Abstract
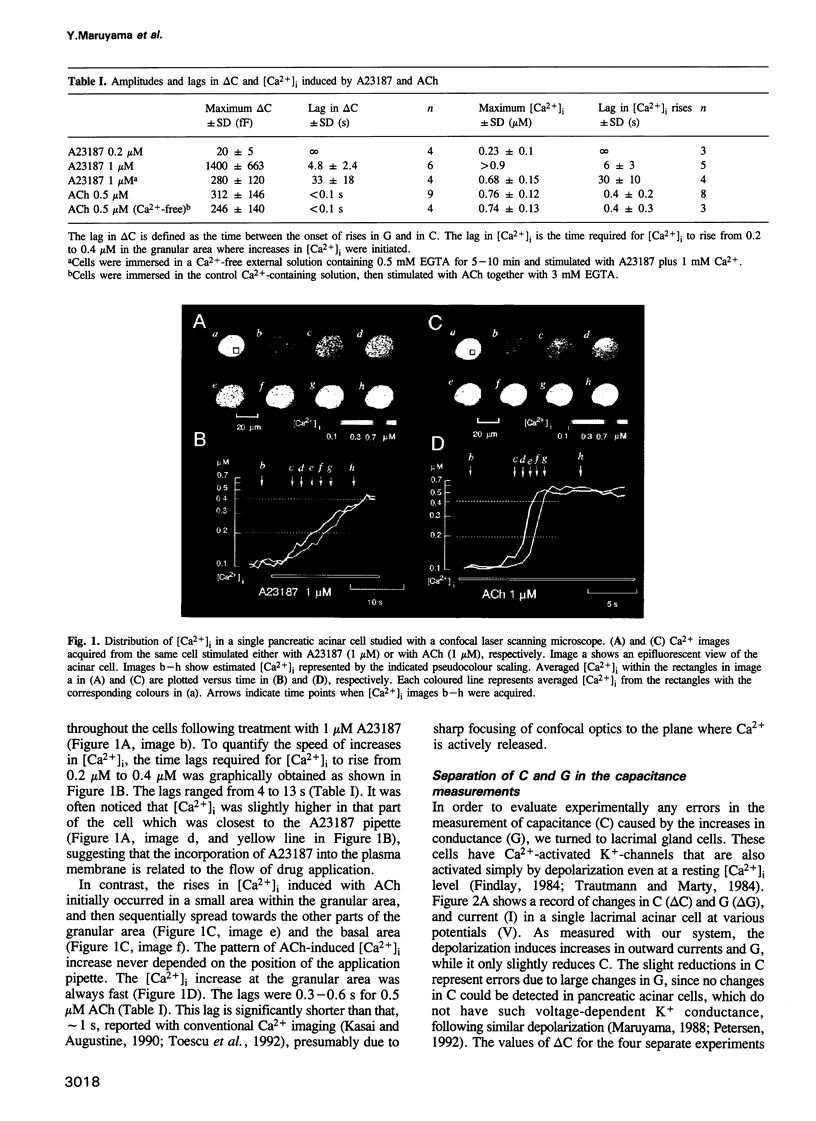
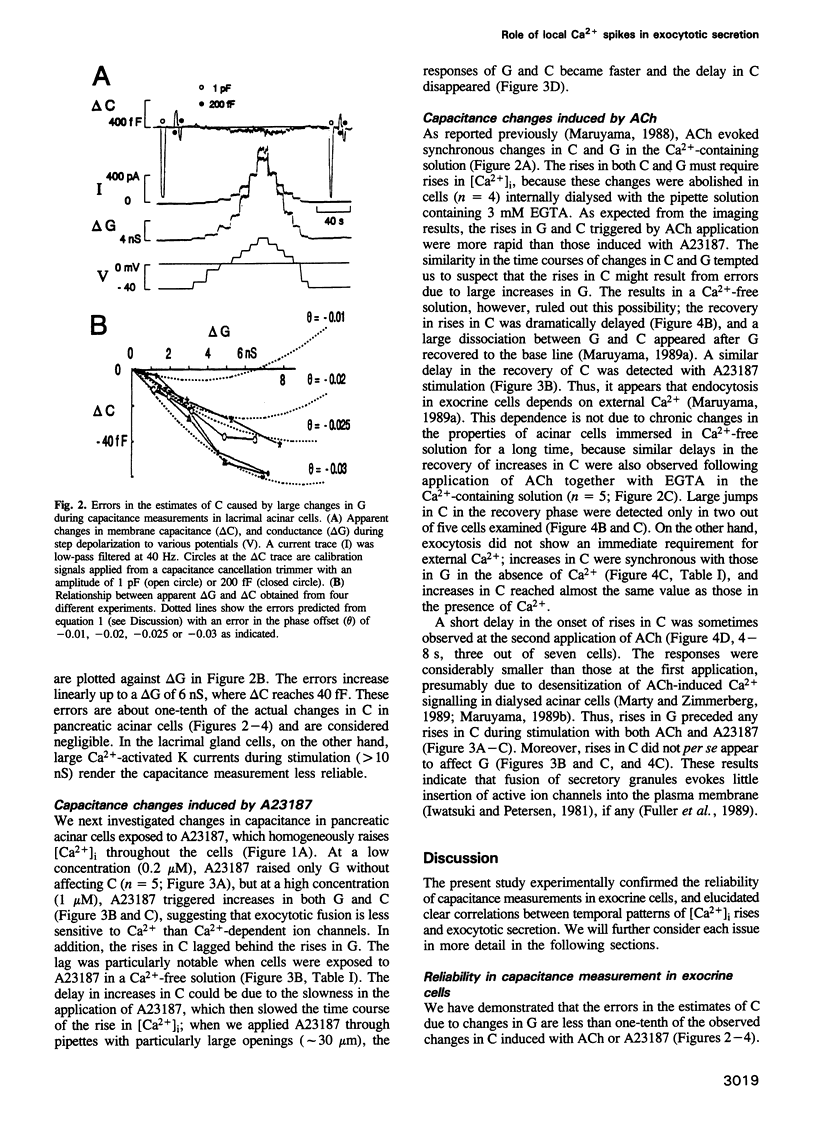
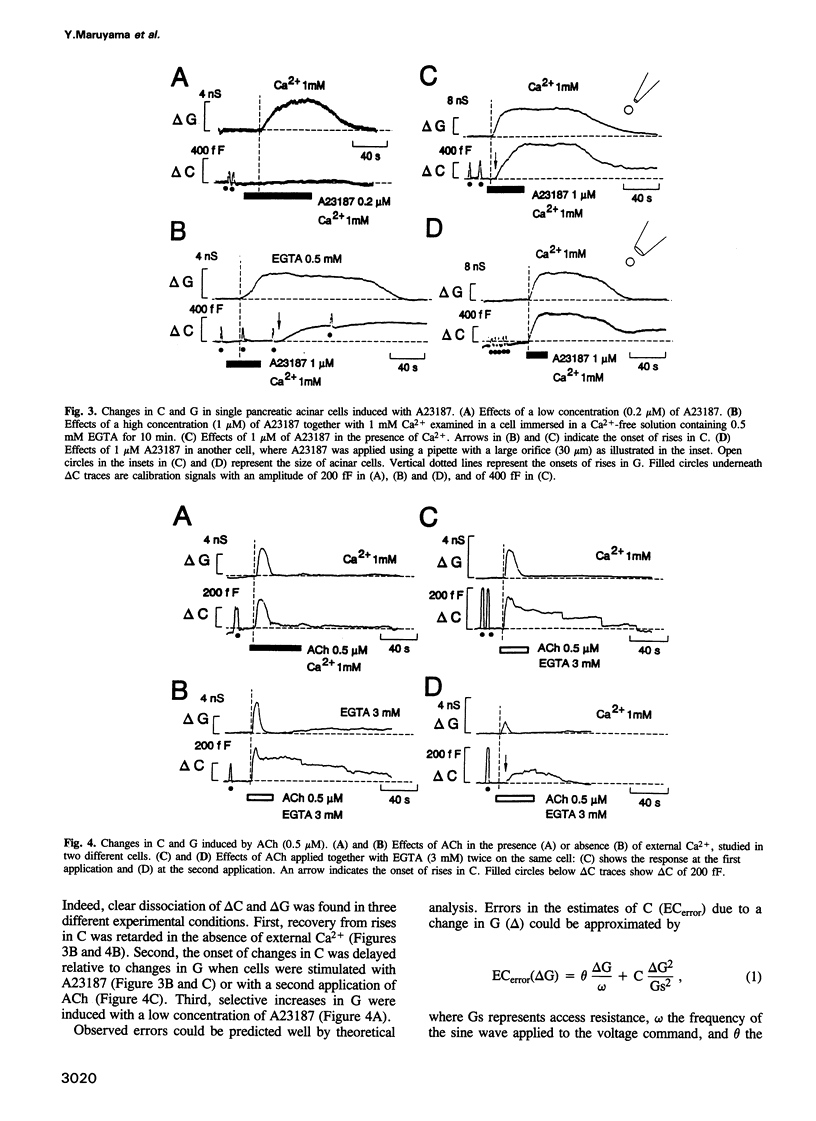
We investigated how agonist-induced patterned rises in cytosolic Ca2+ concentration ([Ca2+]i) regulate exocytotic secretion in the rat pancreatic acinar cell. The distribution of [Ca2+]i was visualized with a confocal microscope, which revealed that a Ca2+ ionophore, A23187, induced slow and homogeneous [Ca2+]i rises, while acetylcholine (ACh) always triggered primary Ca2+ spikes at the granular area which bears secretory granules. Secretion was monitored by measuring capacitance with the patch clamp method. Errors in the estimates of membrane capacitance (C) due to changes in conductance (G) were experimentally as well as theoretically evaluated to be one-tenth of the actual signals. We found that A23187 raised G without changing C at a low concentration, while it triggered asynchronous rises in G and C with lags in C, at a high concentration. By contrast, ACh triggered simultaneous rapid rises in G and C. Our results support the hypothesis that exocytotic secretion is less sensitive to Ca2+ than to ion channels and is directly caused by agonist-induced primary Ca2+ spikes at the granular area. It is therefore suggested that spatio-temporal patterns of Ca2+ oscillations could play a key role in exocytotic secretion from the exocrine acinar cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Brundage R. A., Fogarty K. E., Tuft R. A., Fay F. S. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science. 1991 Nov 1;254(5032):703–706. doi: 10.1126/science.1948048. [DOI] [PubMed] [Google Scholar]
