Abstract
Impaired programmed cell death is an important contributing mechanism in the development of chronic inflammatory and autoimmune diseases. Overexpression of Bcl-2 family proteins in such diseases has led to the concept of targeted suppression of these proteins as a primary therapeutic strategy. However, limited success with this approach has prompted pharmacologists to look at the other side of the coin, with the aim of reactivating jeopardized pro-apoptotic proteins that may neutralize Bcl-2 or other anti-apoptotic molecules. In this effort, BH3-only proteins have gained recent attention as endogenous molecules for the sensitization of resistant cells to undergo apoptosis. Among the BH3-only family, Noxa stands out as exceptional for its specificity to bind Mcl-1 and Bcl-2 and blunt their biological properties. Noxa is now being tested as a promising therapeutic target in cancer biology. Nonetheless, its role and clinical application still lack validation in autoimmune diseases, including rheumatic conditions. This is partly attributed to the significant gap in our understanding of its regulatory role and how either overexpression of Noxa or delivery of BH3 mimetics could be therapeutically exploited. In this review we highlight some recent studies in RA, OA, SLE and SS suggesting that Noxa may be used as a potential therapeutic target to circumvent invasive and tissue destructive processes in these rheumatic diseases.
Keywords: apoptosis, Noxa, Bcl-2, Mcl-1, BH3-only proteins, rheumatoid arthritis, Sjögren’s syndrome, osteoarthritis, therapeutics
Introduction
Abnormalities in apoptosis can be a significant component of diseases such as cancer, autoimmune diseases, AIDS, ischaemia and neurodegenerative diseases [1]. This underlines the reason why the last 25 years have been consumed in developing new therapies that target pro- and anti-apoptotic molecules to specifically influence this mechanism of disease pathogenesis [2]. Although the majority of pharmacological strategies have been aimed at curbing the invasive or tissue destruction processes mediated by anti-apoptotic Bcl-2 family proteins, the results obtained so far have been mixed. Within the last few years, therapeutic approaches have dramatically shifted to deciphering the role of pro-apoptotic Bcl-2 family proteins to overcome the therapeutic challenges and drug resistance. In recent years Noxa, a pro-apoptotic molecule that belongs to the Bcl-2 family proteins [3], has emerged as an exceptionally promising therapeutic target because of its diverse roles in numerous pathologic processes. Dysregulation of Noxa induction can significantly contribute to the pathogenesis of numerous autoimmune diseases involving either an excess or deficiency of apoptosis.
Overview of mechanisms that control apoptosis
Programmed cell death, apoptosis, is an important biological mechanism for maintaining tissue homeostasis stimulated by either the extrinsic (death receptor) pathway or intrinsic (mitochondrial) pathway [4, 5]. The intrinsic pathway is controlled by the B cell lymphocytic-leukaemia proto-oncogene (Bcl-2) family (Table 1) [2].
Table 1.
Classification of Bcl-2 family proteins
| Multidomain |
BH3 only |
|
|---|---|---|
| Anti-apoptotic | Pro-apoptotic | Pro-apoptotic |
| Bcl-2 | Bax | Bid |
| Bcl-xL | Bak | Bad |
| Mcl-1 | — | Noxa |
| — | — | Bim |
| — | — | PUMA |
The Bcl-2 family has been classified into three subfamilies based on their function: anti-apoptotic, pro-apoptotic, and BH3 (Bcl-2 homology 3)-only proteins [6]. The Bcl-2 anti-apoptotic proteins [Bcl-2, Bcl-xL, A1 and myeloid cell leukaemia-1 (Mcl-1)] maintain equilibrium by holding the pro-apoptotic proteins in check. However, induction of the BH3-only proteins leads to inactivation of the anti-apoptotic proteins in order to activate the pro-apoptotic proteins Bax and Bak [7]. The anti-apoptotic proteins contain BH1, BH2 and BH3 domains. These three domains fold into a single globular domain having a hydrophobic groove on its surface, to which an amphipathic α-helix of about 24 residues (BH3 domain) can bind. This coupling neutralizes the pro-survival protein, allowing apoptosis to occur [8].
The BH3-only proteins are pro-apoptotic, but have only one α-helix and possess only the BH3 domain [9]. The BH3-only proteins consist of Bcl-xL/Bcl-2-asociated death promoter (Bad), Bcl-2-interacting domain (Bid), Bim, p53-upregulated modulator of apoptosis (PUMA) and PMA-induced protein (Noxa) [7, 10]. The BH3-only proteins vary in their affinity for the anti- or pro-apoptotic proteins they induce or inhibit [8]. For instance, Bid, Bim and PUMA bind to all of the anti-apoptotic proteins, while Bad and Noxa only bind with specific anti-apoptotic proteins such as Bcl-2 and Mcl-1 [8]. BH3-only proteins can also regulate Bax and Bak activation via direct or indirect mechanisms [8].
