, Icefishes (family Channichthyidae), dominate the fish fauna of the Southern Ocean surrounding Antarctica64. Icefishes are exceptional in that they are the only known vertebrate animals to completely lack the oxygen-carrier HbA in their blood65. Loss of this key respiratory protein has ensued a phenotypic pale, translucent white blood and has earned these animals the common names of ‘white-blooded fishes’ and ‘icefishes’ (Figure 4)66. Absent a gaseous transporter protein, oxygen is carried unassisted in solution of icefish blood and results in an oxygen-carrying capacity that is <10% of that exhibited by red-blooded notothenioid fishes67. Given this consequence and the fact that the HbA-null condition proves lethal in all other examples, both laboratory-based and natural, how has this condition persisted in icefishes then? Although more complex and beyond the scope of what we can present here (for an excellent review, see68), there are several basic considerations that help to answer this question. First, icefishes possess cardiovascular systems with unusually enhanced features as compared to their red-blooded notothenioid relatives; large hearts, large diameter capillaries, and large blood volumes collectively enable icefishes to maintain a high-throughput circulatory design without excessive pressure development69,70. Combined with the abundantly high oxygen content of Antarctic waters and their relatively low metabolic rates, these cardiovascular traits permit icefishes to sufficiently oxygenate their tissues and support their aerobic mode of metabolism71. As one might expect however, the loss of expression of HbA has implications for the metabolism of NO. It was proposed that the loss of NO-oxygenase activity with genetic deletion of HbA may have led to subsequent elevation of NO levels that could explain many, if not all, of the unique cardiovascular and physiological traits that evolved in icefishes68. Substantiating at least part of that hypothesis, Beers et al. (2010) established that NO concentrations in blood plasma appear to be greater in icefishes than in HbA-expressing species72. They also reported that the high NO levels in icefishes were not the result of greater synthesis but, rather, appeared to be due primarily to the loss of the degradative pathway for NO72.
Figure 4. Blood from HbA and HbA null fish.
Blood samples from two Antarctic notothenioid fishes illustrate a striking contrast in level of hemoglobin expression. The test tube on the right contains blood from an HbA-expressing species, Notothenia coriiceps, while the tube on the left depicts the completely HbA-null phenotype of the ‘crocodile’ icefish, Chaenocephalus aceratus.
Building upon the above work, Borley and collaborators subsequently conducted a study in which they induced severe anemia in Notothenia coriiceps, an HbA-expressing notothenioid with a normal hematocrit of 35–40%73. Surgically implanted osmotic pumps were used to treat individuals with a powerful hemolytic agent that resulted in a drastic reduction in hematocrit (>90%) and HbA concentration (>70%). Levels of NO were significantly higher in anemic animals compared to the full HbA-expressing controls and were similar to the levels of NO reported for white-blooded icefishes72,73. Although MEJs have not been identified in these fish, it would be surprising if these anatomical structures weren’t present. The regulation of NO in the arteries of icefish could be one potential avenue for understanding how Hbα regulates NO delivery in arteries with an in vivo model capable of withstanding severe hemoglobin depletion.
Summary
This review describes a new paradigm of localized NO regulation by Hbα in MEJs of resistance arteries. This unique microdomain has the potential for pharmacological targeting and serves as an explanation for several different pathologies associated with Hb α deletion. Although some work has been done on this observation, there is still much to do and we look forward to extending this review in the future.
