Abstract
Multidrug resistance (MDR) is the most common cause of chemotherapy failure in gastric cancer (GC) treatment; however, the underlying molecular mechanisms remain elusive. Long noncoding RNAs (lncRNAs) can be involved in carcinogenesis, but the effects of lncRNAs on MDR are poorly understood. We show here that the lncRNA MRUL (MDR-related and upregulated lncRNA), located 400 kb downstream of ABCB1 (ATP-binding cassette, subfamily B, member 1), was significantly upregulated in two multidrug-resistant GC cell sublines, SGC7901/ADR and SGC7901/VCR. Furthermore, the relative expression levels of MRUL in GC tissues were negatively correlated with in vitro growth inhibition rates of GC specimens treated with chemotherapeutic drugs and indicated a poor prognosis for GC patients. MRUL knockdown in SGC7901/ADR and SGC7901/VCR cells led to increased rates of apoptosis, increased accumulation, and reduced doxorubicin (Adriamycin [ADR]) release in the presence of ADR or vincristine. Moreover, MRUL depletion reduced ABCB1 mRNA levels in a dose- and time-dependent manner. Heterologous luciferase reporter assays demonstrated that MRUL might positively affect ABCB1 expression in an orientation- and position-independent manner. Our findings indicate that MRUL promotes ABCB1 expression and is a potential target to reverse the MDR phenotype of GC MDR cell sublines.
INTRODUCTION
The development of multidrug resistance (MDR) followed by failure to respond to chemotherapy is a crucial problem in gastric cancer (GC) (1, 2). However, to date, the molecular mechanisms underlying MDR have not been fully elucidated.
Chemotherapeutic drugs, such as doxorubicin (Adriamycin [ADR]) and vincristine (VCR), can induce Bax- and Bak-mediated apoptosis of tumor cells through DNA damage. Ribosomal protein (RP) S13 (RPS13) or RPL23 can promote MDR in gastric cancer cells by suppressing drug-induced apoptosis (3), while the activation of c-Jun N-terminal kinase 1 (JNK1) and caspase 3 (CPP32) is associated with enhancement of apoptosis in Bax-transfected gastric cancer cells (4). Among the MDR-related proteins, P-glycoprotein (P-gp), a 170-kDa transmembrane glycoprotein encoded by ABCB1, has been extensively studied (5–7). P-gp is an ATP-dependent efflux pump in the ATP-binding cassette (ABC) family and is expressed constitutively in the liver, kidney, intestine, brain, and many other tissues (5, 8). P-gp exhibits broad substrate specificity and is necessary for the elimination of intracellular toxic metabolic products or materials to maintain body homeostasis. Hence, P-gp can reduce intracellular chemotherapeutic drug concentrations by transporting cytotoxic agents out of the cell. P-gp upregulation often results in a compromised chemotherapy response and a worse prognosis for malignant tumor patients. However, the mechanism by which P-gp is upregulated remains unclear.
Long noncoding RNAs (lncRNAs) refer to RNAs that lack coding potential, are >200 bp in length, and are responsible for at least 80% of all genome transcripts. lncRNAs play important roles in regulating transcriptional processes, splicing, and mRNA translation, as well as organellar biogenesis, subcellular molecular trafficking, and cell development (9, 10). lncRNAs can function as oncogenes or tumor suppressors by silencing or activating the expression of protein-coding genes; abnormal lncRNA expression is involved in the development and metastasis of multiple tumor types (11), such as bladder carcinoma (12), glioma (11), leukemias (13), and colorectal cancer (14).
Based on the function and functional mechanisms of lncRNAs, it is clear that further investigation into the relationship between lncRNAs and MDR gastric cancer development is justified. Recent studies indicate that lncRNAs can be transcribed from specific locus control elements and enhancers, which suggests potential roles of lncRNAs in long-range transcriptional control. For example, HOTTIP, a large intergenic noncoding RNA (lincRNA) transcribed from the 5′ tip of the HOXA locus, can drive histone H3 lysine 4 trimethylation and gene transcription through chromosomal looping to bring HOTTIP into close proximity with its target genes (15). Ørom et al. confirmed that lncRNAs might possess an enhancer-like function to promote neighboring protein-coding gene expression (16–18). Based on these observations, we suggest that P-gp upregulation in MDR gastric cancer cell sublines might be mediated by specific lncRNAs via enhancer-like mechanisms. This study investigated this hypothesis by verifying the presence or absence of a differential lncRNA expression profile between SGC7901 and its MDR sublines using lncRNA microarrays. Because of the importance of P-gp in MDR development, the location of lncRNAs relative to the ABCB1 gene was analyzed in detail. Our results clearly demonstrate enhancer-like roles of the lncRNA MRUL in promoting P-gp upregulation in MDR gastric cancer cell sublines.
MATERIALS AND METHODS
Ethics statement.
The Institutional Review Board of the Fourth Military Medical University approved all experimental procedures. Written informed consent was obtained for all patient samples.
Cell lines, cell cultures, and transfections.
The human GC cell line SGC7901 was obtained from the Academy of Military Medical Sciences (Beijing, China). Multidrug-resistant sublines SGC7901/VCR and SGC7901/ADR were developed in our lab. SGC7901/VCR, SGC7901/ADR, and SGC7901 were cultured in RPMI 1640 medium (Thermo Scientific, USA) supplemented with 10% fetal calf serum (FCS) (Thermo Scientific) in a 5% CO2 humidified incubator at 37°C. Vincristine (VCR; Sigma, Inc., St. Louis, MO; 1.0 μg/ml) and ADR (Sigma, Inc., St. Louis, MO; 0.5 μg/ml) were added to the culture medium of appropriate cell sublines to maintain the drug-resistant phenotype. For transfections, Lipofectamine 2000 (Invitrogen Int., USA), 50 to 100 nM small interfering RNA (siRNA) (Gene Pharma Co., Shanghai, China), lentivirus vector-MRUL(−) (Ribobio Co. Guangzhou, China), and pcDNA3.1(+)-MRUL and pcDNA3.1(+) vector (DingGuoChangSheng Biotechnology Co. Ltd., Beijing, China) were used according to the manufacturer's recommendations as previously described (19). siRNA sequences are as follows: siRNAs targeting NR_024549 (MRUL), siMRUL-1, GGCCUUUGUUUGCAGUUUATT; siMRUL-2, AACACUUUCCUGUUUUGGGUC; siMRUL-3, AGUUUCUACUGUUACUGUGUC; siP-gp (targeting P-gp), GGGACAGGAAUAAUUAUAUTT; siNC (negative control), UUCUCCGAACGUGUCACGUTT.
lncRNA microarray expression analyses.
SGC7901/VCR, SGC7901/ADR, SGC7901/VCR, SGC7901, and GES-1 (immortalized normal gastric epithelial cell line) were used to perform the microarray hybridization, and bioinformatic analysis was performed by KangChen Bio-tech, Shanghai, China. More details are available on request.
MRUL cDNA cloning.
To amplify the 3′ end of lncRNA MRUL cDNA from SGC7901/ADR, 3′ rapid amplification of cDNA ends (RACE) was performed using the 3′-Full RACE Core Set Ver.2.0 (TaKaRa, China). Two primers, P1 and P2, were designed based on the conserved sequence of known MRUL. Nested PCR was performed just as instructed by the manufacturer. To clone the full-length cDNA of MRUL, sense primers P3 and P4 were designed based on the partial sequence amplified and the nested PCR was performed just as instructed by the manufacturer. The PCR products were cloned into the pMD18-T simple vector (TaKaRa) and sequenced bidirectionally with primers M13-47 and RV-M. The sequencing results were verified for cluster analysis.
Primers (5′→3′).
The 3′ RACE adaptor set, provided by TaKaRa, was as follows: 3′ RACE outer primer, TACCGTCGTTCCACTAGTGATTT; 3′ RACE inner primer, CGCGGATCCTCCACTAGTGATTTCACTATAGG; 3′ RACE control outer primer, GAGTGCAGGACATTGTGGTAGGG; 3′ RACE control inner primer, ATCTTTGACTGCCGTTCTCGACC; P1, GCCATCTCTTCTAGCCCCAT; P2, TTCAGCAGACTCTCCTGCTAC; 5′ RACE outer primer, CATGGCTACATGCTGACAGCCTA; 5′ RACE inner primer, CGCGGATCCACAGCCTACTGATGATCAGTCGATG; 5′ RACE control outer primer, AGGTAGGTGATGTTCCGAGAGCGT; 5′ RACE control inner primer, TTGGAGTCGCCCTCAGCAGAGAT; P3, GGTGTGTGACACTGCACTGTAA; P4, CTGTCAGGGTTACTGTGGGA.
