Abstract
Biologic therapies that target pathogenic cytokines such as TNF, IL-1β or IL-6 have greatly improved the treatment of RA. Unfortunately, not all RA patients respond to current biologic therapies and responses are not always maintained, suggesting that there are alternative drivers of RA pathogenesis that might serve as promising therapeutic targets. Discovery of the new Th17 subset of Th cells, and their role in autoimmune disease development, has implicated the proinflammatory IL-12 and IL-17 families of cytokines in RA disease pathogenesis. Members of these cytokine families are elevated in the blood and joints of RA patients and have been shown to remain elevated in patients who do not respond to current biologics. In addition, these cytokines have been shown to play roles in joint destruction and erosion. A new subclass of biologics that target the IL-12 and/or IL-17 signalling pathways are under development. Here we review evidence for a role of Th17 cells as well as IL-12 and IL-17 cytokines in RA pathogenesis as the rationale for a subsequent discussion of the ongoing and completed clinical trials of newly emerging biologic therapies directed at IL-12 or IL-17 pathway inhibition.
Keywords: biologic, Th17 cell, IL-17, IL-17A, IL-12, IL-23, rheumatoid arthritis, autoimmune disease, inflammation, synovitis
Introduction
Management goals for RA are to prevent joint damage and disability, which are best achieved by attaining disease remission [1]. Biologic therapies to inhibit cytokine-driven joint inflammation and damage have increased the likelihood of remission. Currently available biologics for RA target monocyte-derived cytokines, including TNF, IL-1β or IL-6. Biologic therapy, typically initiated with TNF blockers, is effective in most patients. However, approximately 30% of patients have inadequate responses to TNF blockers (TNF-IR), placing them at risk of further cartilage and joint damage [2]. Patients with inadequate responses to one TNF blocker can benefit from treatment with another TNF blocker or another biologic agent. Recently a small molecule Janus kinase (JAK) inhibitor with broad cytokine inhibitory activity was approved for RA [3].
Currently available cytokine blockers were developed when RA was considered a predominantly a T helper (Th)-1–mediated immunoinflammatory disease. Since then, additional subsets of CD4+ T cells, beyond the Th1/Th2 paradigm [4, 5], have been identified that play important roles in autoimmune diseases, including RA [6, 7].
Function and interplay of CD4+ T cell subsets
Th1/Th2 paradigm
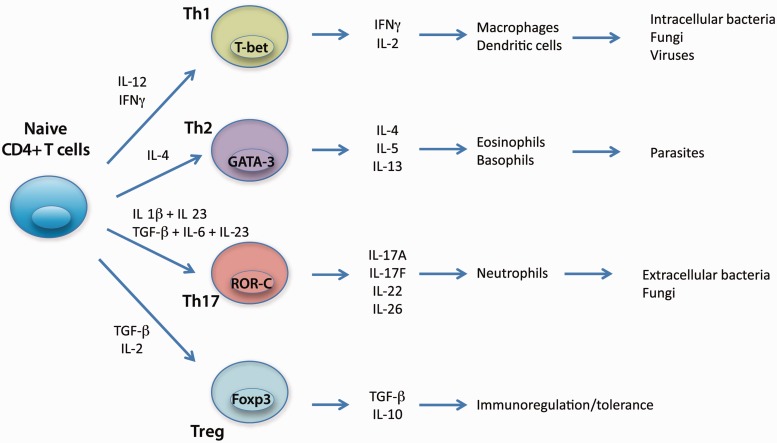
Although an oversimplification, the Th1/Th2 paradigm remains a useful construct to understand progression along the road of T cell differentiation [8, 9]. Under the combined influence of dendritic cells, antigen stimulation and the local cytokine milieu, naive CD4+ T cells differentiate into distinct Th cell subsets with specific effector functions characterized by the cytokines produced (Fig. 1).
Fig. 1.
Differentiation pathways of T helper cells.
Differentiation of naive CD4+ T cells into Th1 cells that secrete IFN-γ and participate in host defence against intracellular pathogens is regulated by the cytokine IL-12 [8, 9]. Th1 cells were originally thought to play a role in the genesis of autoimmune disease because IL-12 and IFN-γ are highly expressed at sites of inflammation [9, 10]. In RA, for example, elevated levels of IL-12 have been identified in both serum and synovial fluid of a substantial portion of patients and correlated with increased disease activity [11]. IL-12 has been shown to be expressed in rheumatoid synovial tissue, mostly by CD68+ cells in the synovial sublining, and to stimulate IFN-γ production by infiltrating T cells [10, 12, 13].
IL-12 and IL-23 and their receptors
IL-12 is a heterodimeric cytokine composed of two disulphide-linked subunits of molecular weights 35 and 40 kDa (p35 and p40) and is produced by cells of the innate immune system as well as by B cells [9, 14–16]. The IL-12 p40 subunit is shared with another structurally related heterodimeric cytokine, IL-23, which is mainly secreted by activated macrophages, dendritic cells and monocytes. In addition to the p40 subunit shared with IL-12, IL-23 is also composed of a p19 subunit. Both IL-12 and IL-23 interact with heterodimeric receptors that share a common IL-12Rβ1 subunit, to which the p40 subunit is thought to bind. The biological specificities of IL-12 and IL-23 reflect binding of IL-12 p35 to its receptor’s IL-12Rβ2 subunit and IL-23 p19 to its IL-23R subunit [17].
Distinguishing the roles of IL-12 and IL-23 in autoimmune disease
Evidence that CIA was exacerbated in mice lacking the p35 subunit, present only in IL-12, whereas mice lacking the p19 subunit, present only in IL-23, were protected from disease, suggested Th1 cells driven by IL-12 signalling were not responsible for CIA. Instead, based on these results, a role for IL-23 in autoimmune disease was recognized [13, 17–19]. Recently a study using anti-IL12 p19 antibodies suggested that IL-23 is not critical for the effector stage of CIA, but instead plays a role in T cell–mediated arthritis flare-up [20].
