Abstract
TM4SF5 overexpressed in hepatocellular carcinoma activates focal adhesion kinase (FAK) during tumor cell migration. However, it remains unknown how TM4SF5 in hepatocellular carcinoma cells compromises with immune actions initiated by extracellular cytokines. Normal and cancerous hepatocytes with or without TM4SF5 expression were analyzed for the effects of cytokine signaling activity on TM4SF5/FAK signaling and metastatic potential. We found that interleukin-6 (IL-6) was differentially expressed in hepatocytes depending on cancerous malignancy and TM4SF5 expression. IL-6 treatment activated FAK and STAT3 and enhanced focal adhesion (FA) formation in TM4SF5-null cells, but it decreased TM4SF5-dependent FAK activity and FA formation in SNU761-TM4SF5 cells. STAT3 suppression abolished the IL-6-mediated effects in normal Chang cells, but it did not recover the TM4SF5-dependent FAK activity that was inhibited by IL-6 treatment in cancerous SNU761-TM4SF5 cells. In addition, modulation of FAK activity did not change the IL-6-mediated STAT3 activity in either the Chang or SNU761 cell system. TM4SF5 expression in SNU761 cells caused invasive extracellular matrix degradation negatively depending on IL-6/IL-6 receptor (IL-6R) signaling. Thus, it is likely that hepatic cancer cells adopt TM4SF5-dependent FAK activation and metastatic potential by lowering IL-6 expression and avoiding its immunological action through the IL-6-STAT3 pathway.
INTRODUCTION
Cell migration and invasion are critical for the homeostatic maintenance of multicellular organisms as well as for cancer metastasis (1), which involves highly complex processes regulated by coordinated signaling pathways responding to extracellular matrix (ECM) or soluble factors (2). As one of the most important signaling molecules activated by cell adhesion, focal adhesion kinase (FAK) plays critical roles in cell migration and invasion (3). FAK is overexpressed in a diverse set of primary and metastatic tumor tissues, including hepatocellular carcinoma (HCC), supporting its protumorigenic and -metastatic roles (4–6).
Tetraspanins (TM4SFs) collaborate with integrins during cell adhesion and migration (7). Similar to tetraspanins, transmembrane 4 L six family member 5 (TM4SF5) is a membrane glycoprotein with four transmembrane domains whose intracellular loop and NH2- and COOH-terminal tails are oriented toward the cytosol (8, 9). TM4SF5 is overexpressed in a diverse set of cancers, and its overexpression in hepatocytes enhances their tumorigenic proliferation, migration, and invasion (8). TM4SF5 binds and activates FAK, thereby directing motility, and this interaction can be the basis for adhesion-dependent FAK activation by TM4SF5 (10). Therefore, TM4SF5 causes abnormal cell growth and enhances the metastatic potential of liver cancer cells (8, 9).
Tumor progression often is driven by inflammatory cells, which produce cytokines that influence the growth and survival of malignant cells. The identification of these cytokines and their mechanisms of action are important, because the inhibition of protumorigenic cytokine actions or the enhancement of antitumorigenic cytokine actions may allow therapeutic strategies (11). Immune cells that often infiltrate tumors produce various cytokines, which propagate a localized inflammatory response and also regulate the growth/survival of premalignant cells (12). Interleukin-6 (IL-6) is a multifunctional cytokine that is important for immune responses, cell fate, and proliferation (13). IL-6 is produced by immune cells and tumor cells (14). IL-6 signaling requires the membrane-bound IL-6 receptor α subunit (mIL-6R; CD126) of the IL-6 receptor and glycoprotein 130 (gp130) on target cells, and the expression of these proteins is limited to hepatocytes and certain leukocytes (15), suggesting autocrine effects by IL-6 on hepatocellular carcinoma cells. By binding to its gp130-associated receptor, IL-6 transduces the signaling pathway that activates JAK1/2-STAT3 (13). The binding of IL-6 to the receptor complex activates the JAK protein tyrosine kinases, leading to the phosphorylation of IL-6R and the recruitment and activation of STAT3. The IL-6/JAKs/STAT3 signaling pathway can be negatively regulated by the actions of the SOCS3 and PIAS proteins (16). The activation of STAT3 induces a diverse group of target genes in diverse tumor types, including HCC (16). In addition, IL-6-independent STAT3 activation (17) or somatic mutation-mediated activation of STAT3 (18) has been reported in hepatocellular tumors. The effect of IL-6-mediated JAKs/STAT3 signaling on breast cancer proliferation can be either inhibitory or stimulatory (19).
We were interested in understanding how TM4SF5-mediated migration/invasion interacts with the cytokine-mediated immune responses. In particular, we examined how TM4SF5/FAK-based signaling, which promotes invasion, might be influenced by IL-6/STAT3 signaling, which could be effective in an autocrine manner. We found that the cross talk between FAK and STAT3 depended on TM4SF5 expression in both normal and cancerous hepatocytes; IL-6/STAT3 signaling activity in Chang cells promoted TM4SF5/FAK activity, whereas IL-6/STAT3 signaling in SNUU761 cells appeared to block TM4SF5/FAK activity. Owing to reduced IL-6 expression, TM4SF5 expression in cancerous cells appears to increase FAK activity, avoiding IL-6/STAT3-mediated inhibition.
MATERIALS AND METHODS
Cell culture.
Control (normal hepatocyte AML12, Chang, hepatocarcinoma SNU449, or SNU761, Huh7-shTM4SF5, non-small-cell lung cancer [NSCLC] HCC827) or TM4SF5 WT-expressing (Chang-TM4SF5, Huh7-shControl, SNU449-TM4SF5, SNU761-TM4SF5, or HCC827-TM4SF5) cells have been described previously (20) or were prepared by G418 (A.G. Scientifics, San Diego, CA) selection following transfection of FLAG-mock or FLAG-TM4SF5 wild type (WT) into the parental cells. Stable cells were maintained in RPMI 1640 (WelGene, Daegu, South Korea) containing 10% fetal bovine serum (FBS), G418 (250 μg/ml), and antibiotics (Invitrogen, Grand Island, NY).
Extract preparation and Western blotting.
Subconfluent cells in normal culture medium or cells transiently transfected with short interfering RNA (siRNA; control or siRNA against STAT3, termed siSTAT3) for 48 h were either kept in suspension or reseeded onto collagen I-, laminin I-, or fibronectin (10 μg/ml; BD Biosciences, San Jose, CA)-precoated dishes with or without IL-6 (50 ng/ml) and/or dimethylsulfoxide (DMSO; vehicle) or diverse pharmacological inhibitors for the indicated periods, as previously explained (10). PF271 (1.0 μM), specifically against FAK (21), and AG490 (100 μM), specifically against JAK2 (LC Laboratories, Woburn, MA), were added in the middle of rocking prior to replating. Cells were either kept in suspension or replated on different extracellular matrix (ECM; 10 μg/ml)-precoated culture dishes for the indicated times. Normal human or cancer liver or colon tissues were obtained after informed consent from each patient according to institutional review board (IRB)-approved methods of the Institute of Laboratory Animal Resources, Seoul National University (ILARSNU). The whole-tissue extracts were prepared as explained previously (20). Whole-cell lysates were prepared with a lysis buffer (1% Brij58, 0.1% SDS, 150 mM NaCl, 20 mM HEPES, pH 7.4, 2 mM MgCl2, 2 mM CaCl2, and protease inhibitors). The primary antibodies included anti-pY397 FAK (Abcam, Cambridge, United Kingdom), anti-pY416 Src, anti-FLAG, anti-Akt, anti-pY165 p130Cas, anti-phospho-extracellular signal-regulated kinases (anti-p-ERKs), anti-ERKs (Cell Signaling Technology, Danvers, MA), anti-pY705 STAT3, anti-pY486 cortactin (Millipore, Billerica, MA), anti-α-tubulin, anti-FLAG (Sigma, St. Louis, MO), anti-c-Src, anti-pY577 FAK, anti-pY118 paxillin, anti-PIAS3, anti-SOCS3, anti-IL-6R, anti-pS473 Akt (Santa Cruz Biotech, Santa Cruz, CA), antipaxillin, anti-FAK (BD Transduction Laboratory, Bedford, MA), and anti-STAT3 (Millipore, Solna, Sweden).
Cytokine antibody array.
Cytokine analysis using whole-cell extracts from the subconfluent cells was performed using RayBio human cytokine antibody array 3 by following the manufacturer's protocols (RayBiotech Inc., Norcross, GA).
Coimmunoprecipitation.
Cells were subjected to mock transfection or were transiently transfected with TM4SF5 WT or the TM4SF5 N138A/N155Q (NANQ) mutant (of N-glycosylation residues) together with or without the extracellular domain of human IL-6R [amino acids 1 to 365; pTarget-hIL-6R(ECD); a kind gift from Soohyun Kim, Medical Immunology Center, KonKuk University, Seoul, South Korea] for 48 h, and whole-cell lysates were prepared as described above. Whole-cell extracts prepared as described above were immunoprecipitated with biotin-precoated beads for 2 h (IBA, Germany) prior to immunoblotting for the indicated molecules.
Indirect immunofluorescence.