- Findlay I. A patch-clamp study of potassium channels and whole-cell currents in acinar cells of the mouse lacrimal gland. J Physiol. 1984 May;350:179–195. doi: 10.1113/jphysiol.1984.sp015195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller C. M., Eckhardt L., Schulz I. Ionic and osmotic dependence of secretion from permeabilised acini of the rat pancreas. Pflugers Arch. 1989 Feb;413(4):385–394. doi: 10.1007/BF00584488. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Dissociation between stimulant-evoked acinar membrane resistance change and amylase secretion in the mouse parotid gland. J Physiol. 1981 May;314:79–84. doi: 10.1113/jphysiol.1981.sp013691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C., Fernandez J. M. Capacitance measurements. An analysis of the phase detector technique used to study exocytosis and endocytosis. Biophys J. 1988 Jun;53(6):885–892. doi: 10.1016/S0006-3495(88)83169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Augustine G. J. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990 Dec 20;348(6303):735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Marty A. Calcium release and internal calcium regulation in acinar cells of exocrine glands. J Membr Biol. 1991 Dec;124(3):189–197. doi: 10.1007/BF01994353. [DOI] [PubMed] [Google Scholar]
- Marty A., Zimmerberg J. Diffusion into the patch-clamp recording pipette of a factor necessary for muscarinic current response. Cell Signal. 1989;1(3):259–268. doi: 10.1016/0898-6568(89)90043-0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y. Activation and desensitization mechanisms of muscarinic current response in single pancreatic acinar cells of rats. J Physiol. 1989 Oct;417:343–359. doi: 10.1113/jphysiol.1989.sp017805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y. Agonist-induced changes in cell membrane capacitance and conductance in dialysed pancreatic acinar cells of rats. J Physiol. 