Acknowledgments
This work was supported by National Institutes of Health grants HL088554 (B.E.I.), HL107963 (B.E.I.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barcroft J, Roberts F. The dissociation curve of haemoglobin. The Journal of physiology. 1909;39:143–148. doi: 10.1113/jphysiol.1909.sp001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schechter AN. Hemoglobin research and the origins of molecular medicine. Blood. 2008;112:3927–3938. doi: 10.1182/blood-2008-04-078188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straub AC, et al. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature. 2012;491:473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgoyne JR, Prysyazhna O, Rudyk O, Eaton P. cGMP-dependent activation of protein kinase G precludes disulfide activation: implications for blood pressure control. Hypertension. 2012;60:1301–1308. doi: 10.1161/HYPERTENSIONAHA.112.198754. [DOI] [PubMed] [Google Scholar]
- 5.Davalos A, et al. Quantitative proteomics of caveolin-1-regulated proteins: characterization of polymerase i and transcript release factor/CAVIN-1 IN endothelial cells. Molecular & cellular proteomics: MCP. 2010;9:2109–2124. doi: 10.1074/mcp.M110.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahaman MM, Straub AC. The emerging roles of somatic globins in cardiovascular redox biology and beyond. Redox biology. 2013;1:405–410. doi: 10.1016/j.redox.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu Y, Sutton L, Riggs AF. Identification of myoglobin in human smooth muscle. The Journal of biological chemistry. 1998;273:23426–23432. doi: 10.1074/jbc.273.36.23426. [DOI] [PubMed] [Google Scholar]
- 8.Galanello R, Cao A. Gene test review. Alpha-thalassemia. Genetics in medicine: official journal of the American College of Medical Genetics. 2011;13:83–88. doi: 10.1097/GIM.0b013e3181fcb468. [DOI] [PubMed] [Google Scholar]
- 9.Origa R, Moi P, Galanello R, Cao A. In: Gene Reviews. Pagon RA, et al., editors. 1993. [Google Scholar]
- 10.Chui DH, Fucharoen S, Chan V. Hemoglobin H disease: not necessarily a benign disorder. Blood. 2003;101:791–800. doi: 10.1182/blood-2002-07-1975. [DOI] [PubMed] [Google Scholar]
- 11.Srisupundit K, Piyamongkol W, Tongsong T. Identification of fetuses with hemoglobin Bart’s disease using middle cerebral artery peak systolic velocity. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2009;33:694–697. doi: 10.1002/uog.6342. [DOI] [PubMed] [Google Scholar]
- 12.Lam YH, Tang MH. Middle cerebral artery Doppler study in fetuses with homozygous alpha-thalassaemia-1 at 12-13 weeks of gestation. Prenatal diagnosis. 2002;22:56–58. doi: 10.1002/pd.237. [DOI] [PubMed] [Google Scholar]
- 13.Lam YH, Ghosh A, Tang MH, Lee CP, Sin SY. Early ultrasound prediction of pregnancies affected by homozygous alpha-thalassaemia-1. Prenatal diagnosis. 1997;17:327–332. [PubMed] [Google Scholar]
- 14.Leung KY, et al. A new strategy for prenatal diagnosis of homozygous alpha(0)-thalassemia. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2006;28:173–177. doi: 10.1002/uog.2720. [DOI] [PubMed] [Google Scholar]
- 15.Leung WC, Oepkes D, Seaward G, Ryan G. Serial sonographic findings of four fetuses with homozygous alpha-thalassemia-1 from 21 weeks onwards. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2002;19:56–59. doi: 10.1046/j.0960-7692.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 16.Vincent L, et al. Remodeling of skeletal muscle microvasculature in sickle cell trait and alpha-thalassemia. Am J Physiol Heart Circ Physiol. 2010;298:H375–H384. doi: 10.1152/ajpheart.00812.2009. [DOI] [PubMed] [Google Scholar]
- 17.Billaud M, et al. Characterization of the thoracodorsal artery: morphology and reactivity. Microcirculation. 2012;19:360–372. doi: 10.1111/j.1549-8719.2012.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulvany MJ, Aalkjaer C. Structure and function of small arteries. Physiological reviews. 1990;70:921–961. doi: 10.1152/physrev.1990.70.4.921. [DOI] [PubMed] [Google Scholar]