In RA patients, plasma IL-23 levels are elevated in early stage RA compared with patients with chronic disease or healthy volunteers [17, 21, 22]. Measured 12 months after treatment for RA, IL-23 levels correlated with disease activity assessed by the 28-joint DAS (DAS28). However, the relevance of IL-23 levels in the rheumatoid synovium is not clear. Although the IL-23 p19 subunit was abundantly expressed by synoviocytes in the synovial lining and at sites of pannus invasion, it was not always accompanied by increased expression of the IL-23 p40 subunit [22, 23]. As a result, IL-23 itself was found at low levels or was undetectable in synovial fluid. IL-17A and TNF acted synergistically to increase p19 expression, but additional inflammatory signals might be necessary for the expression of bioactive IL-23 containing both p19 and p40 subunits [24, 25].
IL-23 and discovery of Th17 cells
Insights provided by studies elucidating the role of IL-23 in autoimmune disease led to the discovery of Th17 cells, a novel T cell population promoted and stabilized by IL-23 and characterized by production of IL-17A, IL-17F, TNF and IL-6, as well as other additional novel factors [6–8] (Fig. 1). Th17 cells coordinate host defence against bacteria and fungi and are required to develop autoimmune disease in several animal models, in addition to CIA [13, 25, 26]. In humans, Th17 cell differentiation is induced by IL-1β plus IL-23, or by TGF-β and IL-6 in the presence of IL-21 or IL-23, leading to expression of retinoic acid-related orphan receptor c (ROR-c), the human counterpart of murine ROR-γt [8, 16, 27] (Fig. 1). Human Th17 cells produce IL-17A, IL-17F, IL-22, IL-26 and the chemokine CCL20, as well as expressing chemokine receptors CCR4 and CCR6. Plasticity of the Th17 cells, and not Th1 cells, is observed in studies demonstrating that a Th1/Th17 subset produces IFN-γ and IL-17A, and can differentiate into Th1 or Th17 cells in the presence of IL-12 or IL-23, respectively [9, 16].
Differentiation of Th17 vs Treg cells and interactions among these cells and selected cytokines
Tregs are a CD4+ T cell subset that have also been hypothesized to play a role in autoimmune disease, based on their ability to maintain immune homeostasis by restraining excessive proinflammatory T cell effector functions [18] (Fig. 1). Tregs are characterized by high expression of CD25 (the alpha chain of the IL-2 receptor) and expression of the transcription factor Forkhead box P3 (Foxp3). Interestingly, Tregs also exhibit plasticity and do not appear to be terminally differentiated, since they can be converted into Th17 cells by IL-2 or IL-15, a process enhanced by inflammatory cytokines such as IL-1β, IL-23 or IL-21 [9, 28]. The transition from defensive inflammatory response to autoimmune disease such as RA might depend on the relative activities of Th17 and Treg cells as well as the cytokine milieu driving differentiation into these subsets.
Role of Th17 cells in RA pathogenesis
In addition to animal models of autoimmune disease, ample clinical evidence exists to support a role for Th17 cells in RA. Th17 cells are increased in the peripheral circulation of RA patients, including those with treatment-naive, early stage disease compared with healthy subjects [29, 30], and have been identified in synovial biopsies and in the synovial fluid of RA patients [31–33]. Among samples taken from patients with early RA, co-culture of Th17 cells with synoviocytes usually stimulated the release of proinflammatory cytokines and MMPs [34], but not Th1 cells or naive T cells [13, 34].
Tregs also accumulate in the joints of patients with RA [35]. Recent evidence suggests that the RA inflammatory cytokine milieu impairs regulatory activity against effector T cells and activated monocytes [36]. Blocking TNF or IL-6 restores Treg function in vitro [36]. It remains to be determined if Tregs in RA have reduced activity against Th17 cells relative to Th1 and Th2 clones, as has been demonstrated in patients with Crohn’s disease [37]. Taken together, the evidence suggests that the inflammatory cytokine environment helps determine the balance between Th17 cells and Tregs in the rheumatoid joint.
IL-17A and its receptors
Th17 cells produce the proinflammatory cytokine IL-17A and many of the inflammatory activities in autoimmune disease have been attributed to this cytokine. IL-17A, a 155 amino acid, 15-kDa glycoprotein, is a member of a unique cytokine family comprising five other members (designated IL-17B through IL-17F). Th17 cells produce IL-17A and IL-17F, each as disulphide-linked homodimers, but IL-17A–IL-17F heterodimers have also been detected [9, 26]. Both IL-17A and IL-17F activate a heterodimeric receptor complex composed of IL-17RA and IL-17RC protein subunits. IL-17F is less potent than IL-17A in rheumatoid synoviocytes and regulates proinflammatory gene expression by a similar, but not identical, signalling pathway downstream of IL-17RA and IL-17RC [38].
IL-17A expression in RA
High concentrations of IL-17A in blood and synovial fluid are associated with disease severity in RA and with disease markers such as anti-citrullinated protein (CCP) antibodies, suggesting elevated IL-17A signifies a more severe clinical course in RA [22, 32, 39, 40]. A longitudinal study of two independent cohorts of RA patients treated with anti-TNF biologics found high baseline circulating Th17 cell levels correlated with a lack of response to anti-TNF therapy [41]. Peripheral blood cells from non-responder patients demonstrated increased stimulated IL-17 production compared with responder patients. These findings suggest that incomplete responses to TNF blockade in patients with inflammatory arthritis associate with the Th17 cells and IL-17 pathways.
