Abstract
All neonates, infants and young children receive multiple priming doses and booster vaccinations in the 1st and 2nd year of life to prevent infections by viral and bacterial pathogens. Despite high vaccine compliance, outbreaks of vaccine-preventable infections are occurring worldwide. These data strongly argue for an improved understanding of the immune responses of neonates, infants and young children to vaccine antigens and further study of the exploitable mechanisms to achieve more robust and prolonged immunity with fewer primary and booster vaccinations in the pediatric population. This review will focus on our recent work involving infant and young child immunity following routine recommended vaccinations. The discussion will address vaccine responses with respect to four areas: (1) systemic antibody responses, (2) memory B-cell generation, (3) CD4 T-cell responses, and (4) APC function.
Keywords: Vaccination, Neonate, Infants, Children, B cells, T cells, Antigen presenting cells, Dendritic cells, immunologic memory, pediatric vaccines, Streptococcus pneumoniae, Haemophilus influenzae, B-cell receptor, MHC II, CD4 T-cells, cytokines, T-cell receptor, Toll-like receptor
Introduction
One of the biggest challenges facing vaccinations is that 19.3 million infants and children throughout the world do not receive the multiple recommended doses of vaccines required to achieve optimal immunity [1–6]. Aside from the many issues facing worldwide vaccination programs, there are environmental and genetic factors that affect the development of the immune system that also contribute to high mortality especially in neonates, infants and young children[3]. We are focusing on the mechanisms involved in the vaccine responses in these youngest of pediatric populations. This review will mainly focus on the work being done in our lab to address this important issue.
Maternal antibody and poor generation of T-cell and B-cell memory in neonates and infants are known to result in inadequate adaptive immunity from vaccinating this population compared to older children and adults [7–20]. Our group has recently identified a subset of infants and young children that fail to generate protective antibody levels to diphtheria (DT), tetanus (TT), pertussis (PT) toxoid, pertussis filamentous hemagglutinin (FHA), and pertussis pertactin (PRN) in DTaP vaccinations, polio serotype 3, and Streptococcus pneumoniae conjugated polysaccharide 23F (Prevnar-CRM) and produce lower geometric mean titers to polio serotypes 1 and 2 and, Streptococcus pneumoniae serotype 14 [21]. However, we did not observe an increase incidence of infections caused by diphtheria, pertussis, tetanus, etc. and reasoned that this could be due to limited-exposure and/or herd immunity. Therefore, we elected to study seasonal influenza infections since they occur as widespread annual community-wide outbreaks. We found that otitis prone, OP, children show inadequate immune responses to influenza vaccination and therefore 10-fold more influenza infections (Verhoeven et al, Vaccine 2014, submitted for publication). These same children have CD4+ T-cell memory recall responses to PT, FHA and PRN that are significantly inferior in quality as compared to adult responses[22]. We are calling these children “low vaccine responders” (LVR), as compared to “normal vaccine responders” (NVR), and have observed that they have features resembling a neonate’s immune system[21–26].
We serendipitously discovered this group of low vaccine responders during our work involving infants and young children prone to recurrent middle ear infections [27–30]. In that research we identified a cohort of young children, 15 (5.9%) of 254, that experienced frequent recurrent middle ear infections, despite individualized care that included tympanocentesis drainage of acute otitis media (AOM) episodes and modification of antibiotic therapy as needed according to the otopathogen isolated and its antibiotic susceptibility [31]. We called these children stringent otitis prone (sOP) due to the stringent requirement of tympanocentesis-proven middle ear infections. Subsequently, we now have over 40 children out of 700 in our prospective study cohort who meet the sOP criteria. We hypothesized and showed that the propensity to recurrent AOM could be attributed to poor adaptive immune responses following infection by the dominant otopathogens Streptococcus pneumoniae and Haemophilus influenzae. Specifically we found low or absent antibody and cellular responses to vaccine candidate antigens PhtD, PhtE, Ply and LytB but less so to PcpA of Streptococcus pneumoniae [24, 27] and to protein D and OMP26 but less so to P6 of Haemophilus influenzae [28, 29]. Also, the children exhibited poor antigen-specific memory T-cell responses to Streptococcus pneumoniae and Haemophilus influenzae antigens, although they responded normally to Staphylococcal enterotoxin B, suggesting the primary immune defect might involve multiple factors such as poor antigen presenting cell (APC) function, altered innate responses or lower toll-like receptor expression [22, 23, 26, 32, 33].
Display of similar immune dysfunction in neonates, infants and young children following vaccination suggests the possibility of involvement of common cell types and mechanisms. Through studying dynamic differences in immune responses over time a better understanding of the state of flux of the immune response should be attainable as neonates and infants rapidly mature from the neonatal regulated state to a metered inflammatory phenotype to protect from disease but limit immunopathology.
Systemic antibody responses
Vaccination produces protective benefits primarily by induction of systemic antibodies [34–38]. Neonates, infants and young children produce lower vaccine-specific IgG serum titers than older children or adults to most vaccines[39].
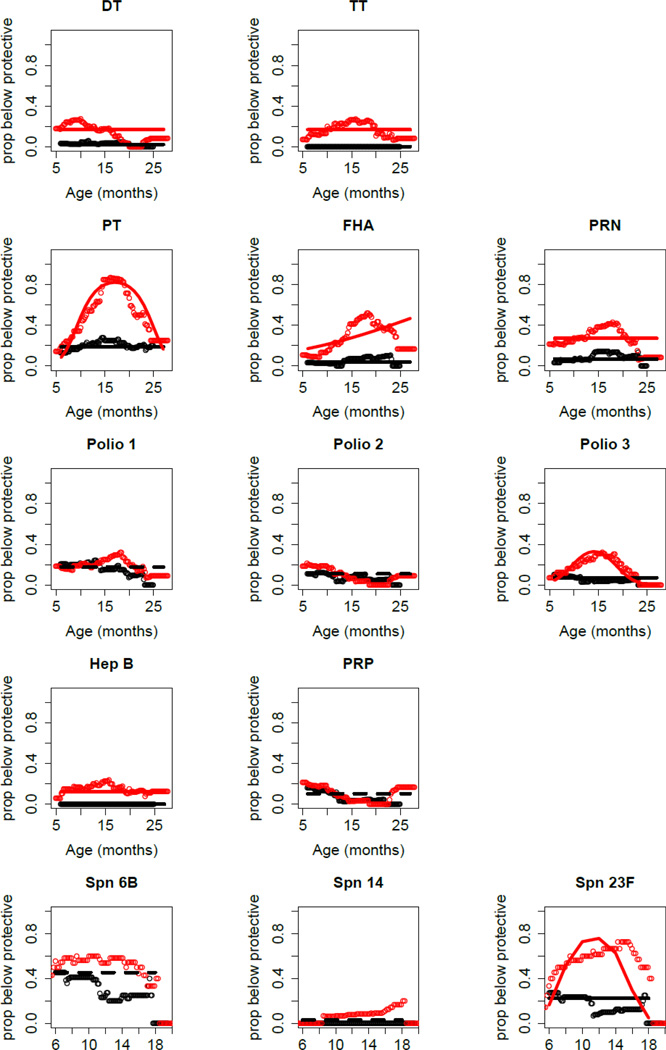
In Figure 1 changes in pediatric vaccine antibody titers over time for 68 age-matched infants and young children from age 6 to 30 months is shown. LVRs (red) selected from a cohort of sOP children and normal vaccine responders (black) selected from a cohort of non-otitis prone children are shown. The nadir of low titers at age 9–15 months old is seen, with improvement after first boosters (measured at 24 months), varying among vaccines. From the results we established an operational classification of children as normal vaccine responder when protective antibody levels to >80% of recommended vaccine antigens tested is achieved. A LVR would be an infant/child with below protective antibody titers to >50% of recommended vaccines tested [40].