- 19.Sandow SL, et al. What’s where and why at a vascular myoendothelial microdomain signalling complex. Clinical and experimental pharmacology & physiology. 2009;36:67–76. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- 20.Segal SS, Bagher P. Regulation of myoendothelial junction formation: bridging the gap. Circulation research. 2010;106:1014–1016. doi: 10.1161/CIRCRESAHA.110.217786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heberlein KR, Straub AC, Isakson BE. The myoendothelial junction: breaking through the matrix? Microcirculation. 2009;16:307–322. doi: 10.1080/10739680902744404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billaud M, et al. Regulation of cellular communication by signaling microdomains in the vascular wall. Pharmacological Reviews. 2014 doi: 10.1124/pr.112.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straub AC, et al. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:399–407. doi: 10.1161/ATVBAHA.110.215939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian J, et al. Role of local production of endothelium-derived nitric oxide on cGMP signaling and S-nitrosylation. American journal of physiology. Heart and circulatory physiology. 2010;298:H112–H118. doi: 10.1152/ajpheart.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwakiri Y, et al. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19777–19782. doi: 10.1073/pnas.0605907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annual review of physiology. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 27.Owusu BY, Stapley R, Patel RP. Nitric oxide formation versus scavenging: the red blood cell balancing act. The Journal of physiology. 2012;590:4993–5000. doi: 10.1113/jphysiol.2012.234906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez MC, Andriantsitohaina R. Reactive nitrogen species: molecular mechanisms and potential significance in health and disease. Antioxidants & redox signaling. 2009;11:669–702. doi: 10.1089/ars.2007.1993. [DOI] [PubMed] [Google Scholar]
- 29.Gross SS, Lane P. Physiological reactions of nitric oxide and hemoglobin: a radical rethink. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9967–9969. doi: 10.1073/pnas.96.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annual review of physiology. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 31.Crawford JH, Chacko BK, Kevil CG, Patel RP. The red blood cell and vascular function in health and disease. Antioxidants & redox signaling. 2004;6:992–999. doi: 10.1089/ars.2004.6.992. [DOI] [PubMed] [Google Scholar]
- 32.Gladwin MT, Lancaster JR, Jr, Freeman BA, Schechter AN. Nitric oxide’s reactions with hemoglobin: a view through the SNO-storm. Nature medicine. 2003;9:496–500. doi: 10.1038/nm0503-496. [DOI] [PubMed] [Google Scholar]
- 33.Isbell TS, et al. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nature medicine. 2008;14:773–777. doi: 10.1038/nm1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortese-Krott MM, et al. Human red blood cells at work: identification and visualization of erythrocytic eNOS activity in health and disease. Blood. 2012;120:4229–4237. doi: 10.1182/blood-2012-07-442277. [DOI] [PubMed] [Google Scholar]
- 35.Cortese-Krott MM, Kelm M. Endothelial nitric oxide synthase in red blood cells: Key to a new erythrocrine function? Redox biology. 2014;2:251–258. doi: 10.1016/j.redox.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Gonon AT, Sjoquist PO, Lundberg JO, Pernow J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15049–15054. doi: 10.1073/pnas.1307058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood KC, et al. Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1861–1871. doi: 10.1161/ATVBAHA.112.301068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin E, Berka V, Tsai AL, Murad F. Soluble guanylyl cyclase: the nitric oxide receptor. Methods in enzymology. 2005;396:478–492. doi: 10.1016/S0076-6879(05)96040-0. [DOI] [PubMed] [Google Scholar]