Pro-survival Bcl-2 proteins: BH3-only domain proteins
The primary role of the BH3-only proteins is to act as a sensor to inhibit anti-apoptotic proteins or to activate pro-apoptotic proteins under physiological conditions [8]. The BH3 proteins, Bad, Bid, Bim, PUMA and Noxa, can be subdivided further into direct activators or sensitizers. The direct activators, such as Bid and Bim, are dependent on their ability to irreversibly bind to Bax or Bak, suggesting they activate oligomerization at the outer mitochondrial membrane to induce apoptosis [11]. On the other hand, the sensitizers, such as Bad and Noxa, neutralize anti-apoptotic Bcl-2 family proteins to displace Bax and Bak [11]. Noxa interacts specifically with Mcl-1 and A1 only, while Bad binds to Bcl-2 and Bcl-xL. The binding of sensitizers facilitates displacement of the pro-apoptotic protein, thereby liberating them to induce apoptosis [10, 11].
Among all BH3-only domain proteins, Noxa stands out for its highly specific binding interactions with anti-apoptotic proteins. Compared with other BH3-only domain proteins, such as Bim and PUMA, that can bind to all pro-survival Bcl-2 proteins, Noxa exhibits high selectivity for Mcl-1, with a lesser affinity for A1 [12]. This notion is further verified by a study testing the selectivity of Bad, Bik, Bim, PUMA and Noxa, where all the members except Noxa had more than one target and Noxa interacted with only Mcl-1 [13]. The selectivity of Noxa to Mcl-1 can be attributed to the structural affinity of critical amino acid residues in its amphipathic α-helical BH3 domain towards the residues in the hydrophobic groove of anti-apoptotic Bcl-2 proteins. The binding properties of BH3-only proteins are so specific that a change of two amino acids in the entire BH3 domain of Noxa, into a mutant Noxa, leads to a 100-fold increase in its affinity to Bcl-xL [12].
Noxa: pathological role
Noxa regulates mitochondrial outer membrane permeabilization by monitoring the release of cytochrome c and activating downstream effector caspases [14]. The paucity in Noxa expression of such proteins has been implicated in the development of various tumour types and resistance to chemotherapeutic regimens [14]. Because of its extensive involvement in T and B cell differentiation, Noxa seems an exceptionally promising therapeutic target in malignant lymphomas [15]. In addition, the loss of Noxa has been shown to accelerate γ-radiation-induced thymic lymphomagenesis [16]. Noxa is also induced via a p53-independent pathway. In this regard, it is transcriptionally activated in hypoxic conditions and mediates hypoxia-inducible factor-1α (HIF-1α)–dependent cell death via hypoxia-responsive element (HRE) on the Noxa promoter [17]. Specifically, Noxa’s ability to displace Bak from Mcl-1 and trigger Mcl-1 degradation makes it a viable therapeutic target in cancer treatment [18].
Toll-like receptors (TLRs) are widely expressed in immune cells and play a crucial role in many aspects of the immune response. TLR3 signalling by apoptosis in human breast cancer cells serves as a potential therapeutic target in clear-cell renal cell carcinoma and melanoma [19]. Polyinosinic-polycytidylic acid [poly(I:C)], a TLR3 ligand, can induce the expression of inflammatory cytokines and type I IFN through multiple pathways [19]. Poly(I:C) can induce caspase-dependent apoptosis of LNCaP cells while increasing the expression of p53 and its target, Noxa [19]. Studies done in mouse lines of colon carcinoma and myeloma cells indicate a consistent down-regulation of Noxa expression in these cancer lines [20]. Therefore it may be beneficial for treatments of these as well as other cancers with similar pathologies to induce expression of Noxa. Bortezomib, a proteasome inhibitor currently used for the treatment of multiple myeloma, induces apoptosis through up-regulation of Noxa followed by inactivation of Mcl-1 [21].
Recent studies suggest the role of Noxa in diseases involving excess apoptosis such as HIV. Following the initial acute systemic infection, a progressive loss of CD4+ T cells occurs, primarily due to apoptosis that is regulated via the Forkhead box transcription factor O class 3a (FOXO3a) transcriptional activator [22]. FOXO3a has been shown to regulate p53 by increasing its half-life but not its transcription [22]. When the stability of p53 increases, more activation of pro-apoptotic Bcl-2 proteins such as PUMA and Noxa, which are overexpressed in HIV-1-infected cells, can occur [22]. Active p53 can also transactivate genes such as PTEN, which are found to be up-regulated in HIV-1. PTEN, in turn, reduces the phosphorylation of Akt1, resulting in reduced phosphorylation of FOXO3a. Unphosphorylated FOXO3a translocates to the nucleus and becomes transcriptionally active, which creates a positive feedback loop that accelerates apoptosis [22].
