Abstract
Bacillus anthracis, the causative agent of anthrax, forms an S-layer atop its peptidoglycan envelope and displays S-layer proteins and Bacillus S-layer-associated (BSL) proteins with specific functions to support cell separation of vegetative bacilli and growth in infected mammalian hosts. S-layer and BSL proteins bind via the S-layer homology (SLH) domain to the pyruvylated secondary cell wall polysaccharide (SCWP) with the repeat structure [→4)-β-ManNAc-(1→4)-β-GlcNAc-(1→6)-α-GlcNAc-(1→]n, where α-GlcNAc and β-GlcNAc are substituted with two and one galactosyl residues, respectively. B. anthracis gneY (BAS5048) and gneZ (BAS5117) encode nearly identical UDP-GlcNAc 2-epimerase enzymes that catalyze the reversible conversion of UDP-GlcNAc and UDP-ManNAc. UDP-GlcNAc 2-epimerase enzymes have been shown to be required for the attachment of the phage lysin PlyG with the bacterial envelope and for bacterial growth. Here, we asked whether gneY and gneZ are required for the synthesis of the pyruvylated SCWP and for S-layer assembly. We show that gneZ, but not gneY, is required for B. anthracis vegetative growth, rod cell shape, S-layer assembly, and synthesis of pyruvylated SCWP. Nevertheless, inducible expression of gneY alleviated all the defects associated with the gneZ mutant. In contrast to vegetative growth, neither germination of B. anthracis spores nor the formation of spores in mother cells required UDP-GlcNAc 2-epimerase activity.
INTRODUCTION
Bacillus anthracis elaborates the secondary cell wall polysaccharide (SCWP), which is comprised of a repeating trisaccharide, [→4)-β-ManNAc-(1→4)-β-GlcNAc-(1→6)-α-GlcNAc-(1→]n, where α-GlcNAc (N-acetylglucosamine) is substituted with α-Gal (galactose) and β-Gal at O-3 and O-4, respectively, and the β-GlcNAc is substituted with α-Gal at O-3 (1). The CsaB protein catalyzes ketal-pyruvylation of the terminal ManNAc (N-acetylmannosamine) of the SCWP (2, 3). This modification supports assembly of the S-layer envelope of B. anthracis that is comprised of the two main S-layer proteins, Sap and EA1, as well as 22 S-layer-associated (BSL) proteins that each bind to pyruvylated SCWP through their S-layer homology (SLH) domains (4, 5). The SCWP is thought to be tethered to the MurNAc (N-acetylmuramic acid) moieties of peptidoglycan via murein linkage units (GlcNAc-ManNAc) (2).
Earlier work demonstrated that bacteriophage lysins, for example, PlyG or PlyL, associate with a cell wall glycopolymer through their C-terminal binding domain, a prerequisite for activation of the N-terminal murein hydrolase domains responsible for peptidoglycan degradation (6–8). The cell wall glycopolymer that serves as the receptor of PlyG or PlyL was originally described as an acid-extractable neutral polysaccharide composed of Gal, GlcNAc, and ManNAc (6, 8). ManNAc is a key residue of the repeating trisaccharide of the well-characterized SCWP, the only polymer known to contain ManNAc in B. anthracis (1). A library of oligosaccharides containing the core trisaccharide motif α-d-GlcNAc-(1→ 4)-β-d-ManNAc-(1→4)-β-d-GlcNAc of the SCWP of B. anthracis was chemically synthesized with various patterns of α-d-Gal and β-d-Gal branching points (9). Measurement of dissociation constants for the cell wall binding domains of the endolysins PlyL and PlyG using surface plasmon resonance established a preferred interaction with a trisaccharide bearing the galactosyl moiety at C-4 of the nonreducing GlcNAc moiety (9). PlyL and PlyG were also found to interact with highly purified SCWP of several B. anthracis isolates via their C-terminal domains but not their N-terminal catalytic domains, thus corroborating the notion that the SCWP serves as a receptor for γ phage lysins (10).
Variations in the galactosylation pattern in the SCWP core structure of bacilli of the Cereus group, but not the terminal pyruvylation, were found to govern specificity toward phage endolysins (7, 9). By conducting a genomic comparison of the PlyG-sensitive B. anthracis Ames and the related PlyG-resistant Bacillus cereus ATCC 10987 strains, Schuch et al. noticed a gene cluster that could be responsible for the biosynthesis of the cell wall glycopolymer receptor of γ phage endolysins (7). Importantly, this cluster designated, sps, encoded a protein (BA5509 in the Ames genome; BAS5117 in the Sterne genome) reported earlier to catalyze the reversible conversion of UDP-GlcNAc into UDP-ManNAc; however, a direct link to SCWP synthesis was not reported (11). Here, we refer to this gene as gneZ (bas5117). As reported earlier (7), a second gene, gneY (bas5048 in the Sterne genome), is predicted to encode a nearly identical protein (98.6% amino acid identity).
UDP-ManNAc is the substrate of TagA, the second enzyme in the biosynthetic pathway of wall teichoic acid (WTA), a polymer tethered to peptidoglycan via the murein linkage unit, GlcNAc-ManNAc-(Gro-P)2-3 (12). Synthesis of the murein linkage unit is initiated by TagO (TarO), which links UDP-GlcNAc and undecaprenyl-phosphate to generate C55-PP-GlcNAc (13–15), followed by TagA-catalyzed addition of ManNAc to generate C55-PP-GlcNAc-ManNAc (12, 16). We reported earlier that B. anthracis carries a tagOBa (bas5050) homologue, a gene located next to gneY (2). tagOBa (bas5050) could not be deleted in B. anthracis; however, expression of tagOBa in trans restored WTA synthesis in a Staphylococcus aureus tagO mutant (2). A model was proposed whereby TagO mediates assembly of linkage units to tether pyruvylated SCWP to the B. anthracis envelope and thereby enable S-layer assembly (2). The B. anthracis genome encodes two TagA homologues, suggesting that ManNAc may be incorporated in both the linkage and repeating units of the SCWP. The finding that tagOBa could not be deleted suggests that disrupting the synthesis of the SCWP in B. anthracis may lead to growth arrest. In agreement with the notion that UDP-GlcNAc 2-epimerases contribute to the synthesis of SCWP, it was reported that a mutant lacking both genes could not be obtained in B. anthracis ΔSterne (a strain that lacks both pXO1 and pXO2 virulence plasmids) unless ba5509 (bas5117) was placed under an inducible promoter (7, 11). Insertional inactivation of either ba5433 (bas5048) or ba5509 (bas5117) could be achieved in B. anthracis ΔSterne (7), although inactivation of ba5509 (bas5117) was reported to affect growth (11).
Here, we asked whether GneY (BAS5048) and GneZ (BAS5117) are required for B. anthracis S-layer assembly and attempted to generate B. anthracis Sterne (pXO1+ pXO2−) variants that lack either one or both genes. We report that deletion of gneY does not affect growth of bacilli, whereas deletion of gneZ is tolerated only under conditions of isopropyl-β-d-thiogalactopyranoside (IPTG)-induced Pspac-gneY expression. Although gneZ is required for vegetative growth, UDP-GlcNAc 2-epimerase expression is dispensable for B. anthracis spore formation and germination. Further, expression of gneZ, but not of gneY, is required for S-layer assembly and for the formation of rod-shaped daughter cells during vegetative growth.
MATERIALS AND METHODS
Media and growth conditions for the propagation of bacteria and phage.
B. anthracis strain Sterne 34F2 (pXO1+ pXO2−) (17) and its variants were cultured in brain heart infusion (BHI) broth or propagated on BHI agar plates at 37°C or, where indicated, at 30 or 42°C. Escherichia coli strains were grown in Luria-Bertani (LB) medium. Phage assays were performed in PA medium as described previously (18). When necessary, growth media were supplemented with spectinomycin (200 μg ml−1) or kanamycin (20 μg ml−1; 50 μg ml−1 for E. coli) for plasmid selection. Expression of gneY from the spac promoter (Pspac) was induced via the addition of 1 to 2 mM IPTG.