- 39.Kukreja RC, Salloum FN, Das A. Cyclic guanosine monophosphate signaling and phosphodiesterase-5 inhibitors in cardioprotection. Journal of the American College of Cardiology. 2012;59:1921–1927. doi: 10.1016/j.jacc.2011.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lancaster JR., Jr Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nature reviews. Immunology. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gladwin MT, Kim-Shapiro DB. Vascular biology: Nitric oxide caught in traffic. Nature. 2012;491:344–345. doi: 10.1038/nature11640. [DOI] [PubMed] [Google Scholar]
- 43.Alayash AI, Cashon RE. Reactions of nitric oxide and hydrogen peroxide with hemoglobin-based blood substitutes. Annals of the New York Academy of Sciences. 1994;738:378–381. doi: 10.1111/j.1749-6632.1994.tb21825.x. [DOI] [PubMed] [Google Scholar]
- 44.Wright R0, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Annals of emergency medicine. 1999;34:646–656. doi: 10.1016/s0196-0644(99)70167-8. [DOI] [PubMed] [Google Scholar]
- 45.Sharma VS, Traylor TG, Gardiner R, Mizukami H. Reaction of nitric oxide with heme proteins and model compounds of hemoglobin. Biochemistry. 1987;26:3837–3843. doi: 10.1021/bi00387a015. [DOI] [PubMed] [Google Scholar]
- 46.Eich RF, et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 47.Alayash AI, Fratantoni JC, Bonaventura C, Bonaventura J, Cashon RE. Nitric oxide binding to human ferrihemoglobins cross-linked between either alpha or beta subunits. Archives of biochemistry and biophysics. 1993;303:332–338. doi: 10.1006/abbi.1993.1292. [DOI] [PubMed] [Google Scholar]
- 48.Hultquist DE, Passon PG. Catalysis of methaemoglobin reduction by erythrocyte cytochrome B5 and cytochrome B5 reductase. Nature: New biology. 1971;229:252–254. doi: 10.1038/newbio229252a0. [DOI] [PubMed] [Google Scholar]
- 49.Percy MJ, Lappin TR. Recessive congenital methaemoglobinaemia: cytochrome b(5) reductase deficiency. British journal of haematology. 2008;141:298–308. doi: 10.1111/j.1365-2141.2008.07017.x. [DOI] [PubMed] [Google Scholar]
- 50.Fregly MJ, Hood CI. Physiologic and anatomic effects of prophylthiouracil on normal and hypertensive rats. Circulation research. 1959;7:486–496. doi: 10.1161/01.res.7.3.486. [DOI] [PubMed] [Google Scholar]
- 51.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarizations: past beliefs and present facts. Annals of medicine. 2007;39:495–516. doi: 10.1080/07853890701491000. [DOI] [PubMed] [Google Scholar]
- 52.Blackwell KA, et al. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287:H2448–H2453. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- 53.d’Uscio LV, Smith LA, Katusic ZS. Differential effects of eNOS uncoupling on conduit and small arteries in GTP-cyclohydrolase I-deficient hph-1 mice. Am J Physiol Heart Circ Physiol. 2011;301:H2227–H2234. doi: 10.1152/ajpheart.00588.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biagioli M, et al. Unexpected expression of alpha- and beta-globin in mesencephalic dopaminergic neurons and glial cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15454–15459. doi: 10.1073/pnas.0813216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dugas JC, Tai YC, Speed TP, Ngai J, Barres BA. Functional genomic analysis of oligodendrocyte differentiation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:10967–10983. doi: 10.1523/JNEUROSCI.2572-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu L, Zeng M, Stamler JS. Hemoglobin induction in mouse macrophages. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6643–6647. doi: 10.1073/pnas.96.12.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marcos-Almaraz MT, Rodriguez-Gomez JA, Lopez-Barneo J, Pascual A. alpha-Haemoglobin regulates sympathoadrenal cell metabolism to maintain a catecholaminergic phenotype. The Biochemical journal. 2012;441:843–850. doi: 10.1042/BJ20111640. [DOI] [PubMed] [Google Scholar]