Cells under normal culture conditions on glass coverslips or transiently transfected for 48 h with siRNA against a control scramble sequence (siControl) or siSTAT3 (Dharmacon, Pittsburgh, PA), together with green fluorescent protein (GFP)-conjugated control siRNA, were immunostained using antibody against pY397 FAK or pY705 STAT3 and subjected to 4′,6-diamidino-2-phenylindole (DAPI) staining for DNA. Immunofluorescent images were acquired on a microscope (BX51TR; Olympus, Japan). Randomly saved images for 10 fields in each experimental condition were visually counted by two independent individuals. Cells with at least similar or increased spreading area and FA numbers upon IL-6 treatment were counted, and the mean ± standard deviation values are presented as a graph.
Transwell migration assay.
Cells were analyzed for migration using Transwell chambers with 8-μm pores (Corning Inc., Corning, NY). The assay was performed with or without IL-6 treatment (50 ng/ml) for 5 h (Chang cells), 7 h (HCC827 cells), or 12 h (SNU761 cells) with serum-free medium containing 10 μg/ml collagen I (Sigma) in the lower chambers. In the case of antibody blocking, anti-IL-6R antibody (20 μg/ml; US Biological, Salem, MA) was incubated with the cells before loading into the chambers. More than at least 5 random images of migrated cells were saved for each experimental condition. After independent visual counts of cells in each image, mean values ± standard deviations were graphed.
ECM degradation analysis.
Cells were transiently transfected with siControl or siSTAT3 (Dharmacon) for 48 h or were treated with DMSO or 1.0 μM PF271 (21) for 1 day. ECM degradation by cells on Oregon Green 488-conjugated gelatin (Invitrogen), with or without IL-6 treatment (50 ng/ml) for 4 h, were analyzed as described previously (22). Antibody neutralizing was performed by a preincubation of the cells using anti-IL-6R antibody (20 μg/ml; US Biological).
Immunohistochemistry.
Immunohistochemistry of human liver tissues was performed with primary antibodies for normal rabbit IgG, TM4SF5 (20), pY397 FAK (Abcam, Cambridge, United Kingdom), or pY705 STAT3 (Millipore, Solna, Sweden).
Statistical methods.
Student's t tests were performed for comparisons of mean values to determine statistical significance. A P value of less than 0.05 was considered statistically significant.
RESULTS
Differential expression of IL-6 in normal and cancerous cells depends on TM4SF5 expression.
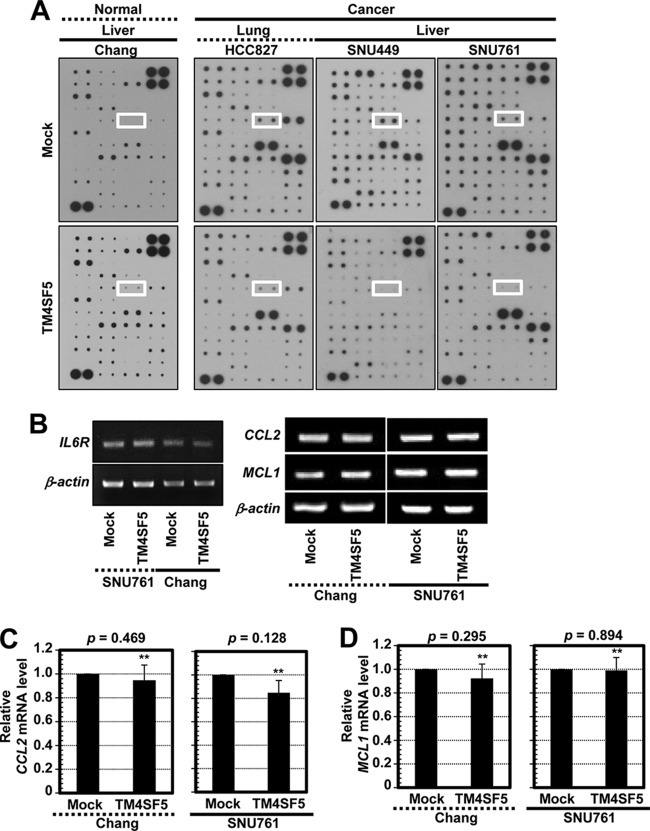
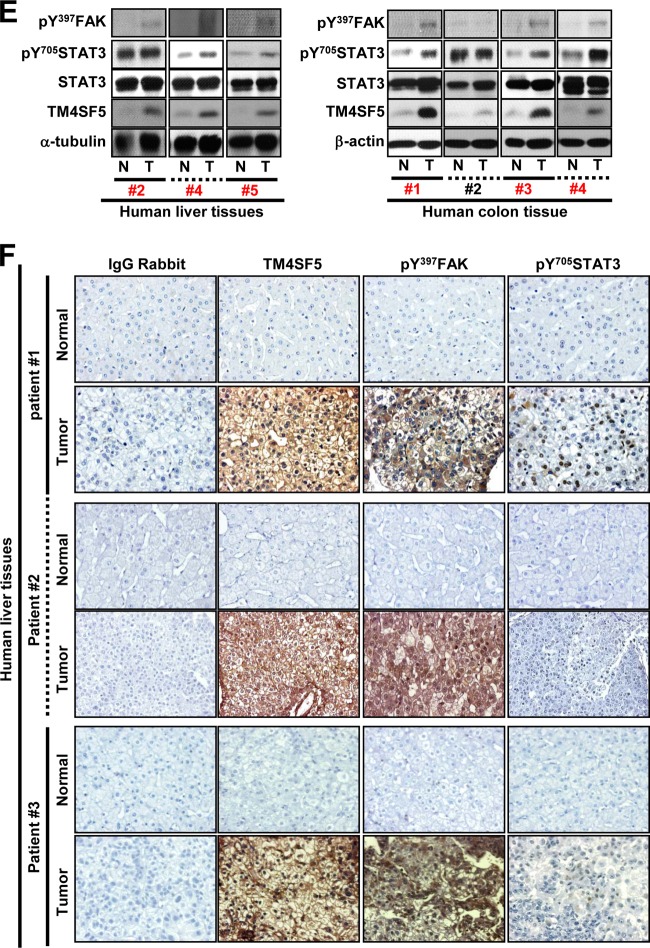
The autocrine effects of cytokines on TM4SF5-mediated tumorigenic functions were studied in normal and cancerous epithelial cells with or without TM4SF5 expression by using an antibody array. Whole-cell lysates from normal Chang hepatocytes, HCC827 lung cancer cells, and SNU449 and SNU761 hepatocellular carcinoma cells lacking or overexpressing TM4SF5 were processed for the array analyses. Interestingly, IL-6 was expressed at higher levels in the Chang-TM4SF5 cells than in parental Chang cells (Fig. 1A, left). However, for the cancer cells, parental TM4SF5-negative cells showed higher IL-6 expression than the TM4SF5-expressing cells (Fig. 1A, right, white boxes). Meanwhile, another cytokine, IL-12 (p40 and p70), which was located 4 rows directly below the IL-6 spots, showed a similar expression pattern, although its intensity was less than that of IL-6 (Fig. 1A). Although IL-12 and other cytokines not tested in this study might be similarly differential in the cellular systems with or without TM4SF5 expression, we focused on IL-6 for further experiments. In contrast, the SNU761 cancer cells and the Chang hepatocytes with or without TM4SF5 expression showed similar IL6R mRNA levels (Fig. 1B). Further, quantitative PCR analyses of downstream target genes in the cells, such as CCL2 (chemokine [C-C motif] ligand 2) and MCL1 (myeloid cell leukemia sequence 1) (23, 24), showed no significant differences in their mRNA levels (Fig. 1B, C, and D). These observations indicated that signaling activities for gene expression by IL-6/IL-6R were comparable between TM4SF5-null and -expressing cells. Thus, the differential effects of IL-6 on TM4SF5-mediated cellular function may be attributed to tumorigenic malignancy. Normal liver or colon tissues were compared to their counterpart cancer tissues for levels of TM4SF5 expression, FAK Tyr397 phosphorylation, or STAT3 Tyr705 phosphorylation. Cancer tissues clearly showed higher levels than those seen in normal tissues for 6 out of 7 TM4SF5-positive tumors (Fig. 1E). Immunohistochemistry analysis of tumor liver tissues resulted in higher TM4SF5, pY397 FAK, and pY705 STAT3 levels (Fig. 1F) than those in normal tissues, indicating that these factors are correlated with liver tumorigenesis. Throughout Western blotting and immunohistochemistry using limited sample numbers, TM4SF5-posiitve liver or colon cancer cells were highly correlated with increased FAK and STAT3 phosphorylation (6/6 and 3/4, respectively) (Fig. 1E and F).
FIG 1.
Differential expression of IL-6 between normal and cancer cells depending on TM4SF5 expression. (A) Cytokine antibody array using whole-cell lysates from diverse mock- or TM4SF5-expressing cells showed differential expression levels of IL-6 (white boxes). (B) Equal expression levels of IL6R mRNA (left) or of certain genes downstream of IL-6/IL-6R (right) in normal Chang and SNU761 cancerous hepatocytes with or without TM4SF5 expression were confirmed by RT-PCR. (C and D) Quantitative real-time PCR analyses for CCL2 (C) or MCL1 (D) were performed using normal Chang and SNU761 cancerous hepatocytes with or without TM4SF5 expression. P values larger than 0.05 (**) depict statistically insignificant differences. (E) Whole extracts prepared from normal (N) or cancer (T) tissues of liver or colon cancer patients were immunoblotted for the indicated molecules. Patient case numbers in red showed a positive relationship among TM4SF5 expression, FAK Tyr397 phosphorylation, and STAT3 Tyr705 phosphorylation. (F) Immunohistochemistry of human liver tissues shows increased TM4SF5, pY397 FAK, or pY705 STAT3 in tumor tissues compared to levels in normal tissues. The data represent three independent experiments.