Figure 1. Proportion of age-matched sOP children (n=34; red color) and non-sOP children (n=34; black color) with antibody protective levels plotted against age of the child.
sOP children more frequently had nonprotective levels of antibody but no time gradient for DT, TT, PRN and HepB. The sOP children more frequently had nonprotective levels of antibody for PT, FHA, Polio 3 and Spn 23F, but the group effect varied with age. sOP and non-sOP children responded similarly to Polio 1, Polio 2, PRP, Spn 6B and Spn 14. Solid lines: Generalized estimating equations (GEEs) were used to fit the statistical models as previously described[21].
We have also analyzed differences in immune response to influenza vaccination and occurrence of infection in LVRs. In that study we found plasma IgG responses to purified hemagglutinin HA1 or HA3 did not correlate with failure to protect against influenza infection. Instead it was the quality of the antibody as determined by hemagglutination inhibition titers and viral neutralizing antibody titers that identified bona fide LVRs who more frequently contracted influenza infection (Verhoeven et al Vaccine 2014, submitted We have also studied immune responses to RSV. sOP children who are LVR, experience higher RSV viral burdens, lower RSV-specific IgG and neutralizing antibody levels that correlate with diminished T-cell responses to RSV. (Verhoeven et al Clin Inf Dis 2014, revision submitted). In addition, these LVR children infected with RSV show lower expression of TLR7 on isolated APCs and lower level of activated HLA-DR expression on B-cells infected with RSV.
Memory B-cell generation
The ability of B-cells to proliferate and differentiate into memory and plasma cells influences the levels of protective antibodies. Infants and young children can elicit adult-like antibody avidity profiles after early-life immunization with protein vaccines[41]. But the underdeveloped B-cell repertoire and the absence of previous antigenic exposure leads to lower level of protective antibody [42].
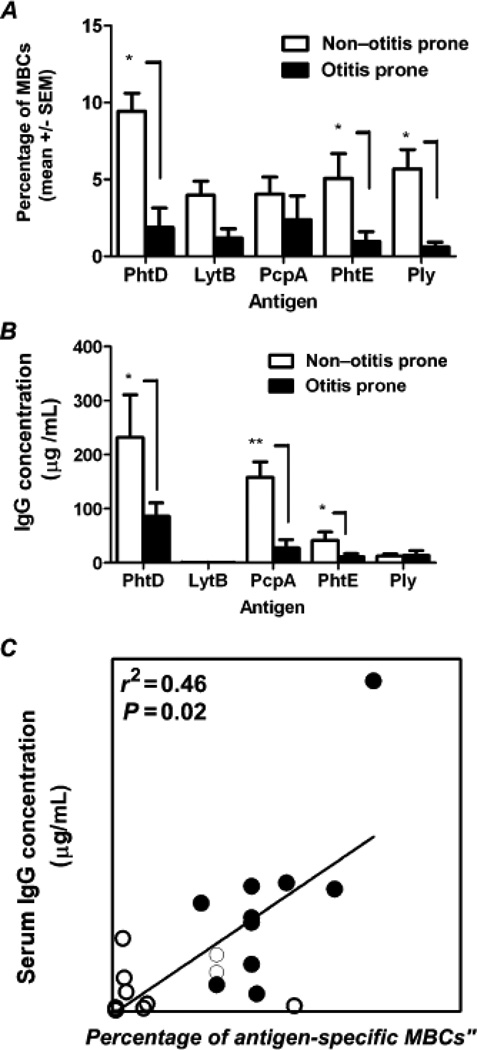
We have recently studied antigen-specific memory B-cells [24] to provide a more precise understanding of dysfunctional mechanism(s) leading to reduced B-cell maturation to IgG- secreting plasma cells in infants and young children. We found that B-cell frequencies in the peripheral circulation correlated with serum levels of antigen-specific Ig responses of sOP infants and young children who were LVRs (Fig. 2) resembled B-cells of neonates. Immaturity in the neonatal B-cell repertoire may include a reduced strength of B-cell receptor (BCR) signaling, under-expressed co-stimulatory receptors and lower activation signals [43, 44].
Figure 2. Correlation of antigen-specific memory B cells and serum IgG titers in LVR versus NOP children.
(A) Frequencies of antigen-specific memory B cells (MBCs). (B) Serum immunoglobulin G (IgG) titers to 5 pneumococcal protein antigens. (C) Correlation between PhtD-specific serum antibody titers and PhtD-specific percentages of antigen-specific MBCs. Data are for 10 LVRsOP children and 12 non–otitis prone (NOP) children. Open circles denote otitis prone and closed circles denote non–otitis prone. *P = 0.05; ** P = 0.005.
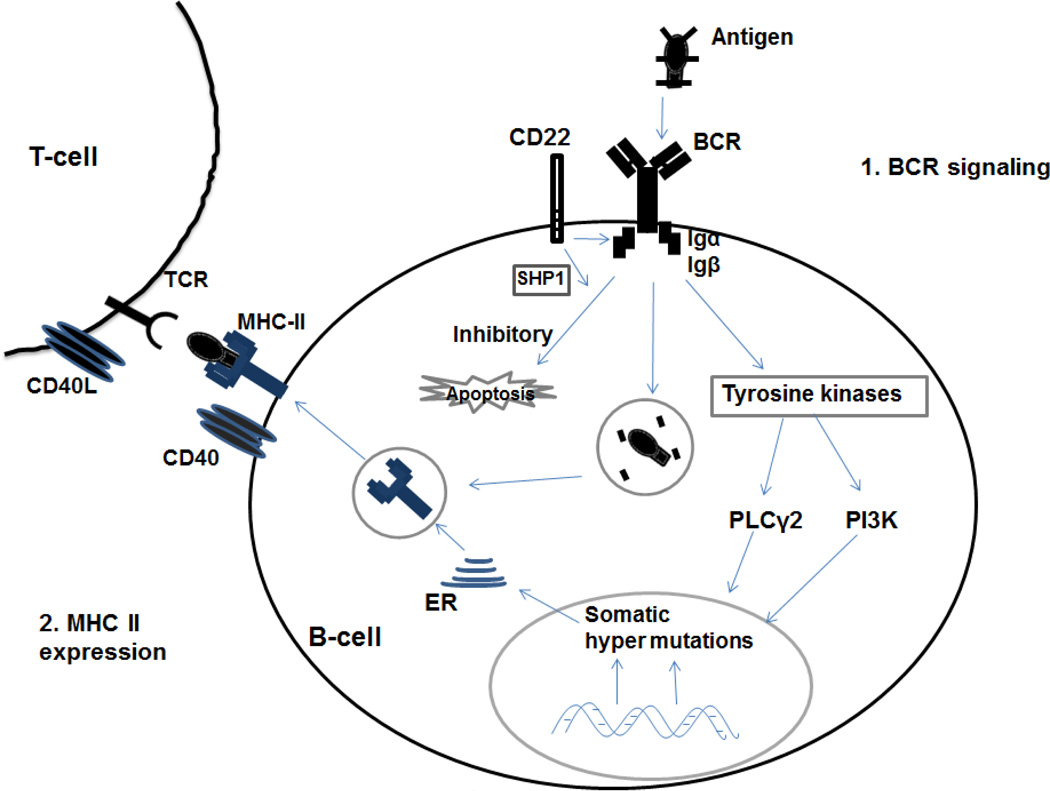
Two mechanisms in B cells likely account for much of the immune dysfunction in neonatal, infant and young children: inadequate B-cell receptor (BCR) signaling and lower levels of MHC class II (MHC II) expression (Fig. 3). CD22 is a surface exposed molecule that affects apoptosis and BCR signaling[44, 45]. Neonatal B cells or cord blood lymphocytes show differential expression of CD22 depending upon antigen stimulation compared to adult B cells, resulting in either apoptosis or impaired B cell activation and differentiation[44]. Expression of MHC II molecules and their ability to present processed antigenic peptides to T helper cells play an important role in B-cell activation, proliferation, Ig isotype switching and somatic hypermutation[46, 47]. Neonatal B-cells express lower levels of MHC II are less effective in antigen processing and presentation to T-cells. CD40 is another important receptor on B cells that interact with CD154 (CD40L), a co-stimulatory molecule on T cells that also regulates B-cell function[48]. The neonatal immune system has showed an immaturity at CD40-CD40L in the T-cell interaction with B cells and monocytes[49]. Lower levels of CD154 on T-cells also results in lower expression of signaling cascade proteins and lower expression of cytokine genes[50].