B. anthracis strains and plasmids.
Three B. anthracis variants were generated to examine the function of gneY and gneZ. These variants carried three distinct alleles, including an in-frame deletion of gneY (ΔgneY allele), an insertion of the gene encoding the LacI repressor along with the Pspac IPTG-inducible promoter recombined in front of the gneY gene (Pspac-gneY allele), and lastly a replacement of the gneZ coding sequence with the spectinomycin resistance marker (gneZ::spc allele). All alleles were recombined on the chromosome of strain Sterne as described earlier (19). Briefly, the ΔgneY allele was generated by cloning two fragments of approximately 1-kb DNA flanking the gneY coding sequence (BAS5048, NC_005945.1) into pLM4 to yield plasmid pYW90 (19). The two DNA fragments were amplified by PCR using B. anthracis Sterne genomic DNA as the template and primer pairs with sequences TTTGGTACCCGAATCCTGAATTTTTACGG/TTTGGATCCTTCATTGCCTCTTTTCTTTCAC and TTTGGATCCCGTGCATCAGAGCGTATTG/TTTGAATTCGAAGATTATGCAACAGACGGTC. The Pspac-gneY allele was generated by cloning the 1-kb DNA region upstream of the gneY coding sequence by PCR using the primer pair with sequences TTTGGTACCCGAATCCTGAATTTTTACGG/TTTGGATCCTTCATTGCCTCTTTTCTTTCAC. Next, the lacI repressor gene sequence along with the Pspac promoter sequence was amplified from plasmid pLM5 (19) with the primer pair TTTGGATCCTATCGATCGGCCGTATCC/TTTGAATTCTAATTGCGTTGCGCTCAC. The lacI repressor-Pspac and the gneY upstream DNA fragments were ligated into pLM4 using the KpnI and EcoRI restriction sites and yielding plasmid pYW82. Next, the coding sequence for the gneY gene amplified using the primer pair with sequence TTTTCTAGAATGACTGAACGTTTAAAAGTAATGAC/TTTCCCGGGTTACTTATTAAAGTGTTTTAAAATTGCTTC was cloned into pYW82, yielding plasmid pYW84, which was used for recombination in the Sterne strain. The gneZ::spc allele was generated by cloning two fragments of approximately 1-kb DNA sequences flanking the gneZ coding region (BAS5117, NC_005945.1) obtained by PCR amplification of strain Sterne genomic DNA and primer pairs with sequences TTTCCCGGGCGTACACGCGTCATTTCAA/TTTTCTAGATTTATTGCCTCTTTCCTTTACCA and TTTTCTAGACGTGCATCAGAGCGTATTG/TTTGAATTCGATCAAACCGGTAGGGAGAG. The DNA fragments were cloned in pLM4, yielding plasmid pYW53. In this construct (pYW53), an XbaI restriction site was placed at the junction of the two DNA segments. This unique restriction site was used to clone the duplicate copy of the aad9 gene specifying spectinomycin resistance, yielding plasmid pYW60, which was used for allelic replacement. Plasmid pJRS312 was used as a template for the amplification of aad9 (20) using the primer pair with sequences TTTTCTAGAATCGATTTTCGTTCGTGAATAC/TTTTCTAGAATATGCAAGGGTTTATTGT. For allelic replacement of gneY and gneZ, plasmids pYW60, pYW84, and pYW90 were propagated in E. coli K1077 (dcm dam mutant) (21) prior to electroporation into wild-type B. anthracis Sterne. Transformants were isolated on BHI agar containing 20 μg ml−1 kanamycin and incubated at 30°C. Isolated colonies were patched on BHI agar containing kanamycin, and plates were incubated at 42°C overnight (restrictive temperature). Isolated colonies were inoculated into BHI broth containing either no antibiotic or 1 mM IPTG or 200 μg ml−1 spectinomycin for recombination of the ΔgneY, Pspac-gneY, and gneY::spc alleles, respectively. This process was repeated 4 times to ensure loss of the plasmids (pYW90, pYW84, and pYW60, respectively). After the last repeat, kanamycin-sensitive colonies were selected and grown to extract genomic DNA for sequence analysis of chromosomal lesions. Strain YW6 harboring both the Pspac-gneY and gneZ::spc mutant alleles was constructed by transforming strain YW29 (Pspac-gneY mutant) with the pLM4-based plasmid pYW60 (carrying the gneZ::spc allele). Curing of pYW60 and allelic replacement of gneZ were performed by growing candidate kanamycin-resistant colonies in the presence of IPTG and spectinomycin until loss of the kanamycin resistance.
Strains YW29 and YW60 expressing gneY under the inducible Pspac promoter on the chromosome were transformed with pJK4, a stable plasmid that expresses lacI (2). This was performed to achieve tighter regulation over the Pspac promoter. The wild-type strain Sterne was also transformed with pJK4 to serve as the isogenic parent control. Cloning of gneZ into vector pWWW412 (pgneZ) was performed by amplifying the coding sequence of the gneZ open reading frame using Sterne DNA as the template and the primer pair bearing the nucleotide sequences TTTCTCGAGACTGAACGTTTAAAAGTAATGACGA/TTTGGATCCTTACTTATTAAAGTGTTTTAAAATTGCTTC. The resulting DNA fragment was cloned by using the restriction sites XhoI and BamHI of pWWW412 such that transcription and translation of gneZ were promoted by the constitutive hprK promoter and ribosome binding site provided on the vector (22).
Phage transduction.
Bacteriophage CP-51 was propagated and assayed by methods described previously (18, 23). To generate a phage lysate, the exponentially growing culture of the target strain was mixed with soft agar and layered on plates and CP-51 phage solution was spotted onto the solidified soft agar. Plaques were observed after 16 h of incubation at 37°C, harvested, and suspended in PA broth to generate phage lysates. For transduction experiments, recipient strains were grown to exponential phase in PA medium before adding the phage lysate at a multiplicity of infection of 1/100. Cultures were incubated at room temperature for 1 h and centrifuged. Bacteria were washed and plated on PA agar at 30°C for 24 h.
B. anthracis vegetative growth, sporulation, and germination.
Growth of vegetative bacilli was compared between strains by monitoring the optical density (absorbance) of cultures at 600 nm (A600). Bacilli grown overnight were washed and diluted 100-fold into 50 ml of BHI medium and incubated at 37°C with shaking. Culture aliquots were withdrawn at timed intervals to record optical densities or acquire microscopic images. B. anthracis sporulation was induced by diluting washed bacilli from overnight cultures 1:5 into modified G medium followed by incubation at 30°C with shaking as described previously (19, 24). Sporulation was visualized by light and fluorescence microscopy with FM4-64 (Invitrogen) staining of fixed cultures (19). Aliquots of cultures were withdrawn after 24 h and heated at 65°C to kill all vegetative cells. The resulting heat-resistant spores were enumerated as CFU following overnight incubation of sample aliquots serially diluted and plated on BHI agar.
Spore suspensions were germinated by inoculation into BHI at 107 spores per ml and grown at 37°C with shaking. Aliquots of cultures were removed at timed intervals to record optical densities. Culture aliquots were also heated for 60 min at 65°C to kill vegetative bacilli. Viable organisms in both nontreated and heat-treated aliquots were subjected to CFU enumeration as described above.
Light and fluorescence microscopy.
Digital micrographs of bacterial samples fixed with 4% formalin were viewed with an Olympus IX81 microscope equipped with 100× or 40× objectives. Phase-contrast images of samples and FM4-64 or boron-dipyrromethene (BODIPY)–vancomycin (Life Technologies) fluorescence images were captured with a charge-coupled device (CCD) camera. Images were merged using ImageJ. To probe for Sap, EA1, and BslR, immunofluorescence microscopy experiments were performed as described previously (25). Briefly, fixed bacilli were sedimented by centrifugation, washed in phosphate-buffered saline (PBS), and incubated with 1% (wt/vol) bovine serum albumin in PBS. Specific rabbit antisera were diluted 1:1,000 into PBS and incubated with the cells for 1 h followed by three washes in PBS and labeling with Alexa-Fluor 594-conjugated goat anti-rabbit IgG (Life Technologies) prior to microscopy. Fluorescent images, as well as differential interference contrast (DIC) images and merge views, were acquired on a Leica SP5 II STED-CW super-resolution laser scanning confocal microscope.