Some studies also suggest an emerging role of Noxa in oculopharyngeal muscular dystrophy (OPMD). OPMD is an autosomal dominant and slowly progressing disease. Patients with the adult onset autosomal dominant form carry a mutation in the PABPN1 gene caused by GCG repeats that predisposes them to the aggregation of the nuclear poly(A)-binding protein 1 (PABPN1) and cell death [23]. In cells expressing PABPN1-A17, pro-apoptotic proteins p53, PUMA and Noxa are up-regulated. This mediates Bax translocation to the mitochondria, release of cytochrome c, activation of caspase 3 and apoptosis [23]. Blocking p53-mediated transcription using pifithrin (a chemical inhibitor of p53) significantly reduced apoptosis.
Modification of Noxa expression
Studies involving the modulation of Noxa have provided important insights into its role and function in numerous pathological states. For instance, treatment of leukaemia cells with the pro-apoptotic agent Gossypol has been shown to increase Noxa levels and displace Bim from Mcl-1, which activates the Bak/Bax pathway and triggers the mitochondrial apoptotic pathway. Conversely, Noxa knockdown in leukaemia cells significantly prevented Gossypol-induced cell death by inhibiting the release of Bim from Mcl-1 and Mcl-1s genesis [14].
Suppression of Noxa in mice has shown a significant effect on bone mass and osteoclast survival. In one study, Noxa-deficient mice exhibited a low bone mass phenotype with an increased number of osteoclasts as the result of decreased osteoclast apoptosis [24]. Bone morphometric analysis indicated an increase in osteoclast number and eroded bone surface, which suggests that osteoclastic bone resorption was enhanced due to an increase in the osteoclast number. However, osteoblast parameters such as the bone formation rate and osteoblast number remained unchanged.
Studies suggest that Noxa is regulated by oncoprotein p53 [24]. Analysis of the osteoclast number in p53-deficient mice showed an increase in bone mass due to enhanced osteoblast differentiation despite the increased osteoclastogenesis-supporting ability of osteoblasts [24]. Glucocorticoids play a pivotal role in the proliferation of osteoblasts and glucocorticoid receptor activation up-regulates the expression of p53 and its downstream molecules p21, PUMA and Noxa, which results in growth inhibition [25]. The discrepancy between Noxa- and p53-deficient mice appears to suggest that Noxa expression is not completely dependent on p53 in osteoclasts. Noxa is reported to be induced by cyclic adenosine monophosphate (cAMP)-responsive element binding protein (CREB) families of transcription factors and as RANK ligand (RANKL) activates the CaMKIV-CREB pathway. It is therefore very tempting to postulate that CREB may be involved in Noxa induction in osteoclasts but not osteoblasts [24].
Noxa is transcriptionally activated in hypoxic conditions and mediates a p53-independent pathway of hypoxic cell death [17]. Myocyte apoptosis induced by Noxa may be attributed in part to the release of both reactive oxygen species (ROS) and reactive nitrogen species (RNS) [26]. Hypoxia and oxidative stress are primary consequences of ischaemia in cardiac myocytes and both ROS and RNS have been suggested to be involved in cardiac ischaemic injury through induction of intracellular Zn2+ (Zn2+i) release [27]. The BH3-only proteins Bnip3 and Noxa are transcriptionally induced by hypoxia through HIF-1α binding sites in their promoters [26]. HIF-1α participates largely in the adaptive process to hypoxia; paradoxically it also mediates hypoxic cell death via interaction with p53 or modulation of effector expression [17]. Inhibition of Noxa expression by antisense (AS) oligonucleotide suppressed cell death and reduced the infarction volumes induced by hypoxia in an ischaemic animal model. Interestingly, suppression of endogenous Noxa expression protected against hypoxic cell death and Noxa-transfected cells with hypoxic exposure showed significantly higher cell death rates than non-transfected cells [17]. Noxa is the first member of a pro-apoptotic gene regulated directly by both HIF-1α and p53, thus connecting two major transactivating systems responsible for cellular response to hypoxia.
Noxa and rheumatic disease
Rheumatoid arthritis
RA is a chronic, systemic autoimmune disease of unknown origin that targets the joints, resulting in inflammation and in the eventual destruction of cartilage and bone [28]. RA is characterized by inflammation and hyperplasia of the synovium, which is the result of an imbalance between proliferation and apoptosis of resident cells, including fibroblast-like synoviocytes (FLSs) [29]. T lymphocytes are suspected to contribute to synovitis and joint destruction in RA through multiple mechanisms. It may be postulated that in autoimmunity, chronic activation of autoreactive T cells may be due in part to down-regulation of Noxa, causing excessive accumulation of primed cells and an exaggerated immune response [30]. T lymphocyte accumulation within the synovial compartment as a result of increased migration, proliferation in situ or inhibition of T cell death leads to consequent failure to resolve the synovial inflammation [31]. The phenotype of synovial T cells suggests their susceptibility to apoptosis, however, these cells were found to possess resistance to apoptosis in vivo. These observations indicate that the synovial compartment in RA is a potent anti-apoptotic environment that promotes synovial T cell survival as a result of fibroblast interactions.