Figure 3. B cell receives proliferation and differentiation signals through the B cell receptor (BCR) and the co-receptors Igα and Igβ.
Following antigen binding to BCR, the co-receptors Igα and Igβ signals to activate downstream molecules phospholipase Cγ2 (PLCγ2) and the phosphoinositide 3-kinase (PI3K) pathway. CD22 is a B cell surface glycoprotein that can negatively regulate BCR signaling. Activation of BCR leads to phosphorylation of CD22, resulting in recruitment of SHP-1 to CD22 by Lyn which finally induces the apoptosis pathway. Defects in PLCγ2 or PI3K impair BCR signaling, class-switching responses and memory B cell formation. CD40 is a co-stimulatory marker expressed on B cells and CD40L (CD154) is the ligand expressed on T cells. CD40-CD40L interaction plays a critical role in T cell-dependent B cell antibody response.
Follicular T-helper, Tfh, cell is another T-helper subset to help B cells differentiate into plasma cells and memory cells[51]. We are interested in the B-cell memory percentages in normal and low vaccine responding infants and whether peripheral blood levels of Tfh cells may reflect the vaccine responses. Preliminary data in our laboratory suggests that circulating blood levels of TFH cells in 6-month and 12-month old infants are significantly lower compared to older children and adults. We also found that the quantity of TFH cells in tonsils increases with age, as would be expected with “outgrowing” the immunological delay of CD4 T-cell activation (unpublished results). Importantly, lower percentages of TFH cells in tonsils of children who are LVRs were measured compared to age-matched normal vaccine responders (data not shown).
CD4 T-cell responses
Although there are many different T-cell cell types, CD8 and CD4 are the main T-cells in adaptive immunity responsible for T-cell killing of infected cells and B-cell help. Function of CD8 T-cells among neonates, infants and young children is similar to older children and adults[52]. However, there are phenotypic changes of CD8 T-cells depending on the vaccine and the age of the person[53] along with neonates, infants and young children having defective DC-induced IL-12 secretion[54].
CD4 T-cells can be broadly divided into different subsets based on their cytokine secretion and function[55–59]. CD4 T-cells that produce mainly IFN-γ, TNFα and IL-2 are designated Th-1 while those that produce predominantly IL-4, IL-5, and IL-10 are designated Th-2. Neonates, infants and young children polarize their vaccine-mediated CD4 T-cell responses to Th-2 whereas adults have a more balanced Th-1/Th-2 response.[3, 60–63] Differences in CD4 T-cell responses to vaccination could dramatically affect the quality of B-cell responses after vaccination[39, 64, 65]. For instance, low vaccine responses in neonates, infants and young children are thought to be due to CD4 T-cell dysfunction [22–24, 66]. Their CD4 T-cells may fail to stimulate antibody-secreting B-cells because of intrinsic T cell-related mechanisms and/or extrinsic effects of deficient APC function[16, 23, 25, 67] failing to properly stimulate naïve CD4 T-cells [66].
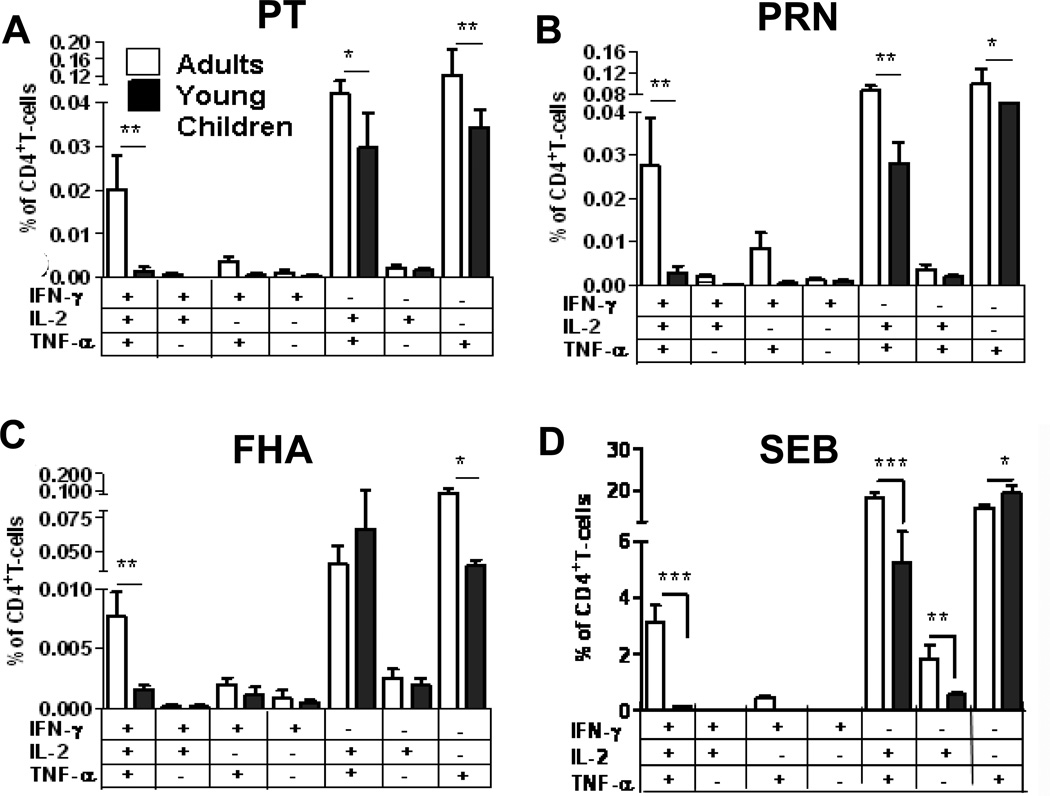
The neonatal T-cell response is marked by poor IFN-γ production, and vaccine-specific responses characterized by a generalized bias towards IL-4 production[3, 68]. We have recently determined that CD4 T-cell responses in normal infants and young children after Staphylococcal enterotoxin B (SEB) mitogenic stimulation exhibit lower polyfunctional cytokine responses as compared to adults (Fig. 4D)[25] and pattern an immunological maturation with age with respect to increasing IFN-γ production (data not shown).