SapSLH-mCherry binding to vegetative bacilli.
B. anthracis Sterne wild-type or mutant strains (YW6 and ΔcsaB strains) were grown in BHI broth to an A600 of 2.5, and vegetative bacilli were sedimented by centrifugation. Bacilli were suspended in PBS supplemented with 3 M urea and heated at 95°C for 10 min to solubilize the S-layer (2). Cells were again sedimented by centrifugation, washed extensively with water, and suspended in PBS. The cell density of samples was normalized to an A600 of 1. These cells (100-μl samples) were incubated with 3 mM SapSLH-mCherry or mCherry alone, purified as described previously (2), for 10 min at room temperature in the dark. After incubation, cells were sedimented by centrifugation, washed extensively with PBS, and imaged by fluorescence or phase-contrast microscopy. Alternatively, fluorescence was quantified using a Biotek Synergy HT microplate reader. The excitation wavelength was 590 (±20) nm, and the emission was read at 645 (±40) nm. Fluorescence measurements were compared using an unpaired, two-tailed Student t test.
RESULTS
B. anthracis gneY and gneZ mutants.
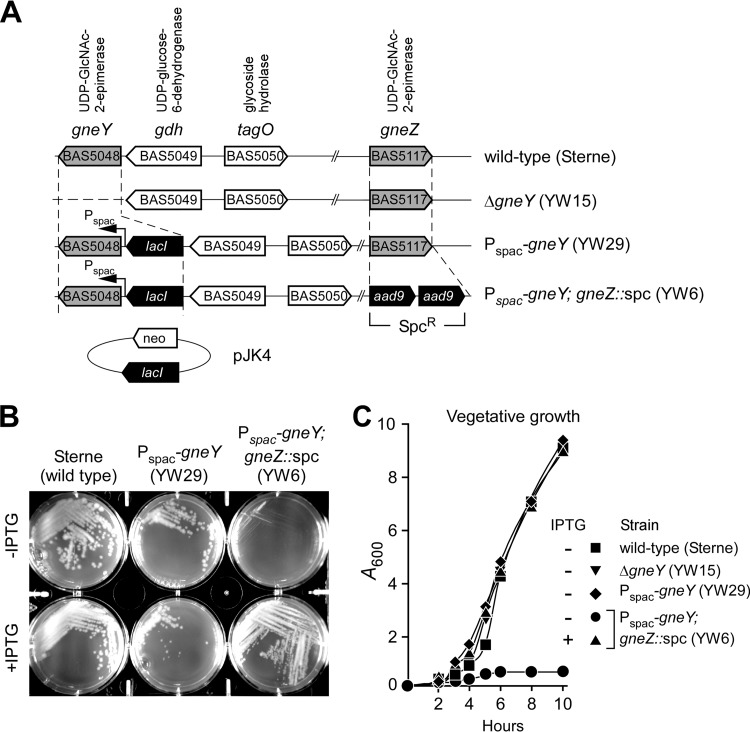
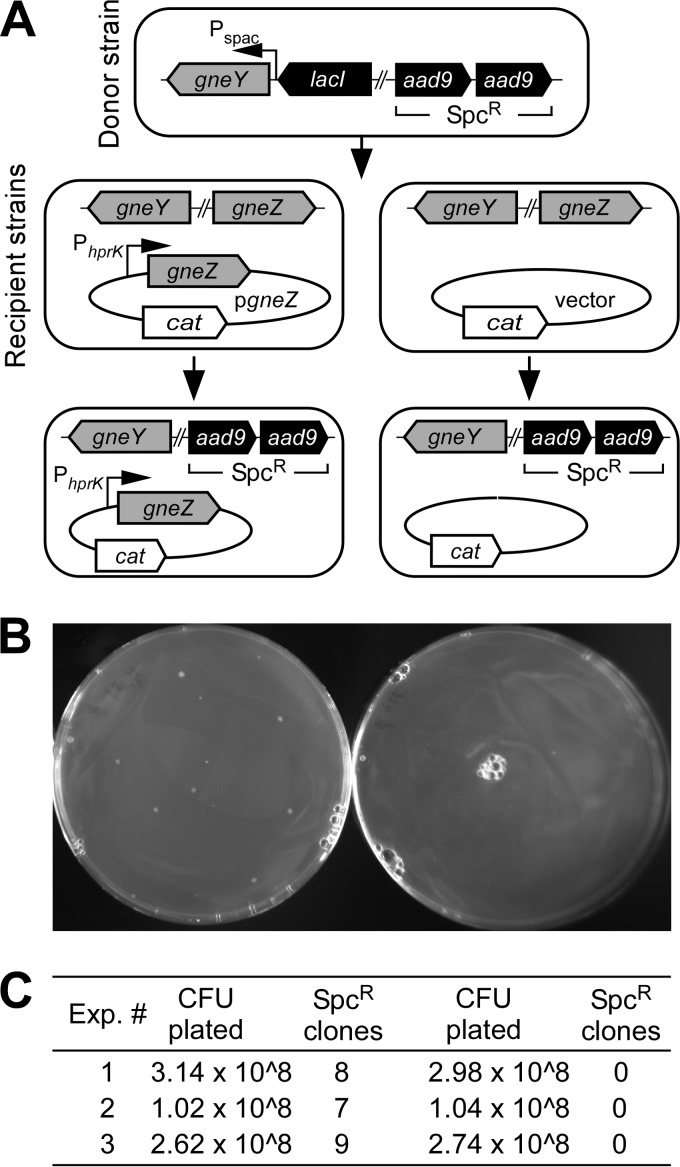
The shuttle vector pLM4 that carries the temperature-sensitive pE194 oriTs-based replicon (26) and kanamycin resistance determinant for plasmid replication and selection in B. anthracis was used for double-crossover allelic replacement experiments (19). Deletion of gneY in B. anthracis Sterne could be readily achieved (ΔgneY) (Fig. 1A). However, attempts to delete gneZ using this method failed. We surmised that the gneY-encoded UDP-GlcNAc 2-epimerase may not be expressed during vegetative growth, causing gneZ to be essential for vegetative growth. Because the transcriptome of B. anthracis has been determined under a variety of growth conditions (27), information about gneY and gneZ transcription could be retrieved from the data set deposited in the NCBI GEO database with accession number GSE13543. Such an analysis revealed that gneZ but not gneY is readily transcribed when bacilli are grown in aerated cultures or in the presence of CO2 (27). We therefore decided to modify the wild-type strain Sterne to allow for inducible expression of gneY. The E. coli lacI repressor gene and the LacI-regulated Pspac promoter were introduced by double crossover upstream of gneY (Fig. 1A). This strain, YW29 (Pspac-gneY), was grown in the presence of IPTG for allelic replacement of the gneZ gene with the aad9 gene encoding the spectinomycin resistance determinant. A new variant, strain YW6 (Pspac-gneY gneZ::spc), was thus obtained (Fig. 1A). However, when plated on BHI agar, B. anthracis YW6 did not require IPTG to form viable colonies (Fig. 1B), suggesting leaky expression from the Pspac promoter. To test this possibility, the strain was transformed with plasmid pJK4, for multicopy expression of lacI (2). B. anthracis YW6(pJK4) formed colonies on BHI agar in the presence but not in the absence of IPTG (Fig. 1B). When strains carried an intact copy of gneZ (Sterne and YW29) along with pJK4, IPTG growth restriction was not observed (Fig. 1B). Growth was also assessed in liquid medium. For this and all subsequent experiments, B. anthracis strains carried the pJK4 (lacI) plasmid and were first grown in BHI supplemented with IPTG to late-logarithmic growth. Next, bacteria were sedimented, washed, and diluted into fresh BHI without IPTG and incubated at 37°C with rotation (Fig. 1C). An increase in turbidity was recorded by monitoring changes in absorbance at 600 nm (A600) for cultures of B. anthracis strains Sterne, YW15 (ΔgneY), and YW29 (Pspac-gneY) but not for strain YW6 (Pspac-gneY; gneZ::spc) (Fig. 1C). Growth of strain YW6 could be restored by addition of 1 mM IPTG into the medium (Fig. 1C). Taken together, these data suggest that gneZ but not gneY is indispensable for growth. Presumably, expression of gneY is not sufficient to promote growth of bacteria lacking gneZ. However, this can be corrected by prompting gneY transcription from the strong Pspac promoter. To further validate this model, a phage lysate was generated using the parent strain YW6 (Pspac-gneY; gneZ::spc) and bacteriophage CP-51. This lysate was used to transduce the gneZ::spc allele into the wild-type Sterne parent transformed with either a plasmid that expresses gneZ constitutively (pgneZ) or a plasmid with no insert (vector control) (Fig. 2A). As expected, gneZ::spc could be crossed only into the merodiploid strain but not in the wild-type strain Sterne (Fig. 2B and C).
