Abstract
The molecular scaffold kinase suppressor of Ras 1 (KSR1) regulates the activation of the Raf/MEK/extracellular signal-regulated kinase (ERK) signal transduction pathway. KSR1 disruption in mouse embryo fibroblasts (MEFs) abrogates growth factor-induced ERK activation, H-RasV12-induced replicative senescence, and H-RasV12-induced transformation. Caveolin-1 has been primarily described as a major component of the coating structure of caveolae, which can serve as a lipid binding adaptor protein and coordinates the assembly of Ras, Raf, MEK, and ERK. In this study, we show that KSR1 interacts with caveolin-1 and is responsible for MEK and ERK redistribution to caveolin-1-rich fractions. The interaction between KSR1 and caveolin-1 is essential for optimal activation of ERK as a KSR1 mutant unable to interact with caveolin-1 does not efficiently mediate growth factor-induced ERK activation at the early stages of pathway activation. Furthermore, abolishing the KSR1–caveolin-1 interaction increases growth factor demands to promote H-RasV12-induced proliferation and has adverse effects on H-RasV12-induced cellular senescence and transformation. These data show that caveolin-1 is necessary for optimal KSR1-dependent ERK activation by growth factors and oncogenic Ras.
INTRODUCTION
Caveolin-1 is a protein constituent of the coating structure of caveolae (1–4) and serves as a lipid binding adaptor protein that interacts with specific lipids and multiple signaling proteins (5, 6). Caveolae are caveolin-containing omega-shaped invaginations located at the plasma membrane. Caveolae and caveolin-1 are implicated in signal transduction, vesicular transport, cell migration, cholesterol homeostasis, cell cycle control, cell polarity, and cellular transformation (7–9). Recent evidence also suggests that noncaveolar caveolin-1 has differential roles in cancer cells as increased tumor aggressiveness correlated to elevated levels of noncaveolar caveolin-1 (10, 11). Caveolin-1 regulates a number of signaling pathways that lead to extracellular signal-regulated kinase (ERK) activation, such as epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), ErbB2, and focal adhesion kinase (FAK) signaling (12–17). An inverse relationship between caveolin-1 expression and Raf/MEK/ERK activity has been reported. Overexpression of caveolin-1 attenuates ERK activity, whereas caveolin-1 deficiency leads to hyperactivation of ERK (12, 18–21). Constitutive activation of Raf/MEK/ERK reduces caveolin-1 mRNA and protein expression (22, 23). However, other reports suggest that caveolin-1 promotes ERK activation. Caveolin-1 interacts with Ras and recruits Raf, MEK, and ERK to caveolae in unstimulated cells, facilitating pathway activation after growth factor stimulation (24, 25). Caveolin-1 recruits Fyn, Shc, and Grb2-SOS to facilitate integrin-induced ERK activation (16). In melanoma, caveolin-1 and Rho-GTPase RhoC promote metastasis by stimulating Src-dependent ERK activation (26). Angiotensin-II promotes persistent ERK activation through caveolin-1/EGFR via induction of reactive oxygen species to promote renal cell dedifferentiation (27). Additionally, fibronectin and l-threonine promote mouse embryonic stem cell (ESC) proliferation through activation of caveola-dependent ERK activation (28, 29). Therefore, these data suggest that the relationship between caveolin-1 and ERK activity is dependent on cellular context.
Kinase suppressor of Ras 1 (KSR1) is a molecular scaffold for the Raf/MEK/ERK cascade (30–32). KSR1 expression regulates the intensity and duration of growth factor-induced ERK activation to modulate cell proliferation, H-RasV12-induced transformation and senescence, and adipogenic potential (30, 33, 34). Importantly, immortalized KSR1−/− mouse embryonic fibroblasts (MEFs) are resistant to oncogenic transformation by H-RasV12, which can be rescued by ectopic expression of KSR1 (30). KSR1−/− mice are normal during development, but resistant to Ras-driven tumors (31, 33, 35). Deletion of KSR1 blocks RasV12-induced ERK activation but not activation of other Ras effector pathways, indicating that KSR1-scaffolded ERK activation is necessary for H-RasV12-induced transformation (30).
Given the importance of KSR1 expression in mediating ERK signaling, the role of KSR1 in mediating caveolin-1/caveola-dependent ERK signaling was assessed. In this study, we show that KSR1 is a caveolin-1-interacting protein; however, the interaction was not restricted to caveolin-1 present in caveolae. The KSR1–caveolin-1 interaction is required for growth factor-stimulated ERK activation, the KSR1–B-Raf and KSR1-MEK interaction, and redistribution of MEK and ERK to caveolin-1-rich subcellular fractions. Disrupting the KSR1–caveolin-1 interaction has adverse effects on H-RasV12-mediated cell fates in KSR1−/− MEFs. Introduction of a KSR1 mutant unable to bind caveolin-1 in KSR1−/− MEFs failed to restore H-RasV12-induced senescence to primary MEFs or loss of contact inhibition in immortalized MEFs, only modestly restored H-RasV12-induced anchorage-independent growth, and limited the proliferation of H-RasV12-expressing MEFs in the presence of low levels of serum. These data demonstrate that the KSR1–caveolin-1 interaction is necessary for growth factor-induced ERK activation, H-RasV12-induced senescence in primary MEFs, and H-RasV12-induced transformation in immortalized MEFs.
MATERIALS AND METHODS
Cell lines and cell culture.
Primary mouse embryo fibroblasts (MEFs) and immortalized KSR1−/− and KSR1+/+ MEFs were previously described (33). MEFs were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 0.1 mM minimal essential medium with nonessential amino acids, and 1% penicillin-streptomycin (P-S). Primary MEF medium contained 55 μM β-mercaptoethanol. 293T cells were maintained in DMEM with 10% FBS and 1% P-S. Cells were incubated at 37°C in 5% CO2.
Plasmids, production of recombinant retroviruses, and generation of cell lines.
KSR1 with the mutations Y755A/F757A/Y762A in the caveolin binding motif (CBM; KSR1.CBM) was generated by site-directed mutagenesis of pCMV5-KSR1-FLAG (36). Murine stem cell virus (MSCV)-KSR1.CBM-IRES-GFP (IRES is internal ribosome entry site, and GFP is green fluorescent protein) was subcloned from the EcoRI and SalI sites of pCMV5-KSR-CBM-FLAG into the EcoRI and XhoI sites of MSCV-IRES-GFP. MSCV-IRES-GFP, MSCV-KSR1-IRES-GFP, MSCV-KSR1.CBM-IRES-GFP, pBabePuroRasV12, or pBabePuro retroviral vector was cotransfected with an ecotropic packaging vector into 293T cells. At 48 to 72 h posttransfection, viral supernatants were collected and filtered. Viral supernatants were then either stored at −80°C or used immediately to infect cells. Puromycin-resistant cells were selected with 4 μg/ml puromycin (Sigma). KSR1−/− MEFs expressing ectopic KSR1 constructs were generated as previously described (34). 293T cells were transfected with pCMV5 vector, pCMV5-KSR1-FLAG, or pCMV5-KSR1.CBM-FLAG using Lipofectamine 2000 as per the manufacturer's recommendation (Life Technologies).
In situ ERK activation assay.
In situ ERK activation assays were performed as previously described (30). Briefly, cells were seeded at 1.5 × 104 cells/well in a 96-well plate 24 h prior to analysis and subjected to an in situ plate assay using a Li-Cor Odyssey infrared imaging system to quantify ERK1/2 activation. Cells at 70% confluence were deprived of serum for 4 h and treated with 100 ng/ml epidermal growth factor (EGF) (R&D Systems) in DMEM–1% bovine serum albumin for the times stated in the figures. Anti-phospho-ERK1/2 (Cell Signaling) (1:100) and anti-ERK1 (Santa Cruz, C-16) (1:100) primary antibodies and anti-mouse Alexa Fluor 680-conjugated (Life Technologies) (1:100) and anti-rabbit IRDye 800-conjugated (infrared dye; Rockland) (1:100) secondary antibodies were used to detect and quantify phosphorylated and total ERK protein levels.
Lysate preparation, immunoprecipitation, and Western blotting.