Figure 4. CD4 T-cell responses in young children and adults.
We assessed the poly-functional cytokine potential CD4 T-cells (PBMC) responses in young children (<12 months, n=20) and adults (mean age 30yr, n=12) to (A) PT, (B) PRN, (C) FHA, or with (D) SEB stimulation. P values *<0.05 and ***<0.005. (Figure is a regraph of figure 3 in reference[25])
To assess the differences in T-cell responses to acellular pertussis vaccination in terms of quality and quantity of the response persisting after vaccination, we stimulated peripheral blood mononuclear cells (PBMCs) from infants, children and adults who were recently vaccinated with DTaP or Tdap, respectively and found lower polyfunctional responses in infants and children compared to adults (Fig. 4A–C)[25]. We also observed lower TNFα/IFN-γ responses to acellular pertussis antigens and SEB in infants (data not shown) suggesting that the strength of the priming TCR signal or homeostatic maintenance of memory subsets after polarization may impact the quality of the recall response. Our results demonstrate a divergence in circulating T-cell memory percentages for vaccine antigens in infants and children compared to adults[25].
Epigenetic modulation of effector cytokines may inhibit CD4 T-cell responses in neonates but whether this persists into early childhood is not known [69, 70]. Low IFN-γ or IL-4 antigen specific recall responses to vaccine antigens persist until at least 1 year of age even with in vitro APC supplementation, suggesting that intrinsic blocks to cytokine responses such as epigenetic regulation of pro-inflammatory responses may persist past the neonatal stage[17]. We have shown that the percentages of CD69+ CD4 T-cells in recall assays to be similar in infants and children compared to adults suggesting that differences in APC stimulatory function may not fully account for diminished normal CD4 T-cell responses in infants and children[25]. The data suggest that CD4 T-cells in infants and children could have intrinsic blocks to robust IFN-γ or IL-4 secretion as compared to older children or adults and we seek to better understand the role of diminished production of these effector cytokines as they relate to regulating vaccine specific memory quality.
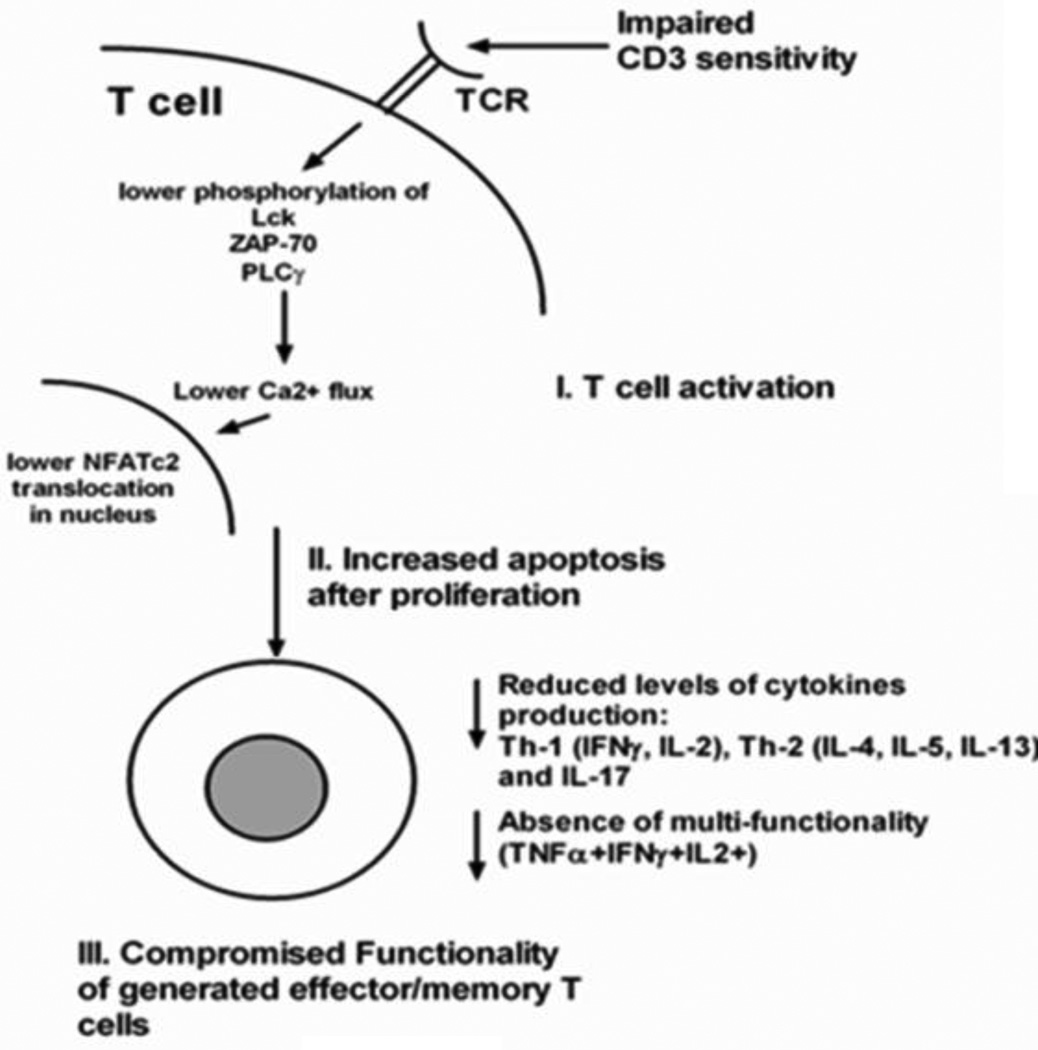
Compared to adult CD4 T-cells, neonatal CD4 T-cells show reduced expression of several transcription factors including signal transducers and activators of transcription factor 4 (STAT4), t-bet, and c-maf during primary stimulation and are less responsive to antigen presentation[50]. Three mechanisms likely predominate to explain the low vaccine-specific memory CD4 T-cells in neonates, infants and young children: 1) Impaired TCR stimulation; 2) Increased apoptosis after proliferation; and 3) Compromised functionality of generated effector and/or memory cells (Fig 5).
Figure 5. Shortcomings of neonatal and young child T-cells.
Various stages in neonatal and young children T-cell activation starting with T-cell receptor (TCR)-mediated activation. T-cells have reduced CD3 sensitivity and lower phosphorylation of several TCR-signaling molecules resulting in low Ca2+ flux induction and reduced NFATc2 translocation in the nucleus. After activation, neonates and young children T-cells demonstrate comparable levels of cellular proliferation compared to adult T-cells. However, neonatal and young children T-cells are prone to apoptosis post-activation. The limited frequencies of activated T-cells that result in effector/memory generation do not produce optimal levels of cytokines and lack multi-functionality.
Impairment of CD4 T-cell activation could be due to a defect in the TCR signaling pathway that activates protein phosphorylation and activation of phospholipases[71]. Experiments with umbilical cord blood T-cells have shown that antigen stimulation does not influence TCR-CD3 surface expression, but initializes TCR internalization and downstream signaling including activation of phospholipase C-γ (PLC) by Src family kinases, Syk, Lck, ZAP70 and Fyn[72]. The low expression of PLC and Syc, Lck and Fyn constitute molecular defects in the signaling pathway that activates the promoters of various cytokines including IL-2[73].