Early studies showed that synovial explants from RA patients, but not OA patients, produced IL-17A ex vivo, as measured by bioassay [32, 42]. Immunostaining revealed that cells secreting IL-17A were localized to T cell–rich areas of the synovium. Although Th17 cells are a prominent source of IL-17A, other cell types can also produce IL-17A, including CD8+ T cells, γδ T cells, NK T cells, NK cells, neutrophils, macrophages, eosinophils and mast cells [9, 43]. Therefore IL-17A found in the rheumatoid joint may be derived from a wide range of cells in the adaptive and innate immune systems.
Biological relevance of IL-17A in RA
Effects on inflammation
IL-17A induced the release of proinflammatory cytokines (e.g. IL-1β, TNF and IL-6) and chemokines (e.g. IL-8 driving neutrophil recruitment; CCL20 driving recruitment of CCR6+ cells, including Th17 cells and dendritic cells) from rheumatoid synovial cells [13, 44, 45]. IL-17A also stimulated CCL2 expression on synovial fibroblasts and macrophages, which resulted in monocyte migration into synovial tissue [45]. In many experimental systems, the effects of IL-17A on cytokine and chemokine production were augmented by the presence of TNF or other inflammatory cytokines [46]. Notably, IL-17A down-regulated expression of IL-12Rβ2, thereby reinforcing commitment of CD4+ T cells to a Th17 rather than Th1 lineage [44].
Effects on joint erosion
IL-17A stimulated production of MMPs by rheumatoid synoviocytes and synovial explants, which enhance matrix turnover and cartilage destruction [13, 47]. IL-17A caused sustained expression of synoviolin in rheumatoid synoviocytes, which is an E3 ubiquitin ligase implicated in synovial hyperplasia and disease chronicity in RA [48]. Finally, IL-17A induced osteoclast differentiation by several mechanisms, including stimulation of RANK ligand (RANKL) expression on osteoblasts, up-regulation of RANK on osteoclast precursors and the production of proinflammatory cytokines from synoviocytes and macrophages, ultimately leading to bone erosion [49, 50].
In experimental models, overexpression of IL-17A induced joint inflammation, cartilage damage and bone erosion, whereas blocking IL-17A protected against these effects [32, 51]. IL-17A inhibition prevented development of arthritis or attenuated arthritis in an established disease paradigm. Moreover, development of CIA was suppressed in IL-17-deficient mice. In a 2-year prospective study in RA patients, synovial expression of IL-17A mRNA predicted progressive joint damage and showed a synergistic interaction with TNF expression [52]. This association was most pronounced in patients with shorter disease durations.
Effects on vasculature
IL-17A induced production of angiogenic factors, microvascular endothelial cell migration and tube formation, suggesting that it serves as an angiogenic mediator in RA [53]. IL-17A caused cytoskeletal rearrangement in rheumatoid synovial fibroblasts and dermal endothelial cells, leading to increased cell migration and invasion [54]. Finally, IL-17A has been associated with impaired microvascular function and arterial compliance, and thereby may contribute to the enhanced cardiovascular risk associated with RA [55].
Biological relevance of IL-23 in RA
In addition to its role in RA pathogenesis mediated by Th17 cell proliferation, maintenance and IL-17A production, IL-23 plays a direct role in RA pathogenesis [15, 17]. Several observations suggest that IL-23 directly promotes osteoclastogenesis and erosive disease. IL-23 induced RANKL expression on CD4+ cells, which was localized to sites where pannus invaded bone in a murine arthritis model [16, 56]. IL-23 also induced RANKL on rheumatoid synoviocytes, which promoted osteoclast differentiation when co-cultured with osteoclast precursors [57]. Finally, IL-23 induced RANK expression on myeloid precursors, thereby committing these cells to RANKL-mediated osteoclast differentiation [17, 58].
Genetic variants of IL-12/-23 linked with RA
A role for IL-12 in RA susceptibility has recently been proposed. There was no direct association between IL-12 gene polymorphisms and RA [59]. However, associations were reported with polymorphisms in the signal transducer and activator of transcription (STAT) 4 gene, encoding a transcription factor involved in the signalling pathways of IL-12 and IL-23 [60].
The frequency of a single nucleotide polymorphism in the gene encoding IL-23R is higher in healthy volunteers compared with patients with autoimmune diseases, suggesting a protective role for this genotype [61]. This particular polymorphism encodes a single amino acid substitution (R381Q) in the cytoplasmic domain of IL-23R. Among patients with RA, those harbouring the protective genotype had lower serum levels of IL-17A compared with patients harbouring the more common genotype [62]. These data suggest that altered function of IL-23R modulates IL-17A production and indicates an integrated IL-23/IL-17 axis.
Outstanding questions
Cytokines play important roles in host defence and therefore cytokine inhibition potentially increases the risk of serious infection and other immune-mediated diseases. TNF blockers, for example, are well recognized to cause tuberculosis reactivation and to generally increase infection risk [63].
IL-17A, a key effector of Th17 cells, is involved in neutrophil-mediated host defence against extracellular bacteria and some fungi [8]. Genetic deficiency in IL-17RA, a receptor subunit involved in both IL-17A and IL-17F signalling, is associated with recurrent or persistent mucocutaneous infections caused by Candida albicans, and to a lesser extent by Staphylococcus aureus, but not with other infections or autoimmune manifestations [64]. Although both IL-17A and IL-17F are necessary for mucocutaneous immunity against C. albicans, they have largely overlapping functions, and therefore blocking only one of these cytokines may have a small effect on infection risk.