Cells were treated with trypsin and pelleted. Pellets were washed twice with phosphate-buffered saline (PBS) and frozen at −80°C. Frozen pellets were sonicated (two times for 7 s) in ice-cold lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 0.1% Tween 20, 1 mM phenylmethylsulfonyl fluoride [PMSF], 0.4 U/ml aprotinin, 1 mM NaF, 10 mM β-glycerophosphate, 0.1 mM sodium orthovanadate). Following sedimentation of undissolved cellular material by centrifugation (Sorvall Biofuge) (at 4°C for 7 min at 14,000 rpm), lysates were assayed for protein concentration by use of a detergent-compatible (DC) protein assay (Bio-Rad). For caveolin-1 immunoprecipitations, cells were stimulated with 100 ng/ml EGF for 2 min and washed once in ice cold 1× PBS. Cells were lysed for 20 min at 4°C in NP-40–octyl glucoside lysis buffer (10 mM Tris [pH 8.0], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 60 mM octyl glucoside, 10 μg/ml aprotinin, 1 mM NaVO3, 1 mM PMSF, 20 μM leupeptin, and 20 mM β-glycerophosphate). Two milligrams of whole-cell lysate (WCL) was incubated with 4 μg of anti-caveolin-1 (610406; BD Transduction Laboratories) for 3 h, followed by incubation with protein G (GE Healthcare) for 30 min at 4°C. Beads were washed and boiled in 4× Laemmli sample buffer. For FLAG immunoprecipitations, cells were stimulated with 100 ng/ml EGF for 5 min, washed once in cold 1× PBS, and lysed in IGEPAL lysis buffer (20 mM Tris, pH 8.0, 137 mM NaCl, 10% glycerol, 1% IGEPAL, 10 μg/ml aprotinin, 1 mM NaVO3, 1 mM PMSF, 20 μM leupeptin, and 20 mM β-glycerophosphate). One milligram of protein extract was incubated with anti-FLAG–agarose (Sigma) for 2 h, followed by incubation of beads with 1 mg/ml FLAG peptide for 30 min at 4°C (Sigma.) FLAG eluate was boiled in 4× Laemmli sample buffer. A 50-μg volume of total protein and immunoprecipitation eluate were loaded per well, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes. Western blot analysis was developed using the following primary and secondary antibodies (antibodies were from Cell Signaling unless otherwise noted): anti-p53 (Ab-7; Calbiochem) (1:2,500), anti-p19ARF (Abcam) (1:400), anti-p15INK4b (Biosource) (1:400), anti-MEK1/2 (1:1,000), anti-phospho-MEK1/2 (1:1,000), anti-phospho-ERK1/2 (1:1,000), anti-Ras (EMD-Biosciences) (1:1,000), anti-caveolin-1 (610059; BD Transduction Laboratories) (1:1000), anti-KSR1 (H-70; Santa Cruz) (1:500), anti-phospho-Ser338 c-Raf (1:500), anti-c-Raf (610151; BD Transduction Laboratories) (1:500), anti-B-Raf (H-145; Santa Cruz) (1:1,000), anti-peroxisome proliferator-activated receptor γ coactivator 1α (anti-PGC-1α) (H-300; Santa Cruz) (1:1,000), anti-estrogen-related receptor α (anti-ERRα) (V-19; Santa Cruz) (1:1,000), and anti-β-actin (Sigma) (1:3,000). Anti-mouse, anti-rabbit, anti-sheep, and anti-goat secondary antibodies conjugated to Alexa Fluor 680 (Life Technologies) (1:3,000), IRDye 680LT (1: 20,000) (Li-Cor), or IRDye 800 (Rockland) (1:3,000) were used to probe primary antibodies. Protein bands were detected and quantified on a Li-Cor Odyssey infrared imaging system.
Cellular fractionation studies.
Subcellular fractionation was performed as previously described (37). Briefly, cells were washed in PBS and lysed in a hypotonic buffer (25 mM HEPES, pH 6.0, 150 mM NaCl, 10 mM NaF, 100 mM sodium pyrophosphate, 1 mM EDTA, 1 mM PMSF, 10 μg/ml aprotinin, 1 mM NaVO3, 1 mM benzamidine). Nuclei and large organelles were pelleted by centrifugation (300 × g at 4°C for 10 min), and the supernatant was taken off as the cytoplasmic fraction. The pellet was then resuspended in 1% Triton X-100 buffer (25 mM Tris [pH 8.0], 1% Triton X-100, 150 mM NaCl, 10 mM NaF, 10 mM Na-pyrophosphate, 1 mM EDTA, 1 mM PMSF, 10 μg/ml aprotinin, 1 mM benzamidine, and 1 mM sodium orthovanadate) for 15 min at 4°C and then centrifuged at 100,000 × g for 1 h. The supernatant (membrane, Triton soluble [MTS]) was then pulled off, and the pellet (membrane, Triton insoluble [MTI]) was then solubilized by boiling in 4× Laemmli sample buffer.
PLA.
MEFs expressing GFP, KSR1, or CBM were serum starved for 4 h. Cells were left untreated or stimulated with 100 ng/ml EGF for 5 min. Cells were washed twice in ice cold 1× PBS and fixed in 3.7% formaldehyde in PBS. Cells were then incubated with cold 0.5% Triton X-100 in PBS for 20 min. Cells were blocked in a 1:1 solution of Li-Cor Odyssey blocking buffer (Li-Cor Biosciences) and 1% Triton X-100 in PBS for 1 h at room temperature. Slides were incubated with anti-FLAG and anti-caveolin-1 antibodies, previously listed, at 1:100 dilutions, overnight at 4°C. To detect KSR1–caveolin-1 interaction, a Duolink proximity ligation assay (PLA) was performed as per the manufacturer's recommendation (Olink Bioscience). Briefly, PLA probes anti-mouse Minus and anti-rabbit Plus were incubated in a 1:1 solution of Li-Cor Odyssey blocking buffer (Li-Cor Biosciences) and 1% Triton X-100 in PBS for 1 h at 37°C. After washes, the ligation-ligase mixture at a 1:40 dilution and Texas Red amplification-polymerase mixture at a 1:80 dilution were added to the slides, and samples were incubated in a preheated humidity chamber for 30 min and 100 min, respectively, at 37°C. Slides were mounted with coverslips using mounting medium with 4′,6′-diamidino-2-phenylindole (DAPI). Images were collected on a Zeiss Axiovert 200 M (Carl Zeiss MicroImaging, Thornwood, NY) equipped with an ORCA-ER (Hamamatsu, Houston, TX) digital camera using 40× and 100× objectives and processed using SlideBook software (Intelligent Imaging Innovations, Santa Monica, CA). To determine the number of cells that demonstrated congregation of PLA bright spots to the periphery of the cells, over 100 cells per slide were counted independently by multiple investigators. The average and standard deviation of those counts were reported.
SA β-galactosidase activity.
Senescence-associated (SA) β-galactosidase activity was measured as previously described (34). Briefly, cells were washed twice with PBS and fixed for 5 min in 2% formaldehyde–0.2% glutaraldehyde, washed twice with PBS, and stained with SA β-galactosidase staining solution (1 mg/ml X-Gal [5-bromo-4-chloro-3-indolyl-β-d-galactosidase; stock of 20 mg/ml in dimethylformamide], 40 mM citric acid-sodium phosphate [pH 6.0], 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, and 2 mM MgCl2). Cells were incubated overnight at 37°C in the absence of CO2. Cells were visualized the following day and quantified (minimum of 50 cells per trial) for the presence or absence of staining.
Transformation assays.
MEFs stably expressing H-RasV12 and KSR1, KSR1.CBM, or control vectors were seeded in 0.32% Noble agar at 1 × 104 cells per 35-mm dish to assess anchorage-independent growth or seeded at 1 × 105 cells per 10-cm dish to assess loss of contact inhibition. Colonies were counted, photomicrographs were taken, and dishes were stained with Wright-Giemsa 14 to 28 days after seeding. Cloning efficiency was assessed by plating cells at 1 × 103 cells per 10-cm dish and counting Wright-Giemsa-stained colonies 21 days after seeding. To assess tumor growth in vivo, 1 × 106 cells were subcutaneously injected in the interscapular area of 6-week-old female athymic NCr-nude/nude mice (National Cancer Institute). Mice were sacrificed when tumors reached a volume of 1 cm3. The animals were housed under pathogen-free conditions, and experiments were carried out under an approved protocol by the University of Nebraska Medical Center Institutional Animal Care and Use Committee.
Proliferation studies.
To assess cellular senescence, cells were seeded at 5 × 104 cells per 35-mm dish. Triplicate dishes were counted 3 h after seeding to account for plating discrepancies and were then assessed every 48 h for total cell number by trypan blue exclusion. To assess proliferation under limiting amounts of serum, cells were washed in serum-free medium and seeded at 2 × 104 cells per 35-mm dish. Three hours after plating, an equal volume of DMEM containing twice the indicated amount of either serum was added to the dish. Triplicate dishes from three independent cultures were counted 3 h after seeding to account for plating discrepancies and were then assessed at 72 h for total cell number on a Beckman Coulter counter.
Statistics.
Statistical significance between the different experimental groups was performed by two-way analysis of variance (ANOVA), followed by a post hoc t test with a Bonferroni adjustment for multiple comparisons. Statistical significance was rejected at a P value of >0.05. All statistical analyses were performed in GraphPad Prism, version 5.
RESULTS
KSR1 binds caveolin-1 and facilitates MEK and ERK assembly at caveolae.
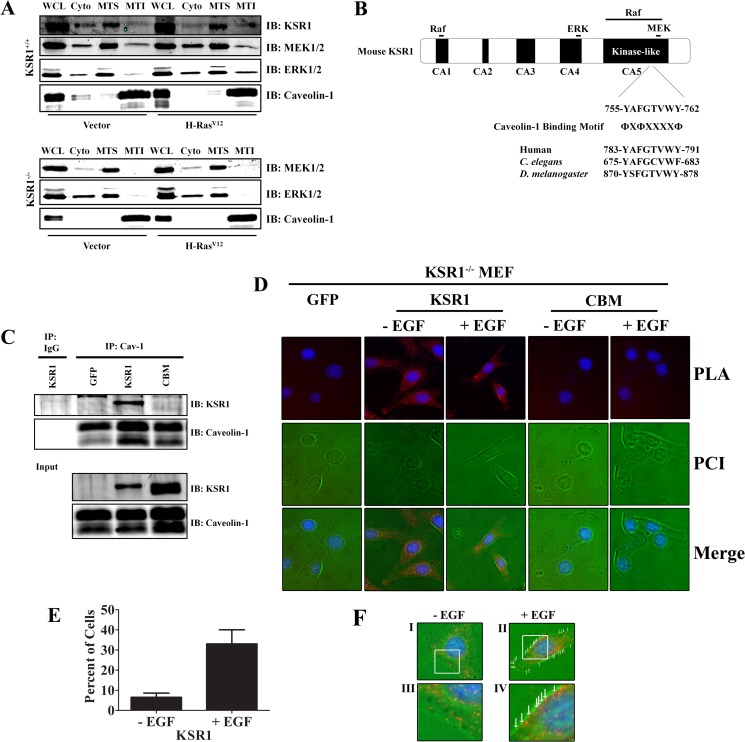
Caveolin-1 recruits and organizes H-Ras, Raf, MEK, and ERK to caveola membranes (25, 38–40) and regulates activation of Raf, MEK, and ERK by growth factors or the Ras oncogene (14, 22, 24, 41, 42). KSR1 localizes to the plasma membrane upon activation of the Raf/MEK/ERK kinase cascade (43). An atypical C1 domain mediates the interaction between KSR1 and the plasma membrane, and KSR1 is directed to specific sites at the plasma membrane by a domain composed of a coiled-coiled (CC) and a sterile α motif (SAM) module (44, 45). Since KSR1 organizes members of the Raf/MEK/ERK kinase cascade at the plasma membrane (43), we hypothesized that KSR1 localized to caveolae upon activation of the Raf/MEK/ERK kinase cascade. To test this hypothesis, the presence of MEK, ERK, and KSR1 was assessed in caveolin-1-rich fractions in cycling wild-type (WT) MEFs. KSR1, MEK, and ERK were detected in the lipid-rich MTI subfraction, which contained caveolin-1 (Fig. 1A). Additionally, expression of H-RasV12 in WT MEFs enhanced the recruitment of KSR1 and ERK in caveolin-1-rich MTI (membrane Triton insoluble) fractions (Fig. 1A).