Previous studies have demonstrated that naïve neonatal CD4 T cells also undergo apoptosis in response to primary TCR-mediated stimulation and also after activation with anti-CD3[74]. This was observed due to the high caspase-mediated cell death as a control mechanism of antigen-independent expansion by cord blood RTEs [75]. However, under appropriate stimulatory conditions and stimulating with anti-CD3/anti-CD28, naïve umbilical cord blood CD4 T-cells can generate competent Th effector cells [17]. IL-12, IFN-γ and IL-4 induce differentiation in newly activated naïve CD4 T-cells and help to stabilize the subset polarity through enforcement of either T-bet or Gata-3 expression[76]. We have data from infants and children that shows low IFN-γ production upon stimulation of memory CD4 T-cells with vaccine antigens[25]. This could be due to an impaired ability to differentiate in response to differentiating signals from cytokines and/or DCs.
Experiments with cord blood cells has shown that neonates have very low frequency Th17 cells due to their intrinsic defect of downstream signaling of transcription factor RORC (Retinoid acid–related Orphan Receptor C)[77]. We have observed delayed generation of IL-17 responses in PBMCs in infants and children to bacterial, vaccine antigens, and SEB stimulation as compared to older children and adults, suggesting differential regulation of this T-cell subtype. Our cumulative studies to date indicate that infants and children have CD4 T-cell dysfunction that extends to Th-17 cells (as compared to adults) whether the type of antigenic exposure is by vaccination or naturally (unpublished data).
APC function
The innate immunity is key to protection against infection in early life but are deficient in newborn and preterm infants[78]. Human myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) are the major producers of IL-12p70 and IFN-α/β respectively[79]. IFN-α/β plays a crucial role in anti-viral immunity and assists the Th1 type immune response. IL-12 plays a major function in co-stimulating Th1 immunity[68]. Available evidence suggests that DC–T-cell interactions are the key determinants of induction of adult-like protective Th1 responses in children[17, 33, 79]. Considering the minimal effects of maternal antibodies on T-cell responses, deficiencies in numbers of DCs and their functional incompetence are potentially the crucial factors limiting the capacity to generate T effector-memory responses in early childhood[15, 79, 80]. Despite the suboptimal levels in neonates, DC derived cytokine IL-12 has been shown to modulate antigen specific T-cell responses by inducing T-cell: IFN-γ secretion[17]. IL-12 also mediates the DC directed T-cell differentiation into T follicular helper cells (TFH) which in turn will generate long term B cell memory response[81]. Therefore, suboptimal activation and functional immaturity of DCs in low vaccine responding infants and young children could directly contribute to defective T-cell function and B-cell memory.
Maturational changes in populations of APCs could have an impact on vaccine-specific memory populations and responses to boosters. Dendritic cells (DC), the most potent antigen presenting cells, are the primary activators of naïve T-cells. DCs and naïve T-cell crosstalk provides the opportunity for antigen recognition through T-cell receptor (TCR) interactions with peptide-MHC complexes at the DC surface[82, 83]. TCRs of naïve CD4 T-cells recognize peptides in context to MHC II[84]. After antigen-uptake, DCs mature and up-regulate several accessory molecules, such as MHC II and CD80 and CD86 (costimulatory markers for CD28 on T-cells) that are required to efficiently prime naïve CD4 T-cells[83, 85, 86] and the strength of the TCR signaling regulates Th1/Th2 polarization[84]. The maturation of naïve CD4 T-cell priming into effector/memory cells is also dependent on the cytokine milieu provided by matured DCs and results from toll like receptor (TLR) triggering. TLRs are the main pathogen recognition receptors that are expressed on the cell surface and within intracellular endosomes. Activation of the TLR results in an internal cascade of signaling events that result in gene expression, cytokine/chemokine secretion and cellular activation[87, 88]. Since DCs link the innate immune system to the adaptive immune system by pathogen associated molecular patterns (PAMPs) and T-cell activation (TLRs, cytokines, MHC), adequate priming of naïve CD4 T-cells and generation of effective memory T-cells may be compromised in neonates, infants and young children by inefficient APC function.
TLR mediated activation of DCs leads to maturation and induction of secreted cytokines. Neonatal DCs show an immature phenotype and are functionally impaired in secreting these essential cytokines in response to various TLR agonists[88, 89]. Moreover, TLR expression plays a role in initiating immune activation and differences in TLR expression have been demonstrated to be a component of immunological maturation and adequate immune responses[90]. Immature neonatal innate immunity is associated with poor sepsis outcome [78] but this can be reversed with TLR agonists in murine studies [91] implying that the capacity to respond to TLR stimulation may be a critical factor in low vaccine responding neonates, infants and children. TLR ligation by vaccine antigens, such as the yellow fever vaccine YF-17D, or TLR ligands, promotes cytokine secretion from APCs that in turn leads to T and B-cell activation [92–94]. TLR4 ligation also plays an important role in whole-cell pertussis-mediated protection[95]. However, strong TLR stimulation during early DC differentiation can also lead to tolerogenic APCs[96]. Therefore, it is important to explore TLR expression from a vaccine response perspective (as long-term immunity may be impaired) that could also model neonatal-like APC responses to vaccination.
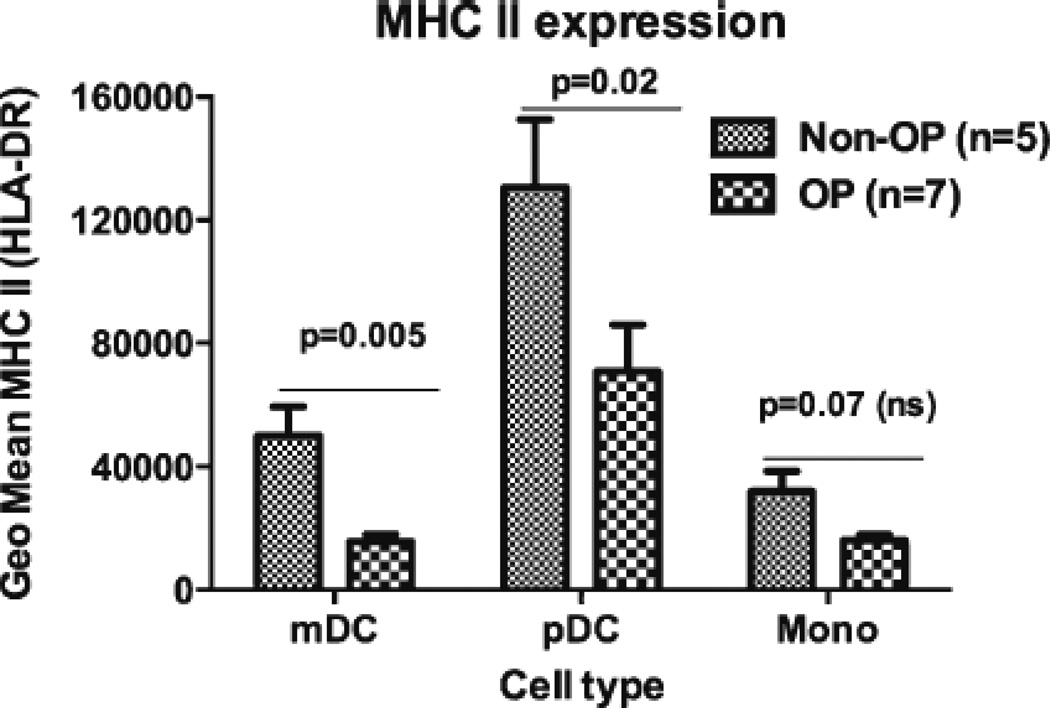
The failure to outgrow the neonatal immune state among low vaccine responding children may be marked by limited APC function[68]. Low CD28 stimulation by CD80/CD86 on APCs drives neonatal CD4 T-cells into singly functional IL-2low producers[8]. We recently found that APCs of low vaccine responding infants modeled a neonatal state in at least two aspects. Specifically, we found lower expression of MHC II, as shown in Figure 6[26], as well as lower levels of CD80/86 also seen by others [79, 97, 98]. We also found significantly impaired uptake of OVA-FITC in low vaccine responding infants’ peripheral blood mDCs and monocytes but not pDCs along with lower APC percentages (data not shown). These data suggest that CD4 T-cell dysfunction in low vaccine responders may be compounded by impaired APCs antigen processing and diminished presentation.