FIG 1.
Bacillus anthracis Sterne variants with mutations in gneY- and gneZ-encoded UDP-GlcNAc 2-epimerase activities. (A) Schematic representation of the B. anthracis Sterne chromosome organization surrounding the gneY (bas5047) and gneZ (bas5117) genes and of variants with a gneY deletion (ΔgneY; YW15) and insertion of lacI and Pspac upstream of gneY either without (Pspac-gneY; YW29) or with (Pspac-gneY; gneZ::spc; YW6) replacement of gneZ. The plasmid pJK4 provides for the constitutive expression of lacI and is selected by growing cells in the presence of kanamycin (neo, neomycin). (B) B. anthracis strains Sterne(pJK4), YW29(pJK4), and YW6(pJK4) were spread on BHI agar plates with 20 μg ml−1 kanamycin for plasmid selection and incubated at 37°C for 16 h either without (−IPTG) or with (+IPTG) 1 mM isopropyl-β-d-thiogalactoside supplement. (C) B. anthracis Sterne(pJK4), YW29(pJK4), and YW6(pJK4) were cultured overnight in BHI with 20 μg ml−1 kanamycin and 1 mM IPTG. Bacteria were centrifuged, washed, and suspended in fresh medium either without (−IPTG) or with (+IPTG) 1 mM IPTG supplement and incubated with rotation at 37°C to monitor vegetative growth. At timed intervals, culture aliquots were withdrawn and absorbance at 600 nm was recorded (A600). Data are representative of three independent experimental determinations.
FIG 2.
Bacteriophage transduction of the gneZ::spc allele. (A) The donor strain B. anthracis YW6(pJK4) was cultured in PA medium with 1 mM IPTG and lysed with bacteriophage CP-51. The lysate was used to cross the gneZ::spc allele into the recipient strain Sterne carrying either a vector control or the isogenic plasmid expressing gneZ from the constitutive hprK promoter (pgneZ). (B) Candidate transductants were selected on plates containing spectinomycin. (C) Spectinomycin-resistant colonies (Spcr clones) were enumerated after incubation of plates at 30°C for 24 h. Insertion of the correct spc allele was verified by DNA sequencing. Data from three independent experiments are shown with the total number of bacteria plated before spectinomycin selection.
Previous studies examined the essentiality of UDP-GlcNAc 2-epimerases of B. anthracis. In this work, Campbell-type insertion mutagenesis with pE194 oriTs-based plasmids, conditionally defective for replication, was used to disrupt either ba5433 (gneY) or ba5509 (gneZ) in the ΔSterne strain that lacks both pXO1 and pXO2 virulence plasmids (7, 11). Insertional inactivation of ba5509 (gneZ) was reported to diminish but not abolish bacterial growth (7). Further, unless placed under an inducible promoter, ba5509 (gneZ) could not be disrupted in the absence of ba5433 (gneY) (7). Of note, plasmids with the pE194 oriTs allele initiate replication and are excised from the bacterial chromosome under permissive conditions (28). We presume that, during incubation at 27°C, a condition that is permissive for the replication of pE194 oriTs-derived plasmids, mixed populations of B. anthracis that carry either wild-type or disrupted alleles of ba5509may have arisen. If so, this may have prompted the conclusion that ba5509 is required, but not essential, for B. anthracis growth (7).
Morphology of B. anthracis vegetative forms grown in the absence of UDG-GlcNAc 2-epimerase.
When diluted into fresh BHI medium, B. anthracis Sterne grows as chains of incompletely separated bacilli that divide to form unit-size cells and separate into new chains of 4 to 8 vegetative bacilli (29). Upon dilution into fresh BHI without IPTG, B. anthracis YW6 (Pspac-gneY; gneZ::spc) carrying pJK4 initially grew at a rate similar to that of wild-type bacilli (Fig. 3). However, 2 h after dilution, this variant formed chains of incompletely separated round cells, and by 3 h, replication of bacteria ceased (Fig. 3). The growth defect and most of the morphological defects of cell division in YW6 were suppressed by IPTG-inducible expression of gneY. Nevertheless, even in the presence of 1 mM IPTG, B. anthracis YW6 displayed morphological defects such as undulating chain morphology and exaggerated chain length at 2 to 6 h following dilution. At later growth stages (8 to 10 h after dilution), B. anthracis YW6 growth and morphology appeared indistinguishable from those of the B. anthracis Sterne parent.
FIG 3.
Expression of the UDP-GlcNAc 2-epimerases is required for B. anthracis vegetative growth. B. anthracis Sterne(pJK4) and YW6(pJK4) were cultured overnight in BHI with 20 μg ml−1 kanamycin and 1 mM IPTG. Bacteria were centrifuged, washed, and suspended in fresh medium either without (−IPTG) or with (+IPTG) 1 mM IPTG supplement and incubated at 37°C. At timed intervals (1 to 10 h), culture aliquots were withdrawn, samples were fixed with glutaraldehyde, and phase-contrast microscopy images of bacteria were acquired at an ×400 or ×1,000 magnification. Bar, 20 μm. Data are representative of three independent experimental determinations.
Contribution of UDP-GlcNAc 2-epimerase toward B. anthracis spore formation.
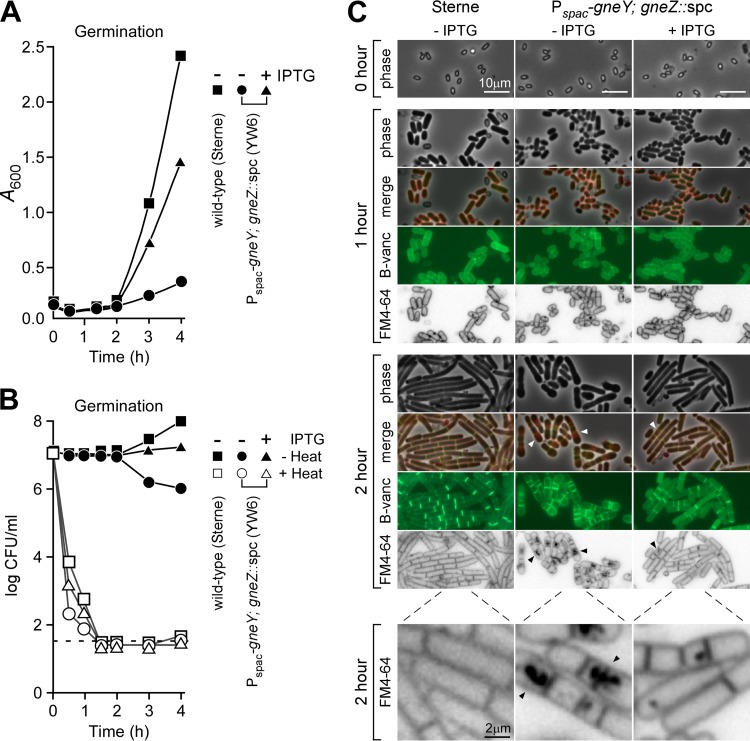
We wondered whether UDP-GlcNAc 2-epimerase enzymes are required for sporulation. To test this possibility, cultures of B. anthracis strains Sterne and YW6 carrying pJK4 were grown in the presence of IPTG prior to inoculation into modified medium G with or without 1 mM IPTG. Bacilli sporulate rapidly when grown in modified medium G (24). Forespore engulfment and endospore formation were monitored by phase-contrast microscopy and by staining membranes with FM4-64 dye (Fig. 4A). Engulfment was manifested by the formation of darker internal structures that became clearly visible as endospores matured. FM4-64 staining and fluorescence microscopy confirmed that polar septation occurred at the same time for both B. anthracis Sterne and YW6 strains (T + 1 h) and did not require IPTG-induced expression of gneY (Fig. 4A). Furthermore, forespore engulfment (T + 2 h) and endospore formation (T + 5 h) commenced with similar speed and efficiency in the two strains and were not impacted by the addition of IPTG to the culture medium (Fig. 4A).
