Abstract
Transcription factor NF-E2 activity is thought to be crucial for the transcriptional regulation of many erythroid-specific genes. The three small Maf family proteins (MafF, MafG, and MafK) that are closely related to the c-Maf protooncoprotein constitute half of the NF-E2 activity by forming heterodimers with the large tissue-restricted subunit of NF-E2 called p45. We have established and characterized murine erythroleukemia cells that conditionally overexpress MafK from a metallothionein promoter. The conditional expression of MafK caused accumulation of hemoglobin, an indication of terminal differentiation along the erythroid pathway. Concomitantly, DNA binding activities containing MafK were induced within the MafK-overexpressing cells. These results demonstrate that MafK can promote the erythroid differentiation program in erythroleukemia cells and suggest that the small Maf family proteins are key regulatory molecules for erythroid differentiation.
Full text
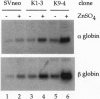
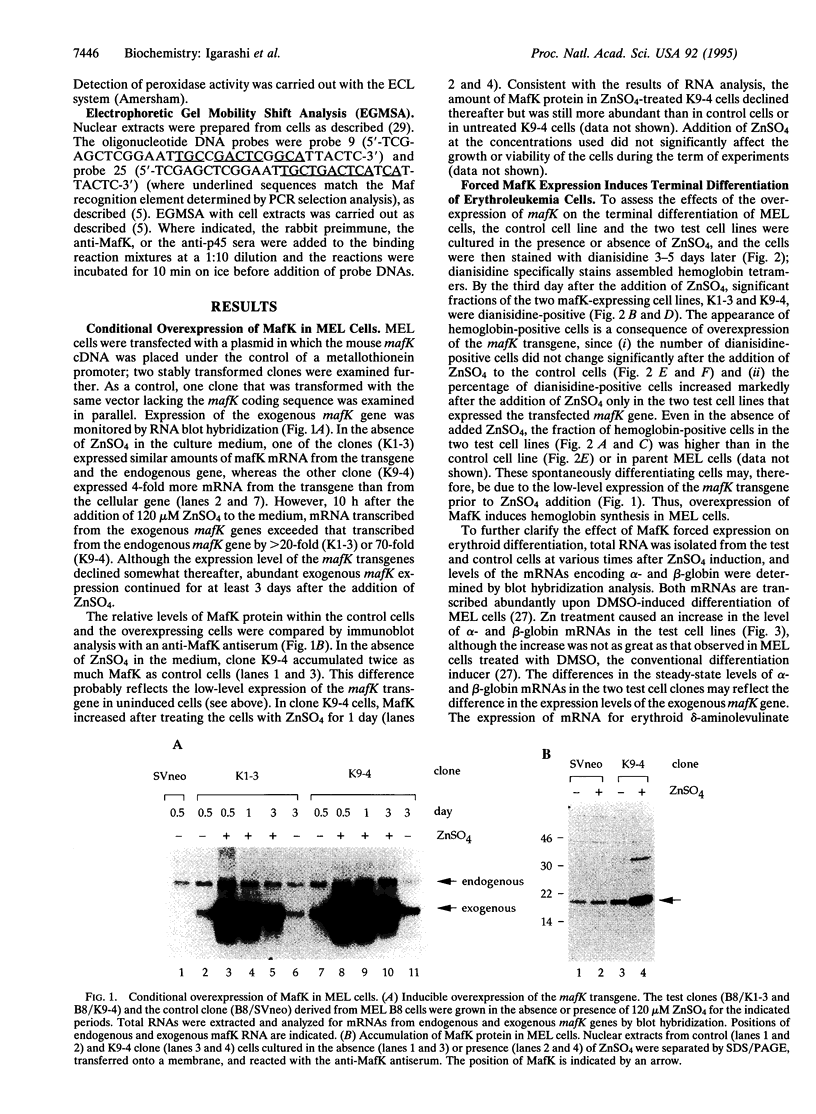
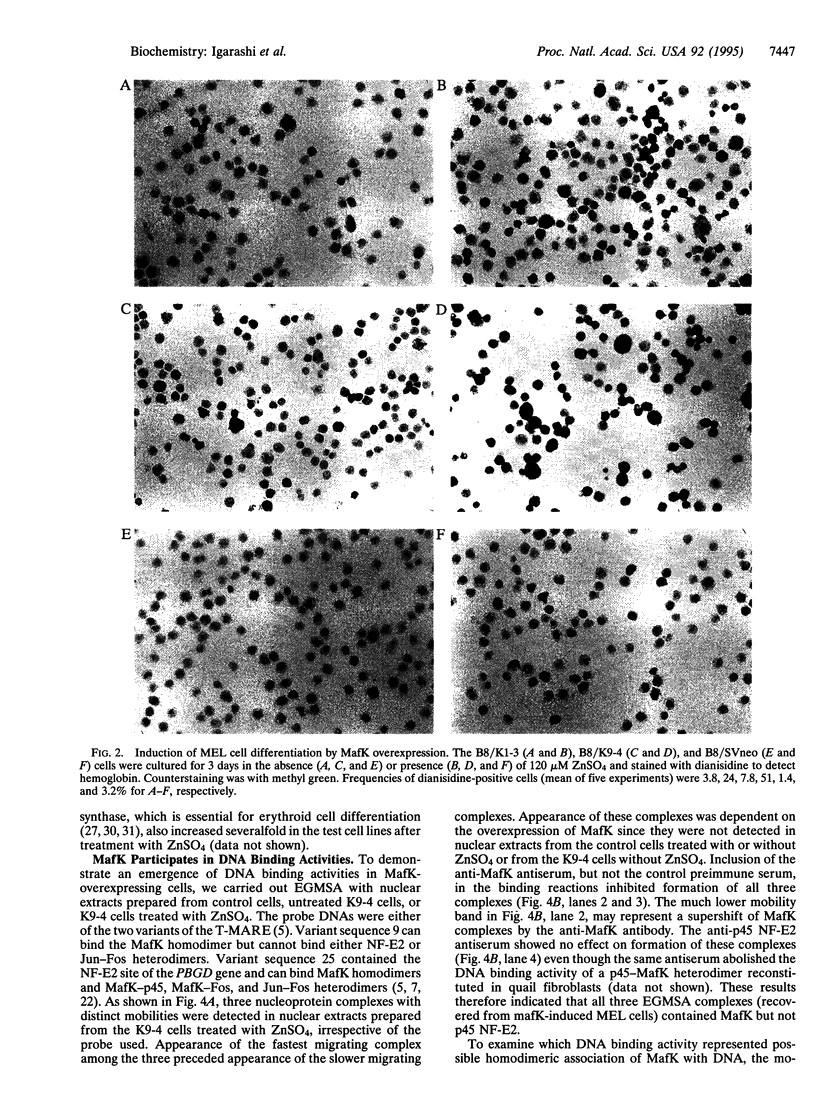
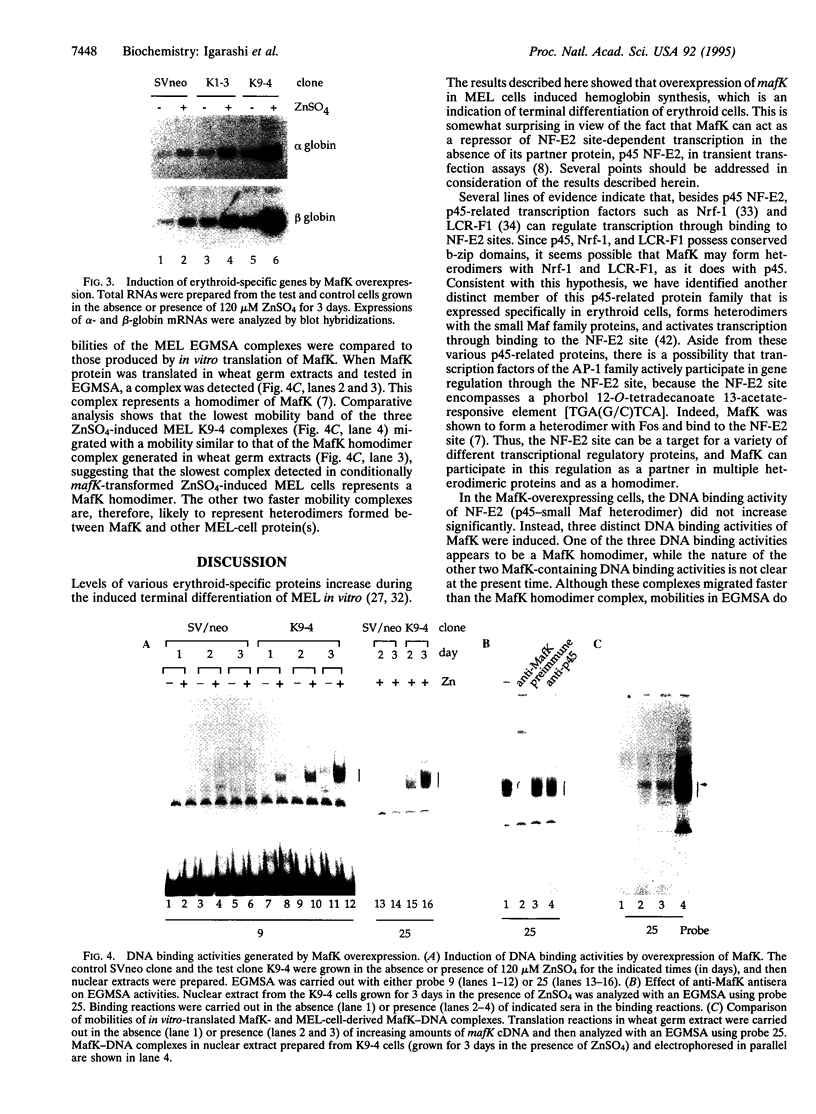
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Erdjument-Bromage H., Davidson M. B., Tempst P., Orkin S. H. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993 Apr 22;362(6422):722–728. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- Andrews N. C., Faller D. V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991 May 11;19(9):2499–2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N. C., Kotkow K. J., Ney P. A., Erdjument-Bromage H., Tempst P., Orkin S. H. The ubiquitous subunit of erythroid transcription factor NF-E2 is a small basic-leucine zipper protein related to the v-maf oncogene. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11488–11492. doi: 10.1073/pnas.90.24.11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplan P. D., Nakahara K., Orkin S. H., Kirsch I. R. The SCL gene product: a positive regulator of erythroid differentiation. EMBO J. 1992 Nov;11(11):4073–4081. doi: 10.1002/j.1460-2075.1992.tb05500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina J. J., Donze D., Sun C. W., Ciavatta D. J., Townes T. M. Cloning and functional characterization of LCR-F1: a bZIP transcription factor that activates erythroid-specific, human globin gene expression. Nucleic Acids Res. 1994 Jun 25;22(12):2383–2391. doi: 10.1093/nar/22.12.