Abstract
The mammalian RNA-binding protein AUF1 (AU-binding factor 1, also known as heterogeneous nuclear ribonucleoprotein D [hnRNP D]) binds to numerous mRNAs and influences their posttranscriptional fate. Given that many AUF1 target mRNAs encode muscle-specific factors, we investigated the function of AUF1 in skeletal muscle differentiation. In mouse C2C12 myocytes, where AUF1 levels rise at the onset of myogenesis and remain elevated throughout myocyte differentiation into myotubes, RNP immunoprecipitation (RIP) analysis indicated that AUF1 binds prominently to Mef2c (myocyte enhancer factor 2c) mRNA, which encodes the key myogenic transcription factor MEF2C. By performing mRNA half-life measurements and polysome distribution analysis, we found that AUF1 associated with the 3′ untranslated region (UTR) of Mef2c mRNA and promoted MEF2C translation without affecting Mef2c mRNA stability. In addition, AUF1 promoted Mef2c gene transcription via a lesser-known role of AUF1 in transcriptional regulation. Importantly, lowering AUF1 delayed myogenesis, while ectopically restoring MEF2C expression levels partially rescued the impairment of myogenesis seen after reducing AUF1 levels. We propose that MEF2C is a key effector of the myogenesis program promoted by AUF1.
INTRODUCTION
The response of mammalian cells to intrinsic and extrinsic cues is carefully orchestrated by changes in gene expression programs. The major posttranscriptional regulators of gene expression are RNA-binding factors: RNA-binding proteins (RBPs) and noncoding RNAs (ncRNAs) (1–3). Together, RBPs and ncRNAs elicit all aspects of posttranscriptional gene regulation, such as pre-mRNA splicing and maturation as well as mRNA transport, storage, turnover, and translation (2, 4, 5). Some RBPs have housekeeping functions and regulate broad groups of mRNAs; for example, ribosomal proteins, translation initiation and elongation factors, and poly(A)-binding protein widely control mRNA stability and translation. However, other RBPs associate with select RNA sequences and control only distinct subsets of mRNAs. The latter group of specialized RBPs includes proteins with key roles in the cellular responses to cell damage, immune agents, energy availability, growth factors, hormones, and developmental signals. Belonging to this group are RBPs of the human antigen family (HuR, HuB, HuC, and HuD), tristetraprolin (TTP), T-cell-restricted intracellular antigen 1 (TIA-1) and related (TIAR) proteins, the CUG triplet repeat RNA-binding proteins (CUGBP), FMRP (fragile X mental retardation protein), and numerous other RBPs (6–10).
One of the RBPs specialized in binding to select target mRNAs is AU-binding factor 1 (AUF1), also known as heterogeneous nuclear ribonucleoprotein D (hnRNP D) (11, 12). AUF1 comprises four different isoforms that arise via alternative splicing (p37, p40, p42, and p45), all of them bearing two RNA recognition motifs (RRMs) through which they bind RNA (13, 14). AUF1 is generally considered to promote the decay of target mRNAs, many of which have been identified over the years and include mRNAs encoding cell cycle-regulatory proteins, oncoproteins, apoptosis regulators, and inflammatory factors (cyclin D1, p21, p27, p16, pRB, c-Fos, JunD, c-Myc, Bcl-2, Bax, interleukin-1β [IL-1β], IL-6, IL-8, and tumor necrosis factor alpha [TNF-α]). Through these and other interactions, AUF1 was implicated in cellular processes such as proliferation, senescence, and the response to immune and stress agents (14, 15).
AUF1 was also recently implicated in developmental and disease processes. Although it suppresses the expression of Bcl-2 and/or cyclin D1, the discovery that AUF1 levels increase in many malignancies has led to the proposal that AUF1 contributes to cancer pathogenesis (16). By helping to maintain appropriately low levels of TNF-α and IL-1β, AUF1 also appears to facilitate the response to inflammatory and immune agents; in this regard, AUF1 knockout mice develop atopic dermatitis and experience severe endotoxic shock following exposure to lipopolysaccharide (17–19). In addition, AUF1 knockout mice display a striking phenotype of accelerated aging, characterized by enhanced telomere erosion, increased levels of inflammatory cytokines, and the accumulation of senescent cells, and numerous developmental defects, such as altered skeletal and muscular systems (20).
In light of the interesting phenotypes of AUF1-deficient mice, we recently performed photoactivatable-ribonucleotide-enhanced cross-linking and immunoprecipitation (PAR-CLIP) analysis of AUF1 (J.-H. Yoon, M. Hafner, S. De, and M. Gorospe, unpublished data), as this method would allow us to identify systematically all the AUF1-interacting RNAs and to map the interactions of AUF1 with its targets at a highly precise sequence resolution (21). The PAR-CLIP method includes a step in which cells are cultured with the modified nucleotide 4-thiouridine, which is incorporated into newly synthesized RNA. Subsequent exposure to UV light cross-links the RNPs and the presence of the modified ribonucleotides provides an internal validation for the binding (22). AUF1 PAR-CLIP analysis (Yoon et al., unpublished) revealed many AUF1-associated mRNAs encoding myogenic factors. This observation led us to inquire whether AUF1 could be implicated in myogenesis. To investigate this possibility, we employed the C2C12 mouse myoblast cell line, widely used to study the molecular mechanisms of muscle cell differentiation. In C2C12 myoblasts, we discovered that among several myogenic factors, AUF1 associated prominently with the Mef2c mRNA and enhanced expression of MEF2C, a key regulator of muscle differentiation. AUF1 bound the 3′ untranslated region (3′UTR) of Mef2c mRNA and increased MEF2C translation, but it did not alter Mef2c mRNA stability. Unexpectedly, AUF1 also associated with the Mef2c promoter and enhanced transcription of Mef2c mRNA. Importantly, silencing AUF1 slowed down myogenesis, but restoring MEF2C expression levels rescued these inhibitory effects. We propose that AUF1 regulates myogenesis at least partly by promoting the expression of MEF2C through a combination of transcriptional and translational mechanisms.
MATERIALS AND METHODS
Cell culture, transfections, and CK activity.
C2C12 mouse myoblasts were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% serum (Invitrogen) and antibiotics (Invitrogen). Differentiation was induced on subconfluent cultures by replacing the growth medium (GM; consisting of DMEM with 10% fetal bovine serum [FBS]) with differentiation medium (DM; consisting of DMEM with 2% horse serum). For silencing experiments, control small interfering RNA (Ctrl siRNA) or AUF1 siRNA was transected twice, 36 h and 12 h before inducing differentiation. For overexpression experiments, 1 μg of myc-DDK-Mef2c (Origene) was transfected into cells along with Ctrl or AUF1 siRNAs. For AUF1 overexpression, 2 μg each of four plasmids, each expressing a Flag-tagged isoform of AUF1 (pFlag-CMV2-AUF1-p37, -p40, -p42, or -p45) (23), was transfected in C2C12 cells and assayed 48 h later. Creatine kinase (CK) activity was determined in cell lysates by the NADPH-coupled assay, using the EnzyChrom creatine kinase assay kit (BioAssay Systems) according to the manufacturer's protocol. The results are expressed as units (μmol per minute) of NADPH formed per μg of total protein.
Cloning and reporter assays.
The 3′UTR of mouse Mef2c mRNA was amplified with specific primers. After XhoI and NotI double digestion, the PCR product was inserted into the psiCHECK2 plasmid downstream of the Renilla open reading frame (ORF). For reporter assays, 600 ng of psiCHECK2-Mef2c-3′UTR plasmid was transfected using Lipofectamine 2000 (Invitrogen) in complete medium; 12 h later, cells were lysed and reporter Renilla luciferase (RL) and firefly luciferase (FL) activities were analyzed using a dual-luciferase assay kit (Promega).
qPCR analysis.
Whole-cell RNA was extracted using TRIzol (Invitrogen), and RNA from RNP immunoprecipitation (RIP) assays was extracted using acidic phenol (Ambion) (24). Reverse transcription (RT) was performed using random hexamers and Maxima reverse transcriptase (Thermo Scientific), and SYBR green was used for real-time quantitative PCR (qPCR) analysis using gene-specific primers for 18S (GTAACCCGTTGAACCCCATT, CCATCCAATCGGTAGTAGCG), for Gapdh mRNA (where GAPDH is glyceraldehyde-3-phosphate dehydrogenase) (AACTTTGGCATTGTGGAAGG, GGATGCAGGGATGATGTTCT), for MyoD mRNA (CTTCTACGCACCTGGACCG, ACTGTAGTAGGCGGTGTCGT), for Myog mRNA (CTTGCTCAGCTCCCTCAACC, GTTGGGACCGAACTCCAGTG), for Mef2c mRNA (TGATCAGCAGGCAAAGATTG, ATCAGACCGCCTGTGTTACC), and for Mef2c pre-mRNA (CTGGCAGCTCTACACCATTG, AAGCCTTCTTCATCAATCCAAA). The quality of the qPCRs was determined by analysis of dissociation curves and by visualization of products on 2% agarose gels. RT-qPCR analysis was performed on Applied Biosystems 7300 and 7900 instruments.