RNA purification, cDNA synthesis, and qRT-PCR.
The RNA purification, cDNA synthesis, quantitative real-time PCR (qRT-PCR), and reverse transcription-PCR (RT-PCR) were performed according to the manufacturer's instructions (TaKaRa). qRT-PCR was performed using the Sybr green reaction mix and a Light480 sequence detection system (Roche, Germany). For all of the qRT-PCRs, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was measured as an internal control. All the primer sequences (Invitrogen) are listed in Table 1. All of the PCR products were separated on an ethidium bromide-stained 1.5% agarose gel and visualized with UV.
TABLE 1.
Primers used for real-time RT-PCRa
| Gene | Sequence |
Size (bp) | |
|---|---|---|---|
| Forward primer | Reverse primer | ||
| MRUL | ACCCACAGACAACTGTGGACCC | GCCGCCCCTATTGTTGCCCA | 225 |
| P-gp | GGCTGATTGGCTGGGCAGGAA | TGGAACGGCCACCAAGACGTG | 203 |
| CROT | ACCTTGAATCAGTCACCAGAAC | CCACCACTCTTCCAGCCAAT | 236 |
| GRM3 | CAGTTGAGTCGCGAGTACGG | TCGGTGAATTTCGGAGTGGG | 249 |
| KIAA1234L | GAGTTGTCGGTGCGAGGAAT | CATGGCTTTGGCCTGGTAGA | 314 |
| ABCB4 | TGTGCAGAAGGCACACATCT | TGACCATCGAGAAGCACTGTC | 504 |
| Uc002hfi | GCTGATGGCTCCACATCTT | TTTTTTTTTTCCTTAACCTTG | 206 |
| NR_026673 | CTGGTGTGAACTTCCGGATT | CAAATGACCACTGCTGGATG | 175 |
| NR_015379 | TTTGCCAGCCTCAGCTTAAT | TTGTCCCCATTTTCCATCAT | 186 |
| NR_027241 | GGGATCTGGGTTTGCTGATA | CCGAATCTTGTGCAGATTGA | 170 |
| Uc003wig | AGGGTTTCAGGTGACCACAG | CACTTGACCCTGCACTCTGA | 192 |
| Uc003whs | CAGTCTGGGCAACAGAGTGA | ACTCAGAGATGCCTGCCAGT | 219 |
| Uc001vtj | TAGGGTTTGGGGCTAGTGTG | CCTGCGCATGACTGTAAAAA | 160 |
| Uc002jmb | TCCTACTGACCCCACCTGAG | CTGGAGACCCAAGAAATCCA | 227 |
| NR_026867 | GAGGATGTGGCTGTGGATTT | CCAGTTGAGGGGTGTCAAGT | 241 |
| NR_027339 | CATCATGCTTTTCCAGCTCA | TGTGGCAAATGAGGAATCAA | 156 |
| NR_027282 | GCACACAGGCATCTAGTGGA | GCTTGGTGGTGTTTTCCTGT | 243 |
| Uc002rvu | TGGTGGGACTTCTGGGTAAG | ATCGTCCTCATCCTCCTCCT | 165 |
| Uc003dhh | ATTCACGGTTATGCCTTTGC | CGTTTCCCTTTGTCCACTGT | 230 |
| Uc010jya | AGACAGCAGCAACAGCTCAA | TTTCATGCCACAACACCAGT | 191 |
| Uc001jtu | TCCCTGCTGGAGTCAGAAGT | TGCTGTCATCTCACGGTCTC | 179 |
| Uc001ras | CCAGTTCACCACTGGAAGGT | TCACAAAGCCTGCATCAAAG | 215 |
| PCNA | CAGGGCTCCATCCTCAAGAA | GAGTCCATGCTCTGCAGGTT | 104 |
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA | 155 |
Melting temperature for all primers was 60°C.
IHC.
Immunohistochemistry (IHC) assays were performed using gastric cancer paraffin-embedded sections and P-glycoprotein antibody as previously described (20).
Assessment of cell survival rate and growth inhibition rate.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT [Sigma, Inc.]; Varioskan Flash [Thermo Scientific]) assay was used to evaluate the growth rate of the cells as previously described (21). Cell growth inhibition rate (%) was calculated as 1 − (mean A490 of treatment group/mean A490 of control group) × 100%. Cell survival rate (%) was calculated as (mean A490 of treatment group/mean A490 of control group) × 100%.
Plating colony formation assay (CFA).
The log-phase cells were harvested and plated into 6-well plates (500 cells per well) followed by addition of chemotherapeutic drugs into the culture medium on the second day. The resulting colonies were stained with Coomassie brilliant blue (Sigma, Inc.), and the visible colonies were counted after 2 weeks.
Histoculture drug response assay (HDRA).
Fresh GC tumor tissue was harvested from each surgically resected specimen and placed in a 24-well plate. Cubes of collagen gel sponge (1 cm3) were immersed in 1 ml RPMI 1640 medium supplemented with 20% fetal bovine serum. Tumor tissues were placed onto the collagen sponge following addition of ADR at a final concentration of 6 μg/ml, and they were incubated for 72 h at 37°C with 5% CO2. Tumor tissues treated with phosphate-buffered saline (PBS) without ADR were used as a control. Evaluation and calculation methods are as previously described (22). The inhibition rate (IR) of tumor growth was calculated as (1 − mean absorbance of treated wells per gram of tumor/mean absorbance of control wells per gram of tumor) × 100%. In this study, the IR cutoff value equal to or greater than 30% (IR30) was defined as chemosensitivity according to preliminary study results (23). More details are available on request.
Fluorescence intensity assay for intracellular ADR.
The fluorescence intensity of intracellular ADR was determined by fluorescent confocal microscopy (FCM) as described previously (24). The experiment was independently done three times. ADR accumulation rate was calculated as (accumulation value − retention value)/accumulation value × 100%. ADR releasing index was calculated as (accumulation value − retention value)/accumulation value × 100%. Details are available on request.
Flow cytometry assay for apoptosis.
Cells were washed and resuspended in 100 μl binding buffer at a concentration of 1 × 106/ml. Then, 5 μl annexin V-fluorescein isothiocyanate (FITC; PharMingen, San Diego, CA) and 10 μl of 20-μg/ml propidium iodide (Sigma, Inc., St. Louis, MO) were added to these cells. After incubation at room temperature for 15 min, 400 μl annexin-binding buffer was added to each sample, and the samples were kept on ice for counting the stained cells by flow cytometry. Annexin V binds to those cells that express phosphatidylserine on the outer layer of the cell membrane, and propidium iodide stains the cellular DNA of those cells with a compromised cell membrane (25).
Western blotting.
Equal amounts of total protein were separated by SDS-PAGE (10% polyacrylamide gel) and transferred to nitrocellulose membranes (Bio-Rad) using an electrotransfer unit (Bio-Rad). The membranes were blocked with 5% nonfat dry milk and 0.1% Tween 20 in Tris-buffered saline (TBS) and then incubated with the primary antibodies overnight at 4°C following incubation with a horseradish peroxidase (HRP)-conjugated sheep anti-mouse IgG antibody. The membranes were scanned and analyzed using a Bio-Rad ChemiDoc XRS+ imaging system with imaging software (version quantity 1). All the antibodies used in the Western blot assay are listed in Table 2.
TABLE 2.