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Han X. L., Kan Y. W. Cloning of Nrf1, an NF-E2-related transcription factor, by genetic selection in yeast. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11371–11375. doi: 10.1073/pnas.90.23.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay P., Maniatis T. Transcriptional regulation of globin gene expression in the human erythroid cell line K562. Science. 1983 Jun 17;220(4603):1281–1283. doi: 10.1126/science.6574602. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clarke M. F., Kukowska-Latallo J. F., Westin E., Smith M., Prochownik E. V. Constitutive expression of a c-myb cDNA blocks Friend murine erythroleukemia cell differentiation. Mol Cell Biol. 1988 Feb;8(2):884–892. doi: 10.1128/mcb.8.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola J. A., Cole M. D. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986 Apr 24;320(6064):760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- Dmitrovsky E., Kuehl W. M., Hollis G. F., Kirsch I. R., Bender T. P., Segal S. Expression of a transfected human c-myc oncogene inhibits differentiation of a mouse erythroleukaemia cell line. Nature. 1986 Aug 21;322(6081):748–750. doi: 10.1038/322748a0. [DOI] [PubMed] [Google Scholar]
- Fujita H., Yamamoto M., Yamagami T., Hayashi N., Bishop T. R., De Verneuil H., Yoshinaga T., Shibahara S., Morimoto R., Sassa S. Sequential activation of genes for heme pathway enzymes during erythroid differentiation of mouse Friend virus-transformed erythroleukemia cells. Biochim Biophys Acta. 1991 Nov 11;1090(3):311–316. doi: 10.1016/0167-4781(91)90195-r. [DOI] [PubMed] [Google Scholar]
- Fujita H., Yamamoto M., Yamagami T., Hayashi N., Sassa S. Erythroleukemia differentiation. Distinctive responses of the erythroid-specific and the nonspecific delta-aminolevulinate synthase mRNA. J Biol Chem. 1991 Sep 15;266(26):17494–17502. [PubMed] [Google Scholar]