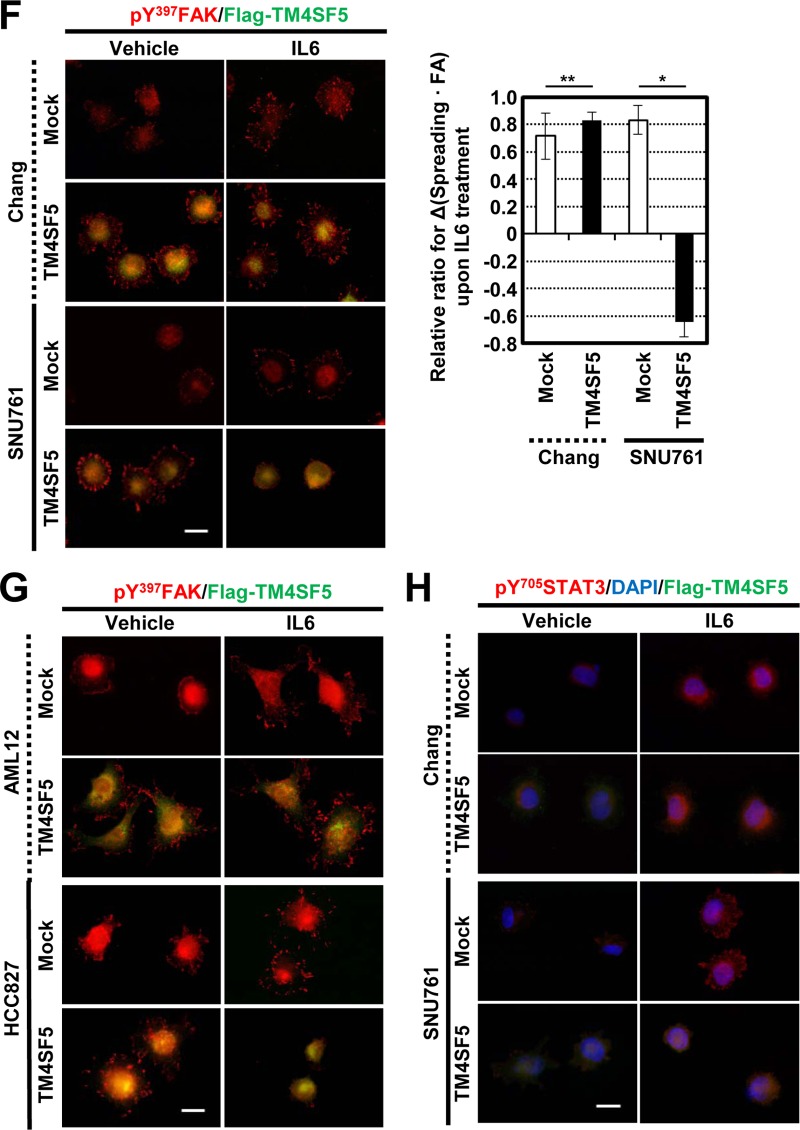
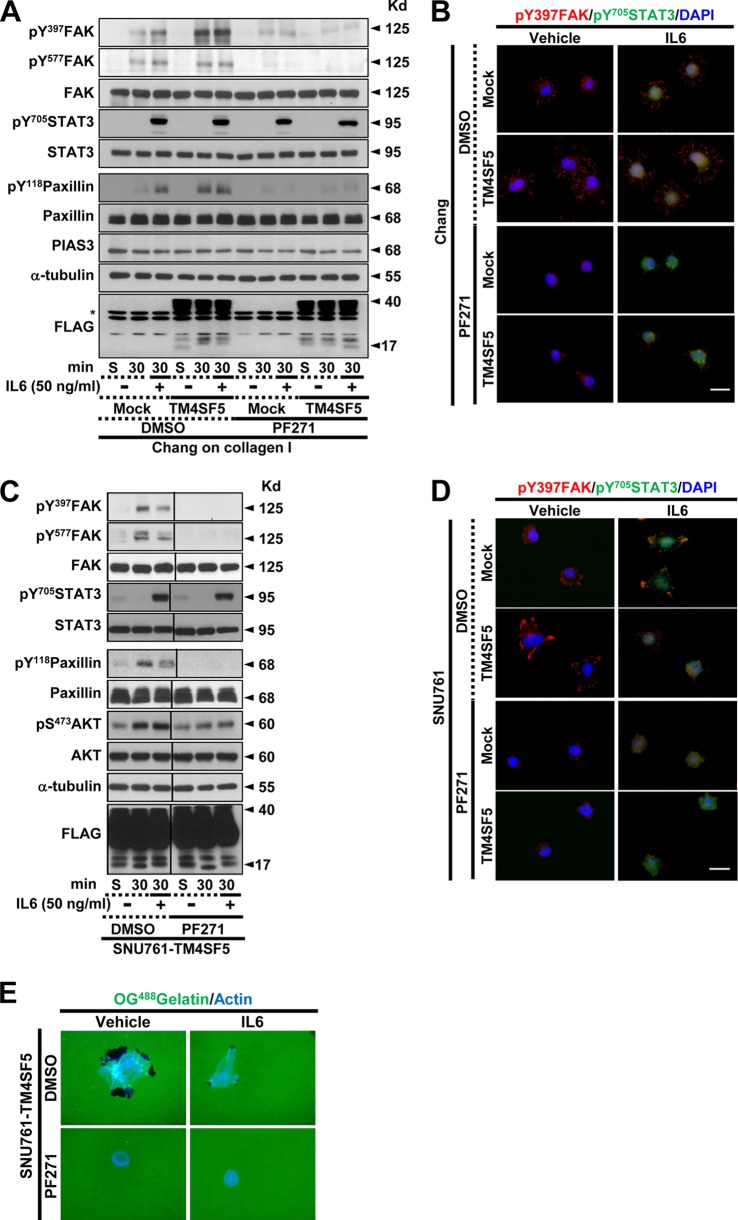
TM4SF5-mediated FAK phosphorylation and focal adhesion formation were maintained or enhanced upon IL-6 treatment of Chang cells but abolished by IL-6 treatment of SNU761 cells.
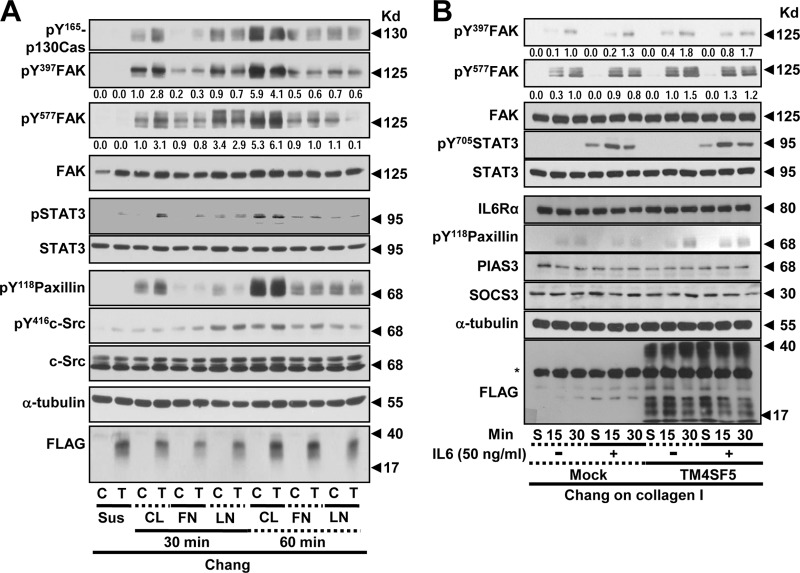
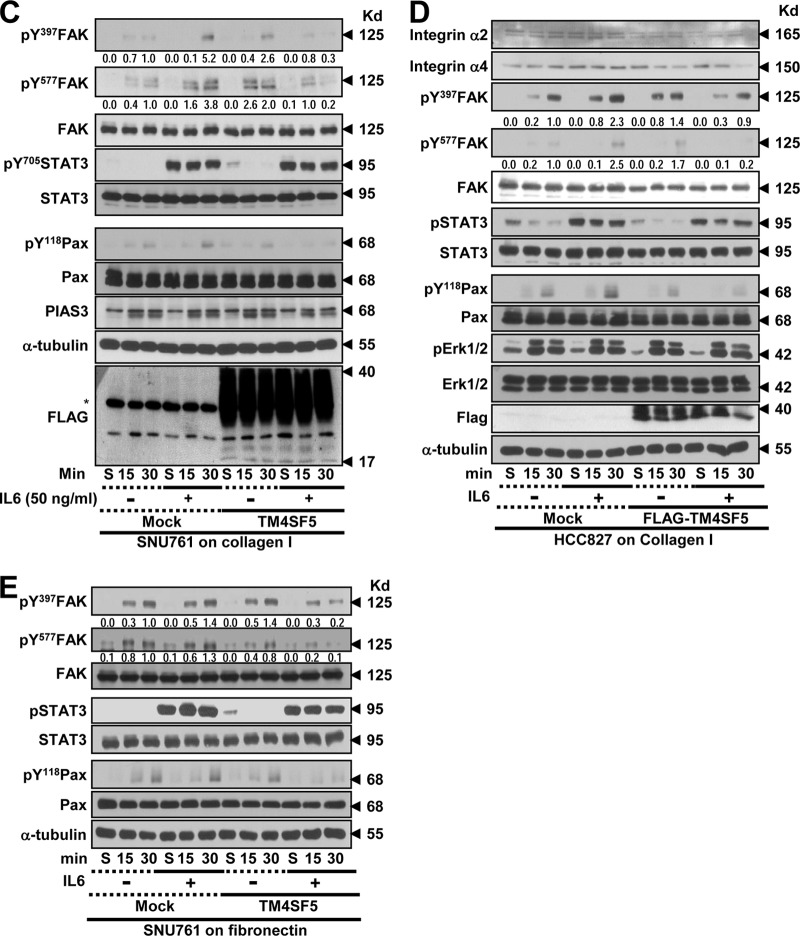
To understand the TM4SF5 dependency of the adhesion-related signaling activities, the cells were reseeded onto a variety of ECM proteins before being analyzed by immunoblotting. Chang cells that were grown on collagen I for 30 min but not 60 min showed obvious TM4SF5-dependent enhancements of the adhesion-related signaling activities compared to cells grown on fibronectin or laminin I (Fig. 2A). Therefore, the cells were analyzed 15 or 30 min after being suspended or reseeded onto collagen I with or without IL-6 treatment. In either the mock-transfected or TM4SF5-expressing cells, the adhesion onto collagen I increased the phosphorylation levels of FAK and paxillin (Pax); this increase was more obvious in cells expressing TM4SF5 than in TM4SF5-null mock-transfected cells (Fig. 2B and C, lanes 1 to 3 and 7 to 9). IL-6 treatment was functional because it increased the Tyr705 phosphorylation of STAT3 (i.e., pY705 STAT3) (Fig. 2B and C). Chang and SNU761 mock-treated cells maintained or increased adhesion-dependent FAK and paxillin phosphorylation upon IL-6 treatment, whereas these phosphorylation levels decreased in the SNU761-TM4SF5 cells; the Chang-TM4SF5 cells maintained the phosphorylation levels (Fig. 2B and C, lanes 4 to 6 and 10 to 12). Thus, IL-6 signaling seemed to negatively affect TM4SF5-dependent FAK activity in SNU761 cancer cells. Such differential TM4SF5-dependent effects on FAK and paxillin phosphorylation following IL-6 treatment also were observed in NSCLC HCC827 cells replated on collagen I (Fig. 2D) and in SNU761 cells replated on fibronectin (Fig. 2E). This point is interesting because the IL-6 levels in TM4SF5-positive cancer cells were lower than those in the TM4SF5-null tumor cells (Fig. 1), which suggests that there was no effective inhibition of TM4SF5 activity by IL-6 signaling in the cancerous cells.
FIG 2.
TM4SF5-mediated FAK phosphorylation and focal adhesion formation were maintained upon IL-6 treatment of Chang cells but was abolished upon IL-6 treatment of SNU761 cells. (A) Chang cells without (C) or with (T) TM4SF5 expression were trypsinized, washed in serum-free medium with 1% bovine serum albumin (BSA), and kept rolling for 1 h prior to being kept in suspension (Sus) for 60 min or reseeding onto collagen I (CL)-, fibronectin (FN)-, or laminin I (LN)-precoated (10 μg/ml) dishes for 30 or 60 min prior to whole-cell lysate preparation. The indicated molecules were immunoblotted using the lysates. (B to H) Chang (B, F, and H), SNU761 (C, F, and H), HCC827 (D and G), or AML12 (G) cells lacking FLAG-TM4SF5 (mock) or overexpressing FLAG-TM4SF5 (TM4SF5) were kept in suspension (S) or were reseeded onto collagen I- or fibronectin (10 μg/ml)-precoated dishes (E) for the indicated times (min) or on cover glasses for 30 