FIG 1.
KSR1 interacts with caveolin-1. (A) KSR1+/+ or KSR1−/− MEFs expressing H-RasV12 or control vectors were either lysed (WCL) or fractionated into cytoplasmic (Cyto), membrane Triton-soluble (MTS), or membrane Triton-insoluble (MTI) fractions (see Materials and Methods). Lysates were then probed with the indicated antibodies to assess whether KSR1 was required to drive MEK1/2 and ERK1/2 into the caveolin-1 signaling compartment. (B) Schematic diagram of murine KSR1 showing a putative caveolar binding motif (CBM) in the kinase-like domain and the regions that mediate Raf, MEK, and ERK interaction. (C) WCLs from immortalized KSR1−/− MEFs expressing control vector, KSR1, or CBM were immunoprecipitated (IP) for caveolin-1, and the immunoprecipitates were probed for KSR1 to assess the KSR1–caveolin-1 interaction. IB, immunoblotting. (D) KSR1–caveolin-1 interaction examined using a proximity ligation assay (PLA) in serum-starved and EGF-stimulated KSR1−/− MEFs expressing KSR1 or CBM. KSR1−/− MEFs expressing GFP were used as a negative control. PCI, phase contrast image. (E) Quantification of cells demonstrating congregation of bright spots along the periphery in serum-starved and EGF-stimulated KSR1−/− MEFs expressing KSR1. (F) Panels I and II show images of PLA-generated fluorescence in KSR1−/− MEFs expressing WT KSR1 before and after EGF stimulation. Panels III and IV show higher magnifications of the boxed regions in panels I and II, respectively.
To assess if KSR1 is required for the localization of MEK and ERK into caveolin-1-rich MTI fractions, KSR1−/− and KSR1−/− MEFs expressing H-RasV12 were fractionated, and the presence of MEK and ERK in the MTI fractions was analyzed. In the absence of KSR1, MEK and ERK remained in the MTS fraction and were not present in the caveolin-1-rich MTI fraction (Fig. 1A). These data indicate that KSR1 mediates the redistribution of MEK and ERK to a compartment containing caveolin-1.
A number of signaling proteins that interact with caveolin-1 were found to contain a caveolin binding motif (CBM). The consensus CBMs are ΦXΦXXXXΦ, ΦXXXXΦXXΦ, or the combined sequence ΦXΦXXXXΦXXΦ, where Φ represents a Phe, Tyr, or Trp residue and X is any amino acid (46). Raf and MEK proteins contain ΦXΦXXXXΦ or ΦXXXXΦXXΦ sequences, whereas the CBM is not present in ERK proteins. Murine KSR1 contains a putative CBM (YAFGTVWY) between amino acids 755 to 762, which is located in the kinase-like domain (Fig. 1B) and is conserved across species from Drosophila melanogaster to Homo sapiens (Fig. 1B) (47). To determine if KSR1 interacts with caveolin-1, caveolin-1 immunoprecipitations were carried out on lysates from KSR1−/− MEFs expressing WT KSR1 or a KSR1 mutant (KSR1.CBM), where the aromatic residues in the CBM have been mutated to alanines. WT KSR1 was detected in caveolin-1 immunoprecipitates. However, mutation of the CBM on KSR1 abolished the KSR1–caveolin-1 interaction, as KSR1.CBM was not detected in caveolin-1 immunoprecipitations (Fig. 1C). To validate this result, proximity ligation assays (PLA) were performed to visualize the KSR1–caveolin-1 interaction. PLA signals were detected in KSR1−/− MEFs expressing WT KSR1, but no PLA signals were detected in KSR1−/− MEFs expressing KSR1.CBM (Fig. 1D), supporting the observation that mutating the CBM on KSR1 abolishes the interaction with caveolin-1. Surprisingly, the PLA signals detected in KSR1−/− MEFs expressing WT KSR1 were not restricted to caveolin-1 present at caveolae as PLA signals were distributed throughout the cytoplasm. However, EGF stimulation increased the presence of PLA signals at the periphery of cells (Fig. 1D to F). These data indicate that KSR1 is a caveolin-1-interacting protein and that the interaction is mediated by the CBM on KSR1.
The KSR1 caveolin-1 binding motif is required for optimal growth factor-induced ERK activation.
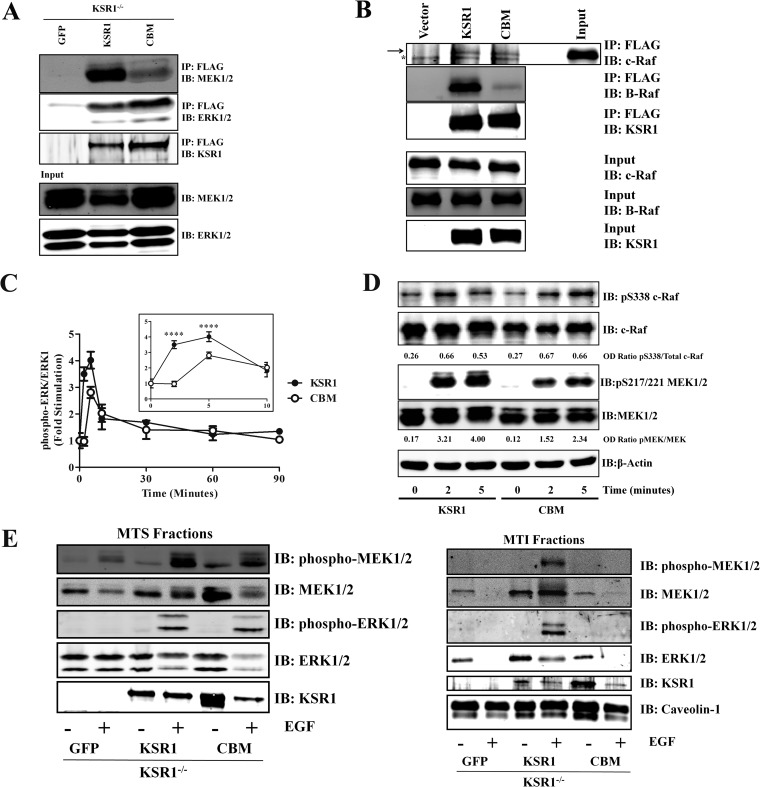
KSR1 immunoprecipitations were performed to determine if mutating CBM on KSR1 affected the interaction of KSR1 with B-Raf, c-Raf, MEK, or ERK. The KSR1-MEK interaction is constitutive, whereas the KSR1-Raf and KSR1-ERK interactions occur during activation of the Raf/MEK/ERK pathway (32, 48, 49). Mutating the CBM on KSR1 did not affect the interaction between KSR1 and ERK as the levels of ERK coimmunoprecipitated with KSR1.CBM were similar to those with WT KSR1 (Fig. 2A). However, mutation of the CBM did lead to a decrease in KSR1-MEK association (Fig. 2A). Moreover, mutation of the CBM reduced the KSR1–B-Raf interaction, but not the KSR1–c-Raf interaction, after EGF stimulation (Fig. 2B). Given the observation that MEK and ERK do not localize to caveolin-1-rich MTI fractions in KSR1−/− MEFs (Fig. 1A) and that the CBM is important for optimal KSR1–B-Raf and KSR1-MEK binding, these data indicate that the CBM is important for KSR1-mediated assembly of the Raf/MEK/ERK kinase cascade.
FIG 2.
The KSR1–caveolin-1 interaction promotes EGF-stimulated ERK1/2 activation. (A) WCLs from immortalized KSR1−/− MEFs expressing control vector, KSR1, or CBM were immunoprecipitated for caveolin-1, and the immunoprecipitates were probed for MEK1/2 and ERK1/2 to assess the KSR1-MEK1/2 and KSR1-ERK1/2 interaction. (B) WCLs from 293T cells transfected with vector, FLAG-tagged KSR1, or FLAG-tagged CBM were immunoprecipitated with anti-FLAG antibodies and subjected to Western blotting for B-Raf and c-Raf to assess KSR1–B-Raf and KSR1–c-Raf interactions. The arrow denotes the band specific for c-Raf. The asterisk indicates s a nonspecific band. (C) Triplicate wells of immortalized KSR1−/− MEFs expressing either KSR1 or KSR1.CBM were treated with 100 ng/ml EGF for the indicated times. ERK1/2 phosphorylation levels were determined in situ for ERK1 and phospho-ERK1/2 with a Li-Cor Odyssey system. Data are expressed as the ratio of phospho-ERK1/2 to ERK1. Data are expressed as means ± standard deviations from three independent experiments. ****, P < 0.001 (D) Immortalized KSR1−/− MEFs expressing either KSR1 or KSR1.CBM were treated with 100 ng/ml EGF for the indicated times. c-Raf and MEK phosphorylation levels were determined via Western blotting. OD, optical density. (E) Immortalized KSR1−/− MEFs expressing GFP alone or the indicated KSR1 construct were stimulated with 100 ng/ml EGF and then fractionated into MTS and MTI fractions (see Materials and Methods). Western blotting for the indicated proteins was performed.