Figure 6. MHC II expression levels on APCs of otitis-prone (OP) and non-otitis-prone (NOP) children.
The MHC II levels of the different cell types from peripheral blood were measured using flow cytometry. mDC, myeloid dendritic cells; pDC, plasmacytoid dendritic cells; Mono, monocytes.
Summary
Our working hypothesis is that LVR children display immune dysfunction and maturational delay similar to neonates and that studying these children and neonates will provide knowledge as to the mechanistic cause of the immune dysfunction in these vulnerable populations eventually resulting in improvements in vaccine strategy. We and others have identified a number of immune targets, such as reduced CD4 T-cell memory and APC function. We see a path forward using TLR agonists or novel adjuvants to stimulate the immune response and overcome poor protective responses in neonates and young children.
Conclusion
Although challenging because of the age of the subjects, more studies to further understand immune dysfunction in neonates, infants and young children are needed. The quality and quantity of systemic and mucosal antibody and memory B-cell generation, adaptive CD4 T-cell vaccine-specific responses and memory recall, and APC – B-cell and CD4 T-cell interactions following recommended vaccinations needs to be more thoroughly characterized. Determining the mechanisms within each cell line responsible for immune maturational delays compared to older children and adults that could be overcome by a more rational approach to modifications in pediatric vaccines such as the addition of novel adjuvants is an achievable goal in the near future.
Finally, a number of groups are using systems biology to identify signatures that can predict immune responses to human vaccines[93, 99, 100] We have initiated experiments to look at gene expression differences among children that suffer from recurrent AOM[101, 102] and are moving to use a systems biology approach to understand the immune defects in children with a neonatal-like immune response to vaccines. The future looks bright that we will able to better understand the immune mechanisms such that newer vaccines will be developed with the goal of being able to offer broader protection against infection to neonates, infants and young children.
Highlights.
Neonates and infants produce lower vaccine-specific IgG antibody than older children to vaccines.
T-cell and B-cell memory in neonates and infants is inadequate for prolonged protection.
Antigen presenting cells function inefficiently in processing vaccines in neonates and infatnts.
Low vaccine responders among infants may have a prolonged neonatal-like immune profile.
Acknowledgments
This work was supported by the U.S. NIH NIDCD RO1 08671. Drs. Robert Zagursky, Naveen Surendran and Saleem Basha, Rochester General Hospital Research Institute, provided useful discussion in preparation of this manuscript.
Abbreviations
- DT
diphtheria
- TT
tetanus
- PT
pertussis toxoid
- FHA
pertussis filamentous hemagglutinin
- PRN
pertussis pertactin
- pDC
plasmacytoid DC
- mDC
myeloid DC
- sOP
stringent otitis prone
- LVR
low vaccine responder
- NOP
non-otitis prone
- APC
antigen presenting cell
- PBMC
peripheral blood mononuclear cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Casey JR, Pichichero ME. Acellular pertussis vaccine safety and efficacy in children, adolescents and adults. Drugs. 2005;65:1367–1389. doi: 10.2165/00003495-200565100-00005. [DOI] [PubMed] [Google Scholar]
- 2.Fay KE, Lai J, Bocchini JA., Jr Update on childhood and adolescent immunizations: selected review of US recommendations and literature: part 1. CurrOpinPediatr. 2011;23:460–469. doi: 10.1097/MOP.0b013e32834877f1. [DOI] [PubMed] [Google Scholar]
- 3.PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, et al. Challenges in infant immunity: implications for responses to infection and vaccines. NatImmunol. 2011;12:189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 4.Rainey JJ, Watkins M, Ryman TK, Sandhu P, Bo A, Banerjee K. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: findings from a systematic review of the published literature, 1999-2009. Vaccine. 2011;29:8215–8221. doi: 10.1016/j.vaccine.2011.08.096. [DOI] [PubMed] [Google Scholar]
- 5.MacNeil A, Dietz V, Cherian T. Vaccine preventable diseases: Time to re-examine global surveillance data? Vaccine. 2014 doi: 10.1016/j.vaccine.2014.02.067. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. World Health Organization Global vaccine action plan 2011-2020. Geneva: World Health Organization; 2013. [Google Scholar]
- 7.Barrios C, Brawand P, Berney M, Brandt C, Lambert PH, Siegrist CA. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol. 1996;26:1489–1496. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- 8.Delespesse G, Yang LP, Ohshima Y, Demeure C, Shu U, Byun DG, et al. Maturation of human neonatal CD4+ and CD8+ T lymphocytes into Th1/Th2 effectors. Vaccine. 1998;16:1415–1419. doi: 10.1016/s0264-410x(98)00101-7. [DOI] [PubMed] [Google Scholar]
- 9.Gans H, DeHovitz R, Forghani B, Beeler J, Maldonado Y, Arvin AM. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine. 2003;21:3398–3405. doi: 10.1016/s0264-410x(03)00341-4. [DOI] [PubMed] [Google Scholar]
- 10.Jonsdottir I. Maturation of mucosal immune responses and influence of maternal antibodies. J Comp Pathol. 2007;137(Suppl 1):S20–S26. doi: 10.1016/j.jcpa.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, et al. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–440. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 12.Petty RE, Hunt DW. Neonatal dendritic cells. Vaccine. 1998;16:1378–1382. doi: 10.1016/s0264-410x(98)00095-4. [DOI] [PubMed] [Google Scholar]
- 13.Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr Res. 2009;65:98R–105R. doi: 10.1203/PDR.0b013e31819f195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prescott SL, Taylor A, King B, Dunstan J, Upham JW, Thornton CA, et al. Neonatal interleukin-12 capacity is associated with variations in allergen-specific immune responses in the neonatal and postnatal periods. Clin Exp Allergy. 2003;33:566–572. doi: 10.1046/j.1365-2222.2003.01659.x. [DOI] [PubMed] [Google Scholar]
- 15.Siegrist CA. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine. 2003;21:3406–3412. doi: 10.1016/s0264-410x(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 16.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 17.Upham JW, Rate A, Rowe J, Kusel M, Sly PD, Holt PG. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun. 2006;74:1106–1112. doi: 10.1128/IAI.74.2.1106-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White OJ, Rowe J, Richmond P, Marshall H, McIntyre P, Wood N, et al. Th2-polarisation of cellular immune memory to neonatal pertussis vaccination. Vaccine. 2010;28:2648–2652. doi: 10.1016/j.vaccine.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Wilson CB, Kollmann TR. Induction of antigen-specific immunity in human neonates and infants. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:183–195. doi: 10.1159/000113493. [DOI] [PubMed] [Google Scholar]