Antibodies used for Western blotting
| Primary antibody | Species in which raised | Supplier (catalog no.) | Dilution |
|---|---|---|---|
| P-gp | Rabbit | Abcam (ab98322) | 1:1,000 |
| Bax | Rabbit | Cell Signaling (2772) | 1:1,000 |
| Bak | Goat | Abcam (ab2273) | 1:500 |
| Bcl-xL | Rabbit | Cell Signaling (2762) | 1:1,000 |
| Bcl-2 | Rabbit | Santa Cruz (sc-25575) | 1:500 |
| RPS13 | Rabbit | Abcam (ab175107) | 1:1,000 |
| RPL23 | Rabbit | Abcam (ab112587) | 1:200 |
| JNK1 | Rabbit | Abcam (ab7949) | 1:200 |
| CPP32 | Rabbit | Abcam (ab2302) | 1:200 |
| Anti-mouse IgG | Goat | Cell Signaling (7076) | 1:5,000 |
| Anti-rabbit IgG | Sheep | Sigma (F7512) | 1:5,000 |
| Actin | Mouse | Santa Cruz (sc-8432) | 1:200 |
In vivo tumor studies.
Four-week-old male nude mice were purchased from the Shanghai National Center for Laboratory Animals, Chinese Academy of Sciences. They were maintained in specific-pathogen-free (SPF) facilities and kept in groups of five mice per cage. The SGC7901/ADR (1 × 107) cells that were transfected with the lentivirus vector-DMTF1v4 or negative-control vectors were injected subcutaneously into both hind limbs of the nude mice (n = 8) for 3 weeks.
Cloning of pGL4.24-minP reporters and luciferase assay.
Construction of vectors was performed by DingGuoChangSheng Biotechnology Co. Ltd. (Beijing, China). pGL4.24[luc2P/minP] (Promega, USA) vector carries the luciferase reporter luc2P gene and contains a multiple cloning region for insertion of a response element of interest upstream of a minimal promoter and the luc2P gene. Full-length cDNA of MRUL (I; length, 4,012 bp) and the 1,500-bp DNA sequence (II) upstream of the transcription start site of MRUL were acquired by gene synthesis through overlapping PCR technique and were verified using sequencing after being linked together into T vector. Then, the fragments including I and II were linked together and cloned into the related sites of the luciferase reporter vector 5′ to the luciferase gene. Assays were performed in 96-well white plates using Dual-Glo (Promega) according to the manufacturer's protocol. The protocols and systems used to construct each vector were described in detail elsewhere (data available on request).
Statistical analyses.
Data were expressed as means ± standard deviations (SD). Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test in SPSS, version 17.0 (SPSS, Inc., Chicago, IL), or a sample-independent t test. The survival times of different groups of patients were analyzed using the Kaplan-Meier method. Differences were considered significant at a P value of <0.05.
RESULTS
MRUL is a potential multidrug resistance-related lncRNA in gastric cancer.
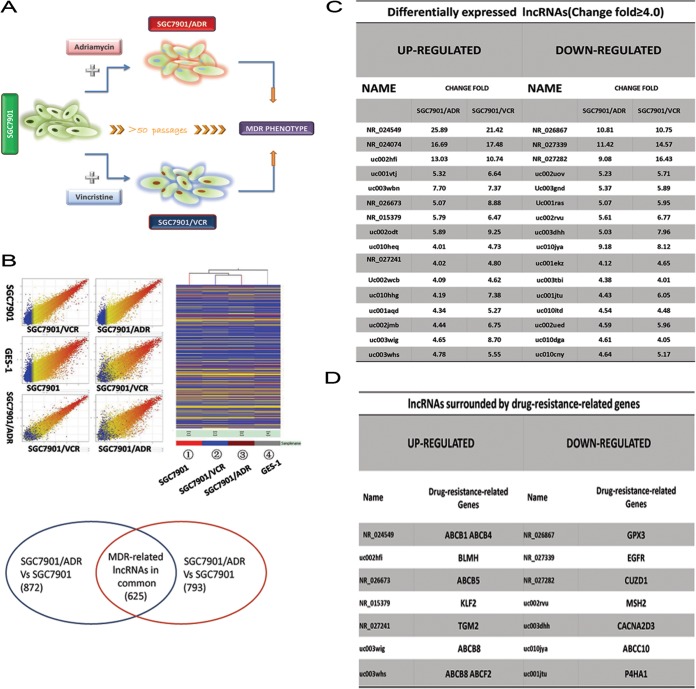
lncRNAs regulate protein-coding gene expression via multiple processes, such as transcription, splicing, mRNA translation, organellar biogenesis, and epigenetic modifications (9, 10). Dysregulation of lncRNAs is involved in the development and metastasis of many malignant tumors (11). Thus, we first determined whether lncRNA dysregulation is directly associated with GC multidrug resistance. SGC7901/ADR and SGC7901/VCR are GC MDR cell sublines derived from the GC cell line SGC7901, using chemotherapy drugs ADR and vincristine (VCR), respectively (Fig. 1A). In this study, we identified potential lncRNAs involved in GC MDR by analyzing the lncRNA expression profiles of SGC7901/ADR, SGC7901/VCR, and SGC7901 cells using microarrays (Fig. 1B). The lncRNAs differentially expressed between the MDR sublines and SGC7901 were subsequently further studied. As shown in Fig. 1B, SGC7901/ADR and SGC7901/VCR shared 625 lncRNAs (lncRNA-Cs) that were differentially expressed with a ≥2.0-fold change relative to SGC7901 cells (Fig. 1B). Among these, 32 lncRNAs exhibited a ≥4.0-fold change (Fig. 1C). Notably, 14 lncRNAs were located near (400 kb upstream or downstream of the lncRNA locus) drug resistance-related protein-coding genes for various tumor types (Fig. 1D). P-glycoprotein is the most studied MDR-related protein (5); therefore, we determined whether there are lncRNAs neighboring the P-gp gene locus. In addition, lncRNA NR_024549 (termed MRUL [MDR-related and upregulated lncRNA]) was located neighboring the MDR1 gene region and was markedly upregulated in both MDR GC cell sublines compared with SGC7901 cells (Fig. 1C; also data not shown). To amplify MRUL cDNA from SGC7901/ADR, 3′ RACE, 5′ RACE, and nested PCR were performed to amplify two fragments corresponding to the 3′ and 5′ ends of the MRUL cDNA. The full-length cDNA sequence of MRUL was determined to be 4,012 bp by cluster analysis of the above fragments (data not shown). These data indicated that MRUL may be a multidrug resistance-related lncRNA in gastric cancer (data not shown).
FIG 1.
Microarray screening of related lncRNAs using SGC7901/ADR and SGC7901/VCR sublines. (A) SGC7901/ADR and SGC7901/VCR sublines were induced by the parental gastric adenocarcinoma cell line SGC7901 using ADR and vincristine, respectively. (B) lncRNA microarrays covered 18,534 lncRNAs and were used to identify MDR-related lncRNAs. When expression profiles were compared between SGC7901/VCR and SGC7901 and between SGC7901/ADR and SGC7901, 1,418 and 1,497 differentially expressed lncRNAs were identified, respectively; of these, SGC7901/VCR and SGC7901/ADR shared 625 differentially expressed lncRNAs (lncRNA-Cs). GES-1 is the immortalized normal gastric epithelial cell line. (C) lncRNA-Cs (fold change, ≥4.0). (D) lncRNA-Cs (fold change, ≥4.0) surrounded by drug resistance-related genes reported in multiple tumors and the corresponding drug resistance-related genes.