min. Vehicle or IL-6 was added as cells were reseeded. The cells were harvested prior to immunoblotting using antibodies against the indicated molecules (B to E) or processed for immunostaining for pY397 FAK (F and G) or pY705 STAT3 in addition to DAPI staining for the nucleus (H). (F) Randomly saved images for 10 fields in each experimental condition were visually counted by two independent individuals. Cells with at least similar or increased spreading areas and FA numbers upon IL-6 treatment were counted, and their mean ± standard deviation values were graphed. Positive y values depict relative ratios of cells with at least similar or increased spreading areas and FA numbers upon IL-6 treatment, and negative y values depict decreased spreading and FA numbers upon IL-6 treatment. Asterisks in panels B and C denote nonspecific bands by the anti-FLAG antibody. One or two asterisks in panel F depict statistically significant or insignificant differences, respectively. The data represent three different experiments.
Although normal Chang hepatocytes showed IL-6-dependent pY705 STAT3 levels (Fig. 2A, B, and C), pY705 STAT3, even without IL-6 treatment (i.e., IL-6-independent pY705 STAT3), might be a characteristic of liver cancer cells. The IL-6-dependent pY705 STAT3 level in Chang cells or in SNU761 mock-treated cells was increased or sustained following cell adhesion, whereas IL-6-independent pY705 STAT3 in SNU761-TM4SF5 cells declined after cell adhesion (Fig. 2C and E, lanes 7 to 9). However, in the case of NSCLC HCC7827, IL-6-independent pY705 STAT3 was observed even without TM4SF5 (Fig. 2D), indicating the presence of tissue-specific IL-6-independent pY705 STAT3 in TM4SF5-positive cells.
The effects of IL-6 treatment on FA formation were examined. TM4SF5 expression in Chang or SNU761 cells resulted in obvious pY397 FAK-enriched FA formation (Fig. 2F, left). Although the mock-transfected Chang (Chang-mock) cells had enhanced FA formation following IL-6 treatment, IL-6-treated Chang-TM4SF5 cells maintained their ability to form FAs and formed more than did the mock cells (Fig. 2F, upper). In addition, SNU761-mock cells showed an IL-6-mediated increase in FA formation similar to that of the Chang cells, but the TM4SF5-dependent FA formation in SNU761-TM4SF5 cells was lost following IL-6 treatment (Fig. 2F, lower). Such observations showing unchanged phospho-Tyr397 FAK-enriched FA formation or decreased FA formation upon IL-6 treatment to TM4SF5-expressing cells also were valid in normal mouse hepatocyte AML12 or NSCLC HCC827 cells that were transiently or stably transfected with TM4SF5, respectively (Fig. 2G). Furthermore, IL-6 treatment enhanced the nuclear translocation of pY705 STAT3, indicating IL-6-mediated STAT3 activation (Fig. 2H). Interestingly, SNU761-mock cells showed obvious localization of pY705 STAT3 at the cellular periphery after IL-6 treatment; it is currently not clear how this occurred (Fig. 2H).
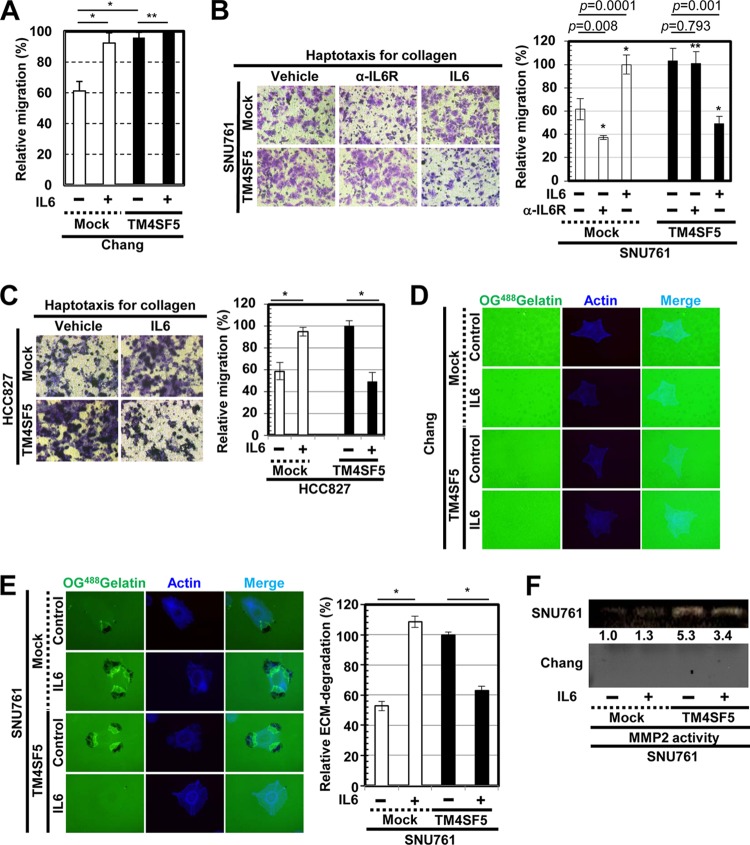
SNU761 cells differentially regulated migration and invasive ECM degradation in a TM4SF5-dependent manner after IL-6 treatment.