To determine whether the KSR1 CBM contributes to ERK activation, EGF-stimulated ERK activation in KSR1−/− MEFs expressing either WT KSR1 or KSR1.CBM was evaluated in situ (Fig. 2C). We observed a marked difference in ERK activation between WT KSR1 and KSR1.CBM immediately after EGF stimulation (Fig. 2C, insert). KSR1−/− MEFs expressing WT KSR1 exhibited elevated ERK activity by 2 min, and the pathway became optimally activated by 5 min. However, KSR1−/− MEFs expressing KSR1.CBM did not show any EGF-induced ERK activation at 2 min. Furthermore, at 5 min EGF-stimulated ERK activation remained approximately 25% lower in KSR1−/− MEFs expressing KSR1.CBM than in those expressing WT KSR1. These data indicate that mutating the CBM on KSR1 affects the ability of KSR1 to mediate the early stages of growth factor-induced ERK activation.
To further discern the contribution of the CBM on KSR1 toward Raf/MEK/ERK pathway activation, EGF-induced c-Raf and MEK activation was evaluated in KSR1−/− MEFs expressing either WT KSR1 or KSR1.CBM via Western blotting. The KSR1 CBM is dispensable for phosphorylation of c-Raf at Ser338 (Fig. 2D). However, the KSR1 CBM mediates MEK phosphorylation as mutation of the CBM reduced phosphorylation of MEK at the 2-min and 5-min time points by approximately 53% and 43%, respectively (Fig. 2D). These data demonstrate that reduced ERK activity due to mutation of the CBM on KSR1 is due to the inability of KSR1.CBM to properly mediate the activation of MEK.
To determine if the effects of the KSR1 CBM mutation on ERK activity were due specifically to reduced localization of MEK and ERK to caveolin-1-rich fractions after EGF stimulation, MEK and ERK distribution and activity in MTS and caveolin-1-rich MTI fractions isolated from either KSR1−/− MEFs expressing GFP, WT KSR1, or KSR1.CBM were assessed. MEK and ERK distribution in the MTS fractions was not affected by the mutation of CBM on KSR1. Moreover, the phosphorylation levels of MEK and ERK were not drastically affected by KSR1.CBM (Fig. 2E). MEK and ERK were present in the MTI fractions isolated from both unstimulated and EGF-stimulated KSR1−/− MEFs expressing ectopic KSR1. Moreover, phosphorylated MEK and ERK were observed in EGF-stimulated samples from these cells (Fig. 2E). In contrast, the presence of MEK and ERK in these MTI fractions was severely reduced in KSR1−/− MEFs expressing GFP or KSR1.CBM prior to and after EGF stimulation (Fig. 2E). These data indicate that KSR1 localizes MEK and ERK to a compartment containing caveolin-1 upon growth factor stimulation, recapitulating the results observed with H-RasV12 (Fig. 1). Additionally, the KSR1–caveolin-1 interaction is necessary and required for KSR1 to efficiently mediate MEK and ERK activation at the early stages of growth factor-induced activation of the Raf/MEK/ERK kinase cascade.
Mutation of the CBM affects KSR1-mediated H-RasV12-induced senescence.
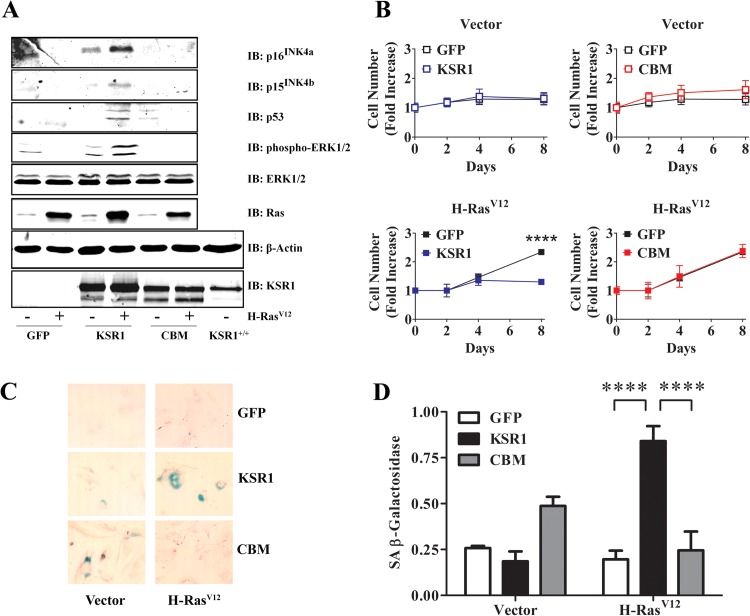
Expression of oncogenic Ras or constitutive activation of the Raf/MEK/ERK kinase cascade promotes activation of INK4/Rb and p19ARF/p53 signaling pathways, causing cellular senescence in primary MEFs (50–56). KSR1 is required for H-RasV12-induced cellular senescence in primary MEFs (34). To determine if the KSR1–caveolin-1 interaction is essential to H-RasV12-induced cellular senescence, early-passage primary KSR1−/− MEFs were transduced with control, WT KSR1, or KSR1.CBM expression vectors in the presence or absence of H-RasV12 retrovirus and were analyzed for activation of INK4A/Rb and p19ARF/p53 pathways (Fig. 3A), cell proliferation (Fig. 3B), and β-galactosidase activity (Fig. 3C and D). Populations of MEFs expressing WT KSR1 or KSR1.CBM at physiological levels were isolated as previously reported (34) (Fig. 3A). Primary MEFs expressing WT KSR1 exhibited sustained ERK activation due to H-RasV12 expression, whereas sustained ERK activation was absent in KSR1−/− MEFs expressing only GFP and H-RasV12 (Fig. 3A). Moreover, mutation of the CBM on KSR1 completely abolished the ability of H-RasV12 to promote ERK activation in primary MEFs (Fig. 3A). Furthermore, H-RasV12 expression promoted induction of p16INK4a, p15INK4b, and p53 in primary KSR1−/− MEFs expressing WT KSR1 but not in MEFs expressing KSR1.CBM or GFP only (Fig. 3A). These data demonstrate that the KSR1–caveolin-1 interaction is required for KSR1-mediated H-RasV12-induced activation of Raf/MEK/ERK, INK4/Rb, and p19ARF/p53 pathways in primary MEFs.
FIG 3.
KSR1–caveolin-1 interaction is required for H-RasV12-induced senescence. (A) WCLs from primary (passage 4 to 6) KSR1−/− MEFs expressing control vector, KSR1, or CBM with or without H-RasV12 were probed with the indicated antibodies to assess induction and activation of each protein by H-RasV12. (B) Cells from the experiment shown in panel A were seeded at 5 × 104 cells per 35-mm dish. Separate duplicate dishes were assessed for cell number at 0, 2, 4, and 8 days after plating. Data are expressed as means ± standard deviations of three independent experiments. (C) Photomicrographs (magnification, ×10) of H-RasV12-expressing cells analyzed in the experiment shown in panel B and stained to visualize SA β-galactosidase activity as described in Materials and Methods. (D) Quantification of SA β-galactosidase activity shown in panel C. Data are representative of three independent experiments. ****, P < 0.001.
As expected, early-passage primary MEFs expressing H-RasV12 and WT KSR1 exhibited characteristics of cellular senescence, such as reduced proliferation in culture (Fig. 3B) and SA β-galactosidase activity (Fig. 3C and D), concomitant with the induction of p16INK4a, p15INK4b, and p53 and sustained ERK activity (Fig. 3A). However, MEFs expressing H-RasV12 and KSR1.CBM did not undergo proliferation arrest (Fig. 3B) or demonstrate SA β-galactosidase activity (Fig. 3C and D), similar to primary MEFs expressing only H-RasV12. These data demonstrate that the KSR1 CBM is required for KSR1-mediated H-RasV12-induced cellular senescence.
The KSR1–caveolin-1 interaction is required for H-RasV12-induced transformation.