The balance between survival and apoptosis in T lymphocytes under homeostatic or inflammatory conditions is tightly regulated by the expression and post-translational modification of Bcl-2 family proteins [32]. Mcl-1 is highly up-regulated in RA, with enhanced expression in the synovial lining and sublining of FLSs in patients with RA. In RA-FLSs, a forced reduction of Mcl-1 expression results in apoptotic cell death mediated in part by Bak, suggesting that Mcl-1 is essential to FLS survival [33]. In particular, Mcl-1 has been implicated as a potential therapeutic target in RA because apoptosis as a result of the reduction of Mcl-1 has been shown to be relatively selective to RA-FLSs over normal FLSs [34].
Apoptotic cell death in RA-FLSs due to the reduction of Mcl-1 is thought to be mediated by Noxa. RA-FLSs express oncogenes as well as somatic mutations of the tumour-suppressor gene p53. Apoptosis mediated by p53 is attributed to transcriptional and non-transcriptional activation of pro-apoptotic Bcl-2 members, including Bax and Noxa [29]. Functional inactivation of p53 is known to result from mutations within the p53 gene. The dysregulated proliferation of RA-FLSs coupled with the lower rates of apoptosis observed in RA synovial tissue indicate that escape from p53-mediated cell cycle control may be a factor in RA pathogenesis.
Noxa induces RA-FLS apoptosis through interaction with Mcl-1, causing dissociation of Bim from Mcl-1 [14]. Bim is unable to induce apoptosis when it is bound to Mcl-1, but once it is liberated from this complex it can cause apoptosis by activation of the Bak/Bax pathway [14]. Bim is reduced in macrophages in RA synovial tissue and its reduction affects not only macrophage survival, but also the state of activation. Bim-deficient mice displayed increased activation of macrophages as compared with the control cells [35]. Arthritic mice treated with a Bim-BH3 mimetic peptide (TAT-BH3) displayed reduced oedema of the ankle, markedly lower histological scores for arthritis and fewer neutrophils and macrophages in the joints. These findings underscore the importance of the notion that therapeutic up-regulation of Noxa may be beneficial in RA patients by liberating more Bim from Mcl-1 and causing apoptosis in RA-FLSs. Additionally, up-regulation of Noxa may be especially promising as a therapeutic option in the treatment of RA because it may prevent further bone damage. Noxa plays an important role in bone homeostasis by minimizing bone loss via enhanced apoptosis of osteoclasts in bone [24]. Decreased osteoclast apoptosis is linked to many pathological conditions associated with bone loss, including osteoporosis, RA and metastatic bone tumours [24].
Osteoarthritis
OA is a common cartilage and joint disease related to age that causes an irregular cartilage structure characterized by a reduction in the number of chondrocytes, loss of extracellular matrix, synovial inflammation and irregular proliferation and death of synoviocytes [36]. Unlike in RA, osteoarthritic cartilage contains a higher percentage of cells undergoing apoptosis than normal cartilage. This is believed to be due to the increased production of nitric oxide (NO) by OA chondrocytes as a consequence of the up-regulation of NO synthase (NOS) induced by IL-1β, TNF-α and other factors [36, 37]. The pathogenic involvement of NO in arthritis was first demonstrated when levels of nitrite, a stable end product of NO metabolism, were shown to be elevated in serum and synovial fluid samples of RA and OA patients [38]. NO is a messenger implicated in the destruction and inflammation of joint tissues by inducing apoptosis in chondrocytes and the inhibition of NO synthesis has been shown to slow down cartilage degeneration [36].
The activity of mitochondrial complexes II and III is lower in OA than in normal human chondrocytes. This produces a decrease in adenosine triphosphate (ATP) levels as well as an increase in ROS generation [36]. In regard to Noxa, accumulation of ROS, including NO, is correlated with an up-regulation of Noxa [39]. It is believed that the generation of ROS is one potential mechanism of Noxa-induced apoptosis in OA chondrocytes. The generation of ROS by Noxa targets mitochondria and induces apoptosis through the loss of the mitochondrial membrane potential [17]. Because the synovial membrane is an aerobic tissue, mitochondrial integrity is likely necessary for synoviocyte survival. Studies have shown that proteins that are regulated by mitochondria, such as the Bcl-2 family, regulate the viability of synoviocytes [36]. The Mcl-1 and Bcl-xL proteins are down-regulated in the presence of NO [38]. Noxa binds specifically to Mcl-1, causing displacement of Bak and the loss of mitochondrial membrane potential through the release of cytochrome c and activation of downstream effector caspases [15].