We next examined how IL-6 treatment affected the TM4SF5-mediated migration and invasive ECM degradation. IL-6 treatment or TM4SF5 expression in Chang cells each increased the migration of the cells, but treating Chang-TM4SF5 cells with IL-6 did not enhance the TM4SF5-mediated migration (Fig. 3A); instead, the migration rate was maintained. In addition, treatment of SNU761-TM4SF5 cells with IL-6 decreased their migration compared to that of untreated SNU761-TM4SF5 cells; however, IL-6 treatment and TM4SF5 expression in SNU761 cells each increased cellular migration (Fig. 3B). Interestingly, preincubation of cells with anti-IL-6R antibody reduced migration of TM4SF5-null SNU761 cells but did not decrease that of SNU761-TM4SF5 cells (Fig. 3B, right, 2nd and 5th bars), supporting endogenous IL-6 expression in SNU761-mock cells but not IL-6 expression in SNU761-TM4SF5 cells (Fig. 1A). Such IL-6 treatment-mediated decreases in migration of TM4SF5-positive cells but increases in TM4SF5-negative cells compared to levels in untreated cells also were valid in NSCLC HCC827 cells (Fig. 3C).
FIG 3.
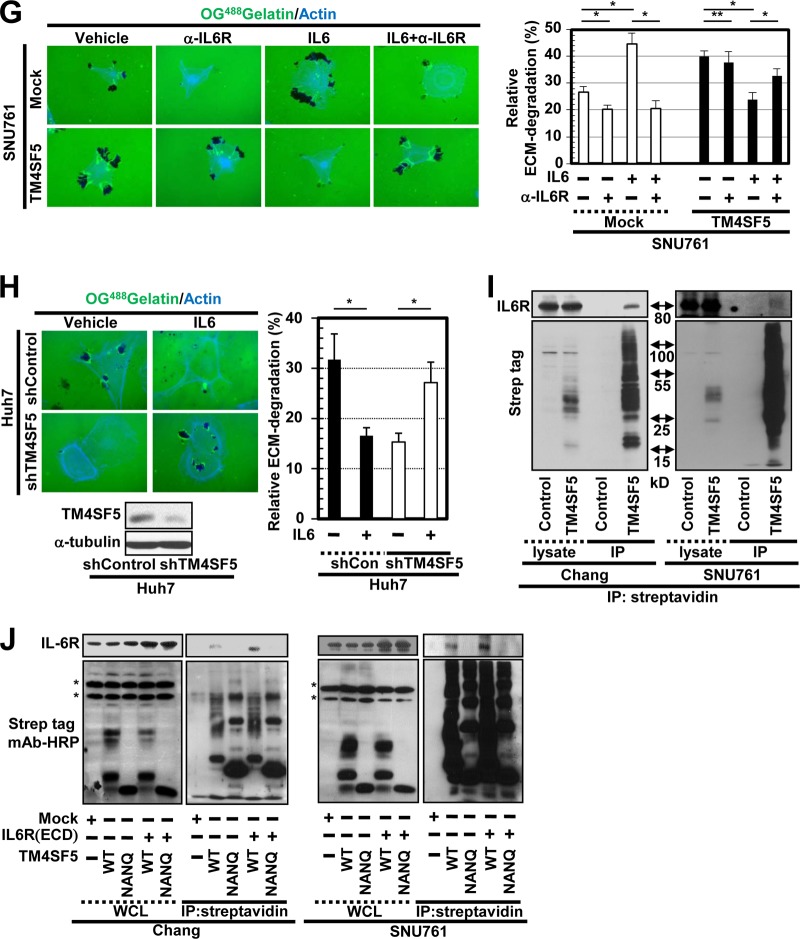
IL-6 treatment of cells differentially regulates migration and invasive ECM degradation depending on TM4SF5 expression. (A to C) The migrations of Chang (A) and SNU761 (B) cells preincubated without or with anti-IL-6R antibody (α-IL-6R; 20 μg/ml) in suspension at 37°C for 1 h or of HCC827 cells (C) stably expressing TM4SF5 or mock transfected were analyzed using Transwell chambers in the presence or absence of IL-6. (D to H) Cells were cultured on cover glasses precoated with Oregon Green 488-conjugated gelatin and were treated with either vehicle or 50 ng/ml IL-6 for 4 h prior to fluorescence imaging. The dark spots indicate ECM degradation, and 10 random regions were imaged under each set of experimental conditions; the means ± standard deviations for the relative ECM degradation are shown in percentages. (E) Conditioned media from the cultures of cells lacking (mock) or ectopically expressing FLAG-TM4SF5 (TM4SF5) in the absence (−) or presence (+) of IL-6 (10 μg/ml) were collected and concentrated before analysis of MMP2 activity by gelatin zymography. (G) Neutralizing anti-IL-6R antibody (20 μg/ml) was added to the cells before analysis. In panels A to D and E to H, one or two asterisks depict statistically significant or insignificant differences, respectively. (I and J) Whole-cell lysates prepared from cells transfected with control-Strep (Control), TM4SF5 WT-Strep (TM4SF5 WT), or TM4SF5 N138A/N155Q-Strep (NANQ) mutant with or without cotransfection of IL-6R(ECD) for 48 h were immunoprecipitated (IP) with streptavidin before immunoblotting using the anti-Strep-tag monoclonal antibody (MAb) conjugated to horseradish peroxidase or an anti-IL-6R antibody. The asterisks in panel J denote nonspecific bands by the anti-Strep tag MAb–horseradish peroxidase (HRP) antibody. The data represent three isolated experiments. shControl, short hairpin control RNA; shTM4SF5, short hairpin TM4SF5 RNA; WCL, whole-cell lysate.
We next examined invasive ECM degradation by culturing cells on OG488-gelatin-precoated cover glasses. Chang hepatocytes did not exhibit ECM degradation under our experimental conditions (Fig. 3D). However, SNU761 cells treated with IL-6 or overexpressing TM4SF5 both exhibited enhanced ECM degradation. Interestingly, treating SNU761-TM4SF5 cells with IL-6 abolished the TM4SF5-enhanced ECM degradation (Fig. 3E). Consistent with these findings, MMP2 activity in the culture medium was not observed in the Chang cells, but the TM4SF5-mediated MMP2 activity of the SNU761-TM4SF5 cells was slightly reduced following IL-6 treatment (Fig. 3F).
Neutralization of the SNU761-TM4SF5 cells with an anti-IL-6R antibody was performed to examine whether IL-6R is critically involved in ECM degradation. Preincubation of the SNU761-mock cells with the anti-IL-6R antibody abolished both basal and IL-6-enhanced ECM degradation, whereas preincubation with the antibody recovered the IL-6-suppressed ECM degradation of the SNU761-TM4SF5 cells to the level of TM4SF5-mediated ECM degradation (Fig. 3G). Again, preincubation of SNU761-mock, but not SNU761-TM4SF5, cells with anti-IL-6R antibody decreased ECM degradation (Fig. 3G), supporting the roles of endogenous IL-6 expression in TM4SF5-negative cells. Further, the IL-6 treatment-mediated decrease in ECM degradation by TM4SF5-positive cancer cells also was observed with Huh7 cells (Fig. 3H).
We then tested whether IL-6R could associate with TM4SF5. Streptavidin (Strep)-tagged TM4SF5 coimmunoprecipitated IL-6R in both Chang and SNU761 cell systems (Fig. 3I). Interestingly, a mutant of TM4SF5 where the N-glycosylation residues were mutated (i.e., NANQ for N138A and N155Q) did not bind to IL-6R (Fig. 3J, 3rd lane in each IP sample). The ectopic extracellular domain of IL-6R (amino acids 1 to 365) resulted in binding to TM4SF5 WT but not to the NANQ mutant (Fig. 3J, 4th and 5th lanes in each IP sample), indicating that their extracellular domains were important for the interaction. Thus, IL-6/IL-6R may differentially regulate adhesion-related signaling and cellular functions of TM4SF5-expressing cells depending on the degree of their malignancy, possibly via extracellular association(s) between IL-6R and TM4SF5.
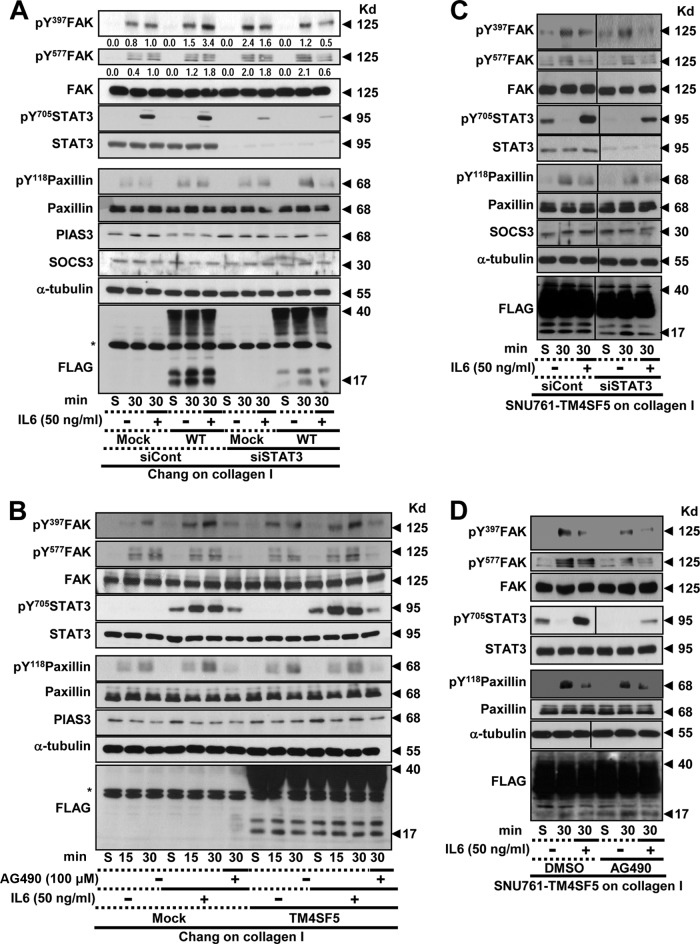
Suppression of JAK/STAT3 signaling inhibited IL-6-mediated FAK phosphorylation in Chang-TM4SF5 cells, but it did not recover the IL-6-suppressed FAK phosphorylation in SNU761-TM4SF5 cells.