Analysis of AUF1 ribonucleoprotein complexes.
To analyze the association of endogenous AUF1 with endogenous mRNAs in C2C12 cells, immunoprecipitation (IP) of endogenous RNP complexes was performed as described previously (24). Briefly, C2C12 cells cultured in GM and DM were lysed in 20 mM Tris-HCl at pH 7.5, 100 mM KCl, 5 mM MgCl2, and 0.5% NP-40 for 10 min on ice and centrifuged at 15,000 × g for 10 min at 4°C. The supernatants were incubated with protein A Dynabeads coated with anti-AUF1 (Millipore) or with control IgG (Santa Cruz Biotechnology) antibodies for 2 h at 4°C. The beads were washed with NT2 buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM MgCl2, 0.05% NP-40), followed by incubation with 20 units of RNase-free DNase I for 15 min at 37°C to remove the DNA. The samples were then incubated for 15 min at 55°C with 0.1% SDS–0.5 mg/ml proteinase K to digest proteins. The RNA from the IP samples was extracted using phenol-chloroform, precipitated, and used for cDNA microarray analysis (RIP chip) or for qPCR analysis. Complete AUF1 PAR-CLIP was performed with Flag-tagged AUF1 (23) isoforms (p37, p40, p42, and p45) using the method described previously (21). A full report of AUF1 PAR-CLIP is in preparation.
Western blot analysis.
Whole-cell extracts were prepared as described previously. Western blot analysis was performed with 10 μg of protein lysates fractionated by electrophoresis through 4 to 12% NuPAGE gels (Invitrogen) and transferred to polyvinylidene difluoride (PVDF) membranes. Proteins were detected with antibodies recognizing AUF1 (Millipore), MYOD, GAPDH, HSP90, myogenin, α-tubulin, HDAC7 (Santa Cruz Biotechnology), and MEF2C (Cell Signaling). After incubation with appropriate secondary antibodies, signals were detected by Immobilon Western chemiluminescent horseradish peroxidase (HRP) substrate (Millipore).
ChIP assay.
For chromatin immunoprecipitation (ChIP) assay, C2C12 cells were cross-linked with formaldehyde and lysed in lysis buffer (50 mM Tris [pH 8], 85 mM KCl, 0.5% NP-40, and protease inhibitors), whereupon the nuclei were collected and lysed using nuclear lysis buffer (50 mM Tris [pH 8], 10 mM EDTA, 1% SDS, and protease inhibitor). The lysate was sonicated and cleared by centrifugation at 12,000 × g for 10 min at 4°C. The lysate was used for IP in the presence of 10 μl of control IgG and AUF1 antibody. The complexes were eluted with buffer containing 1% SDS and 0.1 M NaHCO3, and the cross-linking was reversed by heating at 65°C. The DNA was recovered by phenol-chloroform extraction and analyzed by qPCR using primers specific for the promoters of Tert (CCTCCGCCTACCTAACCTTC and TTGATGGTCACAATGCTGGT), Il6 (GTGTGTGTCGTCTGTCATGC and AGGAAGGGGAAAGTGTGCTT), and Mef2c (TTAAGTGCCATGACCATCCA and GCACACACTTGCTTCATTTCA) genes.
mRNA microarray analysis.
For mRNA expression profiling, total RNA was isolated from C2C12 cells and studied by mRNA microarray analysis. The RNA in AUF1 RIP samples was isolated with phenol-chloroform and also analyzed using mRNA microarrays. Microarray experiments were performed using the MouseRef-8 v2.0 Expression BeadChips (Illumina, San Diego, CA) following the protocols of the Gene Expression and Genomics Unit (http://www.grc.nia.nih.gov/branches/rrb/dna/index/protocols.htm). Raw microarray data were filtered by P values of <0.02, normalized by Z-score transformation, and tested for significant differences in signal intensity by Z-test. The sample quality was analyzed by scatter plot, principal component analysis, and gene sample Z-score-based hierarchy clustering to exclude possible outliers. Transcripts were considered to be significantly changed when they had Z-test P values of ≤0.05, Z-ratio absolute values of ≥1.5 in both directions, a multiple-comparison correction false-discovery rate of ≤0.30, a positive Z-score signal of average intensity, and an independent one-way analysis of variance (ANOVA) on sample group analysis P value of ≤0.05.
Biotin pulldown assay.
DNA templates for in vitro transcription of various regions of the Mef2 mRNA were synthesized by RT-PCR. All of the forward primers contained the T7 RNA polymerase promoter (T7, AGTAATACGACTCACTATAGGG) at their 5′ end. For the amplification of Mef2c 5′UTR, the primers used were (T7) GAGCAGTTCTGTGTTCTTTTGCC and AAGCCTTCTTCATCAATCCAAATTTCC; and for amplification of Mef2c CR, the primers were (T7) AGGTGACTTTTACGAAGAGGAAATTT and ATCATGTTGCCCATCCTTCAGAG. To amplify different fragments of the Mef2c 3′UTR, the primers used were (T7) GTCAAGCGCATGCGACTCTC and ACCGGTAGTCACCACTTGCC (fragment a); (T7) ATAGAGGTTTGGACAGACCC and TCTTGGTTAGCAAAGCATGGAGA (fragment b); (T7) ACCCTAAGCCATGAGGATCT and GAGATCTGAAAGGGGCCCTG (fragment c); (T7) TCTTTTCATTTTATCAAAAGCAGGGC and AAAGGGGGGGGGACATAGAG (fragment d); (T7) AAAAAAAAAAACAAACCTTTCTCTATGTCC and TTTTTAACATATAAAACATTACTACAAGGC (fragment e); (T7) ATGCTAGCCTGCCTTGTAGTAA and GGAGGCCTCTGCTGGCACAT (fragment f); (T7) GCTGAAGCTAGAGTGAACTC and AGCTCACCGTTACTAAAACTCTTTTA (fragment g); (T7) GTATTGTTATACCGAATCCTATTTTAAAAG and TACGCCATGTTTAGGCAACAG (fragment h); (T7) ATAGCATTTTGCTCCCTTGATCC and CCTGCTTACTTCAAGAGTACACT (fragment i); and (T7) GGTATTGCATTTTGCCTTCCCT and ATCACTAAGAAAGCCAGTAACTTTA (fragment j). The PCR products were purified and used for preparing biotinylated transcripts using the MaxiScript T7 kit (Ambion). Five hundred micrograms of cell lysate was incubated with 5 μg of purified biotinylated transcripts for 1 h at room temperature, followed by isolation of RNP complexes using streptavidin-coupled Dynabeads (Invitrogen). The presence of AUF1 in the pulldown complexes was assessed by Western blot analysis.
Polysome analysis.
C2C12 cells were transfected with AUF1 siRNA sequentially (24 h apart) and cultured for an additional 24 h in GM or for another 6 days in DM before they were incubated with cycloheximide (Sigma; 100 μg/ml for 15 min), and the cytoplasmic lysates were prepared by fractionation through 10% to 50% linear sucrose gradients via ultracentrifugation. The RNA in each fraction was prepared using TRIzol (Invitrogen) and quantified by RT-qPCR analysis (7).
Muscle regeneration in mice.
Healthy 12-month-old C57B16 mice were used for the in vivo muscle regeneration experiment. All animal protocols were performed according to methods approved by the NIH Animal Care and Use Committee (ACUC). Hind limb suspension (HS)-induced muscle atrophy was performed as described previously (25, 26). At 5 months of age, mice were housed individually and suspended by the tail by using a strip of adhesive surgical tape attached to a nylon monofilament line via a stainless steel swivel. Mice were suspended at a 30° angle to the floor with only the forelimbs touching the floor. The swivel enabled the animal to explore the cage (360° range of motion) and obtain food and water freely. Food consumption and bodyweight were recorded daily. Fourteen days later, HS was discontinued and mice were allowed to move normally for 3 days, allowing the hind limb muscles to regenerate. Mice were then euthanized, and the gastrocnemius muscles samples were collected for biochemical analysis. Muscle samples were homogenized in radioimmunoprecipitation assay (RIPA) buffer, and the levels of AUF1 and myogenic factors were assessed by Western blotting.
For cardiotoxin-induced muscle regeneration assays, 12-month-old mice were lightly anesthetized with vaporized isoflurane (Forane; Baxter, Deerfield, IL), the surgical site was sterilized, and skin was resected to allow access to the tibialis anterior muscle. Cardiotoxin (0.03 mg/ml in phosphate-buffered saline [PBS], Naja Mossambica, C9759; Sigma, St. Louis, MO) or PBS was administered by submuscular injection (25 μl), and skin was sutured closed. At the indicated time points thereafter, mice were euthanized in accordance with institutional protocols, and the tibialis anterior was excised, covered in Tissue-Tek optimal cutting temperature compound (number 4583; Finetek, Torrance, CA), and frozen in liquid nitrogen-cooled 2-methylbutane (M32631; Sigma, St. Louis, MO) for histological analysis or snap-frozen in liquid nitrogen for protein and mRNA analysis.