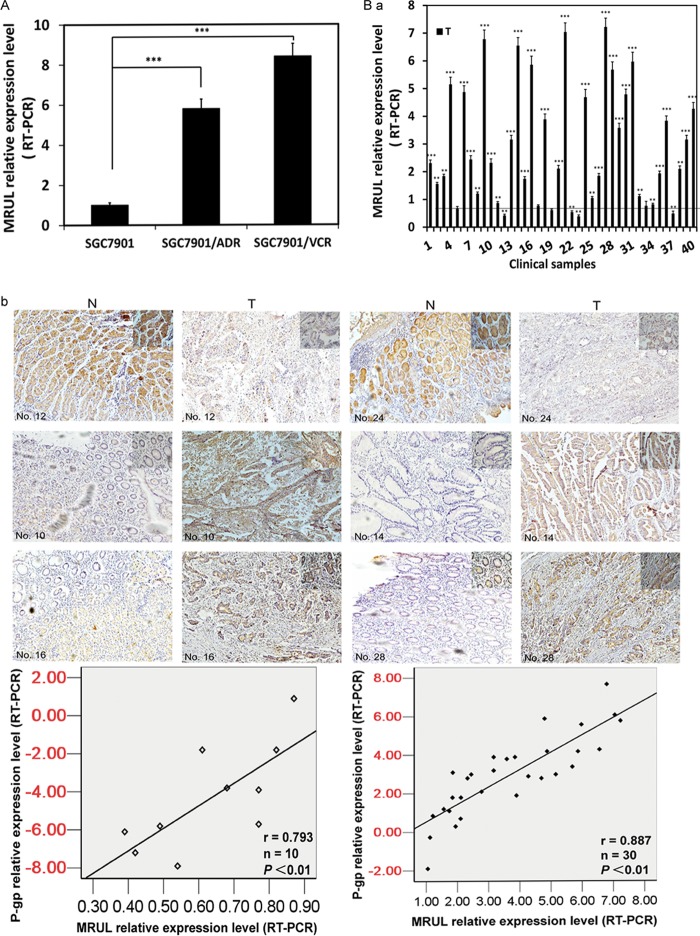
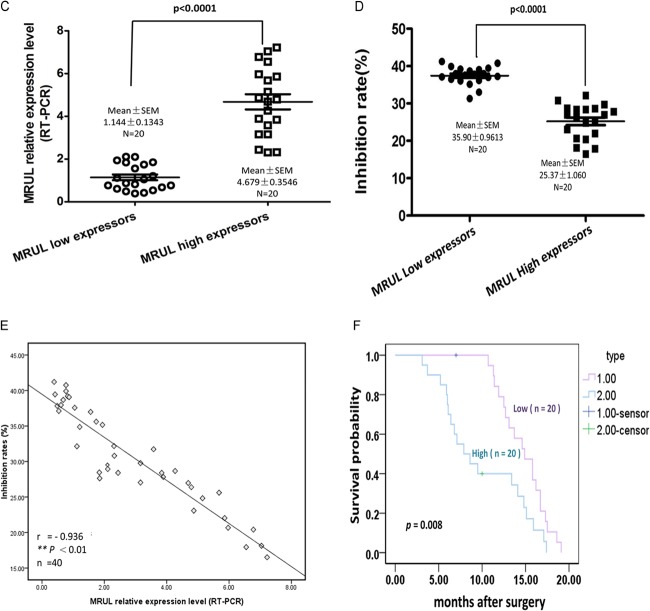
MRUL expression levels were negatively correlated with chemosensitivity of gastric cancer cells to P-gp-related chemotherapy drugs. As shown in Fig. 1C, MRUL was upregulated in two GC MDR sublines, as determined using RT-PCR (Fig. 2A). To assess a potential role of MRUL in GC tissues, 40 primary GC tissues and paired noncancerous gastric mucosa samples were obtained from phase IV patients (Table 3) who had received no chemotherapy and who underwent palliative operations. As shown in Fig. 2B-a, in 25% (6/40) of GC tissues, MRUL expression levels were markedly downregulated, while in 67.5%, levels were significantly upregulated compared with normal gastric mucosa. In addition, MRUL expression was positively correlated with P-gp expression (Fig. 2B-b) (P < 0.01). To evaluate the relationship between MRUL expression level and chemosensitivity of GC tissues, we then performed histoculture drug response assays (HDRAs) using surgically resected GC specimens. The HDRA is a robust method to assess the sensitivity of tumor specimens to chemotherapy drugs in vitro (26, 27). Forty GC specimens were divided into high- and low-MRUL-expression groups according to the fold change median (2.20) (Fig. 2C). Inhibition rates of the two groups were then examined in the presence of ADR using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. As shown in Fig. 2D, the inhibition rate (IR) of the low-MRUL-expression group was markedly higher than that of the high-MRUL-expression group (P < 0.001). We further demonstrated that IR was negatively correlated with MRUL expression level (P < 0.01; Fig. 2E). In addition, the low-MRUL-expression group exhibited a significantly higher chemosensitivity frequency (16/20), i.e., being above the IR threshold (30%), than that of the high-expression group (3/20) (χ2 = 16.942, P < 0.001). Following palliative surgery, all patients received DCF chemotherapy treatment (docetaxel, 75 mg/m2, day 1; cisplatin [CDDP], 20 mg/m2, days 1 to 5; 5-fluorouracil [5-FU], 500 mg/m2, days 1 to 5; every 21 days, 2 to 4 cycles). Docetaxel and ADR are P-gp-related chemotherapy drugs. As shown in Fig. 2F, the survival time of the low-MRUL-expression group was significantly longer than that of the high-expression group (P < 0.01). These results suggested that MRUL expression levels were negatively correlated with chemosensitivity of gastric cancer tissues to P-gp-related chemotherapy drugs.
FIG 2.
lncRNA MRUL expression levels showed a negative linear correlation with chemosensitivity to gastric cancer (GC) cell lines and tissue specimens. (A) MRUL relative expression levels in SGC7901/VCR, SGC7901/ADR, and SGC7901 cells. (B-a) MRUL relative expression levels of clinical samples compared with those of paired noncancerous tissues. (B-b) P-gp expression of 40 primary GC tissues and paired noncancerous tissues. Relative IHC scores of P-gp were positively correlated with MRUL expression levels. T, tumor; N, normal. (C) Forty GC tissues were divided into MRUL high-expressor and low-expressor groups according to the fold change median. (D) Inhibition rates of MRUL high- and low-expressor groups. (E) Negative correlation between inhibition rates and MRUL expression levels. (F) Patient life spans in MRUL low- and high-expressor groups. Data represent means plus standard deviations of three replicates (***, P < 0.001; **, P < 0.01).
TABLE 3.
Clinicopathologic characteristics of 40 GC patients
| Clinicopathologic characteristic | Case distribution |
|---|---|
| Age range, yr (median) | 42–71 (56) |
| Sex (no. male/female) | 29/11 |
| Differentiation by health status (no. well/moderate/poor) | 7/14/19 |
| Tumor location, no. (submucosa/muscularis propria/adventitia) | 6/8/26 |
| Lymph node metastasis (no. negative/positive) | 23/17 |
| Clinical phase/no. of patients | IV/40 |
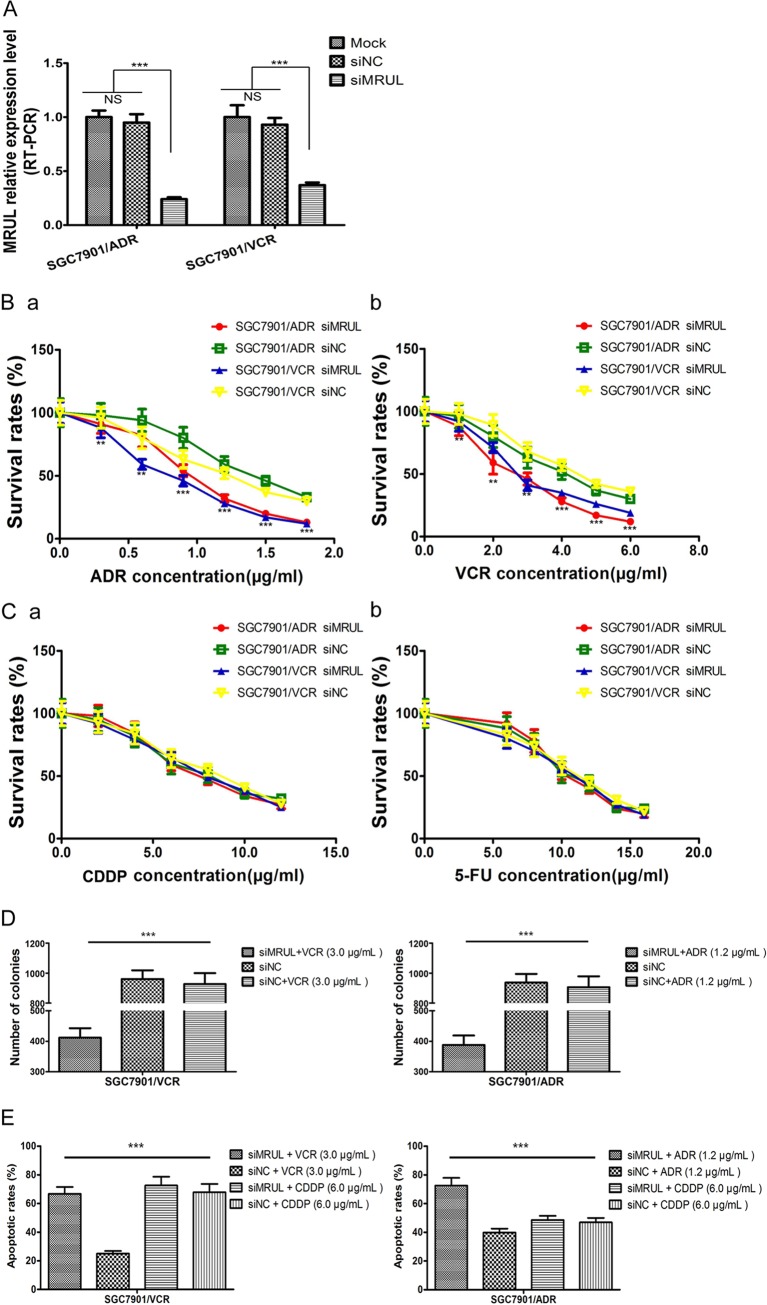
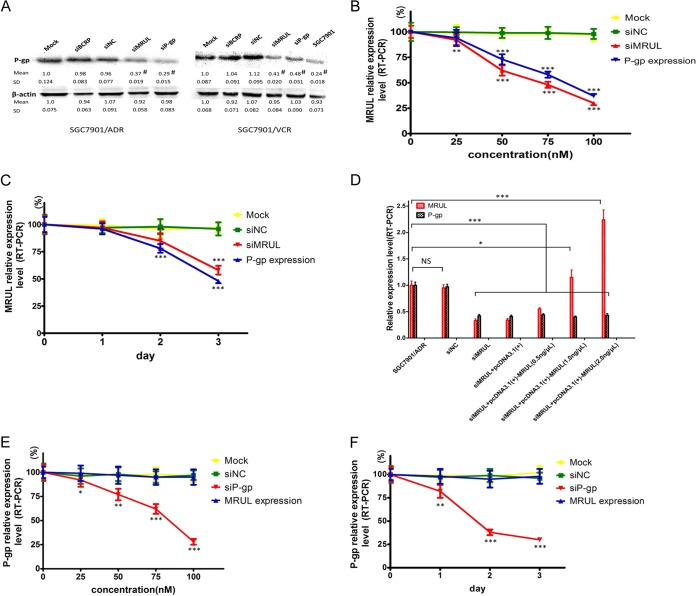
MRUL depletion increases chemosensitivity of MDR GC cell sublines to P-gp-related chemotherapy drugs.
To investigate the effect of MRUL depletion on the chemosensitivity of MDR GC sublines to P-gp-related chemotherapy drugs, such as ADR or VCR, which can be pumped out of cells by P-gp, MRUL expression was knocked down in SGC7901/ADR and SGC7901/VCR cells using siRNA (Fig. 3A). MRUL knockdown significantly reduced survival rates of SGC7901/ADR and SGC7901/VCR cells in the presence of ADR (Fig. 3B-a) or VCR (Fig. 3B-b) but not in the presence of a non-P-gp-related chemotherapy drug, cisplatin (CDDP) (Fig. 3C-a) or 5-fluorouracil (5-FU) (Fig. 3C-b), in a concentration-dependent manner compared with siRNA negative-control (siNC) groups. Moreover, plating colony formation rates of siMRUL groups treated with ADR and VCR were remarkably lower than those of siNC groups (Fig. 3D). Apoptosis is one of the most important mechanisms by which ADR (28–31) and VCR (32, 33) induce cancer cell death. Thus, we further demonstrated that treatment with ADR or VCR increased apoptotic rates in siMRUL-SGC7901/ADR and SGC7901/VCR cells compared with siNC groups (Fig. 3E). Furthermore, to investigate the effects of MRUL upregulation on the MDR GC cell sublines, SGC7901/ADR and SGC7901/VCR cells were transfected with pcDNA3.1(+)-MRUL. However, MRUL upregulation did not significantly increase survival rates or markedly reduce apoptosis rates (data not shown) of SGC7901/ADR or SGC7901/VCR cells in the presence of ADR or VCR. These data indicated that MRUL downregulation increased, while MRUL upregulation did not reduce, the chemosensitivity of MDR GC cell sublines to P-gp-related chemotherapy drugs.
FIG 3.
lncRNA MRUL depletion leads to increased chemosensitivity of MDR GC sublines to P-gp related drugs. (A) MRUL relative expression levels. Following the transfection of SGC7901/ADR and SGC7901/VCR cells with siMRUL for 3 days, RT-PCR was used to assess MRUL expression levels and GAPDH was used as the internal control. (B and C) Survival rates of SGC7901/ADR and SGC7901/VCR cells treated with siMRUL or siNC in the presence of ADR (B-a), VCR (B-b), CDDP (C-a), or 5-FU (C-b). The MTT assay was used to examine cell survival rates at the designated time. (D) Plating colony formation assay of SGC7901/ADR and SGC7901/VCR cells treated with siMRUL or siNC in the presence of ADR or VCR. (E) Apoptosis rates of SGC7901/ADR and SGC7901/VCR cells treated with siMRUL or siNC in the presence of ADR, CDDP, or VCR. Data represent means plus standard deviations of three replicates (***, P < 0.001).
MRUL depletion increases ADR accumulation and ADR-induced apoptosis in MDR GC cell sublines.
As shown in Fig. 3B and E, MRUL downregulation increased chemosensitivity of MDR GC cell sublines to ADR and VCR. It is plausible that MRUL depletion increases intracellular ADR concentration; therefore, we next demonstrated that MRUL knockdown markedly increased ADR accumulation and retention rates in SGC7901/ADR and SGC7901/VCR cells compared with siNC cells (Fig. 4A). ADR and VCR can induce tumor cell apoptosis through DNA damage, and Bax and Bak are proapoptotic proteins involved in apoptosis activation and DNA damage-induced apoptosis. Hence, we showed that MRUL knockdown markedly reduced Bcl-2 expression and increased Bax expression in SGC7901/ADR and SGC7901/VCR cells compared with siNC cells (Fig. 4B). RPS13 or RPL23 can promote MDR in gastric cancer cells by suppressing drug-induced apoptosis (3), and the activation of c-Jun N-terminal kinase 1 (JNK1) and caspase 3 (CPP32) is associated with enhancement of apoptosis in Bax-transfected gastric cancer cells (4). Here, in MRUL-depleted SGC7901/ADR and SGC7901/VCR cells, levels of RPS13 and RPL23 were markedly decreased, while JNK1 and CPP32 levels were significantly increased (Fig. 4C). These data suggested that MRUL depletion might promote ADR-induced apoptosis by increasing ADR accumulation and reducing the Bcl-2/Bax ratio.
FIG 4.

MRUL depletion effects on drug accumulation and apoptosis of MDR GC sublines. (A) ADR accumulation and retention of SGC7901/ADR and SGC7901/VCR cells treated with siMRUL or siNC. (B) Effects of MRUL depletion on apoptosis of SGC7901/ADR and SGC7901/VCR cells. (C) Effects of MRUL depletion on PRL23, RPS13, JNK1, and CPP32 expression of SGC7901/ADR and SGC7901/VCR cells. Data represent means plus standard deviations of three replicates (#, P < 0.01).
MRUL knockdown reduces ABCB1 expression in vitro and in vivo.
ADR is a chemotherapeutic agent that can bind to and be pumped out of cells by P-gp. As shown in Fig. 4, MRUL depletion markedly augmented ADR accumulation in SGC7901/ADR and SGC7901/VCR cells. Therefore, we next investigated whether MRUL knockdown could decrease ABCB1 expression in these cells. As shown in Fig. 5A, MRUL depletion significantly reduced P-gp protein levels in SGC7901/ADR and SGC7901/VCR cells and also decreased ABCB1 mRNA levels in an siMRUL concentration- and time-dependent manner (Fig. 5B and C). Moreover, SGC7901/ADR cells transfected with lentivirus vector-MRUL injected into both hind limbs of nude mice showed that MRUL depletion in vitro resulted in MRUL knockdown in vivo (data not shown). However, MRUL overexpression in SGC7901/ADR cells did not significantly upregulate ABCB1 mRNA levels (Fig. 5D). In addition, P-gp depletion had no effect on MRUL expression levels at different siP-gp concentrations or with time (Fig. 5E and F). We also demonstrated that MRUL depletion significantly reduced GRM3 expression but did not alter ABCB4, CROT, or KIAA1324L expression (data not shown). These data indicated that MRUL downregulation, and not upregulation, significantly inhibit P-gp expression.