- 58.Newton DA, Rao KM, Dluhy RA, Baatz JE. Hemoglobin is expressed by alveolar epithelial cells. The Journal of biological chemistry. 2006;281:5668–5676. doi: 10.1074/jbc.M509314200. [DOI] [PubMed] [Google Scholar]
- 59.Nishi H, et al. Hemoglobin is expressed by mesangial cells and reduces oxidant stress. Journal of the American Society of Nephrology: JASN. 2008;19:1500–1508. doi: 10.1681/ASN.2007101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vinogradov SN, Moens L. Diversity of globin function: enzymatic, transport, storage, and sensing. The Journal of biological chemistry. 2008;283:8773–8777. doi: 10.1074/jbc.R700029200. [DOI] [PubMed] [Google Scholar]
- 61.Feng L, et al. Molecular mechanism of AHSP-mediated stabilization of alpha-hemoglobin. Cell. 2004;119:629–640. doi: 10.1016/j.cell.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 62.Martinell J, Whitney JB, Popp RA, 3rd, Russell LB, Anderson WF. Three mouse models of human thalassemia. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:5056–5060. doi: 10.1073/pnas.78.8.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang J, et al. Inactivation of mouse alpha-globin gene by homologous recombination: mouse model of hemoglobin H disease. Blood. 1996;88:1846–1851. [PubMed] [Google Scholar]
- 64.Eastman JT. The nature of the diversity of Antarctic fishes. Polar Biol. 2005;28:93–107. [Google Scholar]
- 65.Ruud JT. Vertebrates without erythrocytes and blood pigment. Nature. 1954;173:848–850. doi: 10.1038/173848a0. [DOI] [PubMed] [Google Scholar]
- 66.di Prisco G, Cocca E, Parker S, Detrich H. Tracking the evolutionary loss of hemoglobin expression by the white-blooded Antarctic icefishes. Gene. 2002;295:185–191. doi: 10.1016/s0378-1119(02)00691-1. [DOI] [PubMed] [Google Scholar]
- 67.Holeton GF. Oxygen uptake and circulation by a hemoglobinless Antarctic fish (Chaenocephalus aceratus lonnberg) compared with three red-blooded Antartic fish. Comparative biochemistry and physiology. 1970;34:457–471. doi: 10.1016/0010-406x(70)90185-4. [DOI] [PubMed] [Google Scholar]
- 68.Sidell BD, O’Brien KM. When bad things happen to good fish: the loss of hemoglobin and myoglobin expression in Antarctic icefishes. The Journal of experimental biology. 2006;209:1791–1802. doi: 10.1242/jeb.02091. [DOI] [PubMed] [Google Scholar]
- 69.Hemmingsen EA, Douglas EL, Johansen K, Millard RW. Aortic blood flow and cardiac output in the hemoglobin-free fish Chaenocephalus aceratus. Comparative biochemistry and physiology. A, Comparative physiology. 1972;43:1045–1051. doi: 10.1016/0300-9629(72)90176-4. [DOI] [PubMed] [Google Scholar]
- 70.Fitch NA, Johnston IA, Wood RE. Skeletal muscle capillary supply in a fish that lacks respiratory pigments. Respiration physiology. 1984;57:201–211. doi: 10.1016/0034-5687(84)90093-8. [DOI] [PubMed] [Google Scholar]
- 71.Hemmingsen EA, Douglas EL. Respiratory and circulatory responses in a hemoglobin-free fish, Chaenocepahlus aceratus, to changes in temperature oxygen tension. Comparative biochemistry and physiology. A, Comparative physiology. 1972;43:1031–1043. doi: 10.1016/0300-9629(72)90175-2. [DOI] [PubMed] [Google Scholar]
- 72.Beers JM, Borley KA, Sidell BD. Relationship among circulating hemoglobin, nitric oxide synthase activities angiogenic poise in red- and white-blooded Antarctic notothenioid fishes. Comparative biochemistry and physiology. Part A, Molecular & integrative physiology. 2010;156:422–429. doi: 10.1016/j.cbpa.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 73.Borley KA, Beers JM, Sidell BD. Phenylhydrazine-induced anemia causes nitric-oxide-mediated upregulation of the angiogenic pathway in Notothenia coriiceps. The Journal of experimental biology. 2010;213:2865–2872. doi: 10.1242/jeb.043281. [DOI] [PubMed] [Google Scholar]
- 74.Guasch A, et al. Evidence that microdeletions in the alpha globin gene protect against the development of sickle cell glomerulopathy in humans. Journal of the American Society of Nephrology :JASN. 1999;10:1014–1019. doi: 10.1681/ASN.V1051014. [DOI] [PubMed] [Google Scholar]