For histological analysis, muscles were removed at 0, 0.25, 1, 3, 10, and 20 days after cardiotoxin injection and frozen before sectioning. Ten-micrometer-thick transverse sections were prepared with cryostat and stained with hematoxylin and eosin (H&E) to visualize muscle injury and recovery. Endogenous AUF1 was visualized by immunostaining of the sections as described previously (27).
For Western blot analysis, muscle pieces were homogenized with ice-cold buffer containing 0.1 mM Tris-HCl [pH 7.6], 2 mM EDTA, and 0.5% Triton X-100. Lysates were cleared by centrifugation for 10 min at 4°C at 13,000 × g, and 20 μg of supernatant was used for Western blot analysis. For mRNA analysis, muscle was homogenized and total RNA was prepared using TRIzol. Myogenic mRNAs were analyzed using RT-qPCR and the primer pairs listed above.
RESULTS
AUF1 binds to several mRNAs involved in myogenesis.
By using PAR-CLIP analysis (21), we investigated on a transcriptome-wide scale the subset of RNAs directly associated with AUF1 in human embryonic kidney (HEK) 293 cells (Yoon et al., unpublished). Each Flag-tagged AUF1 isoform (23) was expressed separately, and the four libraries of bound RNAs were studied in order to identify mRNAs bound to each isoform. As shown in Table 1, numerous direct AUF1 target mRNAs were found to encode proteins involved in different aspects of muscle development or physiology, including ion transport ATPases (e.g., ATP1A1 mRNA, ATP2A2 mRNA, ATP2B4 mRNA, and other mRNAs), calcium channels (CACNA2D1 mRNA), cell cycle regulators (e.g., CCND1, CCND2, CCNG1, and CDKN1A mRNAs), and myogenic transcription factors (MEF2A, MEF2C, and MYO1E mRNAs). The finding of myogenic factor mRNAs in HEK293 cells was somewhat unexpected, although some of these factors have been found to be expressed in cell types other than muscle cells (data not shown). The regions of interaction (transcript location) spanned the entire mRNA (coding region and UTRs), but most regions of association mapped to the 3′UTR. The number of PAR-CLIP tags (read count) was also found to be varied, in some cases showing very high numbers of interaction sites. Many of the target mRNAs interacted with multiple AUF1 isoforms. Given this evidence, we set out to test if AUF1 might broadly affect the expression of muscle differentiation factors and muscle physiology.
TABLE 1.
Association of AUF1 with several mRNAs involved in myogenesis by PAR-CLIP analysisa
| Transcript ID | Gene name | Sequence | Read countb | Transcript location | AUF1 isoformd |
|---|---|---|---|---|---|
| ATP1A1 | ENST00000445896 | GCGGACACGTGGCAACAGCGGT | 8 | 5′UTR | p37 |
| ATP2A2 | ENST00000539276 | TTTAACTTAATCAATTAATTTTTTTATTG | 8 | 3′UTR | p40 |
| ATP2B4 | ENST00000484746 | TTTTAAAACCAATACACCATACTTTCTTTCTG | 8 | 3′UTR | p40 |
| ATP2C1 | ENST00000508532 | TACAAATACACTATCTATCTTAG | 19 | 3′UTR | p40 |
| CACNA2D1 | ENST00000356860 | TAACACTCATCCCATCAAATTATTACATTACATTTAG | 5 | 3′UTR | p40 |
| CCND1 | ENST00000227507 | TTTTTATACTCTTCCTATTTTTG | 13 | 3′UTR | p40 |
| CCND1 | ENST00000227507 | TTTTTAAACACTAAAATATATAATTTATAG | 5 | 3′UTR | p40 |
| CCND2 | ENST00000261254 | AATTTTTCTTCCTCTCCACTTCTTAG | 5 | 3′UTR | p40 |
| CCNE2 | ENST00000520509 | TATTATATAAACTTAACCTTTTAATACTG | 5 | 3′UTR | p40 |
| CCNE2 | ENST00000520509 | CCATAACACATTTTTTAACTAATAAG | 5 | 3′UTR | p40 |
| CCNG1 | ENST00000393929 | TCATTATTCTAATCCTACTCCTACTTAAATTTTAAG | 79 | 3′UTR | p40 |
| CCNG1 | ENST00000514590 | TAACTCAAAATAAATTATCACTTCG | 12 | 3′UTR | p40 |
| CDKN1A | ENST00000448526 | TTTTAATTTAAACACCTCCTCATG | 6 | 3′UTR | p40 |
| MEF2A | ENST00000354410 | TACCCATATATAATTCTCCCACACTAG | 5 | 3′UTR | p40 |
| MEF2C | ENST00000504921 | CTATCTTTACCCTTATACCTTATCTG | 12 | 3′UTR | p40 |
| MEF2C | ENST00000510942 | CTAACATATTTAATTAAATAAATAAATAAATCTG | 8 | 3′UTR | p40 |
| MYO1E | ENST00000288235 | ACTCTTCTCAACTCTTTCCCTATAG | 5 | 3′UTR | p40 |
| ATP1A1 | ENST00000537345 | TATGAGCCTGCAGCTGTTTCAGAACAAGG | 6 | CRc | p40 |
| ATP2A2 | ENST00000308664 | TCCTGGATCAGAGGTGCTATTTACTACTTTAAAATTG | 6 | CR | p40 |
| MEF2A | ENST00000354410 | ACTCCTTTTCCTAAAAAACTCAAG | 20 | 3′UTR | p42 |
| ATP1A1 | ENST00000369496 | ATCATTTAATCCTTAAAAACATG | 8 | 5′UTR | p42 |
| ATP2A2 | ENST00000395494 | AACATCTGGCTCGTGGGCTCCATCTG | 5 | CR | p42 |
| ATP2A2 | ENST00000395494 | CAACACATCTACCAACCCTG | 6 | 3′UTR | p45 |
| ATP2A2 | ENST00000395494 | CTTATTTATAAATTCATTAAAAACACTACAG | 9 | 3′UTR | p45 |
| ATP2C1 | ENST00000508532 | TACAAATACACTATCTATCTTAG | 11 | 3′UTR | p45 |
| CCND1 | ENST00000227507 | TAACCTCTTCACCTTATTCATG | 6 | 3′UTR | p45 |
| CCNE2 | ENST00000520509 | AATACATTTAATTATTTCCTATG | 6 | 3′UTR | p45 |
| MEF2A | ENST00000354410 | ATTTTTAAATACTCCTTTTCCTAAAAAACTCAAG | 19 | 3′UTR | p45 |
| MEF2A | ENST00000354410 | TACCCATATATAATTCTCCCACACTAG | 5 | 3′UTR | p45 |
| MEF2A | ENST00000354410 | TTTTCTAAAATACTACTACTCAAGGCTCGGAGTTTG | 5 | 3′UTR | p45 |
| MEF2C | ENST00000510942 | CTAACATATTTAATTAAATAAATAAATAAATCTG | 7 | 3′UTR | p45 |
| ATP1A1 | ENST00000537345 | CCTTCCCCTACTCTCTTCTCATCTTCG | 8 | CR | p45 |
A description of the full complement of AUF1 target transcripts is in preparation. MEF2C is highlighted in boldface.
Read count, number of times the tag is found in the PAR-CLIP library.
CR, coding region.
AUF1 isoform binding to the PAR-CLIP tag.
AUF1 levels correlate with skeletal muscle regeneration in vivo.