FIG 5.
MRUL knockdown reduced P-gp expression. (A) P-gp expression examined by Western blotting at the designated time. (B) Effects of MRUL knockdown using different concentrations of siMRUL on MRUL and ABCB1 (P-gp) transcription in SGC7901/ADR cells. (C) Effects of MRUL depletion using 100 nM siMRUL on MRUL and ABCB1 (P-gp) transcription in SGC7901/VCR cells for the indicated times. (D) Effects of MRUL upregulation on MRUL and ABCB1 (P-gp) transcription in SGC7901/ADR cells. (E) Effects of P-gp depletion using different concentrations of siP-gp on MRUL and ABCB1 (P-gp) transcription. (F) Effects of P-gp depletion using 100 nM siP-gp on MRUL and ABCB1 (P-gp) transcription for the indicated times. Data represent means plus standard deviations of three replicates (***, P < 0.001; **, P < 0.01; *, P < 0.05).
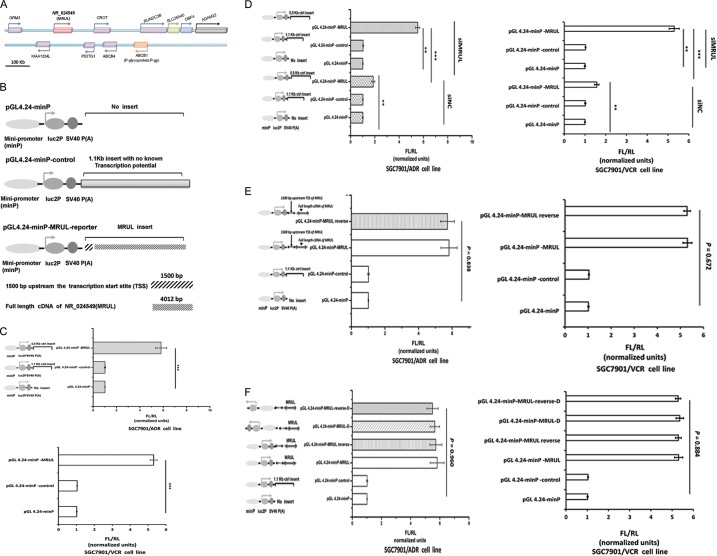
MRUL plays an enhancer-like role in regulating ABCB1 expression.
lncRNAs can perform an enhancer-like role (16, 34) to promote expression of neighboring protein-coding genes. Based on the UCSC (University of California Santa Cruz) human genome 18.0 annotation, MRUL is located approximately 400 kb downstream of the ABCB1 gene locus (Fig. 6A). As mentioned above, silencing of MRUL significantly reduced P-gp mRNA levels, which suggested that MRUL might play an enhancer-like role to regulate ABCB1 transcription. This hypothesis was investigated by performing luciferase assays, because cis-activating enhancers can stimulate transcription when placed adjacent to a heterologous promoter (19). We demonstrated that the MRUL cDNA (data not shown) significantly increased luciferase transcription compared with pGL4.24-minP-control and pGL4.24-minP vectors (Fig. 6C). We also found that MRUL knockdown resulted in a partial abolishment of transcriptional enhancement (Fig. 6D). In addition, reversal (RV) of the MRUL sequence in the reporter construct (data not shown) showed that MRUL exerted its effect in an orientation-independent manner (Fig. 6E). Insertion of MRUL or MRUL-RV (data not shown) downstream of minP in the pGL4.24-minP-luc2P reporter vector did not significantly change transcriptional enhancement (Fig. 6F), indicating that the enhancing activity of MRUL was position independent. These data suggested that MRUL exerts an enhancer-like role in regulating ABCB1 expression.
FIG 6.
lncRNA MRUL plays an enhancer-like role in the activation of a heterologous reporter. (A) Schematic diagram of MRUL and ABCB1 (P-gp) gene locus. (B) Schematic diagram of constructs. SV40, simian virus 40. (C) Enhancement role of MRUL inserts in the heterologous luciferase reporter in MDR GC sublines. (D) Depletion of MRUL using siMRUL reduced markedly the enhancement role of MRUL inserts in the heterologous luciferase reporter. (E) Reversal of MRUL inserts did not affect the enhancement role of MRUL. (F) Alteration of position of MRUL inserts did not affect the enhancement role of MRUL, either. Data represent means + standard deviations of three replicates (***, P < 0.001; **, P < 0.01).
DISCUSSION
Tumor MDR can be fully understood only by elucidating the underlying MDR mechanisms. Previous studies have demonstrated that tumor MDR involves aberrant protein expression, including that for P-gp, which has been extensively studied (5, 35).
Here, we found that two MDR GC cell sublines, SGC7901/ADR and SGC7901/VCR, shared 625 lncRNAs that were differentially expressed with a ≥2.0-fold change (lncRNA-Cs) relative to SGC7901 cells. With the exception of the MDR phenotypes, the two MDR sublines and SGC7901 cells are similar in genetic background; therefore, we expect that the genes that differ between the two sublines and SGC7901 should represent common MDR-related characteristics. Consequently, the occurrence of lncRNA-Cs is intriguing as it indicates that lncRNA dysregulation in MDR GC cell sublines is not a stochastic event but a systemic result. Accumulating evidence indicates that lncRNAs exhibit active roles in regulating the expression of other protein-coding genes, in a cis- or trans-activating (16, 36) or inhibiting (37, 38) way. We also identified the protein-coding genes located in the 800-kb region surrounding each lncRNA locus. These data showed that approximately 5% (31/625), 21% (132/625), and 12.3% (77/625) of the lncRNA-Cs were located in regions near MDR-related genes, oncogenes, and tumor suppressors, respectively (data not shown). Our findings indicate that lncRNAs are not transcriptional noise (39, 40) but are likely to be functional players exerting phenotypic differences. It remains to be investigated whether the lncRNA-Cs analyzed in this study also participate in GC MDR.
P-glycoprotein (P-gp) is a transmembrane transport protein that pumps substrates out of cancer cells via ATP hydrolysis. The protein has been extensively studied over the last several decades. P-gp expression in tumors results in reduced concentrations of intracellular chemotherapeutic agents and consequently reduces the cytotoxicity of chemotherapeutic drugs. Previous studies have suggested that upregulation of P-gp expression is associated with the MDR phenotype of many tumor types and indicated that P-gp silencing could sensitize cancer cells to chemotherapeutic drugs (41, 42). However, the main bottleneck limiting P-gp inhibitor (MDR modulator) application is due to undesirable side effects because of P-gp expression in a diversity of normal organs and tissues (5). Hence, continued investigation of P-gp upregulation mechanisms in tumors is warranted. In the present study, we found that lncRNA MRUL depletion, which was significantly upregulated in two MDR GC cell sublines, led to growth inhibition of these cells under P-gp-related chemotherapeutic drug conditions and decreased P-gp expression. These results shed light on two intriguing areas: the role of MRUL in the development of GC MDR and a possible mechanism for MRUL function.
lncRNAs can promote the epigenetic silencing of protein-coding genes (43, 44). In contrast, lncRNAs can also possess enhancer-like functions to promote expression of neighboring protein-coding genes (40, 45–49). We found that MRUL activated gene expression using a heterologous reporter assay. In addition, MRUL knockdown resulted in a partial abolishment of luciferase transcriptional enhancement, indicating that the observed potentiation of gene expression was mediated through the action of MRUL. One possible mechanism for this observation is that lncRNAs can recruit transcription factors or other complexes necessary for transcription to specific protein-encoding gene promoters by forming a triplex with local DNA double strands (50). We also demonstrated that MRUL depletion significantly reduced GRM3 expression but did not alter ABCB4, CROT, or KIAA1324L expression levels. GRM3, ABCB4, ABCB1, CROT, and KIAA1324L loci are located within the 800-kb region surrounding MRUL. GRM3 and ABCB1 are relatively more distant from MRUL than are the other gene loci (Fig. 6A). lncRNA can regulate long-distance gene transcription by forming loops (51–53); therefore, it is plausible that MRUL affected ABCB1 and GRM3 transcription through looping the chromosome. However, MRUL upregulation did not increase ABCB1 mRNA levels, which might indicate that the MRUL transcript must act on the DNA double helix to play an enhancer-like role.