FIG 4.
B. anthracis spore formation with or without UDP-GlcNAc 2-epimerase expression. B. anthracis Sterne(pJK4) and YW6(pJK4) were cultured overnight in BHI with 20 μg ml−1 kanamycin and 1 mM IPTG. Bacteria were centrifuged, washed, and suspended in modified G medium either without (−IPTG) or with (+IPTG) 1 mM IPTG supplement and incubated at 30°C. (A) At timed intervals (T + 1, T + 2, or T + 5 h), culture aliquots were withdrawn, samples were fixed with formaldehyde, and phase-contrast microscopy or fluorescence microscopy images of FM4-64-stained bacteria were acquired. Polar septa and forespores (black arrowheads) as well as endospores (white arrowheads) were detected. Bar, 10 μm. (B) Twenty-four hours after dilution into modified medium G, samples were either heat treated [60 min at 65°C = Spores (+Heat)] or left untreated [Vegetative bacilli plus spores = Total (−Heat)], spread on BHI agar with 20 μg ml−1 kanamycin and 1 mM IPTG, and incubated at 37°C to enumerate CFU. Data are representative of two independent experimental determinations.
To ensure that expression of gneZ is dispensable for sporulation, culture aliquots (T + 24 h) were serially diluted and heat treated or not for 1 h at 65°C. Heat treatment effectively kills vegetative bacilli but not spores. Bacterial viability in the samples was quantified by plating and enumeration of CFU (Fig. 4B). Similar CFU were obtained for B. anthracis Sterne and strain YW6 whether or not IPTG had been added to modified medium G and regardless of heat treatment (Fig. 4B). We therefore conclude that UDG-GlcNAc 2-epimerase activity, i.e., the expression of either gneY or gneZ, is not required for B. anthracis spore formation.
Contribution of UDP-GlcNAc 2-epimerase toward B. anthracis germination.
Next, we asked whether germination requires the activity of UDP-GlcNAc 2-epimerases. Heat-resistant spore preparations of B. anthracis strains Sterne and YW6 were washed in water, and 107 spores were suspended in BHI with or without IPTG. Rapid germination was accompanied by a small decrease in the optical density (A600) of the suspension within the first 30 min after inoculation into growth medium, which was observed for B. anthracis Sterne and YW6 (Pspac-gneY; gneZ::spc) grown in the presence or absence of IPTG (Fig. 5A). Two hours after dilution, increases in A600 were associated with the completion of germination and vegetative growth of heat-sensitive bacilli as measured by loss of plating efficiency of heat-treated samples (Fig. 5B), which occurred at similar rates for B. anthracis Sterne(pJK4) and YW6(pJK4) grown irrespectively of the presence or absence of IPTG. As expected, vegetative bacilli of strain YW6(pJK4) grown in the absence of IPTG grew poorly, as reflected by both density (A600) and plating (viability) measurements (Fig. 5A and B). When examined by phase-contrast microscopy 1 h after dilution, the small, oval-shaped spores from both strains had germinated to form short cylindrical vegetative forms; this occurred in the presence or absence of IPTG (Fig. 5C). Germinated bacilli could be stained with the membrane dye FM4-64 and with BODIPY-vancomycin, a compound that binds to the peptidoglycan precursors of vegetative forms but not to spores (19) (Fig. 5C). Two hours after dilution into BHI, B. anthracis Sterne formed elongated chains of incompletely separated vegetative forms, which occurred also for B. anthracis YW6 grown in the presence but not in the absence of IPTG (Fig. 5C). After 2 h of growth in the absence of IPTG, strain YW6 accumulated FM4-64-positive membrane material in the cytoplasm of most, but not all, vegetative cells, and BODIPY-vancomycin staining revealed aberrant spacing of septal rings (Fig. 5C). To a lesser extent, this phenotype was also observed with YW6 grown for 2 h in the presence of IPTG (Fig. 5C). These data suggest that the expression of UDG-GlcNAc 2-epimerase, either gneY or gneZ, is not required for the germination of B. anthracis spores, although germinated bacilli require this enzymatic activity to replicate as vegetative forms.
FIG 5.
Expression of the UDP-GlcNAc 2-epimerases is dispensable for B. anthracis spore germination. Spores (107 ml−1) that had been derived from B. anthracis Sterne(pJK4) or YW6(pJK4) grown in modified G medium were washed in water and suspended in brain heart infusion broth without (−IPTG) or with (+IPTG) 1 mM IPTG supplement. (A) Vegetative growth of the spore-inoculated cultures was measured at timed intervals as absorbance at 600 nm (A600). (B) Germination of vegetative bacilli from heat-resistant spores was quantified by enumerating CFU (CFU plotted as log scale) in culture aliquots that were spread on the surface of BHI agar plates at 37°C either without (−Heat) or with (+Heat; 60 min at 65°C) prior heat treatment. Data are representative of two independent experimental determinations. (C) Phase-contrast microscopy images were acquired immediately after spore suspension in BHI (0 h) or after 1 and 2 h of incubation at 37°C. Fluorescence microscopy images were acquired from samples that had been stained with FM4-64, a membrane dye, or BODIPY-vancomycin (B-vanc), which binds to the d-Ala-d-Ala portion of peptidoglycan precursors and cell wall pentapeptides. Bars, 10 μm (2 μm for panels in the bottom row). Data are representative of three independent experimental determinations.
UDP-GlcNAc 2-epimerase activity is essential for B. anthracis S-layer assembly.