It remains to be determined whether blocking IL-17A alone, both IL-17A and IL-17F, or all Th17 cytokines provides the best efficacy vs safety profile in RA. IL-17F shares a similar spectrum of biologic activity with IL-17A and works through the same receptor complex, but is typically less potent, suggesting that dual blockade does not substantially improve efficacy, but as noted above, enhances mucocutaneous infection risk. Both therapeutic approaches—selectively targeting IL-17A vs blocking the effects of both IL-17A and IL-17F—are under clinical evaluation. IL-21 and IL-22 are other Th17 products, and both cytokines and/or their receptors are expressed in rheumatoid synovium [15, 16], but their contribution to joint inflammation and damage are unclear.
Finally, the importance of IL-17A relative to other cytokines in RA (e.g. TNF, IL-6, IL-1β) as well as the relative importance of the Th17 vs Th1 pathways may differ between patients or disease stages. Accordingly, it will be crucial to identify biomarkers in RA patients most likely to respond to IL-17A blockers and at which disease stage they are likely to benefit most.
IL-12, IL-23 and IL-17A as potential therapeutic targets in RA
IL-12/-23 inhibitors in RA
Several compounds have been or are currently in development to inhibit IL-12/IL-23 as therapy for autoimmune disease, some specifically in RA. Currently ustekinumab, a monoclonal antibody directed against the shared p40 subunit of both IL-12 and IL-23, as well as anti-P19 antibody therapies are in development for autoimmune diseases including psoriatic arthritis (where limited but real efficacy was found) and in RA [65].
A potential drawback of IL-12/IL-23 inhibition is broad immune function suppression that might arise due to diminished function of both Th1 and Th17 cells [17]. Clinical studies comparing inhibition of p40 and p19 subunits might clarify whether specifically targeting IL-23 results in less infection risk.
Other approaches to IL-12/IL-23 inhibition
Most cytokines that bind to type I or type II cytokine receptors employ JAK family members [JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2)] and STATs to generate cell-specific responses [66]. Signalling pathways for IL-12 and IL-23 comprise two receptor molecules and signalling proteins: JAK2 and TYK2. Targeting JAK2 is a potential alternative therapeutic approach to inhibiting IL-12/IL-23 signalling in RA [21, 22].
Several orally administered JAK inhibitors are approved or under development for RA (Table 1). The most advanced is tofacitinib, an inhibitor of JAK1, JAK2 and JAK3, which was approved for an RA indication by the US Food and Drug Administration (FDA) in November 2012 [21]. Other JAK inhibitors are at earlier development stages. In a dose-ranging phase 2b study in patients with RA treated with DMARD therapy, the JAK1/JAK2 kinase inhibitor baricitinib (formerly INCB028050), at doses of 1, 2, 4 and 8 mg twice daily for 12 weeks, increased the ACR 20% response rate (ACR20) (57%, 54%, 75% and 78%, respectively, vs 41%), ACR50 (31%, 17%, 35% and 40%, respectively, vs 10%) and ACR70 (12%, 8%, 23% and 20%, respectively, vs 2%) response rates compared with placebo [67]. GLPG0634 is a kinase inhibitor with 30-fold selectivity for JAK1 compared with JAK2 [68]. In a proof-of-concept study including 36 active RA patients, ACR20 responses at 4 weeks were observed in 83% of patients receiving GLPG0634 vs 33% of patients receiving placebo (P < 0.01). VX-509 is a kinase inhibitor with cellular selectivity for JAK3 of 25- to 150-fold relative to other JAKs, but with enzymatic selectivity <10-fold [69]. A 12-week phase 2 trial in patients with moderate to severe RA who failed a non-biologic DMARD and were biologic naive resulted in ACR20 response rates as high as 66% for the highest (150 mg) dose vs 29% for placebo. These compounds have the potential to both inhibit IL-12/IL-23 signalling intracellularly and show efficacy in RA. However, these cytokine effects are indirect and secondary and therefore are not reviewed in detail here.
Table 1.
Current status of investigational agentsa
| Drug (mechanism) | Status in RA | Key ongoing trials in RA | Other indications |
|---|---|---|---|
| Secukinumab (anti-IL-17A mAb) | Phase 3 | 24-week trial in TNF-IR patients on stable MTX, with up to 2-year extension (NCT01377012); 24-week trial in TNF-IR patients on stable dose of conventional DMARD with up to 1-year extension (NCT01350804) |
|
| Ixekizumab (anti-IL-17A mAb) | Phase 2 | 1-year extension in biologic-naive and TNF-IR patients (NCT00966875) | Plaque psoriasis (phase 3) |
| Ustekinumab (anti-p40 mAbb) | Phase 2 | 28-week trial in patients with active RA despite MTX vs CNTO 1959 or placebo (NCT01645280) |
|
| Tofacitinib (JAK1, 2 and 3 inhibitor) | NDA filed | Completed |
|
| Baricitinib (JAK1/2 inhibitor) | Phase 2 | 12-week trial in patients on background MTX, with 12-week extension (NCT01185353) | Plaque psoriasis (phase 2) |
| GLPG0634 (JAK1 inhibitor) | Phase 2 | 4-week trial in patients refractory to MTX completed (NCT01384422); 4-week trial in patients refractory to MTX (NCT01668641) | None |
| VX-509 (JAK3 inhibitor) | Phase 2 | 12-week trial in patients who failed ≥1 DMARD and 0–1 biologic completed (NCT01052194); 24-week trial in patients on stable MTX (NCT01590459) | None |
aStatus based on information available at clinicaltrials.gov as of 13 March 2013. bBlocks IL-12 and IL-23.