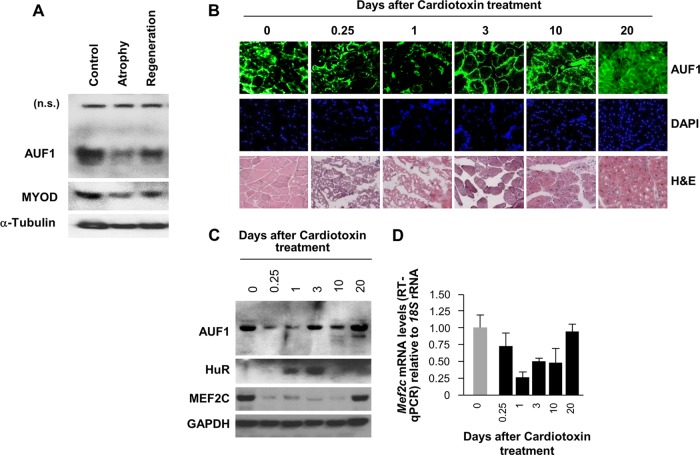
To begin to investigate a possible role of AUF1 in myogenesis, we first examined AUF1 expression levels in two in vivo models of skeletal muscle development. First, we studied AUF1 expression levels in gastrocnemius muscle from 12-month-old mice and compared them to AUF1 levels in the same muscle 14 days after hind limb suspension, an intervention that causes muscle damage (25, 26). As shown, muscle atrophy was accompanied by reduced expression of AUF1 (∼20% of the levels seen in control mice), as well as by reduced levels of the myogenic factor MYOD (30% of the levels in control mice), included here as a control; 3 days after stopping the hind limb suspension to allow muscle regeneration (reloading), AUF1 and MYOD levels were largely restored, reaching 50% and 70% of the levels seen in control mice (Fig. 1A). Second, we investigated AUF1 expression using a different model of muscle regeneration in which muscle damage was induced by an intramuscular injection of cardiotoxin, whereupon the muscle was allowed to regenerate. Injury-induced muscle regeneration was monitored morphologically (Fig. 1B) and by Western blotting (Fig. 1C) of muscle samples prepared before injury (day 0) and 0.25, 1, 3, 10, and 20 days postinjury. As shown, AUF1 levels declined early on following injury but rose again as muscle regeneration was under way (from day 3 on). Levels of HuR as a control RBP were monitored by Western blot analysis during this process, since we had previously reported that HuR levels increased early during regeneration (27). The levels of MEF2C, encoded by Mef2c mRNA, a target of AUF1 (Table 1), were also examined (Fig. 1C). Interestingly, the restoration of MEF2C protein levels lagged behind the restoration of AUF1 levels (Fig. 1C), and the abundance of Mef2c mRNA, as determined by reverse transcription (RT) and real-time qPCR, followed a trend similar to that of MEF2C protein (Fig. 1C and D). These suggestive correlations between muscle regeneration and increased AUF1 levels supported the hypothesis that AUF1 might contribute to the regulation of myogenesis.
FIG 1.
AUF1 levels during mouse skeletal muscle regeneration in vivo. (A) Western blot analysis of the levels of AUF1, MYOD, and loading control protein α-tubulin in mouse gastrocnemius muscle 14 days after suspension of the hind limbs (Atrophy) and 3 days after stopping the hind limb suspension to allow muscle regeneration (Regeneration). n.s., nonspecific. (B) Immunofluorescence detection of AUF1 in frozen muscle sections prepared at different time points during skeletal muscle regeneration in cardiotoxin-injured muscles. Sections were also stained with hematoxylin and eosin (H&E) and the DNA stain DAPI (4′,6-diamidino-2-phenylindole) to monitor the regeneration process. (C and D) Western blot analysis to check the expression of AUF1, HuR, and MEF2C (C) and RT-qPCR analysis to measure the levels of Mef2c mRNA (normalized to the levels of 18S rRNA) (D) during skeletal muscle regeneration in cardiotoxin-injured muscles (E). Data in panel D represent the means and standard errors of the means (SEM) from three different experiments.
Transient AUF1 reduction followed by AUF1 increase during C2C12 muscle cell differentiation.
The mouse myoblast cell line C2C12 has been used extensively to study muscle cell differentiation (28, 29). As shown in Fig. 2A, changing the culture growth medium (GM) of proliferating, undifferentiated C2C12 cells (DMEM + 10% FBS) to a differentiation medium (DM) containing DMEM with 2% horse serum caused myoblasts to differentiate into distinct myotubes over the ensuing 6 days. The degree of muscle differentiation was further assessed by measuring creatine kinase activity (Fig. 2B). As previously reported, Myog and Myod mRNAs, encoding key differentiation markers Myogenin and MYOD, changed dramatically throughout the differentiation period (30), showing peak expression levels by day 2 of culture in DM [DM(2) in Fig. 2C]. Meanwhile, expression of Mef2c mRNA rose later, showing peak abundance at day 6 of culture in DM [DM(6)]. Changes in protein abundance were monitored by Western blotting; as shown in Fig. 2D, AUF1 levels declined early in differentiation, but they increased again later in differentiation [DM(5), DM(6)]. Higher expression of MEF2C was also observed along with the increase of AUF1 at DM(5) (Fig. 2D). The finding that earlier in differentiation, MEF2C levels were low despite high levels of AUF1 suggested that additional factors contributed to the repression of MEF2C biosynthesis in DM(0) to DM(3) and/or to the elevation of MEF2C biosynthesis in DM(4) to DM(6). Taken together, these results agree with the in vivo muscle regeneration data showing that AUF1 levels decline transiently during the first stages of C2C12 differentiation but increase at later stages of myogenesis; in both model systems, MEF2C expression rose during late myogenesis.
FIG 2.
C2C12 myoblast differentiation and expression of myogenic transcription factors. (A) Phase-contrast micrographs of undifferentiated (asynchronously growing) C2C12 myoblasts and C2C12 cells grown to confluence and differentiating into myotubes through culture in DMEM with 2% horse serum for up to 6 days. (B) Creatine kinase assay to quantify the degree of differentiation of C2C12 cells. (C) RT-qPCR analysis to monitor the expression of Myog, Myod, and Mef2c mRNAs, encoding myogenic factors, during C2C12 cell differentiation. The mRNA levels were normalized to the levels of 18S rRNA, measured by RT-qPCR analysis in the same samples. (D) Western blot analysis of the levels of AUF1 and myogenic transcription factors MYOD and MEF2C (as well as loading control GADPH) during differentiation of C2C12 myoblasts into myotubes. Data in panels B and C represent the means and SEM from three different experiments.
AUF1 binds the Mef2c 3′UTR.
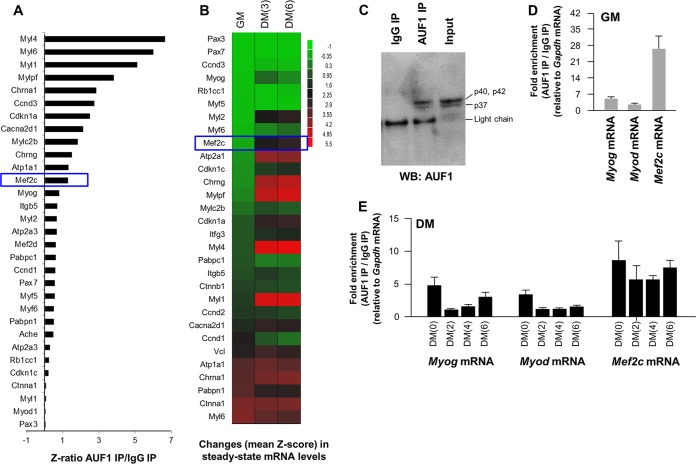
PAR-CLIP analysis using human kidney embryonic cells (HEK293) identified human MEF2C mRNA as a target of AUF1 (specifically, isoforms p40 and p45). Although human AUF1 and mouse AUF1 are highly homologous (97%), we sought to test if mouse AUF1 also interacted with mouse Mef2c mRNA in C2C12 cells using ribonucleoprotein (RNP) immunoprecipitation (RIP) analysis (see Materials and Methods). RIP analysis was carried out using an anti-AUF1 antibody under IP conditions that preserved AUF1:mRNA complexes; the mRNAs present in the complex were then identified in different ways. AUF1 RIP analysis at DM(2) followed by microarray identification of mRNAs revealed numerous mRNAs enriched in AUF1 IP (Fig. 3A), including many that were found to be associated with AUF1 in HEK293 cells (Table 1). The changes in muscle-relevant mRNAs that were measured by microarray analysis and determined by Z-score analysis (Fig. 3B) agreed with the changes observed for individual muscle mRNAs (Fig. 2C). AUF1 RIP (Fig. 3C) followed by RT-qPCR analysis of target mRNAs revealed that in proliferating C2C12 cells (in GM) AUF1 bound Mef2c mRNA, displaying an enrichment >25-fold greater in the AUF1 IP than in a control IP (IP using generic IgG) (Fig. 3D). In DM, AUF1 displayed substantially more binding (as measured by enrichment in AUF1 IP relative to IgG IP) to Mef2c mRNA than to Myod or Myog mRNAs, and the strong enrichment in mRNA persisted throughout the 6-day differentiation period (Fig. 3E). It is important to note that the levels of AUF1-mRNA complexes in DM(2) through DM(6) cannot be accurately compared with those in GM, since GM cells express very low levels of these mRNAs (Fig. 2C). Nonetheless, our findings revealed that AUF1 associates with Mef2c mRNA in C2C12 myoblasts and that AUF1 remains in complex with Mef2c mRNA throughout the differentiation process.
FIG 3.
