Commentary on: Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013;341(6149):1240104.
Mechanics drive tissue formation and regulate tissue repair. Although the previous scientific decade has yielded a virtual flood of insight into how mechanics control tissue-specific gene expression in these biological processes, the basic mechanisms behind mechanobiological regulation remain unclear. This is not surprising given the immense complexity of potential interactions between even a single cell and its local matrix—interactions involving chemistry, mechanics, dimensionality and topology among other factors. This complexity explodes when considering systems of cells and matrix comprising tissues and organs. Such complexity comes not only from signaling cross-talk that plays out over a wide range of spatiotemporal scales but also from the physical complexity of time-dependent mechanical stresses and strains in three dimensions.
The mechanical interactions between a cell's mechanical apparatus and the extracellular environment can be viewed as distinct with regard to the cellular machineries that are able to sense them.1,2 They can also be viewed as distinct in terms of molecular adjustments that a cell makes in response, both within the cell and to its parent tissue.
In compression, cells have some inherent protection from hydrostatic mechanical stresses by nature of their aqueous composition. Cells may also rapidly adjust local osmotic gradients to cope with transient changes in mechanical compression. In tension and shear, a cell embedded within the matrix or among other cells can regulate its instantaneous response to tissue stretch by adjusting its physical coupling to its neighboring environment.3,4 This degree of tensile coupling can also be mediated by polymerization kinetics and/or crosslinking of cytoskeletal actin or other structural filaments. Such adjustments affect not only the loading of mechanosensory elements of the cytoskeleton but also the sensory proteins within the cell membrane and nucleus that are mechanically coupled.5
In the longer term, cells coordinate both the structure and the composition of the extracellular matrix to regulate their mechanical environment. In connective tissues this is primarily achieved by modulating the filamentous composition and structure of collagen networks—often the main task of connective tissue cells. The paradigm of a ‘feed-forward' control loop by which cell-level mechanical stresses drive tissue formation is a central element of mechanoregulation in bone, tendon and other tissues, and the relationship between mechanical stresses and mechanically optimized tissue structure has been recognized for over a century.6
However, between these two temporal extremes (instantaneous adjustment of the cell's mechanical machinery and long-term adjustment of the tissue matrix) a cell can be subjected to substantial and repetitive mechanical stress. Although sensitivity to transient mechanical loading is essential to detect and quickly respond to state changes through rapid modulation of gene expression and transcription, prolonged mechanical stresses can induce long-term, physiologically irreversible changes in the epigenetic composition of a cell.7 In this sense, a mid-term capacity for a cell to desensitize its mechanosensory apparatus until matrix level changes in mechanics are achieved could be useful in regulating tissue development and repair.
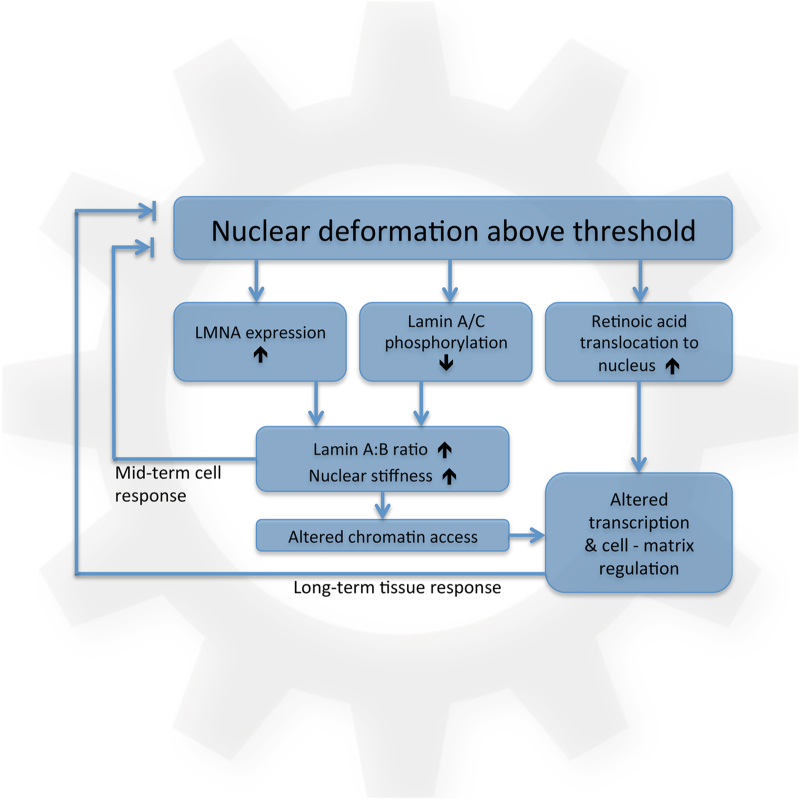
Regardless of timescale, the ability of a cell to sense mechanical loads, retain memory of its loading history and accordingly set or adjust mechanical ‘switches' is a principal theme in models of mechanical regulation: the so-called ‘mechanostat'.8 The study by Swift et al.9 provides evidence for a viable and potentially universal mechanostat model, proposing a mechanistic link between tissue-specific mechanical stresses and the structural composition of the nuclear envelope (Figure 1). The authors show that mechanical distortion of the cell nucleus provokes a relative shift in the balance between lamin A and lamin B, particularly a shift from a soft elastic envelope composed of mostly lamin B to a stiffer but more viscous nuclear envelope consisting of increasing proportions of lamin A.