IL-17A inhibitors in RA
Several biologics targeting IL-17 are in clinical development for RA, including secukinumab (AIN457), ixekizumab (LY2439821) and brodalumab (AMG-827) (Table 2). Secukinumab, a fully human IgG1κ monoclonal antibody directed against IL-17A, was evaluated in a randomized, placebo-controlled, proof-of-concept trial in active RA patients on stable MTX therapy [70]. Secukinumab (10 mg/kg i.v. at baseline and week 3) increased ACR20 response rates and reduced DAS28 scores and CRP levels 1 week after the first infusion; these improvements were maintained until the end of the 16-week study [71]. When analysed by the area under the treatment response–time curve, secukinumab significantly improved ACR20 response rates (P = 0.011), baseline-adjusted DAS28 scores (P = 0.027) and baseline-adjusted CRP (P = 0.002) compared with placebo. The safety profile of secukinumab was comparable to placebo and was consistent with that of an active RA patient population.
Table 2.
Clinical trial results with IL-17 inhibitors in RA
| Reference | Treatment | Patients | Main efficacy results |
|---|---|---|---|
| Hueber et al. (POC trial) [70, 71] | Secukinumab (10 mg/kg i.v. at baseline and week 3) vs placebo | n = 52 on stable MTX ≤ 25 mg/week |
|
| Genovese et al. (phase 2) [72] | Secukinumab (25, 75, 150 or 300 mg s.c. monthly) vs placebo | n = 237 on stable MTX |
|
| Genovese et al. (POC trial) [72, 73] | Ixekizumab (0.2, 0.6 or 2 mg/kg i.v. every 2 weeks for five doses) vs placebo | n = 77 on stable dose of ≥ 1 DMARDa |
|
| Genovese et al. (phase 2) [73] | Ixekizumab (3, 10, 30, 80 or 180b mg at baseline and weeks 1, 2, 4, 6, 8 and 10) vs placebo | n = 260 biologically naive patents and n = 188 TNF-IR patients on stable conventional DMARDs |
|
ACR20: American College of Rheumatology 20% response rate; AUC: area under the response-vs-time curve; DAS28: 28-joint disease activity score; NS: not significant; POC: proof of concept; TNF-IR: TNF blocker inadequate responders. aMTX, HCQ, SSZ, LEF or AZA. bOnly the two highest doses were given to the TNF-IR cohort.
Secukinumab (25, 75, 150 or 300 mg s.c. monthly) was evaluated in a randomized controlled phase 2 trial in RA patients on stable MTX therapy [72]. ACR20 response rates were numerically higher with secukinumab 75–300 mg (47–54%) than placebo (36%) at the 16-week primary endpoint, but response rate differences from placebo did not reach statistical significance. Patients with ACR20 responses at 16 weeks continued on the same dose until week 52, whereas non-responders received escalating doses, and patients initially allocated to placebo received secukinumab 150 mg. Initial responders maintained responses through week 52. For those receiving the 150-mg dose throughout the study, ACR20 response rates were 75% and 90% at weeks 24 and 52, respectively. Responders also had sustained reductions in DAS28-CRP and improvements in HAQ disability index (HAQ-DI). In contrast, non-responders did not benefit from escalation of secukinumab. Secukinumab was well tolerated; adverse events were mostly mild to moderate and led to discontinuation in 6.9% of patients. The overall rate of infection in the 1-year study was 31.9%.
Ixekizumab, a humanized IgG4 monoclonal antibody directed against IL-17A, was evaluated in a two-part, double-blind, proof-of-concept trial in RA patients receiving stable doses of one or more conventional DMARDs [72]. The first part of the study was a dose-escalation phase with single i.v. doses, whereas the second part had a randomized, double-blind, parallel-group design in which patients received ixekizumab (0.2, 0.6 or 2 mg/kg i.v.) or placebo every 2 weeks for a total of five doses. Most patients in the second part received 2 (81%) or 3 (13%) concomitant DMARDs. DAS28 scores declined by week 1 in the ixekizumab groups and remained lower than the placebo group through the end of the 16-week follow-up. At the week 10 primary endpoint, ixekizumab (all doses combined) significantly reduced DAS28 scores from baseline compared with placebo (−2.3 vs −1.7; P ≤ 0.05) [73]. ACR20, ACR50 and ACR70 response rates were numerically higher with ixekizumab compared with placebo at week 10 (78% vs 56%, 39% vs 17% and 24% vs 6%, respectively). Certain dose levels and time points showed significantly increased responses compared with placebo. Adverse events were unrelated to ixekizumab dose; leucopoenia and vertigo (both 6.8%) were common adverse events that occurred more frequently with drug than placebo.
The subsequent phase 2 trial compared ixekizumab vs placebo in 260 biologic-naive patients and 188 TNF-IR patients on stable DMARD therapy [74]. Ixekizumab (3–180 mg s.c. at baseline and weeks 1, 2, 4, 6, 8 and 10) produced a dose-related increase in ACR20 response rates at week 12 in the biologic-naive cohort. Response rates at week 12 were significantly higher, compared with placebo, in the ixekizumab 30-mg dose group. Response rates at earlier time points were significantly higher, compared with placebo, in the group receiving the highest ixekizumab dose. In the TNF-IR cohort, ixekizumab 80 or 180 mg yielded significantly higher ACR20 response rates compared with placebo. At week 12, other efficacy parameters also favoured ixekizumab over placebo. Adverse event rates were similar across treatment groups, with slightly higher infection rates with ixekizumab vs placebo in the biologic-naive cohort (25% vs 19%) and TNF-IR cohort (27% vs 23%).