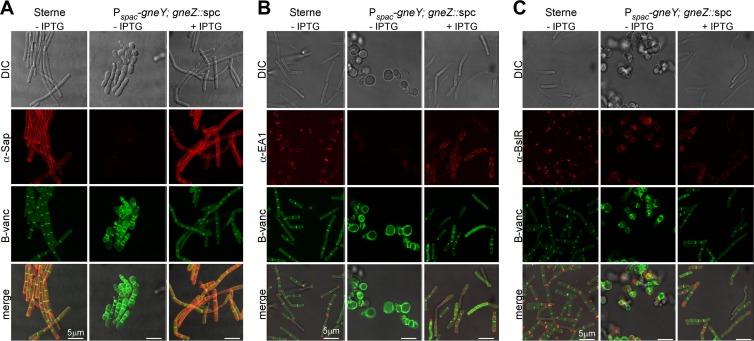
The SCWP and its murein linkage units contain ManNAc residues. If so, expression of UDP-GlcNAc 2-epimerase, which represents the only pathway for de novo synthesis of ManNAc, should be essential for the formation of the bacterial S-layer and the binding of S-layer proteins to the bacterial envelope. This was tested by germinating spores of strains B. anthracis Sterne and YW6 (Pspac-gneY; gneZ::spc) carrying pJK4 and by incubating vegetative forms for 3 h prior to staining with BODIPY-vancomycin and rabbit antibodies raised against purified Sap (αSap), EA1 (αEA1), or BslR (αBslR) (Fig. 6A and B). As expected, B. anthracis Sterne(pJK4) vegetative forms could be stained with BODIPY-vancomycin, which detected peptidoglycan synthesis predominantly at cell division sites but also throughout the cylinder-shaped cell envelope (Fig. 6A). Immunofluorescence microscopy revealed that the S-layer proteins Sap and EA1 were deposited throughout the envelope or near cell separation sites, respectively (Fig. 6A and B). BslR, the S-layer-associated protein, was also deposited at cell separation sites in the envelope of vegetative bacilli (Fig. 6C). When grown in the absence of IPTG, the YW6 (Pspac-gneY; gneZ::spc) variant formed large, coccal cells that could be stained with BODIPY-vancomycin, which suggests that UDP-GlcNAc 2-epimerase activity is not required for peptidoglycan synthesis. Microscopy experiments with αSap or αEA1 did not, however, generate an immunofluorescent signal, suggesting either that the S-layer proteins were not synthesized and secreted or that they could not be retained by the misshapen YW6 cells (Fig. 6A and B). Staining specific for BslR was observed on the surface of misshapen YW6 cells. BslR staining occurred only in small areas and only on some cells and did not colocalize with BODIPY-vancomycin-stained peptidoglycan. When YW6 bacilli were grown in the presence of IPTG, which triggers exogenous expression of gneY, the assembly of Sap and its distribution as a bacterial S-layer protein were restored to wild-type levels (Fig. 6A). Of note, IPTG-induced expression of GneY also restored the assembly of EA1 and BslR into the bacterial envelope; however, it did not support their wild-type distribution. Rather, in the presence of IPTG, YW6 cells incorporated EA1 and BslR along the cylindrical axis of the S-layer in some, but not all, vegetative bacilli (Fig. 6B). Taken together, these data suggest that S-layer and S-layer-associated proteins are not found in the envelope of vegetative bacilli that synthesize peptidoglycan in the absence of GneY and GneZ UDP-GlcNAc 2-epimerases.
FIG 6.
B. anthracis S-layer assembly with and without UDP-GlcNAc 2-epimerase expression. Spores derived from B. anthracis Sterne(pJK4) and YW6(pJK4) were diluted into BHI without (−IPTG) or with (+IPTG) 1 mM IPTG supplement and incubated for 3 h. Differential interference contrast (DIC) and fluorescence microscopy images were acquired from bacilli stained with BODIPY-vancomycin (B-vanc) or with rabbit antibodies against S-layer proteins Sap (α-Sap) (A), EA1 (α-EA1) (B), or BslR (α-BslR) and secondary antibody conjugates to Alexa Fluor 594. Bars, 5 μm. Data are representative of three independent experimental determinations.
UDP-GlcNAc 2-epimerase activity is essential for B. anthracis SCWP synthesis.
The growth defect of YW6 (Pspac-gneY; gneZ::spc) bacilli in medium lacking IPTG precluded a biochemical analysis of SCWP and S-layer protein production. To determine whether YW6 bacilli are unable to synthesize the SCWP, B. anthracis Sterne(pJK4) and YW6(pJK4) were grown in the presence or absence of IPTG, and washed vegetative cells were stripped of S-layer proteins by extraction with urea. Bacilli were then incubated with purified SapSLH-mCherry, a hybrid between the SLH domain of Sap and the mCherry fluorescent protein. Following incubation, bacilli were washed and SapSLH-mCherry binding was quantified by fluorometry or visualized by fluorescence microscopy (Fig. 7A and B). As controls, mCherry alone did not bind to urea-extracted bacilli, whereas SapSLH-mCherry bound the SCWP of B. anthracis strain Sterne but not the SCWP of the isogenic ΔcsaB mutant (2). When grown in the absence of IPTG, the ability of YW6 vegetative bacilli to associate with SapSLH-mCherry was significantly diminished. This phenotypic defect was restored to wild-type levels by growing YW6 in the presence of IPTG. These data indicate that UDP-GlcNAc 2-epimerase activity is required for the synthesis of pyruvylated SCWP and that expression of GneY from an inducible promoter can restore the phenotypic defect in SCWP synthesis of ΔgneZ mutant bacilli (Fig. 7A and B).
FIG 7.
B. anthracis secondary cell wall polysaccharide synthesis with and without UDP-GlcNAc 2-epimerase expression. Spores derived from B. anthracis Sterne(pJK4) and YW6(pJK4) and the ΔcsaB strain were diluted into BHI without (−IPTG) or with (+IPTG) 1 mM IPTG supplement and incubated for 6 h. The S-layer of vegetative forms was extracted with 3 M urea, and bacilli were incubated with purified mCherry or SapSLH-mCherry. (A) Binding of fluorescent protein (mCherry or SapSLH-mCherry) to bacilli was monitored by analyzing the fluorescence intensity of the bacterial sediment. Fluorescence intensity (arbitrary unit [AU]) measurements were normalized to A600 data, i.e., the bacterial densities of each sample. AU/A600 values were averaged, and statistical significance was examined with the unpaired two-tailed Student t test (*, P < 0.001). (B) Images of samples in panel A were acquired by phase-contrast and fluorescence microscopy. Bars, 10 μm. Data are representative of two independent experimental determinations.
DISCUSSION
The structure of the SCWP of Bacillus cereus sensu lato group species, which include B. anthracis (30), is variable, and the corresponding genes that are responsible for the synthesis of these carbohydrate structures are not yet known (31, 32). Using comparative genome analysis, Schuch et al. identified the surface polysaccharide synthesis locus (sps; BAS5116 to BAS5127), which is variable in gene content between different B. cereus species and variable in GC composition from the remainder of the genome (6). The gneZ gene (BAS5117) is located in the sps locus, which also encodes a UDP-ManNAc dehydrogenase as well as the tagGH genes. The TagG and TagH proteins have been proposed to transport undecaprenyl-phosphate-linked precursors of WTA across the plasma membrane (33, 34). gneY (BAS5048) is located in a different locus that includes the genes for TagO, the enzyme that initiates synthesis of the murein linkage units that tether WTA to peptidoglycan (2, 35). Schuch et al. also identified Epimerox, a small molecule that inactivates UDP-GlcNAc 2-epimerase activity and inhibits growth of B. anthracis (7). In agreement with these findings, these authors also reported that gneY and gneZ could not be deleted simultaneously (7). Here, we used double-crossover mutagenesis to generate stable mutations in the bacterial chromosome of strain Sterne and used these technologies to demonstrate that the gneZ gene is essential for B. anthracis growth, whereas the gneY gene is not. Indeed, gneY and gneZ appear not to be expressed under the same conditions. In fact, gneY may not be expressed under laboratory conditions (27). However, when the endogenous gneY promoter is replaced with the inducible Pspac promoter, bacterial growth is no longer dependent on the expression of gneZ (YW6; Pspac-gneY; gneZ::spc). Phenotypes such as stalled vegetative growth and defects in SCWP synthesis and S-layer assembly may be restored by growing YW6 mutant bacteria in the presence of IPTG. Importantly, the gneZ::spc allele of strain YW6 could be crossed by phage transduction into a merodiploid strain carrying two copies of gneZ but not into wild-type B. anthracis Sterne. We therefore conclude that gneZ cannot be deleted under laboratory growth conditions because gneY is poorly expressed. Schuch and colleagues studied the ΔSterne strain (pXO1− pXO2−) (7), whereas work reported here used B. anthracis Sterne (pXO1+ pXO2−). Nevertheless, loss of the pXO1 virulence plasmid from B. anthracis Sterne did not impact SCWP synthesis or S-layer formation (data not shown).