AUF1 interacts with mRNAs encoding skeletal muscle myogenesis factors. (A) AUF1 ribonucleoprotein immunoprecipitation to identify AUF1-bound mRNAs by microarray (RIP chip) analysis in C2C12 cells on DM(2); the levels of mRNAs in AUF1 IP were normalized to the levels of Gapdh mRNA and plotted as fold enrichment relative to the levels seen in control IgG IP samples. The graph shows the interaction of AUF1 with mRNAs encoding proteins involved in myogenesis. (B) Microarray analysis of the levels of mRNAs encoding muscle differentiation factors in C2C12 myoblasts (GM) and C2C12 cells at different stages during differentiation to myotubes [DM(3) and DM(6)]. Data are shown as mean Z-scores. (C) Western blot analysis of AUF1 in AUF1 IP samples used for RIP chip (A) and RIP followed by RT-qPCR analysis (D and E). (D and E) RIP followed by RT-qPCR analysis to assess AUF1-associated Myog, Myod, and Mef2c mRNAs in GM C2C12 myoblasts (D) and in differentiating [DM(0) through DM(6)] C2C12 cells (E). Data in panels D and E represent the means and SEM from three different experiments.
To identify the region(s) of Mef2c mRNA that interacted with AUF1, we prepared partial biotinylated fragments of the Mef2c mRNA, including RNAs spanning the 5′UTR, the coding region (CR), and the 3′UTR (Fig. 4A, schematic). The biotinylated RNAs were incubated with lysates prepared from C2C12 cells [DM(6)] to allow interaction with RBPs; 1 h later, the levels of AUF1 associated with the different biotinylated RNAs were detected by Western blotting. As shown in Fig. 4A, biotinylated Mef2c 5′UTR and CR segments showed very little binding to AUF1. Within the 3′UTR, several partial fragments similarly displayed very little affinity for AUF1 (segments a, h, and i); however, AUF1 interacted strongly with segments b through g as well as with segment j. These findings agree with the PAR-CLIP data showing that AUF1 associates with different sites within the human MEF2C 3′UTR (Table 1). One such PAR-CLIP tag conserved between human and mouse was located within fragment b (not shown); however, the precise identification of all of the AUF1 interaction sites on the Mef2c mRNA will require future AUF1 PAR-CLIP analysis in C2C12 cells.
FIG 4.
AUF1 regulates MEF2C expression by interacting with the Mef2c 3′UTR. (A) Biotinylated RNA fragments spanning the 5′UTR, coding region (CR), and 3′UTR of the Mef2c mRNA used for pulldown identify fragments with affinity for AUF1 using cytoplasmic C2C12 lysates; AUF1 was visualized by Western blotting. (B) Schematic of the dual-luciferase reporter plasmids psiCHECK2, the control vector expressing renilla luciferase (RL) and the internal control firefly luciferase (FL), and psiCHECK2-Mef2c(3′), the test vector bearing the Mef2c 3′UTR downstream of the RL coding region. (C) Influence of AUF1 on the expression of the reporter constructs shown in panel B. Forty-eight hours after transfecting C2C12 cells with AUF1 siRNA or Ctrl siRNA, AUF1 levels were reduced substantially (left), as detected by Western blotting. Twenty-four hours after the transfection of C2C12 cells with either AUF1 siRNA or Ctrl siRNA, each reporter plasmid was transfected, and the ratio of RL activity to FL activity was calculated 24 h after that. The relative RL/FL ratio of AUF1 siRNA-transfected cells relative to the RL/FL of Ctrl siRNA-transfected cells is indicated (right). Data represent the means and SEM from 3 independent experiments. Significance (P) is indicated.
AUF1 promotes MEF2C translation.
The consequences of the AUF1-Mef2c mRNA interaction were assessed using a heterologous luciferase reporter vector (psiCHECK2) to prepare reporter psiCHECK2-Mef2c(3′), bearing the Mef2c 3′UTR (Fig. 4B). Twenty-four hours after transfecting C2C12 cells with AUF1 siRNA to lower AUF1 levels or with Ctrl siRNA (Fig. 4C, left), cells were further transfected with each of the two psiCHECK2 reporters. The ratio of Renilla luciferase (RL), encoded by the reporter transcript bearing the Mef2c 3′UTR, to firefly luciferase (FL), encoded by an internal control reporter transcript, was set as 1 for the parent vector (psiCHECK2). RL/FL ratios in AUF1-silenced transfected cells were studied relative to RL/FL ratios in control cells (Fig. 4C, graph). In this analysis, silencing AUF1 moderately repressed luciferase expression from psiCHECK2-Mef2c(3′) reporter, supporting the hypothesis that AUF1 enhanced MEF2C expression by interacting with the Mef2c 3′UTR.
To ascertain if the repression seen after AUF1 silencing was due to translational inhibition, we quantified the fraction of Mef2c mRNA associated with the translational machinery in cells expressing normal or silenced AUF1. Cytoplasmic extracts from C2C12 cells cultured under DM(0) or DM(6) conditions were fractionated on sucrose gradients (Fig. 5A and C), and the relative abundance of Mef2c mRNA in each fraction was used to quantify the association of Mef2c mRNA with the cellular polysomes (Fig. 5B and D). Under DM(0) conditions, Mef2c mRNA levels were low in nontranslating and low-translating fractions of the gradient (fractions 1 to 5, where free RNA, 40S and 60S subunits, and 80S monosomes are found, and fractions 5 to 7, which contain low-molecular-weight [LMW] polysomes), while the mRNA levels peaked in fractions 8 and 9, which contained actively translating fractions of the gradient (high-molecular-weight [HMW] polysomes) (Fig. 5B). Importantly, silencing AUF1 caused a leftward shift in the distribution of Mef2c mRNA on the gradient, with the major peak appearing between fractions 5 and 8, suggestive of a reduction in Mef2c mRNA translation when AUF1 was silenced.
FIG 5.
AUF1 silencing reduces the size of Mef2c mRNA polysomes. (A and B) Forty-eight hours after siRNA transfection of C2C12 cells, polysomes in cytoplasmic extracts were fractionated through sucrose gradients (the arrow indicates the direction of sedimentation) (A), and the relative distribution of Gapdh mRNA, encoding a housekeeping protein, and Mef2c mRNA was measured by RT-qPCR analysis of RNA in gradient fractions and represented as the percentage of total RNA in the gradient (B). (C and D) Forty-eight hours after siRNA transfection, C2C12 cells were subjected to differentiation; at DM(6), polysomes were analyzed as explained for panels A and B. Data are representative of three independent experiments.
Similarly, under DM(6) conditions, Mef2c mRNA was associated with smaller polysomes after silencing AUF1, shifting from a major peak to a smaller peak at fraction 8 and a relatively greater abundance of Mef2c mRNA in lighter parts of the gradient (as low as fraction 2) (Fig. 5C and D). Under both conditions, Gapdh mRNA (a transcript that encodes a housekeeping protein and is not a target of AUF1) showed only modest shifts in distribution [DM(0)] or distributed in larger polysomes [DM(6)] after silencing AUF1 (Fig. 5B and D). These data support the notion that AUF1 promotes the translation of MEF2C in C2C12 cells throughout differentiation.
AUF1 activates Mef2c transcription.
Since Mef2c mRNA levels changed dramatically during C2C12 differentiation, we sought to determine if AUF1 also affected Mef2c steady-state mRNA levels, not just its association with polysomes. As shown in Fig. 6A and B, silencing AUF1 not only lowered MEF2C protein levels in both DM(0) and DM(6) but also decreased Mef2 mRNA levels in C2C12 cells under DM(6) conditions. It is important to note that the relatively high MEF2C levels in DM(0) are likely due to stress caused by transfection of siRNAs [unlike the cells in Fig. 2, which were otherwise unperturbed, and hence MEF2C was low in DM(0)]. Interestingly, Mef2c mRNA was significantly lower in DM(0) than in DM(6), even though MEF2C protein levels were comparable in DM(0) and DM(6), suggesting that the half-life of MEF2C protein might be higher in DM(0) (Fig. 6A and B). Conversely, AUF1 overexpression (achieved by transfecting proliferating C2C12 cells with a pool of four plasmids, each expressing one AUF1 isoform [23]) revealed that by 48 h after transfection, when C2C12 cells have reached DM(0), MEF2C protein (Fig. 6C, left) and Mef2c mRNA (Fig. 6C, right) were higher in cells overexpressing AUF1. A similar positive influence by AUF1 on MEF2C protein expression was seen after modulating AUF1 levels in HEK293 cells (not shown).
FIG 6.
AUF1 enhances Mef2c gene transcription. (A and B) AUF1 was silenced as described for Fig. 4C; 48 h later [DM(0)] or at DM(6), the levels of AUF1, MEF2C, and loading control α-tubulin were studied by Western blotting (A) and the levels of Mef2 mRNA by RT-qPCR analysis (B). (C) AUF1 was overexpressed by using a pool of four plasmids (derived from pFlag-CMV2), each expressing one AUF1 isoform (see Materials and Methods); 48 h later, when C2C12 cells were in DM(0), the levels of AUF1, MEF2C, and loading control heat shock protein 90 (HSP90) were studied by Western blotting (left) and the levels of Mef2 mRNA by RT-qPCR analysis (right). (D) The stability of Mef2c mRNA (and stable control Gapdh mRNA) was assessed by incubating C2C12 cells prepared as described for panel A with actinomycin D to block de novo transcription, whereupon the Mef2c mRNA and Gapdh mRNA levels were measured by RT-qPCR analysis; half-lives were calculated as the time required for each mRNA to reach one-half (50%, discontinuous line) of its initial abundance at time zero. (E) In cells processed as explained for panel A, de novo transcription of Mef2c was assessed by measuring the levels of Mef2c pre-mRNA using primers that spanned intron-exon junctions. (F) In C2C12 cells transfected as explained for panel C in order to overexpress AUF1, the levels of Mef2c pre-mRNA were assessed by RT-qPCR analysis. (G) Association of promoter regions of Il6 (negative control), Tert (positive control), and Mef2c was analyzed by PCR amplification of DNA present after chromatin IP (ChIP) using AUF1 antibody relative to those in control IgG IP samples. Data in panels B through G represent the means and SEM from three different experiments. Significance (P) is indicated.