Figure 1.
Schematic representation of the model proposed by Swift et al. for the nuclear envelope as a ‘feed-forward' mechanostat that regulates cell and tissue response to mechanical stresses.
Lamins are type V intermediate filament proteins and primary components of the nuclear lamina—a filamentous network that provides structural integrity to the nucleus and anchors nuclear pore complexes. Lamins are known to be important for regulation of gene expression,10 can interact directly with chromatin11 and have an important role in DNA replication, transcription and repair.12 Lamins consist of two distinct subtypes: A-type lamins that are alternative splicing products from the LMNA gene and B-type lamins that are encoded by the separate genes LMNB1 and LMNB2.
Swift et al.9 propose that the ratio of lamin A:B in the nuclear envelope is regulated by mechanical stresses. Here, increasing shear stress is linked to an increase in LMNA gene expression and a concomitant inhibition of lamin A/C phosphorylation and decreased lamin A/C turnover. The consequence is a net increase in lamin A/C (relative to lamin B), and heightened predisposition for its structural integration to the nuclear envelope during dynamic remodeling of the envelope. The precise mechanism by which mechanical stresses inhibit lamin phosphorylation and turnover is not yet fully clear, but could perhaps occur through stretch-induced changes in lamin A/C conformation that expose or obscure molecular docking sites and affect binding affinity of involved signaling factors.13
The model of mechanically regulated nuclear stiffness and viscoelasticity proposed by Swift and colleagues is suggested to regulate downstream cell behaviors via modulation of nuclear deformations. Nuclear stretch has been implicated in locally regulating the spatial organization of chromatin,14 a fact that may have potentially important consequences for transcriptional regulation.15 In this sense, a stretch-regulated stiffening of the nuclear envelope represents a potentially central mechanical ‘switch' with a degree of physical and temporal stability. Such switches represent key elements in whole cell regulation, including state-dependent regulation of cell fate.16
As introduced above, mechanosensitivity of cells is of course not limited to the nucleus, and the authors first screened for candidate universal mechanostat proteins using high-throughput proteomic analysis of a large palette of structural and nuclear proteins from cells extracted from a broad range of hard and soft tissues. The idea was to flag proteins that were present in proportion to ‘matrix micro-stiffness' of the cell origin. The quantification of ‘micro-stiffness' was established (in a somewhat hand-waving manner) from a highly selective composite of reported values from the literature. Using these values of tissue stiffness, the amount of candidate protein lamin A, and even more so the stoichiometric ratio of lamin A to lamin B content, correlated strongly with ‘micro-stiffness'. The authors report that no similarly coherent relationship emerged among the assayed cytoskeletal proteins.
For the biomechanist, it is relevant to note that the authors very roughly equate ‘micro-stiffness' with compressive modulus when the nuclear deformations they experimentally examine are shear induced. They further make only a superficial inferential link between matrix deformation, the corresponding cytoskeletal deformation and eventual transfer of these loads to the nucleus in time and space. This is a critical point because the conceptual framework relies heavily on the concept of tissue micro-stiffness, as do many key relationships the authors attempt to establish between an assumed mechanical environment of tissue origin, cell protein expression and content, and quantified cell behavior. As one example, it may be surprising to the biomechanist that cells derived from cartilage are, without discussion of rationale, associated with tissue micro-stiffness well beyond skeletal and cardiac muscle—an assignment that conflicts with the relatively low tension (or shear) strains in cartilage tissues compared with muscle. How the high hydrostatic stresses in cartilage might translate to nuclear deformation is not discussed.
It may also be important to note that many of the key experimental data utilize progenitor cells derived from bone marrow, with these cells possessing extremely high ratios of lamin A to lamin B. The authors accord human mesenchymal stem cells (MSCs) to the highest level of ‘micro-stiff' environments that they consider. Swift and colleagues quantitatively attribute the MSC microenvironment with a rigidity of 50 kPa (citing their earlier in vitro studies using cell culture model systems20), and discuss this as reflective of the MSC osteogenic niche. They deem MSC-associated micro-stiffness to be more rigid than tissues of either the femur or the skull, without a clear rationale for how these tissue properties were assigned. In any case, given the fact that the MSCs represented the extreme end of both high lamin A:B ratio and high micro-stiffness, these data heavily influence the correlations that are drawn.