Brodalumab (AMG-827) is a fully human monoclonal antibody to IL-17RA that blocks signalling of both IL-17A and IL-17F, and possibly IL-17E [74]. Brodalumab was evaluated in a 16-week, phase 2 study in biologic-naive RA patients receiving a stable dose of MTX (15–25 mg weekly) for ≥4 weeks and results are pending (NCT00950989) [75]. In the study, patients were randomized to receive brodalumab (70, 140 or 210 mg s.c.) or placebo at baseline and weeks 1 and 2, and then every other week. ACR50 at week 12 occurred in 16% (70 mg), 16% (140 mg), 10% (210 mg) and 13% (placebo) of patients. Since the primary endpoint was not met, the brodalumab RA programme has been discontinued.
New biologics compared with existing biologics
It is tempting to compare response rates achieved with the IL-12/IL-23 and IL-17A inhibitors relative to currently available biologics, including TNF blockers, anti-CD20 (rituximab) and anti-IL-6R (tocilizumab). Comparative clinical trials, however, have not yet been performed, and patient populations across trials differed in disease duration and prior treatment history, which in turn influences treatment response. In controlled clinical trials, TNF blockers typically produced ACR20 response rates of approximately 60% at 24 weeks in patients on stable MTX therapy. Meta-analyses of these trials found a relative risk of approximately two for TNF blockers compared with MTX alone [76, 77]. Tocilizumab at the recommended starting dose of 4 mg/kg produced ACR20 response rates of 48% and 61% in two randomized controlled trials (vs 26% and 41%, respectively, for MTX alone) [78, 79], and rituximab produced ACR20 response rates of 50% and 55% in patients on background MTX who had or had not failed previous TNF blocker therapy, respectively (vs 18% and 28%, respectively, for MTX alone) [80, 81]. Response rates achieved in dose-ranging studies with new biologics fall within a range similar to those for currently available agents. This conclusion must be tempered by relatively high background response rates in the control arms of several trials [67, 69, 71, 73]. The safety profile may be a distinguishing feature for new biologics, particularly IL-17A inhibitors.
Conclusions
Advances in understanding of T cell differentiation involved in autoimmunity and cytokines that drive RA pathogenesis have led to the identification of new cytokine therapeutic targets, including IL-12, IL-23 and IL-17A. New biologic or small molecule therapies that block these cytokines or their signalling are at varying stages of clinical development, and some have yielded promising results to date. These agents are likely to be used in patients who have active disease despite the use of MTX or another conventional DMARD, either as an alternative to TNF blockers or as an option in the TNF-IR population.
Rheumatology key messages.
Biologics improved RA remission rates, but response rates are not always complete or long lasting.
Th17 cells and members of the IL-12 and IL-17 cytokine families play a role in RA pathogenesis.
Clinical studies with IL-12 and IL-17 family pathway inhibitors confirm these cytokines are promising targets for inflammatory arthritis treatment.
Acknowledgements
Editorial support was provided by Barry Weichman, PhD, and Hannah Lederman of BioScience Communications, New York, NY, USA.
Funding: This work was supported by Novartis Pharma AG, Basel, Switzerland.
Disclosure statement: P.E. has provided expert advice and undertaken clinical trials for Pfizer, Merck, Abbott, UCB, BMS, Roche and Novartis. D.F. has received grants/research support from AbbVie, Actelion, Amgen, BMS, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech and UCB and has received honoraria as a consultant for AbbVie, Actelion, Amgen, BMS, Janssen, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech and UCB and for participation on speakers’ bureaus (CME only) for AbbVie, Actelion and UCB.
References
- 1.Horton S, Walsh C, Emery P. Established rheumatoid arthritis: rationale for best practice: physicians’ perspective of how to realize tight control in clinical practice. Best Pract Res Clin Rheumatol. 2011;25:509–21. doi: 10.1016/j.berh.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Mewar D, Wilson AG. Treatment of rheumatoid arthritis with tumour necrosis factor inhibitors. Br J Pharmacol. 2011;162:785–91. doi: 10.1111/j.1476-5381.2010.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451–60. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 4.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 5.McInnes I, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 6.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 7.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 9.Zhu S, Qian Y. IL-17/IL-17 receptor system in autoimmune disease: mechanisms and therapeutic potential. Clin Sci (Lond) 2012;122:487–511. doi: 10.1042/CS20110496. [DOI] [PubMed] [Google Scholar]
- 10.Paunovic V, Carroll HP, Vandenbroeck K, et al. Signalling, inflammation and arthritis: crossed signals: the role of interleukin (IL)-12, -17, -23 and -27 in autoimmunity. Rheumatology. 2008;47:771–6. doi: 10.1093/rheumatology/kem352. [DOI] [PubMed] [Google Scholar]
- 11.Kim W, Min S, Cho M, et al. The role of IL-12 in inflammatory activity of patients with rheumatoid arthritis (RA) Clin Exp Immunol. 2000;119:175–81. doi: 10.1046/j.1365-2249.2000.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morita Y, Yamamura M, Nishida K, et al. Expression of interleukin-12 in synovial tissue from patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:306–14. doi: 10.1002/1529-0131(199802)41:2<306::AID-ART15>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Brennan F, McInnes I. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–45. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722–8. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pope RM, Shahrara S. Possible roles of IL-12-family cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2013;9:252–6. doi: 10.1038/nrrheum.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Astry B, Harberts E, Moudgil K. A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J Interferon Cytokine Res. 2011;31:927–40. doi: 10.1089/jir.2011.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rong C, Hu W, Wu F, et al. Interleukin-23 as a potential target for rheumatoid arthritis. Mol Cell Biochem. 2012;361:243–8. doi: 10.1007/s11010-011-1109-6. [DOI] [PubMed] [Google Scholar]