ADR and VCR can induce tumor cell apoptosis through DNA damage. Bax and Bak are proapoptotic proteins, involved in apoptosis activation and DNA damage-induced apoptosis, while Bcl-2 is an antiapoptotic protein. In this study, MRUL knockdown markedly reduced the Bcl-2/Bax ratio in the presence of ADR, further demonstrating that MRUL depletion increased ADR accumulation in SGC7901/ADR and SGC7901/VCR cells. RPS13 or RPL23 can promote MDR in gastric cancer cells by suppressing drug-induced apoptosis (3), and the activation of JNK1 and CPP32 was associated with enhancement of apoptosis in Bax-transfected gastric cancer cells (4). Here, expression of RPS13 and RPL23 was markedly decreased, while expression of JNK1 and CPP32 was significantly increased in MRUL-knockdown SGC7901/ADR and SGC7901/VCR cells, illustrating that MRUL depletion could augment drug-induced apoptosis.
In summary, we identified a group of lncRNAs potentially implicated in GC MDR. We have provided evidence for lncRNA MRUL function in GC MDR development and have revealed enhancer-like roles of MRUL in upregulating P-gp expression. This result indicates that MRUL depletion may be a promising therapeutic strategy for GC MDR reversal. It remains to be investigated whether other lncRNA-Cs identified in this study also participate in GC MDR. More importantly, our results indicate a new mechanism for lncRNA-mediated regulation of multidrug resistance-related proteins.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (no. 81301763 and 81272347), National Program on Key Basic Research Project (973 Program, no. 2010CB529302, no. 2010CB529305, and no. 2010CB529306), Henan Provincial Key Scientific and Technological Projects (no. 142102310473), and Key Program, National Natural Science Foundation of China (no. 81030044).
We are very grateful to Zhe Wang, Pathology Department, Xijing Hospital of Digestive Diseases, for his excellent suggestions regarding the bioinformatic analysis of lncRNAs. We also thank Qingchuan Zhao, Surgical Department, Xijing Hospital of Digestive Diseases, for his efficient help with the collection of the gastric cancer specimens. We thank Ulf Andersson Ørom, Wistar Institute, Philadelphia, PA, and Ken C. Pang, Department of Molecular and Cellular Biology, Harvard University, for their helpful discussions.
We have nothing to disclose.
Y.W. performed all of the bioinformatic evaluations, in vitro experiments, and data analyses for Fig. 1 to 5 and wrote the manuscript. D.Z. performed the cell culture and generated the data for Fig. 6 and cowrote the corresponding legend. K.W. and Q.Z. supervised the in vitro specimen cultures and provided intellectual input. Y.N. cosupervised the project and gave valuable suggestions. D.F. initiated the project, designed the experiments, interpreted the data, and wrote the manuscript. D.F. acted as a guarantor for the data in the manuscript.
Footnotes
Published ahead of print 23 June 2014
REFERENCES
- 1.Zhang D, Fan D. 2007. Multidrug resistance in gastric cancer: recent research advances and ongoing therapeutic challenges. Expert Rev. Anticancer Ther. 7:1369–1378. 10.1586/14737140.7.10.1369 [DOI] [PubMed] [Google Scholar]
- 2.Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. 2008. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int. J. Cancer 123:372–379. 10.1002/ijc.23501 [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Zhai H, Wang X, Han Z, Liu C, Lan M, Du J, Guo C, Zhang Y, Wu K. 2004. Ribosomal proteins S13 and L23 promote multidrug resistance in gastric cancer cells by suppressing drug-induced apoptosis. Exp. Cell Res. 296:337–346. 10.1016/j.yexcr.2004.02.009 [DOI] [PubMed] [Google Scholar]
- 4.Kim R, Ohi Y, Inoue H, Toge T. 1999. Enhancement of chemotherapeutic agents induced-apoptosis associated with activation of c-Jun N-terminal kinase 1 and caspase 3 (CPP32) in bax-transfected gastric cancer cells. Anticancer Res. 20:439–444 [PubMed] [Google Scholar]
- 5.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. 1987. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. U. S. A. 84:7735–7738. 10.1073/pnas.84.21.7735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juranka P, Zastawny R, Ling V. 1989. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 3:2583–2592 [DOI] [PubMed] [Google Scholar]
- 7.Kartner N, Evernden-Porelle D, Bradley G, Ling V. 1985. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. Nature 316:820–823 [DOI] [PubMed] [Google Scholar]
- 8.Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. 1989. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. U. S. A. 86:695–698. 10.1073/pnas.86.2.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilusz JE, Sunwoo H, Spector DL. 2009. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 23:1494–1504. 10.1101/gad.1800909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pang KC, Frith MC, Mattick JS. 2006. Rapid evolution of noncoding RNAs: lack of conservation does not mean lack of function. Trends Genet. 22:1–5. 10.1016/j.tig.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Ren Z, Sun P. 2012. Overexpression of the long non-coding RNA MEG3 impairs in vitro glioma cell proliferation. J. Cell. Biochem. 113:1868–1874. 10.1002/jcb.24055 [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Yu M, Li Z, Kong C, Bi J, Li J, Gao Z, Li Z. 2011. ncRAN, a newly identified long noncoding RNA, enhances human bladder tumor growth, invasion, and survival. Urology 77(2):510.e1-5. 10.1016/j.urology.2010.09.022 [DOI] [PubMed] [Google Scholar]
- 13.Wapinski O, Chang HY. 2011. Long noncoding RNAs and human disease. Trends Cell Biol. 21:354–361. 10.1016/j.tcb.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 14.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S. 2011. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 71:6320–6326. 10.1158/0008-5472.CAN-11-1021 [DOI] [PubMed] [Google Scholar]
- 15.Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta RA. 2011. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472:120–124. 10.1038/nature09819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, Guigo R, Shiekhattar R. 2010. Long noncoding RNAs with enhancer-like function in human cells. Cell 143:46–58. 10.1016/j.cell.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ørom UA, Shiekhattar R. 2011. Long non-coding RNAs and enhancers. Curr. Opin. Genet. Dev. 21:194–198. 10.1016/j.gde.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ørom UA, Shiekhattar R. 2011. Noncoding RNAs and enhancers: complications of a long-distance relationship. Trends Genet. 27:433–439. 10.1016/j.tig.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalby B, Cates S, Harris A, Ohki EC, Tilkins ML, Price PJ, Ciccarone VC. 2004. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods 33:95–103. 10.1016/j.ymeth.2003.11.023 [DOI] [PubMed] [Google Scholar]
- 20.Martin-Broto J, Gutierrez AM, Ramos RF, Lopez-Guerrero JA, Ferrari S, Stacchiotti S, Picci P, Calabuig S, Collini P, Gambarotti M, Bague S, Dei Tos AP, Palassini E, Luna P, Cruz J, Cubedo R, Martinez-Trufero J, Poveda A, Casali PG, Fernandez-Serra A, Lopez-Pousa A, Gronchi A. 2014. MRP1 overexpression determines poor prognosis in prospectively treated patients with localized high-risk soft tissue sarcoma of limbs and trunk wall: an ISG/GEIS study. Mol. Cancer Ther. 13:249–259. 10.1158/1535-7163.MCT-13-0406 [DOI] [PubMed] [Google Scholar]
- 21.van Meerloo J, Kaspers GJ, Cloos J. 2011. Cell sensitivity assays: the MTT assay. Methods Mol. Biol. 731:237–245. 10.1007/978-1-61779-080-5_20 [DOI] [PubMed] [Google Scholar]