We determined if the differential expression of Mef2c mRNA was due to differences in Mef2c mRNA stability by comparing the half-lives of Mef2c mRNA under each of these conditions. In DM(0) and DM(6) C2C12 cells, de novo transcription was blocked by using actinomycin D, whereupon calculation of the mRNA half-life (t1/2; the time required to lower its abundance to 50% of the levels at time zero) revealed that the Mef2c mRNA half-life was lower in DM(0) (∼8 h) than in DM(6) (where it was far greater than 8 h). Despite the documented influence of AUF1 on mRNA decay (13), AUF1 did not appear to affect Mef2c mRNA half-life in either population (Fig. 6D). The half-life of Gapdh mRNA, included as a control long-lived mRNA, was also unaffected by growth conditions or AUF1 abundance (Fig. 6D).
Since AUF1 affected Mef2c mRNA levels (Fig. 6B) but did not appear to influence Mef2c mRNA stability (Fig. 6D), we reasoned that AUF1 might modulate the transcription of Mef2c mRNA. To test this possibility, we measured the concentration of newly transcribed Mef2c mRNA by RT-qPCR using primer pairs that detected only Mef2c pre-mRNA (the primers spanned an intron-exon junction). As shown in Fig. 6E, in DM(0) C2C12 cells, Mef2c pre-mRNA levels were lower than in DM(6) C2C12 cells, suggesting that AUF1 did raise the levels of newly transcribed Mef2c pre-mRNA. Interestingly, while AUF1 silencing did not affect Mef2c pre-mRNA levels significantly in DM(0) cells, it lowered Mef2c pre-mRNA levels in DM(6) cells, suggesting that AUF1 promoted Mef2c gene transcription in differentiating C2C12 cultures, albeit modestly. Conversely, AUF1 overexpression increased the levels of Mef2c pre-mRNA (Fig. 6F). In keeping with the possibility that AUF1 promotes transcription of Mef2c mRNA, AUF1 binding to the promoter region of the Mef2c gene, as determined by chromatin immunoprecipitation (ChIP) analysis, revealed that AUF1 indeed associated with the Mef2c promoter, and this interaction increased moderately in DM(6) cells (Fig. 6G); this analysis included as a positive control the Tert promoter, a target of AUF1, and the Il6 promoter as a negative control, as reported previously (20). Collectively, these findings indicate that AUF1 promotes MEF2C expression during myogenic differentiation by enhancing the transcription and translation of Mef2c mRNA.
AUF1 enhances myogenesis.
Finally, since AUF1 critically enhances MEF2C expression, we investigated whether AUF1 was necessary for C2C12 differentiation into myotubes. As shown in Fig. 7A, silencing AUF1 decreased myogenesis as assessed by measuring creatine kinase activity, a differentiation marker, on differentiation days 3 and 6 [DM(3) and DM(6)]. Analysis using microscopy (phase-contrast and Jenners-Giemsa staining) showed that silencing AUF1 substantially reduced the formation of myotubes by DM(6) (Fig. 7B). Importantly, ectopic overexpression of MEF2C by transfection of a plasmid vector that expressed the coding region of Mef2c mRNA (which migrated with a slightly larger size due to the presence of a tag [Fig. 7C]) partially rescued the differentiation phenotype. Indeed, silencing AUF1 reduced myogenesis significantly by DM(6), as assessed by measuring creatine kinase activity (Fig. 7D), while reexpression of the Mef2c coding region restored creatine kinase activity to levels approaching those of control differentiated C2C12 cells (Fig. 7D). Taken together, these findings indicate that AUF1 promotes myogenesis by enhancing the transcription and translation of MEF2C, as shown schematically in Fig. 8.
FIG 7.
AUF1 is necessary for C2C12 cell differentiation. (A) Forty-eight hours after transfection with AUF1 siRNA or Ctrl siRNA, the differentiation of C2C12 cells at DM(0), DM(3), and DM(6) was monitored by measuring creatine kinase activity. (B) Micrographs of phase-contrast fields and Jenners-Giemsa staining (a dye that stains myotubes) of DM(6) C2C12 cells processed as described for panel A. (C and D) By 24 h after transfection of AUF1 or Ctrl siRNA, cells were further transfected with a plasmid vector plasmid that expressed Myc-tagged MEF2C. Six days later, the levels of AUF1 and MEF2C were assessed by Western blotting (C), and the degree of differentiation was determined by measuring creatine kinase activity (D). Data in panels A and D are the means and SEM from three independent experiments. Significance (P) is indicated.
FIG 8.
Proposed model of AUF1 influence on MEF2C expression. AUF1 can elevate MEF2C expression via two mechanisms: 1, by activating Mef2c gene transcription, and 2, by enhancing translation of Mef2c mRNA. By promoting MEF2C biosynthesis, AUF1 enhances myogenesis.
DISCUSSION
We report that AUF1 promotes myogenesis by enhancing the production of the myogenic transcription factor MEF2C through induced Mef2c gene transcription and increased Mef2c mRNA translation. During differentiation of C2C12 myocytes, AUF1 was found to associate with the Mef2c promoter, as determined by ChIP analysis (Fig. 6G), and enhanced de novo transcription, as determined by measuring the levels of Mef2c pre-mRNA (Fig. 6E). AUF1 was also found to associate with the 3′UTR of mature Mef2c mRNA (Fig. 3 and 4) and elevated MEF2C translation, as assessed by measuring the Mef2c mRNA polysomes (Fig. 5). The joint promotion of Mef2c transcription and translation by AUF1 were essential for the robust expression of MEF2C in differentiated C2C12 cells. Furthermore, while silencing AUF1 significantly delayed C2C12 differentiation into myotubes, ectopic expression of MEF2C largely rescued myogenesis, indicating that MEF2C is a key downstream effector of AUF1-elicited muscle remodeling.
The fact that AUF1 affected Mef2c transcription and translation but not Mef2c mRNA turnover was somewhat unexpected. AUF1 has been shown to modulate the turnover (generally by causing enhanced decay) of many mRNAs, including those that encode TNF-α, cyclin D1, p21, p16, IL-1β, Bcl-2, vascular endothelial growth factor (VEGF), and β-adrenergic receptor. Therefore, we expected to find that AUF1 might affect the half-life of myogenic factors but did not find such an influence for Mef2c mRNA (Fig. 6D). Earlier examples of AUF1 affecting translation are known, but this function of AUF1 is not well understood. AUF1 promoted the translation of MYC mRNA by competing with a translational repressor (TIAR) for binding to MYC 3′UTR (31), and it also promoted the translation of CD83 mRNA via unknown mechanisms (32). At this time, it is unclear if the promotion of Mef2c mRNA translation by AUF1 is linked to the actions of other RBPs or perhaps noncoding RNAs. However, since AUF1 formed strong interactions with eIF4G and poly(A)-binding protein (PABP), it might enhance more generally the translation of certain adenylate/uridylate-rich element (ARE) mRNAs (33).
The transcriptional influence of AUF1 is also poorly understood. AUF1-directed transcription has been reported for the MYC, CD21, and telomerase (TERT) genes (20, 34, 35). While the AUF1 target DNA site is not known, it may be G rich, perhaps including G quadruplex structures, as proposed by Enokizono and colleagues (36). It is interesting that AUF1 affects MYC transcription and translation, just as it affects Mef2c transcription and translation; as en masse studies of AUF1 on gene expression progress, it will be interesting to test if AUF1 jointly affects the transcription and translation (or perhaps turnover) of additional mRNAs. It will also be important to elucidate the specific DNA elements through which AUF1 elicits transcriptional actions.