- 20.Zepp F, Knuf M, Habermehl P, Schmitt JH, Rebsch C, Schmidtke P, et al. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infect Immun. 1996;64:4078–4084. doi: 10.1128/iai.64.10.4078-4084.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pichichero ME, Casey JR, Almudevar A. Nonprotective responses to pediatric vaccines occur in children who are otitis prone. Pediatr Infect Dis J. 2013;32:1163–1168. doi: 10.1097/INF.0b013e31829e887e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma SK, Pichichero ME. Deficiencies in the CD4+T-Helper Cell Arm of the Immune System of Neonates and Young Children. Pediatr Allergy Immunol Pulmonol. 2013;26:4–10. doi: 10.1089/ped.2012.0181. [DOI] [PubMed] [Google Scholar]
- 23.Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis. 2011;204:645–653. doi: 10.1093/infdis/jir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma SK, Casey JR, Pichichero ME. Reduced serum IgG responses to pneumococcal antigens in otitis-prone children may be due to poor memory B-cell generation. J Infect Dis. 2012;205:1225–1229. doi: 10.1093/infdis/jis179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma SK, Pichichero ME. Functional deficits of pertussis-specific CD4+ T cells in infants compared to adults following DTaP vaccination. Clin Exp Immunol. 2012;169:281–291. doi: 10.1111/j.1365-2249.2012.04613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma SK, Pichichero ME. Cellular immune response in young children accounts for recurrent acute otitis media. Current allergy and asthma reports. 2013;13:495–500. doi: 10.1007/s11882-013-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr Infect Dis J. 2011;30:645–650. doi: 10.1097/INF.0b013e31821c2d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine. 2011;29:1023–1028. doi: 10.1016/j.vaccine.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan MN, Kaur R, Pichichero ME. Bactericidal antibody response against P6, protein D, and OMP26 of nontypeable Haemophilus influenzae after acute otitis media in otitis-prone children. FEMS ImmunolMedMicrobiol. 2012;65:439–447. doi: 10.1111/j.1574-695X.2012.00967.x. [DOI] [PubMed] [Google Scholar]
- 30.Verhoeven D, Nesselbush M, Pichichero ME. Lower nasopharyngeal epithelial cell repair and diminished innate inflammation responses contribute to the onset of acute otitis media in otitis-prone children. Med Microbiol Immunol. 2013;202:295–302. doi: 10.1007/s00430-013-0293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichichero ME, Casey JR, Almudevar A. Reducing the Frequency of Acute Otitis Media by Individualized Care. Pediatr Infect Dis J. 2013;32:473–478. doi: 10.1097/INF.0b013e3182862b57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma SK, Roumanes D, Almudervar A, Mosmann TR, Pichichero ME. CD4+ T-cell Responses Among Adults and Young Children In Response to Streptococcus pneumoniae and Haemophilus influenzae Vaccine Candidate Protein Antigens. Boston, Massachusettes: Federation of Clinical Immunology Societies; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velilla PA, Rugeles MT, Chougnet CA. Defective antigen-presenting cell function in human neonates. Clin Immunol. 2006;121:251–259. doi: 10.1016/j.clim.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pichichero ME. Booster vaccinations: can immunologic memory outpace disease pathogenesis? Pediatrics. 2009;124:1633–1641. doi: 10.1542/peds.2008-3645. [DOI] [PubMed] [Google Scholar]
- 35.Pichichero ME. Protein carriers of conjugate vaccines: Characteristics, development and clinical trials. Hum Vaccin Immunother. 2013;9 doi: 10.4161/hv.26109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufmann SH, Juliana McElrath M, Lewis DJ, Del Giudice G. Challenges and responses in human vaccine development. Curr Opin Immunol. 2014;28c:18–26. doi: 10.1016/j.coi.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Koff WC, Burton DR, Johnson PR, Walker BD, King CR, Nabel GJ, et al. Accelerating next-generation vaccine development for global disease prevention. Science. 2013;340:1232910. doi: 10.1126/science.1232910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis. 2013;56:1458–1465. doi: 10.1093/cid/cit048. [DOI] [PubMed] [Google Scholar]
- 39.Bonhoeffer J, Siegrist CA, Heath PT. Immunisation of premature infants. Arch Dis Child. 2006;91:929–935. doi: 10.1136/adc.2005.086306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pichichero ME, Casey JR, Almudervar A. Non- Protective Responses to Pediatric Vaccines Occur in Children Who are Otitis Prone. Boston, Massachusetts: Federation of Clinical Immunology Societies; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schallert N, Pihlgren M, Kovarik J, Roduit C, Tougne C, Bozzotti P, et al. Generation of adult-like antibody avidity profiles after early-life immunization with protein vaccines. Eur J Immunol. 2002;32:752–760. doi: 10.1002/1521-4141(200203)32:3<752::AID-IMMU752>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 42.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 43.Kaur K, Chowdhury S, Greenspan NS, Schreiber JR. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood. 2007;110:2948–2954. doi: 10.1182/blood-2007-01-069245. [DOI] [PubMed] [Google Scholar]
- 44.Viemann D, Schlenke P, Hammers HJ, Kirchner H, Kruse A. Differential expression of the B cell-restricted molecule CD22 on neonatal B lymphocytes depending upon antigen stimulation. Eur J Immunol. 2000;30:550–559. doi: 10.1002/1521-4141(200002)30:2<550::AID-IMMU550>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 45.Somani AK, Yuen K, Xu F, Zhang J, Branch DR, Siminovitch KA. The SH2 domain containing tyrosine phosphatase-1 down-regulates activation of Lyn and Lyn-induced tyrosine phosphorylation of the CD19 receptor in B cells. J Biol Chem. 2001;276:1938–1944. doi: 10.1074/jbc.M006820200. [DOI] [PubMed] [Google Scholar]
- 46.Avalos AM, Ploegh HL. Early BCR Events and Antigen Capture, Processing, and Loading on MHC Class II on B Cells. Front Immunol. 2014;5:92. doi: 10.3389/fimmu.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuseff MI, Pierobon P, Reversat A, Lennon-Dumenil AM. How B cells capture, process and present antigens: a crucial role for cell polarity. Nat Rev Immunol. 2013;13:475–486. doi: 10.1038/nri3469. [DOI] [PubMed] [Google Scholar]
- 48.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 49.Han P, McDonald T, Hodge G. Potential immaturity of the T-cell and antigen-presenting cell interaction in cord blood with particular emphasis on the CD40-CD40 ligand costimulatory pathway. Immunology. 2004;113:26–34. doi: 10.1111/j.1365-2567.2004.01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaminski BA, Kadereit S, Miller RE, Leahy P, Stein KR, Topa DA, et al. Reduced expression of NFAT-associated genes in UCB versus adult CD4+ T lymphocytes during primary stimulation. Blood. 2003;102:4608–4617. doi: 10.1182/blood-2003-05-1732. [DOI] [PubMed] [Google Scholar]
- 51.Mastelic B, Kamath AT, Fontannaz P, Tougne C, Rochat AF, Belnoue E, et al. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J Immunol. 2012;189:5764–5772. doi: 10.4049/jimmunol.1201143. [DOI] [PubMed] [Google Scholar]
- 52.Holt PG. Functionally mature virus-specific CD8(+) T memory cells in congenitally infected newborns: proof of principle for neonatal vaccination? J Clin Invest. 2003;111:1645–1647. doi: 10.1172/JCI18805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He XS, Holmes TH, Mahmood K, Kemble GW, Dekker CL, Arvin AM, et al. Phenotypic changes in influenza-specific CD8+ T cells after immunization of children and adults with influenza vaccines. J Infect Dis. 2008;197:803–811. doi: 10.1086/528804. [DOI] [PubMed] [Google Scholar]