- Fujiwara K. T., Kataoka K., Nishizawa M. Two new members of the maf oncogene family, mafK and mafF, encode nuclear b-Zip proteins lacking putative trans-activator domain. Oncogene. 1993 Sep;8(9):2371–2380. [PubMed] [Google Scholar]
- Igarashi K., Itoh K., Motohashi H., Hayashi N., Matuzaki Y., Nakauchi H., Nishizawa M., Yamamoto M. Activity and expression of murine small Maf family protein MafK. J Biol Chem. 1995 Mar 31;270(13):7615–7624. doi: 10.1074/jbc.270.13.7615. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Kataoka K., Itoh K., Hayashi N., Nishizawa M., Yamamoto M. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature. 1994 Feb 10;367(6463):568–572. doi: 10.1038/367568a0. [DOI] [PubMed] [Google Scholar]
- Johnson P., Chung S., Benchimol S. Growth suppression of Friend virus-transformed erythroleukemia cells by p53 protein is accompanied by hemoglobin production and is sensitive to erythropoietin. Mol Cell Biol. 1993 Mar;13(3):1456–1463. doi: 10.1128/mcb.13.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Fujiwara K. T., Noda M., Nishizawa M. MafB, a new Maf family transcription activator that can associate with Maf and Fos but not with Jun. Mol Cell Biol. 1994 Nov;14(11):7581–7591. doi: 10.1128/mcb.14.11.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Igarashi K., Itoh K., Fujiwara K. T., Noda M., Yamamoto M., Nishizawa M. Small Maf proteins heterodimerize with Fos and may act as competitive repressors of the NF-E2 transcription factor. Mol Cell Biol. 1995 Apr;15(4):2180–2190. doi: 10.1128/mcb.15.4.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Nishizawa M., Kawai S. Structure-function analysis of the maf oncogene product, a member of the b-Zip protein family. J Virol. 1993 Apr;67(4):2133–2141. doi: 10.1128/jvi.67.4.2133-2141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Noda M., Nishizawa M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol Cell Biol. 1994 Jan;14(1):700–712. doi: 10.1128/mcb.14.1.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume T. U., Takada S., Obinata M. Probability that the commitment of murine erythroleukemia cell differentiation is determined by the c-myc level. J Mol Biol. 1988 Aug 20;202(4):779–786. doi: 10.1016/0022-2836(88)90558-x. [DOI] [PubMed] [Google Scholar]