A member of the MADS family of transcription factors (29, 37, 38), defined after its four founding members (Mcm1 from Saccharomyces cerevisiae, Agamous from Arabidopsis thaliana, Deficiens from Antirrhinum majus, and Srf from Homo sapiens), vertebrate MEF2C comprises four proteins with overlapping temporal and spatial expression patterns in embryonic and adult tissues. MEF2C proteins are expressed most highly in striated muscles and brain (39), although they are also found in lymphocytes, smooth muscle, endothelium, bone, and neurons (39–41). In muscle, the MEF2 family of transcription factors promotes the activity-dependent transformation of fast, glycolytic muscle fibers into slow, oxidative muscle fibers (42, 43). Interestingly, MEF2 proteins alone lack myogenic activity but can promote myogenic differentiation in combination with other transcription factors, such as basic helix-loop-helix proteins (MYOD, MYF5, myogenin, and MRF4 [44, 45]) and mastermind-like protein 1 (MAML1 [46]), as well as by posttranslational modification via various signaling pathways (prominently mitogen-activated protein kinases [MAPK]). MEF2 is negatively regulated by class II histone deacetylases (HDACs), which repress MEF2 transcriptional activity (47–49). The balance between these positive and negative regulators drives the relative abundance of fast and slow fibers through the control of expression of muscle structural genes and myogenic transcription factors, such as MRF4, MYOD, myogenin, myoglobin, desmin, MMP10, myosin light chain 2 (mlc-2), Glut-4, BOP, DLX5, DLX6, and aldolase A (28, 50–53). MEF2 also controls expression of microRNAs (miR-1 and miR-133), which in turn modulate expression of other myogenic genes (54, 55). In particular, repression of class II HDACs by miR-1 establishes an important feed-forward mechanism that further enhances MEF2 activity (52).
Mef2c-null mice die around embryonic day 9.5 (E9.5) (56), and skeletal muscle-specific loss of MEF2C causes myofibers to degenerate rapidly after birth due to the disorganization of sarcomeres and the loss of sarcomeric M-line integrity (57). Given its important role in myogenesis, the precise timing of MEF2C production and function is particularly important. In addition to its regulation at the posttranslational level (via activators like myogenic cofactors and repressors like class II HDACs), our results reveal that MEF2C expression is further controlled at the level of transcription and translation by AUF1.
Expression of MEF2C at the level of transcription is also regulated by MyoD and by the forkhead transcription factor Foxj3 (58, 59). Although to our knowledge AUF1 is the first RBP found to bind mature Mef2c mRNA, several microRNAs, including miR-21 and miR-27b (60, 61), can associate with Mef2c mRNA and repress MEF2C production posttranscriptionally. Perhaps the lag between the changes in AUF1 levels and the changes in Mef2c mRNA and MEF2C protein levels during myogenesis (Fig. 2) reflects the actions of such transcription factors and microRNAs regulating MEF2C expression jointly with AUF1. Whether AUF1 controls Mef2c mRNA transcription and translation by cooperating or competing with these factors remains to be studied.
In sum, AUF1 enhances myogenesis by promoting the expression of MEF2C. The influence by AUF1 on muscle differentiation adds to a growing body of evidence that RNA-binding proteins control multiple levels of myogenic gene regulation (reviewed recently in reference 62). Our results suggest that AUF1 contributes to muscle development and maintenance and lend molecular support for the loss of muscle mass seen in the prematurely aged AUF1-null mice (20). The integrated regulation of MEF2C expression by transcriptional and posttranscriptional mechanisms, including those governed by AUF1, illustrates the rich and dynamic control of proteins with rate-limiting functions in complex biological processes such as myogenesis.
ACKNOWLEDGMENTS
We thank Elin Lehrmann, William H. Wood III, and Supriyo De (NIA, NIH) for help with the bioinformatic analysis.
This work was supported by the NIA-IRP, NIH, and by NIH grants GM085693 (R.J.S.) and T32 GM13-A0-S1-090476 (D.M.C.).
Footnotes
Published ahead of print 2 June 2014
REFERENCES
- 1.Glisovic T, Bachorik JL, Yong J, Dreyfuss G. 2008. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 582:1977–1986. 10.1016/j.febslet.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore MJ. 2005. From birth to death: the complex lives of eukaryotic mRNAs. Science 309:1514–1518. 10.1126/science.1111443 [DOI] [PubMed] [Google Scholar]
- 3.Yoon JH, Abdelmohsen K, Gorospe M. 2013. Post-transcriptional gene regulation by long noncoding RNA. J. Mol. Biol. 425:3723–3730. 10.1016/j.jmb.2012.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell P, Tollervey D. 2000. Review mRNA stability in eukaryotes. Curr. Opin. Genet. Dev. 10:193–198. 10.1016/S0959-437X(00)00063-0 [DOI] [PubMed] [Google Scholar]
- 5.Orphanides G, Reinberg D. 2002. A unified theory of gene expression. Cell 108:439–451. 10.1016/S0092-8674(02)00655-4 [DOI] [PubMed] [Google Scholar]
- 6.Hinman MN, Lou H. 2008. Diverse molecular functions of Hu proteins. Cell Mol. Life Sci. 65:3168–3181. 10.1007/s00018-008-8252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee EK, Kim HH, Kuwano Y, Abdelmohsen K, Srikantan S, Subaran SS, Gleichmann M, Mughal MR, Martindale JL, Yang X, Worley PF, Mattson MP, Gorospe M. 2010. hnRNP C promotes APP translation by competing with FMRP for APP mRNA recruitment to P bodies. Nat. Struct. Mol. Biol. 17:732–739. 10.1038/nsmb.1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazan-Mamczarz K, Lal A, Martindale JL, Kawai T, Gorospe M. 2006. Translational repression by RNA-binding protein TIAR. Mol. Cell. Biol. 26:2716–2727. 10.1128/MCB.26.7.2716-2727.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philips AV, Timchenko LT, Cooper TA. 1998. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 280:737–741. 10.1126/science.280.5364.737 [DOI] [PubMed] [Google Scholar]
- 10.Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, Chen S, Gueydan C, Kruys V, Streuli M, Anderson P. 2000. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 19:4154–4163. 10.1093/emboj/19.15.4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loflin P, Chen CY, Shyu AB. 1999. Unraveling a cytoplasmic role for hnRNP D in the in vivo mRNA destabilization directed by the AU-rich element. Genes Dev. 13:1884–1897. 10.1101/gad.13.14.1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Wagner BJ, Ehrenman K, Schaefer AW, DeMaria CT, Crater D, DeHaven K, Long L, Brewer G. 1993. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol. Cell. Biol. 13:7652–7665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gratacós FM, Brewer G. 2010. The role of AUF1 in regulated mRNA decay. Wiley Interdiscip. Rev. RNA 1:457–473. 10.1002/wrna.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White EJ, Brewer G, Wilson GM. 2013. Post-transcriptional control of gene expression by AUF1: mechanisms, physiological targets, and regulation. Biochim. Biophys. Acta 1829:680–688. 10.1016/j.bbagrm.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelmohsen K, Kuwano Y, Kim HH, Gorospe M. 2008. Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence. Biol. Chem. 389:243–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zucconi BE, Wilson GM. 2011. Modulation of neoplastic gene regulatory pathways by the RNA-binding factor AUF1. Front. Biosci. (Landmark Ed.) 16:2307–2325. 10.2741/3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu JY, Sadri N, Schneider RJ. 2006. Endotoxic shock in AUF1 knockout mice mediated by failure to degrade proinflammatory cytokine mRNAs. Genes Dev. 20:3174–3184. 10.1101/gad.1467606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadri N, Lu JY, Badura ML, Schneider RJ. 2010. AUF1 is involved in splenic follicular B cell maintenance. BMC Immunol. 11:1. 10.1186/1471-2172-11-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadri N, Schneider RJ. 2009. Auf1/Hnrnpd-deficient mice develop pruritic inflammatory skin disease. J. Investig. Dermatol. 129:657–670. 10.1038/jid.2008.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pont AR, Sadri N, Hsiao SJ, Smith S, Schneider RJ. 2012. mRNA decay factor AUF1 maintains normal aging, telomere maintenance, and suppression of senescence by activation of telomerase transcription. Mol. Cell 47:5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M, Jr, Jungkamp AC, Munschauer M, Ulrich A, Wardle GS, Dewell S, Zavolan M, Tuschl T. 2010. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141:129–141. 10.1016/j.cell.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ascano M, Hafner M, Cekan P, Gerstberger S, Tuschl T. 2012. Identification of RNA-protein interaction networks using PAR-CLIP. Wiley Interdiscip. Rev. RNA 3:159–177. 10.1002/wrna.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar B, Lu JY, Schneider RJ. 2003. Nuclear import and export functions in the different isoforms of the AUF1/heterogeneous nuclear ribonucleoprotein protein family. J. Biol. Chem. 278:20700–20707. 10.1074/jbc.M301176200 [DOI] [PubMed] [Google Scholar]