- 22.Lee S-W, Kim Y-M, Kim M-B, Kim D-Y, Kim J-H, Nam J-H, Kim Y-T. 2012. In vitro chemosensitivity using the histoculture drug response assay in human epithelial ovarian cancer. Acta Med. Okayama 66:271–277 http://www.lib.okayama-u.ac.jp/www/acta/pdf/66_3_271.pdf [DOI] [PubMed] [Google Scholar]
- 23.Gwak H-S, Park HJ, Yoo H, Youn SM, Rhee CH, Lee SH. 2011. Chemotherapy for malignant gliomas based on histoculture drug response assay: a pilot study. J. Korean Neurosurg. Soc. 50:426–433. 10.3340/jkns.2011.50.5.426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Han Y, Wang X, Zhao Y, Ning X, Xiao B, Fan D. 2002. MGr1-Ag is associated with multidrug-resistant phenotype of gastric cancer cells. Gastric Cancer 5:154–159. 10.1007/s101200200027 [DOI] [PubMed] [Google Scholar]
- 25.Perkins CL, Fang G, Kim CN, Bhalla KN. 2000. The role of Apaf-1, caspase-9, and bid proteins in etoposide-or paclitaxel-induced mitochondrial events during apoptosis. Cancer Res. 60:1645–1653 [PubMed] [Google Scholar]
- 26.Furukawa T, Kubota T, Hoffman RM. 1995. Clinical applications of the histoculture drug response assay. Clin. Cancer Res. 1:305–311 [PubMed] [Google Scholar]
- 27.Jung P-S, Kim D-Y, Kim M-B, Lee S-W, Kim J-H, Kim Y-M, Kim Y-T, Hoffman RM, Nam J-H. 2013. Progression-free survival is accurately predicted in patients treated with chemotherapy for epithelial ovarian cancer by the histoculture drug response assay in a prospective correlative clinical trial at a single institution. Anticancer Res. 33:1029–1034 [PubMed] [Google Scholar]
- 28.Shi R, Peng H, Yuan X, Zhang X, Zhang Y, Fan D, Liu X, Xiong D. 2013. Down-regulation of c-fos by shRNA sensitizes adriamycin-resistant MCF-7/ADR cells to chemotherapeutic agents via P-glycoprotein inhibition and apoptosis augmentation. J. Cell. Biochem. 114:1890–1900. 10.1002/jcb.24533 [DOI] [PubMed] [Google Scholar]
- 29.Zhang J-Y, Lin M-T, Yi T, Tang Y-N, Fan L-L, He X-C, Zhao Z-Z, Chen H-B. 2013. Apoptosis sensitization by euphorbia factor L1 in ABCB1-mediated multidrug resistant K562/ADR cells. Molecules 18:12793–12808. 10.3390/molecules181012793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Kuroda M, Gao X-S, Asaumi J-I, Shibuya K, Kawasaki S, Akaki S, St Clair D, Hiraki Y, Kanazawa S. 2005. Hydrogen peroxide overload increases adriamycin-induced apoptosis of SaOS2FM, a manganese superoxide dismutase-overexpressing human osteosarcoma cell line. Int. J. Oncol. 26:1291–1300. 10.3892/ijo.26.5.1291 [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, Koh E. 2000. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats in vivo study. Circulation 102:572–578. 10.1161/01.CIR.102.5.572 [DOI] [PubMed] [Google Scholar]
- 32.Tang XQ, Bi H, Feng JQ, Cao JG. 2005. Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol. Sin. 26:1009–1016. 10.1111/j.1745-7254.2005.00149.x [DOI] [PubMed] [Google Scholar]
- 33.Groninger E, Boer M-D, De Graaf S, Kamps W, De Bont E. 2002. Vincristine induced apoptosis in acute lymphoblastic leukaemia cells: a mitochondrial controlled pathway regulated by reactive oxygen species? Int. J. Oncol. 21:1339–1345. 10.3892/ijo.21.6.1339 [DOI] [PubMed] [Google Scholar]
- 34.Yang F, Zhang L, Huo XS, Yuan JH, Xu D, Yuan SX, Zhu N, Zhou WP, Yang GS, Wang YZ, Shang JL, Gao CF, Zhang FR, Wang F, Sun SH. 2011. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology 54:1679–1689. 10.1002/hep.24563 [DOI] [PubMed] [Google Scholar]
- 35.Cordon-Cardo C, O'Brien J, Boccia J, Casals D, Bertino J, Melamed M. 1990. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J. Histochem. Cytochem. 38:1277–1287. 10.1177/38.9.1974900 [DOI] [PubMed] [Google Scholar]
- 36.Xu D, Yang F, Yuan JH, Zhang L, Bi HS, Zhou CC, Liu F, Wang F, Sun SH. 2013. LncRNA-LALR1 accelerates hepatocyte proliferation during liver regeneration by activating Wnt/β-Catenin signaling. Hepatology 58:739–751. 10.1002/hep.26361 [DOI] [PubMed] [Google Scholar]
- 37.Nagano T, Fraser P. 2009. Emerging similarities in epigenetic gene silencing by long noncoding RNAs. Mamm. Genome 20:557–562. 10.1007/s00335-009-9218-1 [DOI] [PubMed] [Google Scholar]
- 38.Beisel C, Paro R. 2011. Silencing chromatin: comparing modes and mechanisms. Nat. Rev. Genet. 12:123–135. 10.1038/nrg2932 [DOI] [PubMed] [Google Scholar]
- 39.Jia H, Osak M, Bogu GK, Stanton LW, Johnson R, Lipovich L. 2010. Genome-wide computational identification and manual annotation of human long noncoding RNA genes. RNA 16:1478–1487. 10.1261/rna.1951310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rinn JL, Chang HY. 2012. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 81:145–166. 10.1146/annurev-biochem-051410-092902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, Nel AE. 2010. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano 4:4539–4550. 10.1021/nn100690m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi Z, Liang YJ, Chen ZS, Wang XW, Wang XH, Ding Y, Chen LM, Yang XP, Fu LW. 2006. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol. Ther. 5:39–47. 10.4161/cbt.5.1.2236 [DOI] [PubMed] [Google Scholar]
- 43.Santoro F, Mayer D, Klement RM, Warczok KE, Stukalov A, Barlow DP, Pauler FM. 2013. Imprinted Igf2r silencing depends on continuous Airn lncRNA expression and is not restricted to a developmental window. Development 140:1184–1195. 10.1242/dev.088849 [DOI] [PubMed] [Google Scholar]
- 44.Davalos V, Esteller M. 2014. Unraveling the complex network of interactions between noncoding RNAs and epigenetics in cancer, p 125–148 In Fabbri M. (ed), Non-coding RNAs and cancer. Springer, Philadelphia, PA [Google Scholar]
- 45.Huang Y, Liu N, Wang JP, Wang YQ, Yu XL, Wang ZB, Cheng XC, Zou Q. 2012. Regulatory long non-coding RNA and its functions. J. Physiol. Biochem. 68:611–618. 10.1007/s13105-012-0166-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lam MT, Cho H, Lesch HP, Gosselin D, Heinz S, Tanaka-Oishi Y, Benner C, Kaikkonen MU, Kim AS, Kosaka M. 2013. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature 498:511–515. 10.1038/nature12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, Merkurjev D, Zhang J, Ohgi K, Song X. 2013. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 498:516–520. 10.1038/nature12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mousavi K, Zare H, Dell'Orso S, Grontved L, Gutierrez-Cruz G, Derfoul A, Hager GL, Sartorelli V. 2013. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol. Cell 51:606–617. 10.1016/j.molcel.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai F, Orom UA, Cesaroni M, Beringer M, Taatjes DJ, Blobel GA, Shiekhattar R. 2013. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature 494:497–501. 10.1038/nature11884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai F, Shiekhattar R. 2014. Enhancer RNAs: the new molecules of transcription. Curr. Opin. Genet. Dev. 25:38–42. 10.1016/j.gde.2013.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krivega I, Dean A. 2012. Enhancer and promoter interactions—long distance calls. Curr. Opin. Genet. Dev. 22:79–85. 10.1016/j.gde.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vance KW, Sansom SN, Lee S, Chalei V, Kong L, Cooper SE, Oliver PL, Ponting CP. 2014. The long non-coding RNA Paupar regulates the expression of both local and distal genes. EMBO J. 33:296–311. 10.1002/embj.201386225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bergmann JH, Spector DL. 2014. Long non-coding RNAs: modulators of nuclear structure and function. Curr. Opin. Cell Biol. 26:10–18. 10.1016/j.ceb.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]