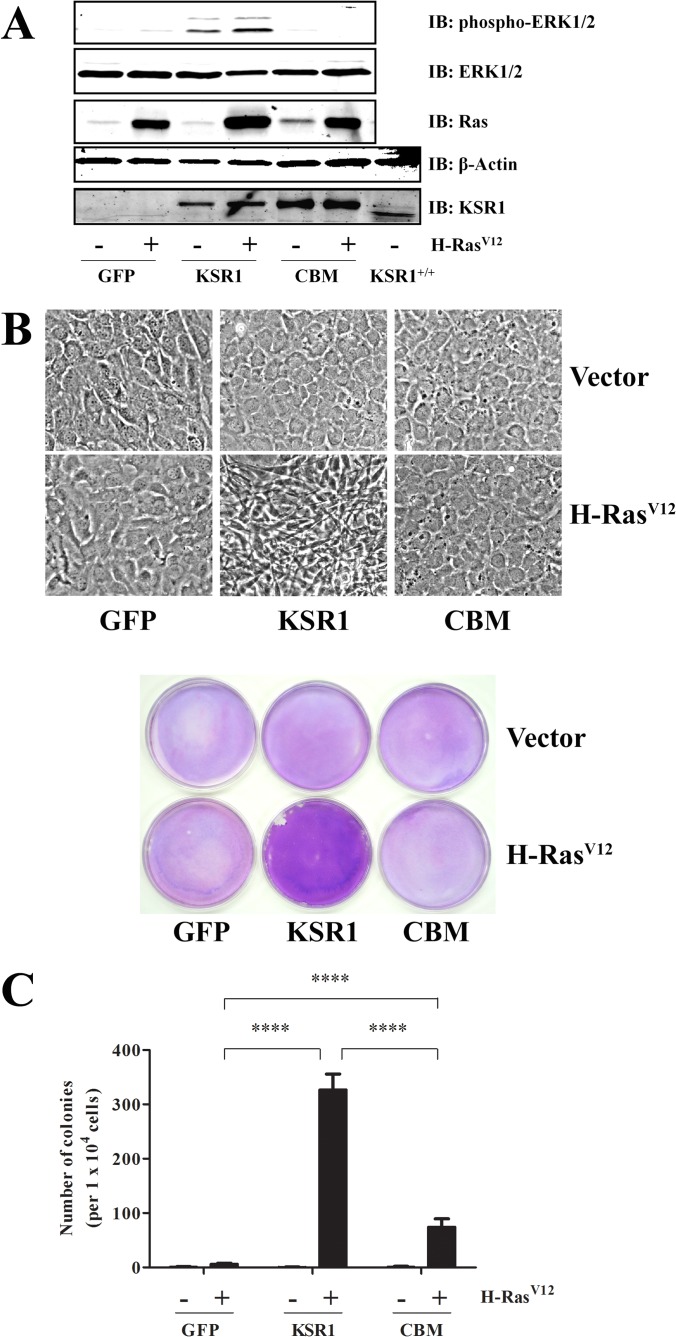
KSR1 is required for H-RasV12-induced transformation of immortalized MEFs in vitro (30) and oncogenic Ras-mediated tumor formation in vivo (31, 35). To determine if the KSR1 CBM is required for KSR1-mediated H-RasV12-induced transformation, immortalized KSR1−/− MEFs were transduced with WT KSR1, KSR1.CBM, or control retrovirus in the presence or absence of H-RasV12 retrovirus. Populations of MEFs expressing WT KSR1 or KSR1.CBM to physiological levels were isolated (Fig. 4A). The reintroduction of KSR1 in KSR1−/− MEFs expressing H-RasV12 promoted sustained ERK activation (Fig. 4A) and demonstrated two properties of cell transformation in vitro, loss of contact inhibition (Fig. 4B) and the ability to grow in an anchorage-independent manner (Fig. 4C). As previously reported (30), H-RasV12 was not able to promote sustained ERK activity, loss of contact inhibition, or anchorage-independent growth in the absence of KSR1 (Fig. 4A to C). Moreover, mutation of the CBM on KSR1 abolished the ability of H-RasV12 to promote both sustained ERK activation (Fig. 4A) and loss of contact inhibition (Fig. 4B). Interestingly, expression of KSR1.CBM did support some H-RasV12-induced anchorage-independent growth in KSR1−/− MEFs but to a significantly lesser extent than was observed in KSR1−/− MEFs expressing comparable levels of WT KSR1 (Fig. 4C).
FIG 4.
KSR1 CBM is required to promote H-RasV12-induced anchorage-independent growth and loss of contact inhibition in immortalized MEFs. (A) WCLs from immortalized KSR1−/− MEFs expressing control vector, KSR1, or CBM ± H-RasV12 were probed with the indicated antibodies to assess induction and activation of each protein by H-RasV12. For the KSR1 and β-actin blots, KSR1+/+ MEFs are shown for comparison. Cells were assessed for transformation by focus formation to assess loss of contact inhibition (B) and growth on soft agar (C) as described in Materials and Methods. ****, P < 0.001.
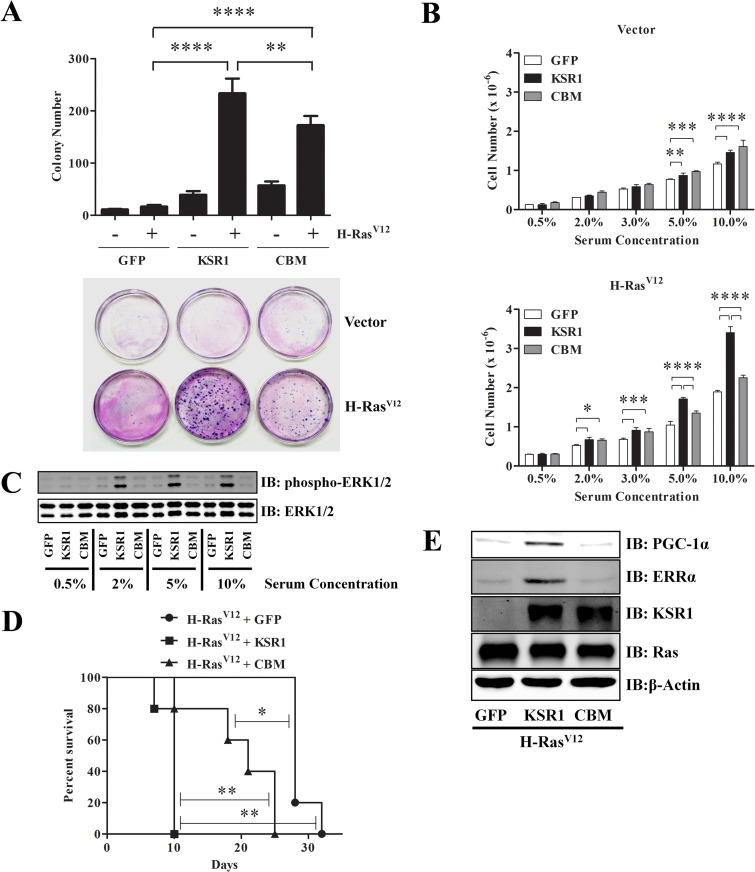
Since the KSR1–caveolin-1 interaction was absolutely required for only one of two classic models of cellular transformation in situ, we sought additional means of assessing the requirements of this interaction to the H-RasV12-transformed phenotype. In addition to showing enhanced proliferative rates, transformed cells can proliferate at extremely low densities and show reduced growth factor requirements. To assess proliferation at low density, KSR1−/− MEFs expressing H-RasV12 and either GFP, KSR1, or KSR1.CBM were plated at 102 cells/10-cm dish and assessed for clonal proliferation after 21 days by assessment of colony formation. The presence of KSR1 increased the clonal efficiency of KSR1−/− MEFs expressing H-RasV12 in comparison to KSR1−/− MEFs expressing GFP and H-RasV12. Colony formation was also observed in KSR1−/− MEFs expressing KSR1.CBM and H-RasV12, although not to levels observed with WT KSR1. Moreover, the sizes of the colonies observed in KSR1−/− MEFs expressing KSR1.CBM and H-RasV12 were smaller than those observed in KSR1−/− MEFs expressing WT KSR1 and H-RasV12 (Fig. 5A).
FIG 5.
KSR1 CBM optimizes H-RasV12-induced cell growth and proliferation to promote transformation and tumorigenesis. KSR1−/− MEFs expressing control vector, KSR1, or CBM with or without H-RasV12 were assessed for transformation by cloning efficiency (A) and growth under limiting serum concentrations (B) and after subcutaneous injection into athymic mice (D) as described in Materials and Methods. Immortalized KSR1−/− MEFs expressing H-RasV12 and either GFP, KSR1, or KSR1.CBM were analyzed for ERK phosphorylation levels 72 h after plating in limiting serum concentrations (C) and for PGC-1α and ERRα protein expression via Western blotting (E). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.001.
Given the observation that KSR1−/− MEFs expressing KSR1.CBM and H-RasV12 exhibited reduced ERK activity (Fig. 4A) and that KSR1.CBM did not optimally mediate growth factor-induced ERK activation (Fig. 2C), the proliferative rate of KSR1−/− MEFs expressing H-RasV12 and GFP, WT KSR1, or KSR1.CBM was examined under various serum concentrations. Serum depletion leads to growth arrest and mimics metabolic stresses encountered in solid tumors (57). Increased cell proliferation was observed in KSR1−/− MEFs expressing H-RasV12 and WT KSR1 or KSR1.CBM at serum concentrations of 2% and 3% in comparison to KSR1−/− MEFs expressing H-RasV12 alone. At 5% and 10% serum concentrations, KSR1−/− MEFs expressing H-RasV12 and WT KSR1 exhibited faster proliferative rates than KSR1−/− MEFs expressing H-RasV12 and KSR1.CBM (Fig. 5B). The increased proliferative rates of KSR1−/− MEFs expressing H-RasV12 and KSR1 correlated with the elevated levels of ERK activity observed in these cells (Fig. 5C). These data demonstrate that the KSR1–caveolin-1 interaction may be dispensable to promote survival under metabolic stress but is required for optimal response to nutrient-rich conditions.
To further discern the importance of the KSR1–caveolin-1 interaction in H-RasV12-induced transformation, KSR1−/− MEFs expressing H-RasV12 and either GFP, KSR1, or KSR1.CBM were subcutaneously injected in the interscapular area of NCr-nude/nude mice, and tumor growth in vivo was analyzed over time. Mice were sacrificed when tumor volume reached 1 cm3. Recapitulating the results in vitro, all mice injected with MEFs expressing H-RasV12 and KSR1 obtained the maximum allowed tumor volume in 10 days, whereas the absence of KSR1 delayed maximal tumor formation to 28 days after transplantation (Fig. 5D). Consistent with the intermediate phenotype observed in H-RasV12-induced anchorage-independent growth, 50% of the tumors arising from KSR1−/− MEFs expressing H-RasV12 and KSR1.CBM reached a 1-cm3 tumor volume at 21 days postinjection, indicating approximately twice the latency of tumors expressing WT KSR1 (Fig. 5D). These data indicate that the KSR1 CBM is necessary to optimally mediate H-RasV12-induced transformation and tumorigenesis.