- 24.Abdelmohsen K, Pullmann R, Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. 2007. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol. Cell 25:543–557. 10.1016/j.molcel.2007.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morey ER, Sabelman EE, Turner RT, Baylink DJ. 1979. A new rat model simulating some aspects of space flight. Physiologist 22:S23–S24 [PubMed] [Google Scholar]
- 26.Park E, Schultz E. 1993. A simple hindlimb suspension apparatus. Aviat. Space Environ. Med. 64:401–404 [PubMed] [Google Scholar]
- 27.Figueroa A, Cuadrado A, Fan J, Atasoy U, Muscat GE, Muñoz-Canoves P, Gorospe M, Muñoz A. 2003. Role of HuR in skeletal myogenesis through coordinate regulation of muscle differentiation genes. Mol. Cell. Biol. 23:4991–5004. 10.1128/MCB.23.14.4991-5004.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molkentin JD, Olson EN. 1996. Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 6:445–453. 10.1016/S0959-437X(96)80066-9 [DOI] [PubMed] [Google Scholar]
- 29.Molkentin JD, Olson EN. 1996. Combinatorial control of muscle development by basic helix-loop-helix and MADS-box transcription factors. Proc. Natl. Acad. Sci. U. S. A. 93:9366–9373. 10.1073/pnas.93.18.9366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson E. 1992. Activation of muscle-specific transcription by myogenic helix-loop-helix proteins. Symp. Soc. Exp. Biol. 46:331–241 [PubMed] [Google Scholar]
- 31.Liao B, Hu Y, Brewer G. 2007. Competitive binding of AUF1 and TIAR to MYC mRNA controls its translation. Nat. Struct. Mol. Biol. 14:511–518. 10.1038/nsmb1249 [DOI] [PubMed] [Google Scholar]
- 32.Ehlers C, Schirmer S, Kehlenbach RH, Hauber J, Chemnitz J. 2013. Post-transcriptional regulation of CD83 expression by AUF1 proteins. Nucleic Acids Res. 41:206–219. 10.1093/nar/gks1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu JY, Bergman N, Sadri N, Schneider RJ. 2006. Assembly of AUF1 with eIF4G-poly(A) binding protein complex suggests a translation function in AU-rich mRNA decay. RNA 12:883–893. 10.1261/rna.2308106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dempsey LA, Hanakahi LA, Maizels N. 1998. A specific isoform of hnRNP D interacts with DNA in the LR1 heterodimer: canonical RNA binding motifs in a sequence-specific duplex DNA binding protein. J. Biol. Chem. 273:29224–29229. 10.1074/jbc.273.44.29224 [DOI] [PubMed] [Google Scholar]
- 35.Tolnay M, Vereshchagina LA, Tsokos GC. 1999. Heterogeneous nuclear ribonucleoprotein D0B is a sequence-specific DNA-binding protein. Biochem. J. 338:417–425. 10.1042/0264-6021:3380417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enokizono Y, Konishi Y, Nagata K, Ouhashi K, Uesugi S, Ishikawa F, Katahira M. 2005. Structure of hnRNP D complexed with singlestranded telomere DNA and unfolding of the quadruplex by heterogeneous nuclear ribonucleoprotein D. J. Biol. Chem. 280:18862–18870. 10.1074/jbc.M411822200 [DOI] [PubMed] [Google Scholar]
- 37.Blais A, Tsikitis M, Acosta-Alvear D, Sharan R, Kluger Y, Dynlacht BD. 2005. An initial blueprint for myogenic differentiation. Genes Dev. 19:553–569. 10.1101/gad.1281105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shore P, Sharrocks AD. 1995. The MADS-box family of transcription factors. Eur. J. Biochem. 229:1–13. 10.1111/j.1432-1033.1995.tb20430.x [DOI] [PubMed] [Google Scholar]
- 39.Edmondson DG, Lyons GE, Martin JF, Olson EN. 1994. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development 120:1251–1263 [DOI] [PubMed] [Google Scholar]
- 40.Arnold MA, Kim Y, Czubryt MP, Phan D, McAnally J, Qi X, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. 2007. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev. Cell 12:377–389. 10.1016/j.devcel.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 41.Black BL, Lu J, Olson EN. 1997. The MEF2A 3′ untranslated region functions as a cis-acting translational repressor. Mol. Cell. Biol. 17:2756–2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu H, Naya FJ, McKinsey TA, Mercer B, Shelton JM, Chin ER, Simard AR, Michel RN, Bassel-Duby R, Olson EN, Williams RS. 2000. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 19:1963–1973. 10.1093/emboj/19.9.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, Bassel-Duby R, Williams RS. 2001. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 20:6414–6423. 10.1093/emboj/20.22.6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molkentin JD, Black BL, Martin JF, Olson EN. 1995. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 83:1125–1136. 10.1016/0092-8674(95)90139-6 [DOI] [PubMed] [Google Scholar]
- 45.Wang DZ, Valdez MR, McAnally J, Richardson J, Olson EN. 2001. The Mef2c gene is a direct transcriptional target of myogenic bHLH and MEF2 proteins during skeletal muscle development. Development 128:4623–4633 [DOI] [PubMed] [Google Scholar]
- 46.Shen H, McElhinny AS, Cao Y, Gao P, Liu J, Bronson R, Griffin JD, Wu L. 2006. The Notch coactivator, MAML1, functions as a novel coactivator for MEF2C-mediated transcription and is required for normal myogenesis. Genes Dev. 20:675–688. 10.1101/gad.1383706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang S, McKinsey TA, Zhang CL, Richardson JA, Hill JA, Olson EN. 2004. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol. Cell. Biol. 24:8467–8476. 10.1128/MCB.24.19.8467-8476.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKinsey TA, Zhang CL, Lu J, Olson EN. 2000. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408:106–111. 10.1038/35040593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKinsey TA, Zhang CL, Olson EN. 2002. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem. Sci. 27:40–47. 10.1016/S0968-0004(01)02031-X [DOI] [PubMed] [Google Scholar]
- 50.Liu ML, Olson AL, Edgington NP, Moye-Rowley WS, Pessin JE. 1994. Myocyte enhancer factor 2 (MEF2) binding site is essential for C2C12 myotube-specific expression of the rat GLUT4/muscle-adipose facilitative glucose transporter gene. J. Biol. Chem. 269:28514–28521 [PubMed] [Google Scholar]
- 51.Olson EN, Perry M, Schulz RA. 1995. Regulation of muscle differentiation by the MEF2 family of MADS box transcription factors. Dev. Biol. 172:2–14. 10.1006/dbio.1995.0002 [DOI] [PubMed] [Google Scholar]
- 52.Potthoff MJ, Olson EN. 2007. MEF2: a central regulator of diverse developmental programs. Development 134:4131–4140. 10.1242/dev.008367 [DOI] [PubMed] [Google Scholar]
- 53.Richardson JM, Pessin JE. 1993. Identification of a skeletal muscle-specific regulatory domain in the rat GLUT4/muscle-fat gene. J. Biol. Chem. 268:21021–21027 [PubMed] [Google Scholar]
- 54.Liu N, Williams AH, Kim Y, McAnally J, Bezprozvannaya S, Sutherland LB, Richardson JA, Bassel-Duby R, Olson EN. 2007. An intragenic MEF2-dependent enhancer directs muscle-specific expression of microRNAs 1 and 133. Proc. Natl. Acad. Sci. U. S. A. 104:20844–20849. 10.1073/pnas.0710558105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sokol NS, Ambros V. 2005. Mesodermally expressed Drosophila microRNA-1 is regulated by Twist and is required in muscles during larval growth. Genes Dev. 19:2343–2354. 10.1101/gad.1356105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin Q, Schwarz J, Bucana C, Olson EN. 1997. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276:1404–1407. 10.1126/science.276.5317.1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potthoff MJ, Olson EN, Bassel-Duby R. 2007. Skeletal muscle remodeling. Curr. Opin. Rheumatol. 19:542–549. 10.1097/BOR.0b013e3282efb761 [DOI] [PubMed] [Google Scholar]
- 58.Alexander MS, Shi X, Voelker KA, Grange RW, Garcia JA, Hammer RE, Garry DJ. 2010. Foxj3 transcriptionally activates Mef2c and regulates adult skeletal muscle fiber type identity. Dev. Biol. 337:396–404. 10.1016/j.ydbio.2009.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dodou E, Xu SM, Black BL. 2003. Mef2c is activated directly by myogenic basic helix-loop-helix proteins during skeletal muscle development in vivo. Mech. Dev. 120:1021–1032. 10.1016/S0925-4773(03)00178-3 [DOI] [PubMed] [Google Scholar]
- 60.Chinchilla A, Lozano E, Daimi H, Esteban FJ, Crist C, Aranega AE, Franco D. 2011. MicroRNA profiling during mouse ventricular maturation: a role for miR-27 modulating Mef2c expression. Cardiovasc. Res. 89:98–108. 10.1093/cvr/cvq264 [DOI] [PubMed] [Google Scholar]
- 61.Yelamanchili SV, Chaudhuri AD, Chen LN, Xiong H, Fox HS. 2010. MicroRNA-21 dysregulates the expression of MEF2C in neurons in monkey and human SIV/HIV neurological disease. Cell Death Dis. 1:e77. 10.1038/cddis.2010.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blech-Hermoni Y, Ladd AN. 2013. RNA binding proteins in the regulation of heart development. Int. J. Biochem. Cell Biol. 45:2467–2478. 10.1016/j.biocel.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]