In a disease state such as OA, where apoptosis is up-regulated in affected cells, it may be useful to develop a Noxa inhibitor to decrease the amount of NO-induced apoptosis. In one study, cells that were transfected with antioxidant N-acetylcysteine (NAC) showed reduced ROS levels and protected against cell death in Noxa-transfected cells in a concentration-dependent manner [17]. A recent report showed that the intra-articular administration of the pan-caspase inhibitor zVAD-FMK into the knees of rabbits with OA led to a significant reduction in chondrocyte apoptosis [44]. In another study, injection of hyaluronic acid (HA) in one knee of rabbits with OA in both knees showed a significant decrease in both the severity of arthritis and the production of NO [37].
Systemic lupus erythematosus
SLE is a multifactorial autoimmune disease characterized by the presence of autoantibodies, especially against nuclear components. The assortments of autoantibodies produced are broad and the consequential manifestations of the disease are diverse [40]. The oxidative damage mediated by ROS resulting in the defect in control of apoptosis and delayed clearance of apoptotic cells. This may prolong interaction between ROS and apoptotic cell macromolecules, generating neo-epitopes that subsequently create a broad spectrum of autoantibody formation leading to the tissue damage in SLE [40]. Superoxide dismutase (SOD) is a metalloprotein, considered to be the first line of defence against free radicals. It catalyses the dismutation of superoxide radical into oxygen and hydrogen peroxide. It has been suggested that antibodies to SOD are potentially responsible for increased oxidative damage in SLE patients [40]. Tolerance of self-antigens requires the deletion of autoreactive T and B cells by apoptosis. Therefore defects in inducing apoptosis could lead to the persistence of autoreactive T or B cells. Thus defective apoptosis leading to prolonged survival of pathogenic lymphocytes could be another cause of SLE [40].
Novel treatments for SLE aim to specifically target these autoreactive lymphocytes. Bz-423, a pro-apoptotic 1,4-benzodiazepine with therapeutic properties in murine models of lupus, has been linked to specifically inducing apoptosis of disease-causing lymphocytes [41]. Bz-423 selectively kills splenic CD4+ T cells, which is the lymphoid subset responsible for disease in this model, by a parallel apoptotic cascade marked by increased levels of Noxa and Bak, leading to preferential activation of Bak [41]. Activation of Bak, as a result of Mcl-1 neutralization by Noxa, overcomes Bcl-xL-dependent cell survival [41].
Sjögren’s syndrome
SS is a chronic organ-specific autoimmune disease characterized by lymphocytic and large mononuclear cell (MNC) infiltration into the salivary and lacrimal glands, resulting in keratoconjunctivitis sicca and xerostomia, B cell hyperreactivity and various serum autoantibodies [42]. SS can develop alone or in association with other autoimmune disorders such as SLE and RA [43]. In primary SS (pSS) salivary glands, expression of the anti-apoptotic proteins Bcl-2 and Bcl-xL is greater than that of pro-apoptotic proteins [44]. Down-regulation of pro-apoptotic Noxa may also play a role in pathogenesis. Studies with Noxa−/− mice indicate that Noxa plays a pivotal role in the survival of memory T cells as well as the selection of high-affinity clones in B cell expansion [30, 45]. Knockout of Noxa in mice causes increased diversity and survival of memory T cells as well as B cells [30, 45]. In SS, this may allow the survival of autoreactive T and B lymphocytes and may increase the production of pro-inflammatory cytokines, which enhances the ability of these immune effector cells to damage glandular tissue [44].
Interestingly, it has been found that excessive apoptosis may also contribute to the pathogenesis of SS [44]. Overexpression of pro-apoptotic molecules in the epithelial cells of submandibular glands has been recently shown in an animal model of SS [44]. In addition, expression of Fas and CD40 was shown to be significantly higher in SS salivary epithelial cells than in normal cells [46]. CD40 signals promote Fas-dependent death of SS salivary epithelial cells by down-regulating cellular FLICE (FADD-like IL-1β-converting enzyme) inhibitory protein (c-FLIP) expression [46]. It has been suggested that increased apoptosis in ductal and acinar epithelial cells is the main mechanism of tissue damage in primary SS [44]. Chronic and persistent immunologic activation appears to be involved in the induction of apoptotic cell death in affected glandular epithelial cells [46]. In contrast, the infiltrating MNC and autoreactive lymphocytes are resistant to apoptosis [44].
It is well recognized that a number of autoimmune conditions, including SS, predispose to the development of lymphomas [42]. Hyperactivation of autoreactive B cells is thought to be responsible in part for the development of lymphomas in SS [15]. Immunohistochemical examination of SS lymphomas has shown that the neoplastic cells are CD20+ B cells, which proliferate in the lymphoepithelial lesions caused by primary SS, and also include infiltration of a significant T cell population. Various cytokines and chemokines may also be involved in the pathogenesis of these lymphomas [42]. These interactions may allow these cells to live cooperatively in lymphoproliferative lesions and not undergo apoptosis.