- 54.McCarron MJ, Reen DJ. Neonatal CD8+ T-cell differentiation is dependent on interleukin-12. Hum Immunol. 2010;71:1172–1179. doi: 10.1016/j.humimm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan MH. Th9 cells: differentiation and disease. Immunol Rev. 2013;252:104–115. doi: 10.1111/imr.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Basu R, O'Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramiscal RR, Vinuesa CG. T-cell subsets in the germinal center. Immunol Rev. 2013;252:146–155. doi: 10.1111/imr.12031. [DOI] [PubMed] [Google Scholar]
- 59.Kara EE, Comerford I, Fenix KA, Bastow CR, Gregor CE, McKenzie DR, et al. Tailored immune responses: novel effector helper T cell subsets in protective immunity. PLoS Pathog. 2014;10:e1003905. doi: 10.1371/journal.ppat.1003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am J Perinatol. 2013;30:105–112. doi: 10.1055/s-0032-1333412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, et al. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133:228–237. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marodi L. Innate cellular immune responses in newborns. Clin Immunol. 2006;118:137–144. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 63.Vosters O, Lombard C, André F, Sana G, Sokal EM, Smets F. The interferon-alpha and interleukin-10 responses in neonates differ from adults, and their production remains partial throughout the first 18 months of life. Clin Exp Immunol. 2010;162:494–499. doi: 10.1111/j.1365-2249.2010.04267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klein NP, Gans HA, Sung P, Yasukawa LL, Johnson J, Sarafanov A, et al. Preterm infants' T cell responses to inactivated poliovirus vaccine. J Infect Dis. 2010;201:214–222. doi: 10.1086/649590. [DOI] [PubMed] [Google Scholar]
- 65.Klein Klouwenberg P, Bont L. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clin Dev Immunol. 2008;2008:628963. doi: 10.1155/2008/628963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma SK, Roumanes D, Almudevar A, Mosmann TR, Pichichero ME. CD4(+) T-cell responses among adults and young children in response to Streptococcus pneumoniae and Haemophilus influenzae vaccine candidate protein antigens. Vaccine. 2013;31:3090–3097. doi: 10.1016/j.vaccine.2013.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 68.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 70.Upham JW, Lee PT, Holt BJ, Heaton T, Prescott SL, Sharp MJ, et al. Development of interleukin-12- producing capacity throughout childhood. Infect Immun. 2002;70:6583–6588. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palin AC, Ramachandran V, Acharya S, Lewis DB. Human neonatal naive CD4+ T cells have enhanced activation-dependent signaling regulated by the microRNA miR-181a. J Immunol. 2013;190:2682–2691. doi: 10.4049/jimmunol.1202534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miscia S, Di Baldassarre A, Sabatino G, Bonvini E, Rana RA, Vitale M, et al. Inefficient phospholipase C activation and reduced Lck expression characterize the signaling defect of umbilical cord T lymphocytes. J Immunol. 1999;163:2416–2424. [PubMed] [Google Scholar]
- 73.Schneider OD, Weiss AA, Miller WE. Pertussis toxin utilizes proximal components of the T-cell receptor complex to initiate signal transduction events in T cells. Infect Immun. 2007;75:4040–4049. doi: 10.1128/IAI.00414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Canto E, Rodriguez-Sanchez JL, Vidal S. Distinctive response of naive lymphocytes from cord blood to primary activation via TCR. J Leukoc Biol. 2003;74:998–1007. doi: 10.1189/jlb.0303098. [DOI] [PubMed] [Google Scholar]
- 75.O'Neill RM, Hassan J, Reen DJ. IL-7-regulated homeostatic maintenance of recent thymic emigrants in association with caspase-mediated cell proliferation and apoptotic cell death. J Immunol. 2003;170:4524–4531. doi: 10.4049/jimmunol.170.9.4524. [DOI] [PubMed] [Google Scholar]
- 76.Smeets RL, Fleuren WW, He X, Vink PM, Wijnands F, Gorecka M, et al. Molecular pathway profiling of T lymphocyte signal transduction pathways; Th1 and Th2 genomic fingerprints are defined by TCR and CD28- mediated signaling. BMC Immunol. 2012;13:12. doi: 10.1186/1471-2172-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Roock S, Stoppelenburg AJ, Scholman R, Hoeks SB, Meerding J, Prakken BJ, et al. Defective TH17 development in human neonatal T cells involves reduced RORC2 mRNA content. J Allergy Clin Immunol. 2013;132:754–756. e3. doi: 10.1016/j.jaci.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 78.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med. 2011;24:25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- 79.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009;39:26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- 80.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, et al. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 82.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 83.Huang G, Wang Y, Chi H. Regulation of TH17 cell differentiation by innate immune signals. Cell Mol Immunol. 2012;9:287–295. doi: 10.1038/cmi.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev. 2013;252:12–23. doi: 10.1111/imr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, et al. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J Immunol. 2001;166:2444–2450. doi: 10.4049/jimmunol.166.4.2444. [DOI] [PubMed] [Google Scholar]
- 87.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 88.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37:771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, Kallos A, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One. 2010;5:e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burl S, Townend J, Njie-Jobe J, Cox M, Adetifa UJ, Touray E, et al. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS One. 2011;6:e18185. doi: 10.1371/journal.pone.0018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kimman TG, Banus S, Reijmerink N, Reimerink J, Stelma FF, Koppelman GH, et al. Association of interacting genes in the toll-like receptor signaling pathway and the antibody response to pertussis vaccination. PLoS One. 2008;3:e3665. doi: 10.1371/journal.pone.0003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wolfle SJ, Strebovsky J, Bartz H, Sahr A, Arnold C, Kaiser C, et al. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41:413–424. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- 97.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002;128:118–123. doi: 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, Goldman M, et al. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–281. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 99.Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol. 2014;15:195–204. doi: 10.1038/ni.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kennedy RB, Oberg AL, Ovsyannikova IG, Haralambieva IH, Grill D, Poland GA. Transcriptomic profiles of high and low antibody responders to smallpox vaccine. Genes Immun. 2013;14:277–285. doi: 10.1038/gene.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu K, Chen L, Kaur R, Pichichero M. Transcriptome signature in young children with acute otitis media due to Streptococcus pneumoniae. Microbes and infection / Institut Pasteur. 2012;14:600–609. doi: 10.1016/j.micinf.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu K, Chen L, Kaur R, Pichichero ME. Transcriptome signature in young children with acute otitis media due to non-typeable Haemophilus influenzae. Int Immunol. 2013;25:353–361. doi: 10.1093/intimm/dxs154. [DOI] [PMC free article] [PubMed] [Google Scholar]