- Lake-Bullock H., Dailey H. A. Biphasic ordered induction of heme synthesis in differentiating murine erythroleukemia cells: role of erythroid 5-aminolevulinate synthase. Mol Cell Biol. 1993 Nov;13(11):7122–7132. doi: 10.1128/mcb.13.11.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. J., Rowan S., Bani M. R., Ben-David Y. Retroviral integration within the Fli-2 locus results in inactivation of the erythroid transcription factor NF-E2 in Friend erythroleukemias: evidence that NF-E2 is essential for globin expression. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8398–8402. doi: 10.1073/pnas.91.18.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- McClinton D., Stafford J., Brents L., Bender T. P., Kuehl W. M. Differentiation of mouse erythroleukemia cells is blocked by late up-regulation of a c-myb transgene. Mol Cell Biol. 1990 Feb;10(2):705–710. doi: 10.1128/mcb.10.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte V., Eleouet J. F., Raich N., Romeo P. H. Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6548–6552. doi: 10.1073/pnas.86.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte V., Wall L., deBoer E., Grosveld F., Romeo P. H. Two tissue-specific factors bind the erythroid promoter of the human porphobilinogen deaminase gene. Nucleic Acids Res. 1989 Jan 11;17(1):37–54. doi: 10.1093/nar/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi N., Minegishi M., Tsuchiya S., Fujie H., Nagai T., Hayashi N., Yamamoto M., Konno T. Erythropoietin-dependent induction of hemoglobin synthesis in a cytokine-dependent cell line M-TAT. J Biol Chem. 1994 Nov 4;269(44):27700–27704. [PubMed] [Google Scholar]
- Ney P. A., Andrews N. C., Jane S. M., Safer B., Purucker M. E., Weremowicz S., Morton C. C., Goff S. C., Orkin S. H., Nienhuis A. W. Purification of the human NF-E2 complex: cDNA cloning of the hematopoietic cell-specific subunit and evidence for an associated partner. Mol Cell Biol. 1993 Sep;13(9):5604–5612. doi: 10.1128/mcb.13.9.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney P. A., Sorrentino B. P., Lowrey C. H., Nienhuis A. W. Inducibility of the HS II enhancer depends on binding of an erythroid specific nuclear protein. Nucleic Acids Res. 1990 Oct 25;18(20):6011–6017. doi: 10.1093/nar/18.20.