In a study of long-term follow-up of 31 patients with primary SS, 52% of patients showed further lymphocytic organ damage, including lymphomas and other types of cancers [42]. Three types of lymphoproliferative disorders were seen in the SS patients that developed lymphomas [42]. It has been reported that ∼5% of patients with SS develop malignant lymphomas [42]. A secondary event, such as a mutation of p53, may transform a low-grade B cell lymphoma into a high-grade, large B cell lymphoma [15].
When lymphoepithelial lesions are stained with anti-Bcl-2 antibody, a distinct expression of Bcl-2 protein can be seen, mainly in B lymphocytes [42]. The p53 gene is thought to play an important role in the evolution of monoclonal lymphoproliferation. Partial loss of p53 tumour suppressor activity is associated with the development of low-grade lymphoma, whereas complete loss of the function is related to high-grade transformation [15]. It has been observed in other B cell lymphomas that the induction of Noxa by ROS and activation of the p53 pathway improves patient conditions and increases apoptosis in affected cells by the release of Bak from Mcl-1 through interaction with Noxa [47]. This suggests that patients with SS may also benefit from up-regulation of Noxa by controlling the dysregulation of B cell survival.
Conclusions
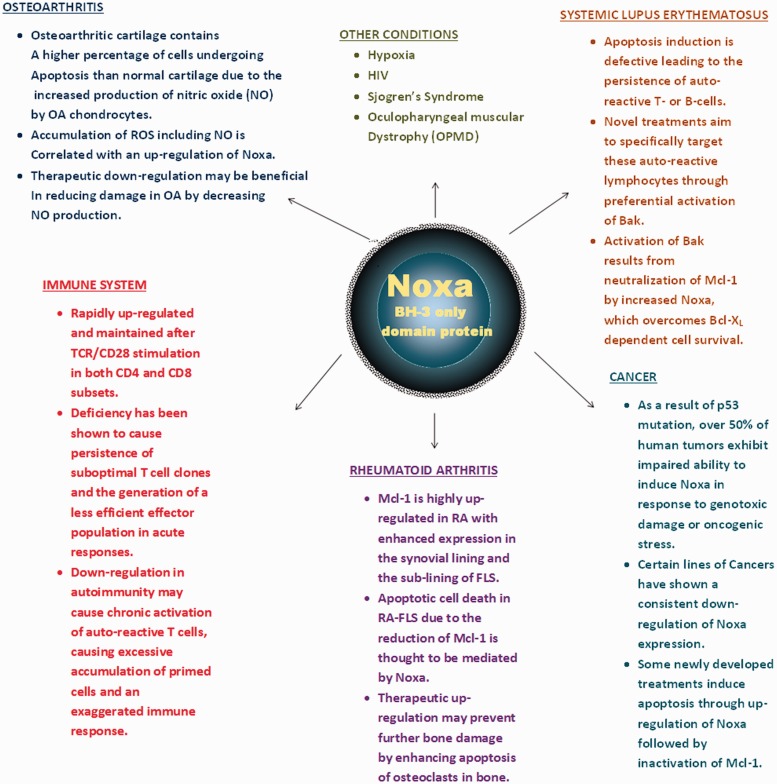
The studies summarized in this review provide novel insights into the physiological and pathological roles of Noxa in autoimmune diseases, including rheumatic conditions (summarized in Fig. 1). Although further studies are warranted to validate the fundamental role of Noxa in these diseases using experimental and/or therapeutic tools, the results from recent findings provide promise and a rationale to target Noxa for improved therapeutic outcomes in autoimmune diseases. While these findings are open to interpretation, it could be suggested that the specificity of these endogenous proteins, e.g. Mcl-1 in the case of Noxa, may serve as a valuable tool to combat a very aggressive battery of anti-apoptotic mechanisms in order to achieve optimum therapeutic benefits with minimal adverse effects. These efforts may also be complementary in overcoming the current limitations wherein the increased expression of Bcl-2 family members commonly occurs and is associated with disease progression, resistance to therapies and poor clinical outcomes.
Fig. 1.
Schematic diagram describing the involvement of Noxa in various pathological conditions
In summary, these studies provide evidence for the development of potential therapeutic strategies targeting Noxa, among the BH3-only protein family, for the amelioration of chronic rheumatic diseases. Developing a clearer understanding by modulating the expression of Noxa in the primary human diseased cells or using the preclinical models of specific rheumatic conditions will be very important in creating the foundation for designing additional therapeutic strategies in order to achieve better clinical outcomes.
Rheumatology key messages.
Emerging evidence suggests that Noxa plays an important immunological role in rheumatic diseases.