We next explored whether the suppression or inhibition of JAK/STAT3 signaling affected the TM4SF5-dependent FAK phosphorylation. STAT3 suppression, through introduction of siSTAT3, did not significantly alter the FAK activity in the Chang-mock cells compared to that of the nonsuppressed cells (Fig. 4A, lanes 1 to 3 and 7 to 9). However, STAT3 suppression in the Chang-TM4SF5 cells abolished IL-6-mediated enhancement of FAK activity (Fig. 4A, lane 6 versus 12); the IL-6/STAT3 signaling pathway did not affect the basal FAK activity (TM4SF5 independent) of the Chang-mock cells but enhanced the TM4SF5-dependent FAK activity of the Chang-TM4SF5 cells. Additionally, pharmacological inhibition of JAK2 using the AG490 inhibitor blocked the IL-6-mediated FAK activity in both the Chang-mock and Chang-TM4SF5 cell lines (Fig. 4B, lanes 3, 6, 7, 10, 13, and 14). This discrepancy in FAK activities between IL-6-treated Chang-mock cells following STAT3 suppression (Fig. 4A lane 9) and IL-6-tretaed Chang-mock cells following JAK2 inhibition (Fig. 4B. lane 7) may reflect nonspecific effects of AG490 on FAK activity, in contrast to the effects observed after siRNA-based specific suppression of STAT3. Furthermore, STAT3 suppression or AG490 treatment in the SNU761-TM4SF5 cells did not recover the FAK activity that was inhibited by IL-6 treatment (Fig. 4C and D). IL-6-independent pY705 STAT3 was undetectable in the SNU761-TM4SF5 cells kept in suspension after STAT3 suppression or AG490 treatment (Fig. 4C and D, lanes 1 and 4), indicating that the IL-6-independent STAT3 activity in the SNU761 cancer cells was obvious, dependent on STAT3 level and JAK2 activity, and presumably competitive with adhesion-dependent TM4SF5/FAK activity.
FIG 4.
Suppression of STAT3/JAK signaling inhibited IL-6-mediated FAK phosphorylation of Chang-TM4SF5 cells but did not recover IL-6-suppressed FAK phosphorylation of SNU761-TM4SF5 cells. Cells were manipulated, as explained in the legend to Fig. 2, after transfection of siRNA against control sequence (siCont) or siSTAT3 for 48 h or after AG490 (a specific JAK2 inhibitor) treatment in the middle of the rolling step. The asterisks in panels A and C depict nonspecific bands to anti-FLAG antibody. S, suspension. Data represent three independent experiments.
STAT3 suppression inhibited IL-6-mediated FA formation by Chang-TM4SF5 cells but did not recover the IL-6-suppressed FA formation and ECM degradation by SNU761-TM4SF5 cells.
The effects of STAT3 suppression on FA formation and ECM degradation were examined next. To mark the siSTAT3-transfected cells, siSTAT3 was cotransfected with a GFP-conjugated siRNA control that forms foci in perinuclear regions (25). Transfection of Chang cells with siControl did not alter FA formation, regardless of IL-6 treatment, compared to FA formation by untransfected cells (Fig. 5A, upper), and the Chang-mock cells after STAT3 suppression did not alter FA formation regardless of IL-6 treatment (Fig. 5A, lower). However, STAT3 suppression in the Chang-TM4SF5 cells decreased FA formation upon IL-6 treatment (Fig. 5A, lower, cells marked by an arrow), although the siSTAT3-untransfected Chang-TM4SF5 cells showed an obvious increase in TM4SF5-mediated FA formation upon IL-6 treatment (Fig. 5A, lower, cells without an arrow).
FIG 5.
STAT3 suppression inhibited IL-6-mediated FA formation by Chang-TM4SF5 cells but did not recover the IL-6-suppressed FA formation and ECM degradation by SNU761-TM4SF5 cells. (A to E) Normal Chang (A and C), AML12-TM4SF5 (D), cancerous SNU761 (B and C), or HCC827-TM4SF5 (E) cells transiently transfected with siRNAs (either siControl or siSTAT3 together with the GFP-tagged siControl) were immunostained for pY397 FAK (red) following treatment with vehicle or IL-6, as explained in the legend to Fig. 2. The arrowheads indicate the GFP-tagged siRNA cotransfected with siControl or siSTAT3. (C) Randomly saved images for 10 fields in each experimental condition were quantitated as explained in the legend to Fig. 2E. One or two asterisks depict statistically significant or insignificant differences, respectively. (F) SNU761-TM4SF5 cells transfected with siControl or siSTAT3 were analyzed for ECM degradation following vehicle or IL-6 treatment for 4 h, as explained in the legend to Fig. 3. The data shown represent three different experiments.
In addition, SNU761-mock cells that were transfected with siControl still exhibited enhanced FA formation in response to IL-6 treatment, whereas siControl-transfected SNU761-TM4SF5 cells exhibited reduced FA formation upon IL-6 treatment (Fig. 5B, upper), as shown in Fig. 2F. STAT3 suppression in the SNU761-mock cells did not alter IL-6-mediated FA formation compared with FA formation by nonsuppressed cells (Fig. 5B, 3rd row). STAT3 suppression in SNU761-TM4SF5 cells did not alter the IL-6-mediated decrease in FA formation; in other words, IL-6 treatment also decreased FA formation in the cells in which STAT3 had been suppressed (Fig. 5B, lower right, arrow), similar to nonsuppressed cells (Fig. 5B, lower, no arrow). Statistically, STAT3 silencing in Chang-TM4SF5 blocked and decreased spreading and FA formation upon IL-6 treatment (Fig. 5C, upper, bars 7 and 8), but STAT3 silencing in SNU761-TM4SF5 did not change the IL-6-mediated decrease in spreading and FA formation (Fig. 5C, lower, bars 7 and 8). The importance of STAT3 for the IL-6-mediated FA formation in normal hepatocytes was confirmed with mouse normal AML12 hepatocytes (Fig. 5D), whereas a STAT3-independent decrease in FA formation upon IL-6 treatment of TM4SF5-expressing cancer cells also was verified with NSCLC HCC827 cells stably expressing TM4SF5 (Fig. 5E). Furthermore, STAT3 suppression in the SNU761-TM4SF5 cells resulted in no ECM degradation following IL-6 treatment, again similar to the behavior of nonsuppressed cells (Fig. 5F).
Inhibition of FAK activity did not alter IL-6-mediated pY705 STAT3 in Chang or SNU761 cells.
We next wondered whether FAK affected STAT3 phosphorylation. To test this, we used the specific FAK inhibitor PF271 (10). FAK inhibition in Chang cells did not alter pY705 STAT3 or its translocation into the nucleus under any circumstances, although FAK activity and FA formation were decreased (Fig. 6A and B). Furthermore, the SNU761-TM4SF5 cells did not show any change in pY705 STAT3 levels or nuclear localization, even after FAK inhibition; however, FAK activity, FA formation, and ECM degradation were diminished after treatment with the inhibitor (Fig. 6C, D, and E). These data support the hypothesis that FAK does not function in the regulation of IL-6/STAT3 signaling.
FIG 6.
Inhibition of FAK activity did not alter IL-6-mediated pY705 STAT3 in Chang or SNU761 cells. The cells were manipulated as explained in the legend to Fig. 2 and were treated with or without PF271 (a specific FAK inhibitor) prior to being kept in suspension (S) or reseeding onto collagen I-precoated dishes. The cells were treated with IL-6 when they were reseeded. The cells were harvested for immunoblot analysis (A and C) or were immunostained for pY397 FAK (red) and pY705 STAT3 (green); the nuclei were counterstained with DAPI (blue) (B and D). The asterisk in panel A denotes nonspecific bands by the anti-FLAG antibody. (E) SNU761-TM4SF5 cells were cultured on OG488-conjugated gelatin for 4 h in the presence or absence of DMSO or PF271 together with vehicle or IL-6. Visualization of ECM-degraded black spots around cells (stained with actin; blue) using an immunofluorescence microscope then was performed to save at least 10 independent images for each experimental condition. Representative images are shown. The data shown represent three independent experiments.