We believe that many of the genetic determinants for SCWP synthesis in B. anthracis remain to be identified. The sps locus may not be the sole gene cluster providing for SCWP synthesis, and B. anthracis may harbor two or more gene clusters that are involved in the synthesis of the SCWP and of the murein linkage units. Under laboratory growth conditions, GneZ activity supplies the ManNAc substrate for both the murein linkage unit and SCWP synthesis; however, the enzymes that synthesize the glycosidic bonds for the polymer [→4)-β-ManNAc-(1→4)-β-GlcNAc-(1→6)-α-GlcNAc] or its galactosyl substitutions remain unknown. Both GneY and GneZ encompass the conserved WecB domain first described for the Bacillus subtilis MnaA epimerase that catalyzes stereochemical inversion at the C-2 position of UDP-GlcNAc to provide UDP-ManNAc for WTA synthesis (36). We propose a model whereby B. anthracis homologues of tagO, tagA, and tagGH synthesize the undecaprenyl-phosphate-linked disaccharide (GlcNAc-ManNAc) of the murein linkage unit onto which the SCWP is synthesized and modified and suggest that such precursor molecules are ultimately transported across the plasma membrane for incorporation into the cell wall envelope.
Earlier work implicated murein linkage units in WTA synthesis and demonstrated that tagO and tagA are dispensable for growth of S. aureus or B. subtilis (37, 38). In agreement with this model, mutants lacking functional tagO or tagA are devoid of WTA. In S. aureus, loss of WTA leads to increased deposition of the major autolysin Atl on the cell surface; bacterial cells are more prone to lysis and display aberrant sizes and septation patterns (39–41). In wild-type staphylococci, deposition of secreted Atl in the envelope is limited to sites of septation that are devoid of WTA. The WTA occupancy of the remainder of the cell surface physically prevents the engagement of peptidoglycan binding domains of Atl with its ligand (39). In contrast, deletion of late-stage WTA synthesis genes, tagBDFGH and tarIJL, results in lethality, which can be rescued through genetic inactivation of tagO or tagA or chemical inhibition of TagO by tunicamycin (37). This phenomenon of synthetic viability also occurs in B. subtilis and probably most Gram-positive bacteria that elaborate WTA (37). Synthetic viability has been attributed to the limited availability of undecaprenyl and its phosphate derivatives that also serve as precursor for lipid II in peptidoglycan synthesis (37). It is not clear why early-stage SCWP synthesis genes, including tagOBa and now gneZ, are required for B. anthracis growth. It is interesting that in group B streptococci (GBS), a TagO homologue (GbsO) is involved in the synthesis of the peptidoglycan-anchored (Lancefield group B) carbohydrate and is essential for streptococcal growth (42). Perhaps, the TagO enzymes of B. anthracis and GBS catalyze only the forward but not the reverse reaction, as has been described for S. aureus or B. subtilis; if so, accumulation of stalled SCWP precursors may affect bacterial viability. Alternatively, the SCWP may be essential for the cell division process, although the specific mechanism(s) remains to be identified.
The finding that the SCWP and S-layer proteins of B. anthracis are dispensable for spore formation and germination was expected. Liu et al. had monitored gene expression during B. anthracis sporulation with full-genome DNA microarrays (43). Neither gneY nor gneZ was expressed during sporulation (43). On the other hand, vegetative bacilli require the SCWP for subsequent cell division events, which also require dedicated functions of S-layer-associated proteins such as the BslO murein hydrolase and the S-layer protein Sap (29, 44). Certainly during sporulation, which occurs as an asymmetric cell division event in the cytoplasm of the mother cell (19), the SCWP and ManNAc do not appear to contribute physiological functions.
ACKNOWLEDGMENTS
We thank So-Young Oh and other laboratory members for experimental advice and discussion.
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases, Infectious Diseases Branch (AI069227), to O.S. We acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (National Institute of Allergy and Infectious Diseases award 1-U54-AI-057153).
Footnotes
Published ahead of print 9 June 2014
REFERENCES
- 1.Choudhury B, Leoff C, Saile E, Wilkins P, Quinn CP, Kannenberg EL, Carlson RW. 2006. The structure of the major cell wall polysaccharide of Bacillus anthracis is species specific. J. Biol. Chem. 281:27932–27941. 10.1074/jbc.M605768200 [DOI] [PubMed] [Google Scholar]
- 2.Kern J, Ryan C, Faull K, Schneewind O. 2010. Bacillus anthracis surface-layer proteins assemble by binding to the secondary cell wall polysaccharide in a manner that requires csaB and tagO. J. Mol. Biol. 401:757–775. 10.1016/j.jmb.2010.06.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forsberg LS, Abshire TG, Friedlander A, Quinn CP, Kannenberg EL, Carlson RW. 2012. Localization and structural analysis of a conserved pyruvylated epitope in Bacillus anthracis secondary cell wall polysaccharides and characterization of the galactose deficient wall polysaccharide from avirulent B. anthracis CDC 684. Glycobiology 22:1103–1117. 10.1093/glycob/cws080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kern JW, Schneewind O. 2008. BslA, a pXO1-encoded adhesin of Bacillus anthracis. Mol. Microbiol. 68:504–515. 10.1111/j.1365-2958.2008.06169.x [DOI] [PubMed] [Google Scholar]
- 5.Kern J, Wilton R, Zhang R, Binkowski TA, Joachimiak A, Schneewind O. 2011. Structure of surface layer homology (SLH) domains from Bacillus anthracis surface array protein. J. Biol. Chem. 286:26042–26049. 10.1074/jbc.M111.248070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuch R, Nelson D, Fischetti VA. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884–889. 10.1038/nature01026 [DOI] [PubMed] [Google Scholar]
- 7.Schuch R, Pelzek AJ, Raz A, Euler CW, Ryan PA, Winer BY, Farnsworth A, Bhaskaran SS, Stebbins CE, Xu Y, Clifford A, Bearss DJ, Vankayalapati H, Goldberg AR, Fischetti VA. 2013. Use of a bacteriophage lysin to identify a novel target for antimicrobial development. PLoS One 8:e60754. 10.1371/journal.pone.0060754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low LY, Yang C, Perego M, Osterman A, Liddington RC. 2005. Structure and lytic activity of a Bacillus anthracis prophage endolysin. J. Biol. Chem. 280:35433–35439. 10.1074/jbc.M502723200 [DOI] [PubMed] [Google Scholar]
- 9.Mo KF, Li X, Li H, Low LY, Quinn CP, Boons GJ. 2012. Endolysins of Bacillus anthracis bacteriophages recognize unique carbohydrate epitopes of vegetative cell wall polysaccharides with high affinity and selectivity. J. Am. Chem. Soc. 134:15556–15562. 10.1021/ja3069962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganguly J, Low LY, Kamal N, Saile E, Forsberg LS, Gutierrez-Sanchez G, Hoffmaster AR, Liddington R, Quinn CP, Carlson RW, Kannenberg EL. 2013. The secondary cell wall polysaccharide of Bacillus anthracis provides the specific binding ligand for the C-terminal cell wall-binding domain of two phage endolysins, PlyL and PlyG. Glycobiology 23:820–832. 10.1093/glycob/cwt019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velloso LM, Bhaskaran SS, Schuch R, Fischetti VA, Stebbins CE. 2008. A structural basis for the allosteric regulation of non-hydrolysing UDP-GlcNAc 2-epimerases. EMBO Rep. 9:199–205. 10.1038/sj.embor.7401154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoyama K, Miyashita T, Araki Y, Ito E. 1986. Structure and functions of linkage unit intermediates in the biosynthesis of ribitol teichoic acids in Staphylococcus aureus H and Bacillus subtilis W23. Eur. J. Biochem. 161:479–489. 10.1111/j.1432-1033.1986.tb10469.x [DOI] [PubMed] [Google Scholar]