Noxa modulation in rheumatic diseases may have the potential for rapid clinical impact.
Noxa may be used as a potential therapeutic target in rheumatic diseases.
Acknowledgements
S.A. was supported in part by NIH grants R01 AR-063104 and AR-055741 and start-up funds from the University of Toledo. The authors thank Charisse Montgomery for her editorial help.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Placzek WJ, Wei J, Kitada S, et al. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010;1:40. doi: 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Thompson CB. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol. 1999;1:209–16. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- 4.Kale J, Liu Q, Leber B. Shedding light on apoptosis at subcellular membranes. Cell. 2012;151:1179–84. doi: 10.1016/j.cell.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 6.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardwick JM, Youle RJ. SnapShot: BCL-2 proteins. Cell. 2009;138:404. doi: 10.1016/j.cell.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkholi R, Floros KV, Chipuk JE. The role of BH3-only proteins in tumor cell development, signaling, and treatment. Genes Cancer. 2011;2:523–37. doi: 10.1177/1947601911417177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishna S, Low ICC, Pervaiz S. Regulation of mitochondrial metabolism: yet another facet in the biology of the oncoprotein Bcl-2. Biochem J. 2011;435:545–51. doi: 10.1042/BJ20101996. [DOI] [PubMed] [Google Scholar]
- 11.Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2008;27:S2–19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ploner C, Kofler R, Villunger A. Noxa: at the tip of the balance between life and death. Oncogene. 2008;27:S84–92. doi: 10.1038/onc.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Willis SN, Wei A, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Mazumder S, Choudhary GS, Al-Harbi S, et al. Mcl-1 phosphorylation defines ABT-737 resistance that can be overcome by increased NOXA expression in leukemic B cells. Cancer Res. 2012;72:69–3079. doi: 10.1158/0008-5472.CAN-11-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariette X. Lymphomas complicating Sjogren’s syndrome and hepatitis C virus infection may share a common pathogenesis: chronic stimulation of rheumatoid factor B cells. Ann Rheum Dis. 2001;60:1007–10. doi: 10.1136/ard.60.11.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Happo L, Strasser A, Cory S. BH3-only proteins in apoptosis at a glance. J Cell Sci. 2012;125:1081–7. doi: 10.1242/jcs.090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JY, Ahn HJ, Ryu JH, et al. BH3-only protein noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1 alpha. J Exp Med. 2004;199:113–23. doi: 10.1084/jem.20030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-x(L), but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harashima N, Inao T, Imamura R, et al. Roles of the PI3K/Akt pathway and autophagy in TLR3 signaling-induced apoptosis and growth arrest of human prostate cancer cells. Cancer Immunol Immunother. 2012;61:667–76. doi: 10.1007/s00262-011-1132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piya S, Moon AR, Song PI, et al. Suppression of IRF4 by IRF1, 3, and 7 in Noxa expression is a necessary event for IFN-gamma-mediated tumor elimination. Mol Cancer Res. 2011;9:1356–65. doi: 10.1158/1541-7786.MCR-11-0185. [DOI] [PubMed] [Google Scholar]
- 21.Ri M, Iida S, Ishida T, et al. Bortezomib-induced apoptosis in mature T-cell lymphoma cells partially depends on upregulation of Noxa and functional repression of Mcl-1. Cancer Sci. 2009;100:341–8. doi: 10.1111/j.1349-7006.2008.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabrowska A, Kim N, Aldovini A. Tat-induced FOXO3a is a key mediator of apoptosis in HIV-1-infected human CD4(+) T lymphocytes. J Immunol. 2008;181:8460–77. doi: 10.4049/jimmunol.181.12.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharjee RB, Zannat T, Bag J. Expression of the polyalanine expansion mutant of nuclear poly(A)-binding protein induces apoptosis via the p53 pathway. Cell Biol Int. 2012;36:697–704. doi: 10.1042/CBI20110348. [DOI] [PubMed] [Google Scholar]
- 24.Idrus E, Nakashima T, Wang L, et al. The role of the BH3-only protein Noxa in bone homeostasis. Biochem Biophys Res Commun. 2011;410:620–5. doi: 10.1016/j.bbrc.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Qian WW, Weng XS, et al. Glucocorticoid receptor and sequential p53 activation by dexamethasone mediates apoptosis and cell cycle arrest of osteoblastic MC3T3-E1 cells. PLoS One. 2012;7:e37030. doi: 10.1371/journal.pone.0037030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webster KA, Graham RM, Thompson JW, et al. Redox stress and the contributions of BH3-only proteins to infarction. Antioxid Redox Signal. 2006;8:1667–76. doi: 10.1089/ars.2006.8.1667. [DOI] [PubMed] [Google Scholar]