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney P. A., Sorrentino B. P., McDonagh K. T., Nienhuis A. W. Tandem AP-1-binding sites within the human beta-globin dominant control region function as an inducible enhancer in erythroid cells. Genes Dev. 1990 Jun;4(6):993–1006. doi: 10.1101/gad.4.6.993. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Kataoka K., Goto N., Fujiwara K. T., Kawai S. v-maf, a viral oncogene that encodes a "leucine zipper" motif. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7711–7715. doi: 10.1073/pnas.86.20.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori Y., Tanabe J., Takada S., Lee W. M., Obinata M. Functional domains of c-Myc involved in the commitment and differentiation of murine erythroleukemia cells. Oncogene. 1993 Feb;8(2):379–386. [PubMed] [Google Scholar]
- Shoji W., Yamamoto T., Obinata M. The helix-loop-helix protein Id inhibits differentiation of murine erythroleukemia cells. J Biol Chem. 1994 Feb 18;269(7):5078–5084. [PubMed] [Google Scholar]
- Swaroop A., Xu J. Z., Pawar H., Jackson A., Skolnick C., Agarwal N. A conserved retina-specific gene encodes a basic motif/leucine zipper domain. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):266–270. doi: 10.1073/pnas.89.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot D., Philipsen S., Fraser P., Grosveld F. Detailed analysis of the site 3 region of the human beta-globin dominant control region. EMBO J. 1990 Jul;9(7):2169–2177. doi: 10.1002/j.1460-2075.1990.tb07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters M., Martin D. I. Functional erythroid promoters created by interaction of the transcription factor GATA-1 with CACCC and AP-1/NFE-2 elements. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10444–10448. doi: 10.1073/pnas.89.21.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Yew N. S., Federspiel M., Dodgson J. B., Hayashi N., Engel J. D. Isolation of recombinant cDNAs encoding chicken erythroid delta-aminolevulinate synthase. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3702–3706. doi: 10.1073/pnas.82.11.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]