- 13.Soldo B, Lazarevic V, Karamata D. 2002. tagO is involved in the synthesis of all anionic cell-wall polymers in Bacillus subtilis 168. Microbiology 148:2079–2087 [DOI] [PubMed] [Google Scholar]
- 14.Wyke AW, Ward JB. 1977. Biosynthesis of wall polymers in Bacillus subtilis. J. Bacteriol. 130:1055–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia G, Kohler T, Peschel A. 2010. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int. J. Med. Microbiol. 300:148–154. 10.1016/j.ijmm.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 16.D'Elia MA, Henderson JA, Beveridge TJ, Heinrichs DE, Brown ED. 2009. The N-acetylmannosamine transferase catalyzes the first committed step of teichoic acid assembly in Bacillus subtilis and Staphylococcus aureus. J. Bacteriol. 191:4030–4034. 10.1128/JB.00611-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne M. 1937. Avirulent anthrax vaccine. Onderstepoort J. Vet. Sci. Anim. Ind. 21:41–43 [PubMed] [Google Scholar]
- 18.Green BD, Battisti L, Koehler TM, Thorne CB, Ivins BE. 1985. Demonstration of a capsule plasmid in Bacillus anthracis. Infect. Immun. 49:291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marraffini LA, Schneewind O. 2006. Targeting proteins to the cell wall of sporulating Bacillus anthracis. Mol. Microbiol. 62:1402–1417. 10.1111/j.1365-2958.2006.05469.x [DOI] [PubMed] [Google Scholar]
- 20.Saile E, Koehler TM. 2002. Control of anthrax toxin gene expression by the transition state regulator abrB. J. Bacteriol. 184:370–380. 10.1128/JB.184.2.370-380.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulford W, Model P. 1984. Specificity of translational regulation by two DNA-binding proteins. J. Mol. Biol. 173:211–226. 10.1016/0022-2836(84)90190-6 [DOI] [PubMed] [Google Scholar]
- 22.Bubeck Wardenburg J, Williams WA, Missiakas D. 2006. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 103:13831–13836. 10.1073/pnas.0603072103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruhfel RE, Robillard NJ, Thorne CB. 1984. Interspecies transduction of plasmids among Bacillus anthracis, B. cereus, and B. thuringiensis. J. Bacteriol. 157:708–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HU, Goepfert JM. 1974. A sporulation medium for Bacillus anthracis. J. Appl. Bacteriol. 37:265–267. 10.1111/j.1365-2672.1974.tb00438.x [DOI] [PubMed] [Google Scholar]
- 25.Wang YT, Oh SY, Hendrickx AP, Lunderberg JM, Schneewind O. 2013. Bacillus cereus G9241 S-layer assembly contributes to the pathogenesis of anthrax-like disease in mice. J. Bacteriol. 195:596–605. 10.1128/JB.02005-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youngman P. 1987. Plasmid vectors for recovering and exploiting Tn917 transpositions in Bacillus and other Gram-positive bacteria, p 79–103 In Hardy KG. (ed), Plasmids: a practical approach. IRL Press, Oxford, United Kingdom [Google Scholar]
- 27.Passalacqua KD, Varadarajan A, Ondov BD, Okou DT, Zwick ME, Bergman NH. 2009. Structure and complexity of a bacterial transcriptome. J. Bacteriol. 191:3203–3211. 10.1128/JB.00122-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novick RP, Projan SJ, Rosenblum W, Edelman I. 1984. Staphylococcal plasmid cointegrates are formed by host- and phage-mediated general rec systems that act on short regions of homology. Mol. Gen. Genet. 195:374–377. 10.1007/BF00332777 [DOI] [PubMed] [Google Scholar]
- 29.Anderson VJ, Kern JW, McCool JW, Schneewind O, Missiakas D. 2011. The SLH-domain protein BslO is a determinant of Bacillus anthracis chain length. Mol. Microbiol. 81:192–205. 10.1111/j.1365-2958.2011.07688.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen GB, Hensen BM, Eilenberg J, Mahillon J. 2003. The hidden lifestyles of Bacillus cereus and relatives. Environ. Microbiol. 5:631–640. 10.1046/j.1462-2920.2003.00461.x [DOI] [PubMed] [Google Scholar]
- 31.Leoff C, Saile E, Sue D, Wilkins PP, Quinn CP, Carlson RW, Kannenberg EL. 2008. Cell wall carbohydrate compositions of strains from Bacillus cereus group of species correlate with phylogenetic relatedness. J. Bacteriol. 190:112–121. 10.1128/JB.01292-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forsberg LS, Choudhury B, Leoff C, Marston CK, Hoffmaster AR, Saile E, Quinn CP, Kannenberg EL, Carlson RW. 2011. Secondary cell wall polysaccharides from Bacillus cereus strains G9241, 03BB87 and 03BB102 causing fatal pneumonia share similar glycosyl structures with the polysaccharides from Bacillus anthracis. Glycobiology 21:934–948. 10.1093/glycob/cwr026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazarevic V, Karamata D. 1995. The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol. Microbiol. 16:345–355. 10.1111/j.1365-2958.1995.tb02306.x [DOI] [PubMed] [Google Scholar]
- 34.Campbell J, Singh AK, Swoboda JG, Gilmore MS, Wilkinson BJ, Walker S. 2012. An antibiotic that inhibits a late step in wall teichoic acid biosynthesis induces the cell wall stress stimulon in Staphylococcus aureus. Antimicrob. Agents Chemother. 56:1810–1820. 10.1128/AAC.05938-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan YG, Frankel MB, Dengler V, Schneewind O, Missiakas D. 2013. Staphylococcus aureus mutants lacking the LytR-CpsA-Psr family of enzymes release cell wall teichoic acids into the extracellular medium. J. Bacteriol. 195:4650–4659. 10.1128/JB.00544-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soldo B, Lazarevic V, Pooley HM, Karamata D. 2002. Characterization of a Bacillus subtilis thermosensitive teichoic acid-deficient mutant: gene mnaA (yvyH) encodes the UDP-N-acetylglucosamine 2-epimerase. J. Bacteriol. 184:4316–4320. 10.1128/JB.184.15.4316-4320.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Elia MA, Pereira MP, Chung YS, Zhao W, Chau A, Kenney TJ, Sulavik MC, Black TA, Brown ED. 2006. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J. Bacteriol. 188:4183–4189. 10.1128/JB.00197-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown S, Santa Maria JP, Jr, Walker S. 2013. Wall teichoic acids of gram-positive bacteria. Annu. Rev. Microbiol. 67:313–336. 10.1146/annurev-micro-092412-155620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlag M, Biswas R, Krismer B, Kohler T, Zoll S, Yu W, Schwarz H, Peschel A, Gotz F. 2010. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol. Microbiol. 75:864–873. 10.1111/j.1365-2958.2009.07007.x [DOI] [PubMed] [Google Scholar]
- 40.Campbell J, Singh AK, Santa Maria JP, Jr, Kim Y, Brown S, Swoboda JG, Mylonakis E, Wilkinson BJ, Walker S. 2011. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem. Biol. 6:106–116. 10.1021/cb100269f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan YG, Frankel MB, Dengler V, Schneewind O, Missiakas D. 2013. Staphylococcus aureus mutants lacking the LytR-CpsA-Psr family of enzymes release cell wall teichoic acids into the extracellular medium. J. Bacteriol. 195:4650–4659. 10.1128/JB.00544-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caliot E, Dramsi S, Chapot-Chartier MP, Courtin P, Kulakauskas S, Pechoux C, Trieu-Cuot P, Mistou MY. 2012. Role of the group B antigen of Streptococcus agalactiae: a peptidoglycan-anchored polysaccharide involved in cell wall biogenesis. PLoS Pathog. 8:e1002756. 10.1371/journal.ppat.1002756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu H, Bergman NH, Thomason B, Shallom S, Hazen A, Crossno J, Rasko DA, Ravel J, Read TD, Peterson SN, Yates J, III, Hanna PC. 2004. Formation and composition of the Bacillus anthracis endospore. J. Bacteriol. 186:164–178. 10.1128/JB.186.1.164-178.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kern VJ, Kern JW, Theriot JA, Schneewind O, Missiakas D. 2012. Surface-layer (S-layer) proteins sap and EA1 govern the binding of the S-layer-associated protein BslO at the cell septa of Bacillus anthracis. J. Bacteriol. 194:3833–3840. 10.1128/JB.00402-12 [DOI] [PMC free article] [PubMed] [Google Scholar]