DISCUSSION
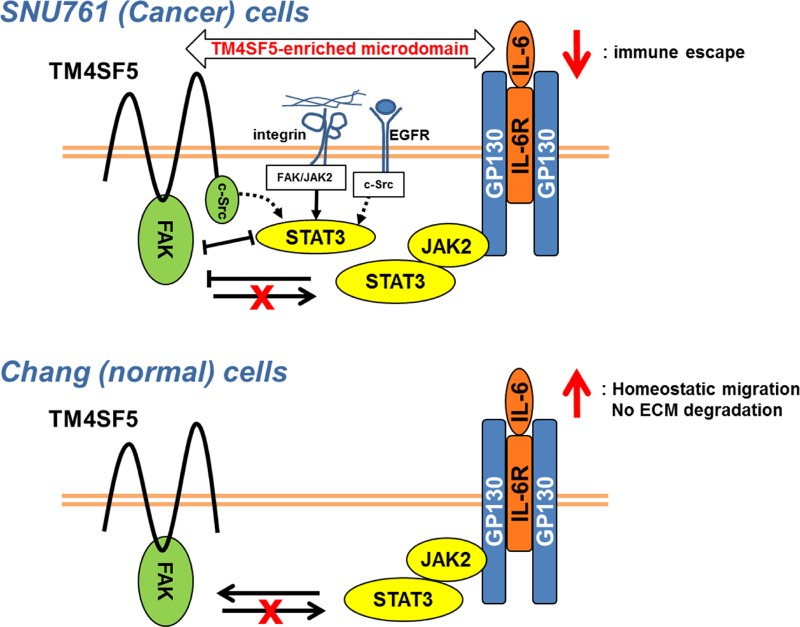
This study shows that forced expression of TM4SF5 reduced expression of the cytokine IL-6 in cancerous hepatocytes compared to TM4SF5-null cancer cells but increased IL-6 expression in normal Chang hepatocytes. IL-6/STAT3 signaling activity appeared to promote FAK activity in Chang or AML12 normal cells but blocked FAK activity in SNU761, Huh7, and HCC827 cancer cells. Furthermore, TM4SF5 expression in cancer cells but not in normal cells caused an increase in IL-6-independent pY705 STAT3 under suspended conditions which declined following cell adhesion to ECM, indicating TM4SF5-mediated aberrant STAT3 activation that is competitive with adhesion-dependent FAK activity. However, in both the Chang and SNU761 cell lines, FAK activity did not affect IL-6-dependent pY705 STAT3. The enhanced IL-6 expression in the Chang cells might regulate homeostatic cell functions, such as migration, whereas reduced IL-6 expression and enhanced FAK activity upon TM4SF5 expression in the SNU761 cancer cells might allow the cells to escape from IL-6/STAT3-mediated immune activity. Thus, in cancer cells, TM4SF5 plays a tumorigenic role by promoting immune escape (Fig. 7).
FIG 7.
Cross talk between the TM4SF5/FAK and IL-6/STAT3 pathways. In normal and cancerous hepatocytes lacking TM4SF5 expression, IL-6 treatment causes FAK activation (not depicted). TM4SF5-expressing cancer cells exhibited IL-6-independent and -dependent STAT3 activity. IL-6-independent pY705 STAT3 presumably is influenced by the FAK/c-Src signaling activity caused by the membrane receptors within the TM4SF5-enriched microdomain and is competitive with TM4SF5-mediated FAK activity in adhered cells (10). (Top) IL-6-dependent STAT3 activity in the TM4SF5-positive cancer cells negatively regulates adhesion- and TM4SF5-dependent FAK activity. However, TM4SF5-expressing cancer cells express less IL-6 than TM4SF5-null cells, presumably leading to TM4SF5-mediated immune escape. (Bottom) In normal Chang-TM4SF5 cells, IL-6-dependent STAT3 activity results in TM4SF5/FAK activity and migration, suggesting a regulatory role for IL-6 in homeostatic migration but not in invasive ECM degradation. In both cell lines, FAK activity does not modulate IL-6-dependent STAT3 activity.
TM4SF5, a member of the tetraspan(in) family (9), is overexpressed in various cancers, including liver cancers (20). TM4SF5 can localize to tetraspanin-enriched microdomains (TEMs) (26), where tetraspan(in)s form complexes with other tetraspan(in)s, integrins, or growth factor receptors (27) and transduce signals that lead to a diverse set of cellular functions. TM4SF5 collaborates with integrins and regulates cell migration and invasion (28). In addition, the cytosolic region of TM4SF5 interacts with and activates FAK and c-Src, promoting directional migration and invasive protrusions (10, 29). Therefore, TM4SF5-mediated FAK activation can be critical during tumor progression (8).
Given the influence of extracellular cues on cancer cell behavior, the TM4SF5-mediated activation of FAK/c-Src can be compromised by the activity of soluble extracellular factors. Although immune cell-produced cytokines can affect the behavior of cancer cells, it is also known that cancer cells themselves express cytokines that can exert autocrine effects (14). The current study showed that IL-6/STAT3 signaling might play different roles depending on the degree of malignancy of the hepatocytes. IL-6 has either pro- or anti-inflammatory roles during liver disease (30), and IL-6-mediated JAK/STAT3 signaling activity either inhibits or promotes breast tumorigenesis (19). Therefore, reduced IL-6 expression could be a strategy by which TM4SF5-positive tumors avoid IL-6-mediated antitumorigenic immunological actions. Furthermore, enhanced IL-6 levels in the Chang-TM4SF5 cells correlated with adhesion-mediated FAK activity and migration, so that cross talk between the (ectopic) TM4SF5-FAK and IL-6-STAT3 signaling pathways in normal hepatocytes supports homeostatic migration-related signaling activity, although normal hepatocytes only minimally express TM4SF5 (20). The preincubation of TM4SF5-null SNU761 cells with anti-IL-6R antibody reduced migration and invasive ECM degradation, but the antibody preincubation of SNU761-TM4SF5 cells did not change the TM4SF5-dependent migration and invasion capacities. These observations suggested roles of IL-6 endogenously expressed in TM4SF5-null cells that are more important than those in TM4SF5-positive cancer cells, which is shown in the antibody array in Fig. 1. Meanwhile, normal or cancer cells with or without TM4SF5 expression showed comparable signaling activities for expression of certain genes downstream of IL-6/IL-6R, suggesting that certain signaling pathways in the cells emanated by the IL-6/IL-6R system were comparably intact. In contrast, adhesion-enhanced FAK activity in the SNU761-TM4SF5 cells was inhibited by IL-6 treatment, indicating that IL-6-mediated regulation of TM4SF5-FAK signaling would not occur because of the reduced IL-6 expression, leading to immunological escape; however, the roles played by immune cell-secreted IL-6 should be considered further.
Diverse tumors have enhanced STAT3 activity (31). Here, STAT3 was shown to be overactivated in suspended SNU761 cells even in the absence of IL-6 but not in normal Chang hepatocytes. Furthermore, TM4SF5 was shown to interact with IL-6R, and the interaction appeared to be through their extracellular domains, especially extracellular loop 2, containing N-glycosylation residues, in the case of TM4SF5. These results suggest that the IL-6R/STAT3 pathway is activated by TM4SF5 even in the absence of ligand binding. TM4SF5 causes Tyr845 phosphorylation of enhanced growth factor receptor (EGFR) via TM4SF5-mediated c-Src activation even in the absence of EGF (29). The ligands for most TM4SFs, including TM4SF5, currently are not known. TM4SF5 associates with integrins (8), and interactions between tetraspanins and integrins can regulate integrin functions (7). These observations suggest that interaction with TM4SF5 mimics ligand binding to IL-6R. Interestingly, the IL-6-independent STAT3 activity appeared to be negatively correlated with FAK activity in the SNU761-TM4SF5 cells. FAK is also hyperactivated in TM4SF5-positive liver cancer cells (10, 20). Therefore, FAK downstream of TM4SF5 might compete with IL-6-independent STAT3 activity; however, FAK did not affect IL-6-dependent STAT3 activity. Growth hormone-mediated FAK activity in CHO cells is not required for STAT3-mediated transcription (32). Therefore, during TM4SF5-mediated tumorigenesis, a balance between TM4SF5-mediated FAK and STAT3 signaling activity can be subtly modulated.
It is currently unknown how TM4SF5 in liver cancer cells activates STAT3 in the absence of IL-6. The negative regulators of STAT3, such as PIAS3 and SOCS3 (31), do not seem to be involved in IL-6-dependent and -independent STAT3 activation in SNU761 cells under the experimental conditions we used, as PIAS3 and SOCS3 levels did not correlate with pY705 STAT3 levels. Therefore, instead of the negative regulators, the upstream tyrosine kinases may contribute to the overactivation of STAT3 in TM4SF5-positive cancers. EGF/EGFR activates STAT3, but it does not lead to mitogenic effects (33), and fibronectin-initiated adhesion activates STAT3, leading to epithelial-mesenchymal transition (EMT) by EGFR-dependent and -independent mechanisms (34). Interestingly, both FAK and EGFR can associate with TM4SF5 (10, 35). TM4SF5-mediated c-Src activity (29) also may be a candidate for causing STAT3 overactivation, as c-Src is upstream of STAT3 (36). Therefore, IL-6-independent STAT3 activation may involve various molecules in TM4SF5-expressing hepatic cancer cells. Because JAK2 inhibition abolished IL-6-independent pY705 STAT3 in the SNU761-TM4SF5 cells, presumably integrin/ECM engagement-mediated FAK/JAK2 activity would lead to STAT3 activity (34).
FAK and STAT3 activities in Chang cells did not promote ECM degradation, although the activities in SNU761 cells correlated with migration and invasive ECM degradation. Thus, during TM4SF5-mediated tumor progression, FAK and STAT3 activities can regulate metastatic potentials, presumably via their cross talk during communication between TM4SF5-positive cancer cells and extracellular cues, such as IL-6.
ACKNOWLEDGMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant for the Tumor Microenvironment Global Core Research Center (GCRC; 2011-0030001) and the senior researcher program (Leap Research; 2012-0005606/2013-035235) and by the Medicinal Bioconvergence Research Center (NRF-2012M3A6A4054271), funded by the Korean government (Ministry of Science, ICT & Future Planning), to J.W.L.
We have no competing interests to declare.
Footnotes
Published ahead of print 9 June 2014
REFERENCES
- 1.Yamaguchi H, Condeelis J. 2007. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 1773:642–652. 10.1016/j.bbamcr.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedl P, Wolf K. 2009. Proteolytic interstitial cell migration: a five-step process. Cancer Metastasis Rev. 28:129–135. 10.1007/s10555-008-9174-3 [DOI] [PubMed] [Google Scholar]
- 3.Luo M, Guan JL. 2010. Focal adhesion kinase: a prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett. 289:127–139. 10.1016/j.canlet.2009.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JS, Huang XH, Wang Q, Chen XL, Fu XH, Tan HX, Zhang LJ, Li W, Bi J. 2010. FAK is involved in invasion and metastasis of hepatocellular carcinoma. Clin. Exp. Metastasis 27:71–82. 10.1007/s10585-010-9306-3 [DOI] [PubMed] [Google Scholar]
- 5.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. 2005. The role of focal-adhesion kinase in cancer–a new therapeutic opportunity. Nat. Rev. Cancer 5:505–515. 10.1038/nrc1647 [DOI] [PubMed] [Google Scholar]
- 6.Zhao J, Guan JL. 2009. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 28:35–49. 10.1007/s10555-008-9165-4 [DOI] [PubMed] [Google Scholar]
- 7.Berditchevski F. 2001. Complexes of tetraspanins with integrins: more than meets the eye. J. Cell Sci. 114:4143–4151 [DOI] [PubMed] [Google Scholar]
- 8.Lee SA, Park KH, Lee JW. 2011. Modulation of signaling between TM4SF5 and integrins in tumor microenvironment. Front. Biosci. 16:1752–1758. 10.2741/3818 [DOI] [PubMed] [Google Scholar]
- 9.Wright MD, Ni J, Rudy GB. 2000. The L6 membrane proteins–a new four-transmembrane superfamily. Protein Sci. 9:1594–1600. 10.1110/ps.9.8.1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung O, Choi S, Jang SB, Lee SA, Lim ST, Choi YJ, Kim HJ, Kim DH, Kwak TK, Kim H, Kang M, Lee MS, Park SY, Ryu J, Jeong D, Cheong HK, Park KH, Lee BJ, Schlaepfer DD, Lee JW. 2012. Tetraspan TM4SF5-dependent direct activation of FAK and metastatic potential of hepatocarcinoma cells. J. Cell Sci. 125:5960–5973. 10.1242/jcs.100586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dougan M, Dranoff G. 2009. Immune therapy for cancer. Annu. Rev. Immunol. 27:83–117. 10.1146/annurev.immunol.021908.132544 [DOI] [PubMed] [Google Scholar]
- 12.Sansone P, Bromberg J. 2011. Environment, inflammation, and cancer. Curr. Opin. Genet. Dev. 21:80–85. 10.1016/j.gde.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Kishimoto T. 2005. IL-6: from laboratory to bedside. Clin. Rev. Allergy Immunol. 28:177–186. 10.1385/CRIAI:28:3:177 [DOI] [PubMed] [Google Scholar]
- 14.Waldner MJ, Foersch S, Neurath MF. 2012. Interleukin-6–a key regulator of colorectal cancer development. Int. J. Biol. Sci. 8:1248–1253. 10.7150/ijbs.4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto K, Rose-John S. 2012. Therapeutic blockade of interleukin-6 in chronic inflammatory disease. Clin. Pharmacol. Ther. 91:574–576. 10.1038/clpt.2012.11 [DOI] [PubMed] [Google Scholar]
- 16.Carbia-Nagashima A, Arzt E. 2004. Intracellular proteins and mechanisms involved in the control of gp130/JAK/STAT cytokine signaling. IUBMB Life 56:83–88. 10.1080/15216540410001668064 [DOI] [PubMed] [Google Scholar]
- 17.Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, Izard T, Balabaud C, Bioulac-Sage P, Zucman-Rossi J. 2009. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature 457:200–204. 10.1038/nature07475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilati C, Amessou M, Bihl MP, Balabaud C, Van Nhieu JT, Paradis V, Nault JC, Izard T, Bioulac-Sage P, Couchy G, Poussin K, Zucman-Rossi J. 2011. Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. J. Exp. Med. 208:1359–1366. 10.1084/jem.20110283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dethlefsen C, Hojfeldt G, Hojman P. 2013. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res. Treat. 138:657–664. 10.1007/s10549-013-2488-z [DOI] [PubMed] [Google Scholar]
- 20.Lee SA, Lee SY, Cho IH, Oh MA, Kang ES, Kim YB, Seo WD, Choi S, Nam JO, Tamamori-Adachi M, Kitajima S, Ye SK, Kim S, Hwang YJ, Kim IS, Park KH, Lee JW. 2008. Tetraspanin TM4SF5 mediates loss of contact inhibition through epithelial-mesenchymal transition in human hepatocarcinoma. J. Clin. Investig. 118:1354–1366. 10.1172/JCI33768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. 2010. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol. 188:877–890. 10.1083/jcb.200906012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SA, Kim TY, Kwak TK, Kim H, Kim S, Lee HJ, Kim SH, Park KH, Kim HJ, Cho M, Lee JW. 2010. Transmembrane 4 L six family member 5 (TM4SF5) enhances migration and invasion of hepatocytes for effective metastasis. J. Cell. Biochem. 111:59–66. 10.1002/jcb.22662 [DOI] [PubMed] [Google Scholar]
- 23.Bournazou E, Bromberg J. 2013. Targeting the tumor microenvironment: JAK-STAT3 signaling. JAKSTAT 2:e23828. 10.4161/jkst.23828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H, Pardoll D, Jove R. 2009. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9:798–809. 10.1038/nrc2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H, Kang M, Lee SA, Kwak TK, Jung O, Lee HJ, Kim SH, Lee JW. 2010. TM4SF5 accelerates G1/S phase progression via cytosolic p27(Kip1) expression and RhoA activity. Biochim. Biophys. Acta 1803:975–982. 10.1016/j.bbamcr.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 26.Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. 2009. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 19:434–446. 10.1016/j.tcb.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 27.Zoller M. 2009. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer 9:40–55. 10.1038/nrc2543 [DOI] [PubMed] [Google Scholar]
- 28.Lee SA, Kim YM, Kwak TK, Kim HJ, Kim S, Kim SH, Park KH, Cho M, Lee JW. 2009. The extracellular loop 2 of TM4SF5 inhibits integrin alpha2 on hepatocytes under collagen type I environment. Carcinogenesis 30:1872–1879. 10.1093/carcin/bgp234 [DOI] [PubMed] [Google Scholar]
- 29.Jung O, Choi YJ, Kwak TK, Kang M, Lee MS, Ryu J, Kim HJ, Lee JW. 2013. The COOH-terminus of TM4SF5 in hepatoma cell lines regulates c-Src to form invasive protrusions via EGFR Tyr845 phosphorylation. Biochim. Biophys. Acta 1833:629–642. 10.1016/j.bbamcr.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 30.Tilg H, Kaser A, Moschen AR. 2006. How to modulate inflammatory cytokines in liver diseases. Liver Int. 26:1029–1039. 10.1111/j.1478-3231.2006.01339.x [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Crowe PJ, Goldstein D, Yang JL. 2012. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers. Int. J. Oncol. 41:1181–1191. 10.3892/ijo.2012.1568 [DOI] [PubMed] [Google Scholar]
- 32.Zhu T, Goh EL, Lobie PE. 1998. Growth hormone stimulates the tyrosine phosphorylation and association of p125 focal adhesion kinase (FAK) with JAK2. Fak is not required for stat-mediated transcription. J. Biol. Chem. 273:10682–10689. 10.1074/jbc.273.17.10682 [DOI] [PubMed] [Google Scholar]
- 33.Guren TK, Abrahamsen H, Thoresen GH, Babaie E, Berg T, Christoffersen T. 1999. EGF-induced activation of Stat1, Stat3, and Stat5b is unrelated to the stimulation of DNA synthesis in cultured hepatocytes. Biochem. Biophys. Res. Commun. 258:565–571. 10.1006/bbrc.1999.0684 [DOI] [PubMed] [Google Scholar]
- 34.Balanis N, Wendt MK, Schiemann BJ, Wang Z, Schiemann WP, Carlin CR. 2013. Epithelial to mesenchymal transition promotes breast cancer progression via a fibronectin-dependent STAT3 signaling pathway. J. Biol. Chem. 288:17954–17967. 10.1074/jbc.M113.475277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee MS, Kim HP, Kim TY, Lee JW. 2012. Gefitinib resistance of cancer cells correlated with TM4SF5-mediated epithelial-mesenchymal transition. Biochim. Biophys. Acta 1823:514–523. 10.1016/j.bbamcr.2011.11.017 [DOI] [PubMed] [Google Scholar]
- 36.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. 1998. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol. Cell. Biol. 18:2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]