KSR1−/− MEFs expressing H-RasV12 and KSR1 promote the induction of peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1) α and estrogen-related receptor α (ERRα) in an ERK-independent manner. Both transcriptional coactivators are required to promote H-RasV12-induced anchorage-independent growth in a KSR1-dependent manner (58). Therefore, it was postulated that the anchorage-independent growth mediated by KSR1.CBM in KSR1−/− MEFs expressing H-RasV12 could be due to PGC-1α and ERRα protein expression. However, analysis via Western blotting indicated that KSR1−/− MEFs expressing H-RasV12 and KSR1.CBM did not promote the induction of PGC-1α and ERRα (Fig. 5E). These data suggest that H-Ras-induced anchorage-independent growth mediated by KSR1.CBM is dependent on the limited ERK activity present in these cells.
DISCUSSION
As a molecular scaffold, KSR1 facilitates Ras activation of the Raf/MEK/ERK kinase cascade (30, 31, 48, 49). Disruption of ksr1 in D. melanogaster and Caenorhabditis elegans reverts the activated Ras phenotype, and KSR1 disruption in mammalian models inhibits oncogenic Ras-induced senescence and transformation in vitro and in vivo (30, 31, 34, 35, 47, 59, 60). KSR1 translocates to the plasma membrane in response to growth factor treatment and Ras activation, where it facilitates the activation of MEK by Raf and of ERK by MEK (34, 61). Caveolin-1 is a component of caveolae, which are pits in the plasma membrane implicated in endocytosis, calcium signaling, and other signal transduction events (9, 62, 63). Members of the Ras/Raf/MEK/ERK kinase cascade are present in caveolae, which modulate pathway activation. In this study, we show that KSR1 is a caveolin-1 binding protein, that caveolin-1 facilitates the formation of the KSR1/MEK/ERK complex and is a regulator of KSR1-mediated ERK activation, and that the KSR1–caveolin-1 interaction is required for H-RasV12-induced cellular senescence, transformation, and tumorigenesis.
Caveolin-1 has been primarily described as an inhibitor of Raf/MEK/ERK signaling (12, 20). Higher Raf/MEK/ERK activity has been ascribed to caveolin-1 knockdown or knockout in fibroblasts (18, 21). Constitutive activation of the Raf/MEK/ERK pathway promotes downregulation of caveolin-1 transcription and protein expression in mouse fibroblasts but not human fibroblasts (22, 23). Moreover, overexpression of caveolin-1 attenuates Raf/MEK/ERK activation (12, 19). However, other studies have demonstrated that components of the Raf/MEK/ERK kinase cascade are present in caveolae in unstimulated cells, which are activated by growth factors (24). Studying the relationship between caveolin-1 and ERK activity through KSR1 revealed that caveolin-1 may serve as an organizing node for pathway activation. KSR1 is essential and required for optimal activation of the Raf/MEK/ERK signaling pathway (30, 31). Disrupting the interaction between KSR1 and caveolin-1 attenuated ERK activation. Members of the Raf/MEK/ERK kinase cascade were absent from caveolin-1-rich fractions in cells lacking KSR1 or expressing a KSR1 unable to interact with caveolin-1. These data demonstrate that the KSR1–caveolin-1 interaction is required for the localization and activation of MEK and ERK to caveolin-1-dependent compartments in the cell. KSR1 also mediates ERK-dependent negative feedback signaling on Raf, MEK, and KSR1 (64). In full serum, KSR1−/− MEFs expressing KSR1.CBM proliferated at rates similar to those expressing WT KSR1 (Fig. 5B), indicating that ERK activity in KSR1.CBM-expressing MEFs is sufficient to mediate proliferation or that ERK-dependent negative feedback signaling is disrupted, thereby sustaining the limited ERK activity. Further studies should reveal the extent to which KSR1–caveolin-1 interactions contribute to the negative feedback loops emanating from ERK activation. Such studies might explain why cells deficient in caveolin-1 exhibit higher ERK activation (18, 21, 65).
While preventing interaction with caveolin-1, mutation of the CBM on KSR1 did not affect the localization of KSR1.CBM to the MTI fraction. Interestingly, more WT KSR1 and KSR1.CBM were observed in the MTI fractions in serum-starved cells than in EGF-stimulated cells (Fig. 2E). Moreover, the KSR1 paralog, KSR2, does not contain a CBM. Expressing extremely low levels of KSR2 in KSR1−/− MEFs was sufficient to promote growth factor-induced ERK activation, whereas KSR1 had to be expressed nine times higher in order to give a similar response (66). Previous reports described the presence of Raf, MEK, and ERK in caveola fractions prior to growth factor stimulation (24, 25), and mutating the CBM on KSR1 drastically reduced ERK activation during the early response to growth factor stimulation (Fig. 2C). Combined with the results observed with the PLA (Fig. 1D), in which the KSR1–caveolin-1 interaction was not limited to the periphery of the cell, these data suggest that caveolin-1 may be mediating the preassembly of KSR1 with members of the Raf/MEK/ERK kinase cascade prior to growth factor stimulation and tightly regulating ERK signaling activation. Additionally, these data suggest that the CBM does not dictate KSR1 localization and that reduced ERK signaling in KSR1−/− MEFs expressing KSR1.CBM is likely due to its inability to interact with caveolin-1 and properly assemble the Raf/MEK/ERK signaling cassette. While the CBM is required for the interaction between KSR1 and caveolin-1, the proximity of these two proteins throughout the cell was striking. Further experimentation should reveal the affinity of the KSR1–caveolin-1 interaction in unstimulated and growth factor-stimulated cells.
KSR1 is the major scaffold that mediates ERK activation at lipid rafts (67). While disruption of the KSR1–caveolin-1 interaction affected the early response due to growth factor signaling, mutation of the CBM on KSR1 did not completely abolish ERK activity in MEFs. These data demonstrate that KSR1-mediated ERK signaling is not solely based on its interaction with caveolin-1 as cells expressing the KSR1.CBM did exhibit moderate levels of ERK activation after the initial burst of KSR1 CBM-dependent EGF-stimulated ERK phosphorylation observed at 2 min. However, translation of ERK activation into a fully competent biological response is defective in cells expressing KSR1.CBM.
Disruption of the KSR1–caveolin-1 interaction attenuated the ability of H-RasV12 to sustain ERK activation in MEFs. Although KSR1.CBM did not rescue H-RasV12-induced senescence in primary MEFs (Fig. 3), it showed a weak and variable ability to restore the H-RasV12-transformed phenotype in immortalized MEFs (Fig. 4 to 5). These data suggest that KSR1 mediates ERK activation at other compartments within the cell that contribute to H-RasV12-induced transformation and is sufficient to promote proliferation in the presence of high serum concentrations. The data also show that the interaction between KSR1 and caveolin-1 is required for optimal induction of H-RasV12-mediated senescence transformation and xenograft tumor formation. These data are in accord with other reports indicating that caveolin-1-dependent ERK signaling is essential to activate Ras-to-ERK signaling, which is necessary to promote the epithelial-to-mesenchymal transition in renal epithelial cells and proliferation in ESCs (27–29).
Through these studies, the dynamic relationship between MEK and KSR1 was further elucidated. Mutation of the CBM on KSR1 did not affect the KSR1-ERK interaction, but did reduce MEK interaction. The reduced binding or lack of binding between KSR1 and MEK did not affect the activation of ERK. This is consistent with observations that mutations R589M and C809Y in KSR, or analogous mutations in brain-specific KSR1, that reduce or abolish the KSR1-MEK interaction do not affect the KSR1-ERK interaction (61, 68). Moreover, mutations that disrupt MEK interaction with KSR1 enhance ERK binding in PC12 cells and ERK activation in KSR1−/− MEFs (34, 68), showing that KSR1-MEK binding is dispensable to activate ERK. These mutations affected the temporal activation of Raf/MEK/ERK as they impaired differentiation but enhanced H-RasV12-induced transformation (34, 68). Therefore, it is unlikely that the reduced MEK binding to KSR1.CBM is contributing to reduced ERK activity. Moreover, our data show that MEK is not present in fractions containing caveolin-1 in the absence of KSR1 or in the presence of KSR1.CBM. This was an unexpected result as MEK contains a CBM. However, previous data demonstrated that MEK affects subcellular KSR1 localization and vice versa (61, 69). These data suggest that KSR1 and MEK may work in concert to localize with caveolin-1 during pathway activation.
Advances in bioinformatics and structural analyses have cast doubt on whether the CBM indeed mediates the interaction between caveolin-1 and interacting signal proteins (70, 71). These models propose that the aromatic residues contained within the CBM are hydrophobic and will not be accessible at the surface to other proteins. Furthermore, the models argue that mutating these aromatic residues may lead to protein misfolding and affect protein localization. However, the models cannot eliminate the possibility that caveolin-1 interacts with other proteins before they take on a fully folded conformation. KSR1 binding to ATP and the interaction between the kinase domains of KSR1 and Raf lead to a fully closed conformational change in the kinase domain of KSR1 (72, 73). Therefore, it is possible that the interaction between KSR1 and caveolin-1 occurs before KSR1 adapts a fully closed conformation. These data raise the possibility that caveolin-1 plays a role in the recruitment of KSR1 to caveolae, while the critical step in the localization of KSR1 to the plasma membrane is strictly regulated by the CC-SAM motif and the CA3 domain of KSR1 (44). Detailed structural studies between the CBM of proteins and the scaffolding domain of caveolin-1 will conclusively determine the extent to which the CBM directly mediates the caveolin-1 interaction.
These data reveal a model in which assembly and activation of KSR1-scaffolded Raf/MEK/ERK complexes are modulated by caveolin-1 to transduce critical biological actions of growth factors and oncogenic Ras. Further characterization of these molecular mechanisms central to oncogenic Ras signaling may reveal novel therapeutic targets of Ras-driven tumorigenesis.