- 27.Lin CL, Tseng HC, Chen RF, et al. Intracellular zinc release-activated ERK-dependent GSK-3beta-p53 and Noxa-Mcl-1 signaling are both involved in cardiac ischemic-reperfusion injury. Cell Death Differ. 2011;18:1651–63. doi: 10.1038/cdd.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutcheson J, Perlman H. BH3-only proteins in rheumatoid arthritis: potential targets for therapeutic intervention. Oncogene. 2008;27:S168–75. doi: 10.1038/onc.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leech M, Lacey D, Xue JR, et al. Regulation of p53 by macrophage migration inhibitory factor in inflammatory arthritis. Arthritis Rheum. 2003;48:1881–9. doi: 10.1002/art.11165. [DOI] [PubMed] [Google Scholar]
- 30.Wensveen FM, Klarenbeek PL, van Gisbergen KPJM, et al. Pro-apoptotic protein Noxa regulates memory T cell population size and protects against lethal immunopathology. J Immunol. 2013;190:1180–91. doi: 10.4049/jimmunol.1202304. [DOI] [PubMed] [Google Scholar]
- 31.Salmon M, Scheel-Toellner D, Huissoon AP, et al. Inhibition of T cell apoptosis in the rheumatoid synovium. J Clin Invest. 1997;99:439–46. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abreu JRF, Grabiec AM, Krausz S, et al. The presumed hyporesponsive behavior of rheumatoid arthritis T lymphocytes can be attributed to spontaneous ex vivo apoptosis rather than defects in T cell receptor signaling. J Immunol. 2009;183:621–30. doi: 10.4049/jimmunol.0803278. [DOI] [PubMed] [Google Scholar]
- 33.Liu HT, Eksarko P, Temkin V, et al. Mcl-1 is essential for the survival of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2005;175:8337–45. doi: 10.4049/jimmunol.175.12.8337. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed S, Silverman MD, Marotte H, et al. Down-regulation of myeloid cell leukemia 1 by epigallocatechin-3-gallate sensitizes rheumatoid arthritis synovial fibroblasts to tumor necrosis factor alpha-induced apoptosis. Arthritis Rheum. 2009;60:1282–93. doi: 10.1002/art.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scatizzi JC, Hutcheson J, Pope RM, et al. Bim-Bcl-2 homology 3 mimetic therapy is effective at suppressing inflammatory arthritis through the activation of myeloid cell apoptosis. Arthritis Rheum. 2010;62:441–51. doi: 10.1002/art.27198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cillero-Pastor B, Martin MA, et al. Effect of nitric oxide on mitochondrial activity of human synovial cells. BMC Musculoskeletal Dis. 2011;12:42. doi: 10.1186/1471-2474-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz-Gallego L, Prieto JG, Coronel P, et al. Apoptosis and nitric oxide in an experimental model of osteoarthritis in rabbit after hyaluronic acid treatment. J Orthop Res. 2005;23:1370–6. doi: 10.1016/j.orthres.2005.05.003.1100230619. [DOI] [PubMed] [Google Scholar]
- 38.Kim HA, Lee KB, Bae SC. The mechanism of low-concentration sodium nitroprusside-mediated protection of chondrocyte death. Arthritis Res Ther. 2005;7:R526–35. doi: 10.1186/ar1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonino SH, van Laar J, van Oers MH, et al. ROS-mediated upregulation of Noxa overcomes chemoresistance in chronic lymphocytic leukemia. Oncogene. 2011;30:701–13. doi: 10.1038/onc.2010.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah D, Sah S, Nath SK. Interaction between glutathione and apoptosis in systemic lupus erythematosus. Autoimmun Rev. 2013;12:741–51. doi: 10.1016/j.autrev.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundberg TB, Swenson L, Wahl DR, et al. Apoptotic signaling activated by modulation of the F0F1-ATPase: implications for selective killing of autoimmune lymphocytes. J Pharmacol Exp Ther. 2009;331:437–44. doi: 10.1124/jpet.109.156422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masaki Y, Sugai S. Lymphoproliferative disorders in Sjogren’s syndrome. Autoimmun Rev. 2004;3:175–82. doi: 10.1016/S1568-9972(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 43.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manganelli P, Fietta P. Apoptosis and Sjogren syndrome. Semin Arthritis Rheum. 2003;33:49–65. doi: 10.1053/sarh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 45.Alves NL, Derks IAM, Berk E, et al. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity. 2006;24:703–16. doi: 10.1016/j.immuni.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Ping L, Ogawa N, Sugai S. Novel role of CD40 in Fas-dependent apoptosis of cultured salivary epithelial cells from patients with Sjogren’s syndrome. Arthritis Rheum. 2005;52:573–81. doi: 10.1002/art.20789. [DOI] [PubMed] [Google Scholar]
- 47.Perez-Galan P, Roue G, Villamor N, et al. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–64. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]