- 18.Mai J, Wang H, Yang X-F. Th 17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity. Front Biosci. 2010;15:986–1006. doi: 10.2741/3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–7. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornelissen F, Asmawidjaja PS, Mus AM, et al. IL-23 dependent and independent stages of experimental arthritis: no clinical effect of therapeutic IL-23p19 inhibition in collagen-induced arthritis. PLoS One. 2013;8:e57553. doi: 10.1371/journal.pone.0057553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmussen TK, Andersen T, Hvid M, et al. Increased interleukin 21 (IL-21) and IL-23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J Rheumatol. 2010;37:2014–20. doi: 10.3899/jrheum.100259. [DOI] [PubMed] [Google Scholar]
- 22.Tang C, Chen S, Qian H, et al. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112–24. doi: 10.1111/j.1365-2567.2011.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brentano F, Ospelt C, Stanczyk J, et al. Abundant expression of the interleukin (IL)23 subunit p19, but low levels of bioactive IL23 in the rheumatoid synovium: differential expression and Toll-like receptor-(TLR) dependent regulation of the IL23 subunits, p19 and p40, in rheumatoid arthritis. Ann Rheum Dis. 2009;68:143–50. doi: 10.1136/ard.2007.082081. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg M, Nadiv O, Luknar-Gabor N, et al. Synergism between tumor necrosis factor α and interleukin-17 to induce IL-23 p19 expression in fibroblast-like synoviocytes. Mol Immunol. 2009;46:1854–9. doi: 10.1016/j.molimm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanov S, Linden A. Interleukin-17 as a drug target in human disease. Trends Pharmacol Dis. 2008;30:95–103. doi: 10.1016/j.tips.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Annunziato F, Romagnani S. Heterogeneity of human effector CD4+ T cells. Arthritis Res Ther. 2009;11:257. doi: 10.1186/ar2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koenen HJ, Smeets RL, Vink PM, et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–52. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 29.Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–56. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 30.Leipe J, Grunke M, Dechant C, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–85. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 31.Pène J, Chevalier S, Preisser L, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423–30. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 32.van den Berg W, Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:549–553. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 33.Gullick NJ, Evans HG, Church LD, et al. Linking power Doppler ultrasound to the presence of Th17 cells in the rheumatoid arthritis joint. PLoS One. 2010;5:e12516. doi: 10.1371/journal.pone.0012516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Hamburg JP, Asmawidjaja P, Davelaar N, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- 35.Möttönen M, Heikkinen J, Mustonen L, et al. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–7. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrath J, Müller M, Amoudruz P, et al. The inflammatory milieu in the rheumatoid joint reduces regulatory T-cell function. Eur J Immunol. 2011;41:2279–90. doi: 10.1002/eji.201041004. [DOI] [PubMed] [Google Scholar]
- 37.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hot A, Zrioai S, Toh ML, et al. IL-17A- vs. IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341–8. doi: 10.1136/ard.2010.132233. [DOI] [PubMed] [Google Scholar]
- 39.Suurmond J, Dorjée AL, Boon MR, et al. Mast cells are the main interleukin 17-positive cells in anticitrullinated protein antibody-positive and -negative rheumatoid arthritis and osteoarthritis synovium. Arthritis Res Ther. 2011;13:R150. doi: 10.1186/ar3466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Metawi SA, Abbas D, Kamal MM, et al. Serum and synovial fluid levels of interleukin-17 in correlation with disease activity in patients with RA. Clin Rheumatol. 2011;30:1201–7. doi: 10.1007/s10067-011-1737-y. [DOI] [PubMed] [Google Scholar]
- 41.Alzabin S, Abraham S, Taher T, et al. Incomplete response of inflammatory arthritis to TNFα blockade is associated with Th17 pathway. Ann Rheum Dis. 2012;71:1741–8. doi: 10.1136/annrheumdis-2011-201024. [DOI] [PubMed] [Google Scholar]
- 42.Ziolkowska M, Koc A, Luszczykiewicz G, et al. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164:2832–8. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- 43.Hueber AJ, Asquith DL, Miller AM, et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184:3336–40. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 44.Toh ML, Kawashima M, Hot A, et al. Role of IL-17 in the Th1 systemic defects in rheumatoid arthritis through selective IL-12Rbeta2. Ann Rheum Dis. 2010;69:1562–7. doi: 10.1136/ard.2009.111757. [DOI] [PubMed] [Google Scholar]
- 45.Shahrara S, Pickens SR, Mandelin AM, II, et al. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol. 2010;184:4479–87. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koenders MI, Joosten LA, van den Berg WB. Potential new targets in arthritis therapy: interleukin (IL)-17 and its relation to tumor necrosis factor and IL-1 in experimental arthritis. Ann Rheum Dis. 2006;65(Suppl 3):29–33. doi: 10.1136/ard.2006.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moran EM, Mullan R, McCormick J, et al. Human rheumatoid arthritis tissue production of IL-17A drives matrix and cartilage degradation: synergy with tumour necrosis factor-alpha, Oncostatin M and response to biologic therapies. Arthritis Res Ther. 2009;11:R113. doi: 10.1186/ar2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toh ML, Gonzales G, Koenders MI, et al. Role of interleukin 17 in arthritis chronicity through survival of synoviocytes via regulation of synoviolin expression. PLoS One. 2010;5:e13416. doi: 10.1371/journal.pone.0013416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotake S, Yago T, Kawamoto M, et al. Role of osteoclasts and interleukin-17 in the pathogenesis of rheumatoid arthritis: crucial ‘human osteoclastology’. J Bone Miner Metab. 2012;30:125–35. doi: 10.1007/s00774-011-0321-5. [DOI] [PubMed] [Google Scholar]