Regardless of difficulties in quantifying extracellular matrix mechanical properties or predicting how these translate to the mechanical deformation of the cell nucleus, the model proposed and supported by Swift and colleagues is intriguing and potentially very important. It describes a novel, single-cell-level, ‘feed-forward' mechanism by which the history of mechanical deformations can be stored in the structural composition of a cell. Importantly, these structural changes act through a protein of the nuclear envelope that is directly positioned to regulate transcription. The study clearly demonstrates that increased cell deformation (shear in a rheometer; aspiration by micropipette) reduces the turnover of lamin A, with downstream effects on Yes-associated protein (YAP) and the serum response factor (SRF) signaling pathway. Beyond these well-known mechanosensitive regulators of transcription, lamin A was also demonstrated to drive translocation of retinoic acid to the nucleus, provoking additional transcription of LMNA, potentially regulating RUNX2 expression in bone progenitor cells, and more broadly modulating epigenetic regulation of lamina-associated chromatin in the cell nucleus. This latter mechanism has potentially vast implications for transcription of a wide range of genes.
Rather than providing a unifying framework to explain the response of a cell to mechanical stresses, the study by Swift et al. illuminates an additional layer of complexity. This layer is likely to be involved in epigenetic regulation of gene transcription, and could be essential in guiding phenotypic cell and tissue differentiation. It may also be important, or even essential, in maintaining phenotypic stability. This mechanical switch also holds a large potential for manifold regulatory interactions with other mechanically sensitive machinery in the nucleus, cytoskeleton, cell membrane and extracellular matrix itself. As such, the study by Swift and colleagues may add an important piece to the puzzle of understanding how mechanical loads drive our bodies forward.
Footnotes
The author declares no conflict of interest.
References
- Vogel V, Sheetz MP. Cell fate regulation by coupling mechanical cycles to biochemical signaling pathways. Curr Opin Cell Biol 2009;21:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Booth-Gauthier EA, Ladoux B. In the middle of it all: mutual mechanical regulation between the nucleus and the cytoskeleton. J Biomech 2010;43:2–8. [DOI] [PubMed] [Google Scholar]
- Renkawitz J, Schumann K, Weber M, Lammermann T, Pflicke H, Piel M et al. Adaptive force transmission in amoeboid cell migration. Nat Cell Biol 2009;11:1438–1443. [DOI] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science 2009;323:642–644. [DOI] [PubMed] [Google Scholar]
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 2009;10:75–82. [DOI] [PubMed] [Google Scholar]
- Wolff J. Das gesetz der transformation der knochen. DMW-Deutsche Medizinische Wochenschrift 1892;19:1222–1224. [Google Scholar]
- Vlcek S, Foisner R. Lamins and lamin-associated proteins in aging and disease. Curr Opin Cell Biol 2007;19:298–304. [DOI] [PubMed] [Google Scholar]
- Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec 1987;219:1–9. [DOI] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013;341:1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaburn KJ, Misteli T. Cell biology: chromosome territories. Nature 2007;445:379–781. [DOI] [PubMed] [Google Scholar]
- Taniura H, Glass C, Gerace L. A chromatin binding site in the tail domain of nuclear lamins that interacts with core histones. J Cell Biol 1995;131:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman HJ. Nuclear lamins and laminopathies. J Pathol 2012;226:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell 2006;10:11–20. [DOI] [PubMed] [Google Scholar]
- Verstraeten VL, Lammerding J. Experimental techniques for study of chromatin mechanics in intact nuclei and living cells. Chromosome Res 2008;16:499–510. [DOI] [PubMed] [Google Scholar]
- Coulon A, Chow CC, Singer RH, Larson DR. Eukaryotic transcriptional dynamics: from single molecules to cell populations. Nat Rev Genet 2013;14:572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell JE Jr. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol 2002;14:140–148. [DOI] [PubMed] [Google Scholar]
- Madden R, Han SK, Herzog W. Chondrocyte deformation under extreme tissue strain in two regions of the rabbit knee joint. J Biomech 2013;46:554–560. [DOI] [PubMed] [Google Scholar]
- Bray MA, Adams WJ, Geisse NA, Feinberg AW, Sheehy SP, Parker KK. Nuclear morphology and deformation in engineered cardiac myocytes and tissues. Biomaterials 2010;31:5143–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest 2004;113:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 2005;310:1139–1143. [DOI] [PubMed] [Google Scholar]