1988 Dec;406:299–313. doi: 10.1113/jphysiol.1988.sp017381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y. Effects of external calcium on acetylcholine-evoked increases in membrane capacitance in rat pancreatic acinar cells. Pflugers Arch. 1989 Feb;413(4):438–440. doi: 10.1007/BF00584496. [DOI] [PubMed] [Google Scholar]
- Maruyama Y. Inhibitory effects of arachidonic acid on muscarinic current response in single pancreatic acinar cells of rats. J Physiol. 1990 Nov;430:471–482. doi: 10.1113/jphysiol.1990.sp018302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson M. H., Padfield P. J., O'Sullivan A. J., Burgstahler A. D., Jamieson J. D. Mechanism of Ca2+ wave propagation in pancreatic acinar cells. J Biol Chem. 1992 Sep 5;267(25):18118–18121. [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. Stimulus-secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J Physiol. 1992 Mar;448:1–51. doi: 10.1113/jphysiol.1992.sp019028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Tan Y. P., Marty A., Trautmann A. High density of Ca(2+)-dependent K+ and Cl- channels on the luminal membrane of lacrimal acinar cells. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11229–11233. doi: 10.1073/pnas.89.23.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu E. C., Lawrie A. M., Petersen O. H., Gallacher D. V. Spatial and temporal distribution of agonist-evoked cytoplasmic Ca2+ signals in exocrine acinar cells analysed by digital image microscopy. EMBO J. 1992 Apr;11(4):1623–1629. doi: 10.1002/j.1460-2075.1992.tb05208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann A., Marty A. Activation of Ca-dependent K channels by carbamoylcholine in rat lacrimal glands. Proc Natl Acad Sci U S A. 1984 Jan;81(2):611–615. doi: 10.1073/pnas.81.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]