- 50.Adamopoulos IE, Chao CC, Geissler R, et al. Interleukin-17A upregulates receptor activator of NF-κB on osteoclast precursors. Arthritis Res Ther. 2010;12:R29. doi: 10.1186/ar2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lubberts E, Joosten LA, Oppers B, et al. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–13. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 52.Kirkham BW, Lassere MN, Edmonds JP, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54:1122–31. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 53.Pickens SR, Volin MV, Mandelin AM, II, et al. IL-17 contributes to angiogenesis in rheumatoid arthritis. J Immunol. 2010;184:3233–41. doi: 10.4049/jimmunol.0903271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moran EM, Connolly M, Gao W, et al. Interleukin-17A induction of angiogenesis, cell migration, and cytoskeletal rearrangement. Arthritis Rheum. 2011;63:3263–73. doi: 10.1002/art.30582. [DOI] [PubMed] [Google Scholar]
- 55.Marder W, Khalatbari S, Myles JD, et al. Interleukin 17 as a novel predictor of vascular function in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1550–5. doi: 10.1136/ard.2010.148031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ju JH, Cho ML, Moon YM, et al. IL-23 induces receptor activator of NF-kappaB ligand expression on CD4+ T cells and promotes osteoclastogenesis in an autoimmune arthritis model. J Immunol. 2008;181:1507–18. doi: 10.4049/jimmunol.181.2.1507. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Kim KW, Cho ML, et al. IL-23 induces receptor activator of NF-kappaB ligand expression in fibroblast-like synoviocytes via STAT3 and NF-kappaB signal pathways. Immunol Lett. 2010;127:100–7. doi: 10.1016/j.imlet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 58.Chen L, Wei XQ, Evans B, et al. IL-23 promotes osteoclast formation by up-regulation of receptor activator of NF-kappaB (RANK) expression in myeloid precursor cells. Eur J Immunol. 2008;38:2845–54. doi: 10.1002/eji.200838192. [DOI] [PubMed] [Google Scholar]
- 59.Orozco G, González-Gay MA, Paco L, et al. Interleukin 12 (IL12B) and interleukin 12 receptor (IL12RB1) gene polymorphisms in rheumatoid arthritis. Hum Immunol. 2005;66:710–5. doi: 10.1016/j.humimm.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Liang YL, Wu H, Shen X, et al. Association of STAT4 rs7574865 polymorphism with autoimmune diseases: a meta-analysis. Mol Biol Rep. 2012;39:8873–82. doi: 10.1007/s11033-012-1754-1. [DOI] [PubMed] [Google Scholar]
- 61.Di Meglio P, Di Cesare A, Laggner U, et al. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th-17 effector response in humans. PLoS One. 2011;6:e17160. doi: 10.1371/journal.pone.0017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hazlett J, Stamp LK, Merriman T, et al. IL-23R re11209026 polymorphism modulates IL-17A expression in patients with rheumatoid arthritis. Genes Immun. 2012;13:282–7. doi: 10.1038/gene.2011.80. [DOI] [PubMed] [Google Scholar]
- 63.Furst D. The risk of infections with biologic therapies for rheumatoid arthritis. Semin Arthritis Rheum. 2010;39:327–46. doi: 10.1016/j.semarthrit.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–8. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toussirot E. The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm Allergy Drug Targets. 2012;11:159–68. doi: 10.2174/187152812800392805. [DOI] [PubMed] [Google Scholar]
- 66.Seavey M, Dobrzanski P. The many faces of Janus kinase. Biochem Pharmacol. 2012;83:1136–45. doi: 10.1016/j.bcp.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 67.Genovese M, Keystone E, Taylor P, et al. 24-week results of a blinded phase 2b dose-ranging study of baricitinib, an oral Janus kinase 1/Janus kinase 2 inhibitor, in combination with traditional disease modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64(Suppl):S1049. [Google Scholar]
- 68.Vanhoutte F, Mazur M, van der Aa A, et al. Selective JAK1 inhibition in the treatment of rheumatoid arthritis: proof of concept with GPLG0634. Arthritis Rheum. 2012;64(Suppl):S1051. [Google Scholar]
- 69.Fleischmann R, Spender-Green G, Fan F, et al. Dose ranging study of VX-509, an oral selective JAK3 inhibitor, as monotherapy in patients with active rheumatoid arthritis (RA) Arthritis Rheum. 2011;63(Suppl):L3. [Google Scholar]
- 70.Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 71.Genovese MC, Durez P, Richards HB, et al. One year efficacy and safety results of a phase II trial of secukinumab in patients with rheumatoid arthritis. Arthritis Rheum. 2011;63(Suppl):S149–50. [Google Scholar]
- 72.Genovese MC, Van den Bosch F, Roberson SA, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–39. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 73.Genovese MC, Greenwald MW, Cho CS, et al. A phase 2 study of multiple subcutaneous doses of LY2439821, an anti-IL-17 monoclonal antibody, in patients with rheumatoid arthritis in two populations: naïve to biologic therapy or inadequate responders to tumor necrosis factor alpha inhibitors. Arthritis Rheum. 2011;63(Suppl):S1017. [Google Scholar]
- 74.Papp K, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–98. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 75.Pavelka K, Chon Y, Newmark R, et al. A randomized, double-blind, placebo-controlled, multiple-dose study to evaluate the safety, tolerability, and efficacy of brodalumab (AMG 827) in subjects with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheum. 2012;64(Suppl):S362. doi: 10.3899/jrheum.141271. [DOI] [PubMed] [Google Scholar]
- 76.Aaltonen KJ, Virkki LM, Malmivaara A, et al. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS One. 2012;7:e30275. doi: 10.1371/journal.pone.0030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alonso-Ruiz A, Pijoan JI, Ansuategui E, et al. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metanalysis of efficacy and safety. BMC Musculoskelet Disord. 2008;9:52. doi: 10.1186/1471-2474-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maini RN, Taylor PC, Szechinski J, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–29. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 79.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–97. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 80.Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicentre, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 81.Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-bind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54:1390–400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]