ACKNOWLEDGMENTS
We thank members of the Lewis laboratory for their constructive comments. We gratefully acknowledge the assistance of Tom Dao in acquisition of images for the PLA and technical assistance by Alexander Chan. We thank Charles A. Kuszynski, Victoria Smith, and Linda M. Wilkie of the University of Nebraska Medical Center Cell Analysis Facility for their technical expertise in the generation of GFP-expressing cell lines. We thank Janice A. Taylor and James R. Talaska of the Confocal Laser Scanning Microscope Core Facility at the University of Nebraska Medical Center for providing assistance with confocal microscopy.
This research was supported by NIH grants R01 CA90040 and R01 DK52809 (R.E.L.). R.L.K. and K.W.F. were supported by Physician-Scientist Training Grants from the American Diabetes Association. M.R.F. was supported by the NCI Minority Supplement through NIH grant R01 CA90040, the Skala Fellowship, and training grant T32 CA09476 to the Eppley Institute for Research in Cancer and Allied Diseases. We thank the Nebraska Research Initiative and the Eppley Cancer Center for their support of the Core Facility.
Footnotes
Published ahead of print 7 July 2014
REFERENCES
- 1.Anderson RG. 1998. The caveolae membrane system. Annu. Rev. Biochem. 67:199–225. 10.1146/annurev.biochem.67.1.199 [DOI] [PubMed] [Google Scholar]
- 2.Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV. 1995. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol. Biol. Cell 6:911–927. 10.1091/mbc.6.7.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sargiacomo M, Scherer PE, Tang Z, Kubler E, Song KS, Sanders MC, Lisanti MP. 1995. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc. Natl. Acad. Sci. U. S. A. 92:9407–9411. 10.1073/pnas.92.20.9407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP. 1997. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J. Biol. Chem. 272:29337–29346. 10.1074/jbc.272.46.29337 [DOI] [PubMed] [Google Scholar]
- 5.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. 1998. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 273:5419–5422. 10.1074/jbc.273.10.5419 [DOI] [PubMed] [Google Scholar]
- 6.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. 1999. Caveolins, liquid-ordered domains, and signal transduction. Mol. Cell. Biol. 19:7289–7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarro A, Anand-Apte B, Parat MO. 2004. A role for caveolae in cell migration. FASEB J. 18:1801–1811. 10.1096/fj.04-2516rev [DOI] [PubMed] [Google Scholar]
- 8.Parton RG, Simons K. 2007. The multiple faces of caveolae. Nat. Rev. Mol. Cell. Biol. 8:185–194. 10.1038/nrm2122 [DOI] [PubMed] [Google Scholar]
- 9.Williams TM, Lisanti MP. 2005. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am. J. Physiol. Cell Physiol. 288:C494–C506. 10.1152/ajpcell.00458.2004 [DOI] [PubMed] [Google Scholar]
- 10.Aung CS, Hill MM, Bastiani M, Parton RG, Parat MO. 2011. PTRF-cavin-1 expression decreases the migration of PC3 prostate cancer cells: role of matrix metalloprotease 9. Eur. J. Cell Biol. 90:136–142. 10.1016/j.ejcb.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 11.Steffens S, Schrader AJ, Blasig H, Vetter G, Eggers H, Trankenschuh W, Kuczyk MA, Serth J. 2011. Caveolin 1 protein expression in renal cell carcinoma predicts survival. BMC Urol. 11:25. 10.1186/1471-2490-11-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, Kohtz DS, Lisanti MP. 1998. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 428:205–211 [DOI] [PubMed] [Google Scholar]
- 13.Engelman JA, Lee RJ, Karnezis A, Bearss DJ, Webster M, Siegel P, Muller WJ, Windle JJ, Pestell RG, Lisanti MP. 1998. Reciprocal regulation of Neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo. Implications for human breast cancer. J. Biol. Chem. 273:20448–20455. 10.1074/jbc.273.32.20448 [DOI] [PubMed] [Google Scholar]
- 14.Engelman JA, Wykoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. 1997. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J. Biol. Chem. 272:16374–16381. 10.1074/jbc.272.26.16374 [DOI] [PubMed] [Google Scholar]
- 15.Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. 1996. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87:733–743. 10.1016/S0092-8674(00)81392-6 [DOI] [PubMed] [Google Scholar]
- 16.Wary KK, Mariotti A, Zurzolo C, Giancotti FG. 1998. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94:625–634. 10.1016/S0092-8674(00)81604-9 [DOI] [PubMed] [Google Scholar]
- 17.Wei Y, Yang X, Liu Q, Wilkins JA, Chapman HA. 1999. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 144:1285–1294. 10.1083/jcb.144.6.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, Shirani J, Pereira de Souza A, Kitsis RN, Russell RG, Weiss LM, Tang B, Jelicks LA, Factor SM, Shtutin V, Tanowitz HB, Lisanti MP. 2003. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am. J. Physiol. Cell Physiol. 284:C457–C474. 10.1152/ajpcell.00380.2002 [DOI] [PubMed] [Google Scholar]
- 19.Fiucci G, Ravid D, Reich R, Liscovitch M. 2002. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene 21:2365–2375. 10.1038/sj.onc.1205300 [DOI] [PubMed] [Google Scholar]
- 20.Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, Unruh H, Gounni AS, Zaagsma J, Halayko AJ. 2006. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 291:L523–L534. 10.1152/ajplung.00013.2006 [DOI] [PubMed] [Google Scholar]
- 21.Williams TM, Lee H, Cheung MW, Cohen AW, Razani B, Iyengar P, Scherer PE, Pestell RG, Lisanti MP. 2004. Combined loss of INK4a and caveolin-1 synergistically enhances cell proliferation and oncogene-induced tumorigenesis: role of INK4a/CAV-1 in mammary epithelial cell hyperplasia. J. Biol. Chem. 279:24745–24756. 10.1074/jbc.M402064200 [DOI] [PubMed] [Google Scholar]
- 22.Engelman JA, Zhang XL, Razani B, Pestell RG, Lisanti MP. 1999. p42/44 MAP kinase-dependent and -independent signaling pathways regulate caveolin-1 gene expression. Activation of Ras-MAP kinase and protein kinase a signaling cascades transcriptionally down-regulates caveolin-1 promoter activity. J. Biol. Chem. 274:32333–32341. 10.1074/jbc.274.45.32333 [DOI] [PubMed] [Google Scholar]
- 23.Sasai K, Kakumoto K, Hanafusa H, Akagi T. 2007. The Ras-MAPK pathway downregulates caveolin-1 in rodent fibroblast but not in human fibroblasts: implications in the resistance to oncogene-mediated transformation. Oncogene 26:449–455. 10.1038/sj.onc.1209792 [DOI] [PubMed] [Google Scholar]
- 24.Liu P, Ying Y, Anderson RG. 1997. Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae. Proc. Natl. Acad. Sci. U. S. A. 94:13666–13670. 10.1073/pnas.94.25.13666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. 1996. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J. Biol. Chem. 271:9690–9697 [DOI] [PubMed] [Google Scholar]
- 26.Arpaia E, Blaser H, Quintela-Fandino M, Duncan G, Leong HS, Ablack A, Nambiar SC, Lind EF, Silvester J, Fleming CK, Rufini A, Tusche MW, Brustle A, Ohashi PS, Lewis JD, Mak TW. 2012. The interaction between caveolin-1 and Rho-GTPases promotes metastasis by controlling the expression of α5-integrin and the activation of Src, Ras and Erk. Oncogene 31:884–896. 10.1038/onc.2011.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Chen JK, Harris RC. 2012. Angiotensin II induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway. Mol. Cell. Biol. 32:981–991. 10.1128/MCB.06410-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JH, Ryu JM, Han HJ. 2011. Involvement of caveolin-1 in fibronectin-induced mouse embryonic stem cell proliferation: role of FAK, RhoA, PI3K/Akt, and ERK 1/2 pathways. J. Cell Physiol. 226:267–275. 10.1002/jcp.22338 [DOI] [PubMed] [Google Scholar]
- 29.Ryu JM, Han HJ. 2011. L-threonine regulates G1/S phase transition of mouse embryonic stem cells via PI3K/Akt, MAPKs, and mTORC pathways. J. Biol. Chem. 286:23667–23678. 10.1074/jbc.M110.216283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kortum RL, Lewis RE. 2004. The molecular scaffold KSR1 regulates the proliferative and oncogenic potential of cells. Mol. Cell. Biol. 24:4407–4416. 10.1128/MCB.24.10.4407-4416.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen A, Burack WR, Stock JL, Kortum R, Chaika OV, Afkarian M, Muller WJ, Murphy KM, Morrison DK, Lewis RE, McNeish J, Shaw AS. 2002. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol. Cell. Biol. 22:3035–3045. 10.1128/MCB.22.9.3035-3045.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy F, Laberge G, Douziech M, Ferland-McCollough D, Therrien M. 2002. KSR is a scaffold required for activation of the ERK/MAPK module. Genes Dev. 16:427–438. 10.1101/gad.962902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kortum RL, Costanzo DL, Haferbier J, Schreiner SJ, Razidlo GL, Wu MH, Volle DJ, Mori T, Sakaue H, Chaika NV, Chaika OV, Lewis RE. 2005. The molecular scaffold kinase suppressor of Ras 1 (KSR1) regulates adipogenesis. Mol. Cell. Biol. 25:7592–7604. 10.1128/MCB.25.17.7592-7604.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kortum RL, Johnson HJ, Costanzo DL, Volle DJ, Razidlo GL, Fusello AM, Shaw AS, Lewis RE. 2006. The molecular scaffold kinase suppressor of Ras 1 is a modifier of RasV12-induced and replicative senescence. Mol. Cell. Biol. 26:2202–2214. 10.1128/MCB.26.6.2202-2214.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lozano J, Xing R, Cai Z, Jensen HL, Trempus C, Mark W, Cannon R, Kolesnick R. 2003. Deficiency of kinase suppressor of Ras1 prevents oncogenic ras signaling in mice. Cancer Res. 63:4232–4238 [PubMed] [Google Scholar]
- 36.Joneson T, Fulton JA, Volle DJ, Chaika OV, Bar-Sagi D, Lewis RE. 1998. Kinase suppressor of Ras inhibits the activation of extracellular ligand-regulated (ERK) mitogen-activated protein (MAP) kinase by growth factors, activated Ras, and Ras effectors. J. Biol. Chem. 273:7743–7748. 10.1074/jbc.273.13.7743 [DOI] [PubMed] [Google Scholar]
- 37.Sordella R, Jiang W, Chen GC, Curto M, Settleman J. 2003. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell 113:147–158. 10.1016/S0092-8674(03)00271-X [DOI] [PubMed] [Google Scholar]
- 38.Li S, Couet J, Lisanti MP. 1996. Src tyrosine kinases, Gα subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J. Biol. Chem. 271:29182–29190. 10.1074/jbc.274.45.32333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mineo C, James GL, Smart EJ, Anderson RG. 1996. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J. Biol. Chem. 271:11930–11935. 10.1074/jbc.271.20.11930 [DOI] [PubMed] [Google Scholar]
- 40.Rybin VO, Xu X, Steinberg SF. 1999. Activated protein kinase C isoforms target to cardiomyocyte caveolae: stimulation of local protein phosphorylation. Circ. Res. 84:980–988. 10.1161/01.RES.84.9.980 [DOI] [PubMed] [Google Scholar]
- 41.Agelaki S, Spiliotaki M, Markomanolaki H, Kallergi G, Mavroudis D, Georgoulias V, Stournaras C. 2009. Caveolin-1 regulates EGFR signaling in MCF-7 breast cancer cells and enhances gefitinib-induced tumor cell inhibition. Cancer Biol. Ther. 8:1470–1477. 10.4161/cbt.8.15.8939 [DOI] [PubMed] [Google Scholar]
- 42.Furuchi T, Anderson RG. 1998. Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK). J. Biol. Chem. 273:21099–21104. 10.1074/jbc.273.33.21099 [DOI] [PubMed] [Google Scholar]
- 43.Muller J, Ory S, Copeland T, Piwnica-Worms H, Morrison DK. 2001. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell 8:983–993. 10.1016/S1097-2765(01)00383-5 [DOI] [PubMed] [Google Scholar]
- 44.Koveal D, Schuh-Nuhfer N, Ritt D, Page R, Morrison DK, Peti W. 2012. A CC-SAM, for coiled coil-sterile alpha motif, domain targets the scaffold KSR-1 to specific sites in the plasma membrane. Sci. Signal. 5:ra94. 10.1126/scisignal.2003289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou M, Horita DA, Waugh DS, Byrd RA, Morrison DK. 2002. Solution structure and functional analysis of the cysteine-rich C1 domain of kinase suppressor of Ras (KSR). J. Mol. Biol. 315:435–446. 10.1006/jmbi.2001.5263 [DOI] [PubMed] [Google Scholar]
- 46.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. 1997. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 272:6525–6533. 10.1074/jbc.272.10.6525 [DOI] [PubMed] [Google Scholar]
- 47.Therrien M, Chang HC, Solomon NM, Karim FD, Wassarman DA, Rubin GM. 1995. KSR, a novel protein kinase required for RAS signal transduction. Cell 83:879–888. 10.1016/0092-8674(95)90204-X [DOI] [PubMed] [Google Scholar]
- 48.Cacace AM, Michaud NR, Therrien M, Mathes K, Copeland T, Rubin GM, Morrison DK. 1999. Identification of constitutive and ras-inducible phosphorylation sites of KSR: implications for 14-3-3 binding, mitogen-activated protein kinase binding, and KSR overexpression. Mol. Cell. Biol. 19:229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Therrien M, Michaud NR, Rubin GM, Morrison DK. 1996. KSR modulates signal propagation within the MAPK cascade. Genes Dev. 10:2684–2695. 10.1101/gad.10.21.2684 [DOI] [PubMed] [Google Scholar]
- 50.Ferbeyre G, de Stanchina E, Lin AW, Querido E, McCurrach ME, Hannon GJ, Lowe SW. 2002. Oncogenic ras and p53 cooperate to induce cellular senescence. Mol. Cell. Biol. 22:3497–3508. 10.1128/MCB.22.10.3497-3508.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferbeyre G, de Stanchina E, Querido E, Baptiste N, Prives C, Lowe SW. 2000. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 14:2015–2027 [PMC free article] [PubMed] [Google Scholar]
- 52.Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. 1998. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12:3008–3019. 10.1101/gad.12.19.3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malumbres M, Perez De Castro, I, Hernandez MI, Jimenez M, Corral T, Pellicer A. 2000. Cellular response to oncogenic ras involves induction of the Cdk4 and Cdk6 inhibitor p15INK4b. Mol. Cell. Biol. 20:2915–2925. 10.1128/MCB.20.8.2915-2925.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmero I, Pantoja C, Serrano M. 1998. p19ARF links the tumour suppressor p53 to Ras. Nature 395:125–126. 10.1038/25870 [DOI] [PubMed] [Google Scholar]
- 55.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593–602. 10.1016/S0092-8674(00)81902-9 [DOI] [PubMed] [Google Scholar]
- 56.Zhu J, Woods D, McMahon M, Bishop JM. 1998. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12:2997–3007. 10.1101/gad.12.19.2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pirkmajer S, Chibalin AV. 2011. Serum starvation: caveat emptor. Am. J. Physiol. Cell Physiol. 301:C272–C279. 10.1152/ajpcell.00091.2011 [DOI] [PubMed] [Google Scholar]
- 58.Fisher KW, Das B, Kortum RL, Chaika OV, Lewis RE. 2011. Kinase suppressor of Ras 1 (KSR1) regulates PGC1α and estrogen-related receptor alpha to promote oncogenic Ras-dependent anchorage-independent growth. Mol. Cell. Biol. 31:2453–2461. 10.1128/MCB.05255-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Downward J. 1995. KSR: a novel player in the RAS pathway. Cell 83:831–834. 10.1016/0092-8674(95)90198-1 [DOI] [PubMed] [Google Scholar]
- 60.Sundaram M, Han M. 1995. The C. elegans ksr-1 gene encodes a novel Raf-related kinase involved in Ras-mediated signal transduction. Cell 83:889–901. 10.1016/0092-8674(95)90205-8 [DOI] [PubMed] [Google Scholar]
- 61.Stewart S, Sundaram M, Zhang Y, Lee J, Han M, Guan KL. 1999. Kinase suppressor of Ras forms a multiprotein signaling complex and modulates MEK localization. Mol. Cell. Biol. 19:5523–5534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurzchalia TV, Parton RG. 1999. Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11:424–431. 10.1016/S0955-0674(99)80061-1 [DOI] [PubMed] [Google Scholar]
- 63.Lisanti MP, Tang Z, Scherer PE, Kubler E, Koleske AJ, Sargiacomo M. 1995. Caveolae, transmembrane signalling and cellular transformation. Mol. Membr. Biol. 12:121–124. 10.3109/09687689509038506 [DOI] [PubMed] [Google Scholar]
- 64.McKay MM, Ritt DA, Morrison DK. 2009. Signaling dynamics of the KSR1 scaffold complex. Proc. Natl. Acad. Sci. U. S. A. 106:11022–11027. 10.1073/pnas.0901590106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capozza F, Williams TM, Schubert W, McClain S, Bouzahzah B, Sotgia F, Lisanti MP. 2003. Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation. Am. J. Pathol. 162:2029–2039. 10.1016/S0002-9440(10)64335-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernandez MR, Henry MD, Lewis RE. 2012. Kinase suppressor of Ras 2 (KSR2) regulates tumor cell transformation via AMPK. Mol. Cell. Biol. 32:3718–3731. 10.1128/MCB.06754-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casar B, Arozarena I, Sanz-Moreno V, Pinto A, Agudo-Ibanez L, Marais R, Lewis RE, Berciano MT, Crespo P. 2009. Ras subcellular localization defines extracellular signal-regulated kinase 1 and 2 substrate specificity through distinct utilization of scaffold proteins. Mol. Cell. Biol. 29:1338–1353. 10.1128/MCB.01359-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muller J, Cacace AM, Lyons WE, McGill CB, Morrison DK. 2000. Identification of B-KSR1, a novel brain-specific isoform of KSR1 that functions in neuronal signaling. Mol. Cell. Biol. 20:5529–5539. 10.1128/MCB.20.15.5529-5539.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brennan JA, Volle DJ, Chaika OV, Lewis RE. 2002. Phosphorylation regulates the nucleocytoplasmic distribution of kinase suppressor of Ras. J. Biol. Chem. 277:5369–5377. 10.1074/jbc.M109875200 [DOI] [PubMed] [Google Scholar]
- 70.Byrne DP, Dart C, Rigden DJ. 2012. Evaluating caveolin interactions: do proteins interact with the caveolin scaffolding domain through a widespread aromatic residue-rich motif? PLoS One 7:e44879. 10.1371/journal.pone.0044879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collins BM, Davis MJ, Hancock JF, Parton RG. 2012. Structure-based reassessment of the caveolin signaling model: do caveolae regulate signaling through caveolin-protein interactions? Dev. Cell 23:11–20. 10.1016/j.devcel.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu J, Yu H, Kornev AP, Zhao J, Filbert EL, Taylor SS, Shaw AS. 2011. Mutation that blocks ATP binding creates a pseudokinase stabilizing the scaffolding function of kinase suppressor of Ras, CRAF and BRAF. Proc. Natl. Acad. Sci. U. S. A. 108:6067–6072. 10.1073/pnas.1102554108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajakulendran T, Sahmi M, Lefrancois M, Sicheri F, Therrien M. 2009. A dimerization-dependent mechanism drives RAF catalytic activation. Nature 461:542–545. 10.1038/nature08314 [DOI] [PubMed] [Google Scholar]