Abstract
Competence for natural DNA transformation is a tightly controlled developmental process in streptococci. In mutans and salivarius species, the abundance of the central competence regulator σX is regulated at two levels: transcriptional, by the ComRS signaling system via the σX/ComX/SigX-inducing peptide (XIP), and posttranscriptional, by the adaptor protein MecA and its associated Clp ATPase, ClpC. In this study, we further investigated the mechanism and function of the MecA-ClpC control system in the salivarius species Streptococcus thermophilus. Using in vitro approaches, we showed that MecA specifically interacts with both σX and ClpC, suggesting the formation of a ternary σX-MecA-ClpC complex. Moreover, we demonstrated that MecA ultimately targets σX for its degradation by the ClpCP protease in an ATP-dependent manner. We also identify a short sequence (18 amino acids) in the N-terminal domain of σX as essential for the interaction with MecA and subsequent σX degradation. Finally, increased transformability of a MecA-deficient strain in the presence of subinducing XIP concentrations suggests that the MecA-ClpCP proteolytic complex acts as an additional locking device to prevent competence under inappropriate conditions. A model of the interplay between ComRS and MecA-ClpCP in the control of σX activity is proposed.
INTRODUCTION
Several bacteria are able to enter a transitory physiological state known as “competence for natural transformation,” enabling the natural capture and chromosomal integration of exogenous naked DNA, a key process for genome plasticity and virulence (for a recent review, see reference 1). In Gram-positive bacteria, competence development is under the control of a master regulator that transcriptionally activates the so-called late competence (com) genes, encoding DNA uptake, protection, and homologous recombination machineries, among others (1). In bacilli, this master regulator is a transcriptional activator (ComK), while in streptococci, it is an alternative sigma factor (σX or ComX/SigX) that transiently associates with the core of the RNA polymerase. Both regulators were shown to specifically bind to specific DNA motifs located in the promoters of late com genes (1). Since DNA transformation is an energy-expensive process, with possible deleterious effects on genome integrity and cell division, a strict control of the production of the master regulator is essential to avoid its activation under inappropriate conditions (1). This control takes place during the so-called early phase of competence at two major levels: transcriptional, by a pheromone-based signaling system, and posttranslational, through the active degradation/stabilization of the master regulator (1).
In Bacillus subtilis, competence is activated at the entry into the stationary growth phase by the quorum-sensing peptide pheromone ComXBsu (2). This pheromone is sensed by the ComAP phosphorelay system, which regulates the production of ComSBsu, a short peptide encoded within the surfactin A-encoding operon (3–5). ComSBsu is necessary for ComK release from degradation. ComK then autoactivates its production and activates those of late com genes (3–5). The posttranslational control of ComK abundance has been investigated in detail and relies on the adaptor protein MecABsu (for a review, see reference 6). To prevent competence development under unfavorable conditions, ComK is sequestered by MecABsu, which specifically interacts with ClpC, resulting in ComK degradation by the ClpCP proteolytic machinery (7–11). In contrast, under competence-inducing conditions, ComK is released from this inhibitory complex by the antiadaptor peptide ComSBsu through a direct competitive interaction with MecABsu (9, 12). Short core sequences sharing some similarities were identified in ComK and ComSBsu (FMLYPK and IILYPR, respectively) as important for their interaction with MecABsu (9). Thus, in B. subtilis, the pheromone-based signaling system indirectly controls the abundance of the master regulator ComK at the posttranslational level, a control level of key importance for competence development in this species (5, 6).
In streptococci, two alternative signaling systems, ComCDE and ComRS, directly control comX transcription via distinct activation mechanisms (for recent reviews, see references 1 and 13). Both systems activate competence transiently during the early logarithmic growth phase. The ComCDE system, present in the mitis and anginosus groups, has been extensively studied in Streptococcus pneumoniae (1, 14, 15). In this species, the extracellular pheromone competence-stimulating peptide (CSP) encoded by comC is sensed by the ComDE phosphorelay system, which in turn activates comX and comCDE (positive feedback loop) (1, 14) and other early com genes, including comW (16). comW encodes the small protein ComW, which stabilizes and activates σX at the posttranslational level (17). In addition, the abundance of σX is controlled by the ClpEP and ClpCP proteolytic machineries, which degrade σX and ComW, respectively (18). Thus, in S. pneumoniae, the pheromone-based system controls the master regulator σX at both transcriptional and posttranslational levels. The recently discovered ComRS system, present in the salivarius (19–21), mutans (22–24), pyogenic (24, 25), and bovis (24, 26) groups, was experimentally shown to be the signaling system that directly controls comX transcription in at least one representative species of all the above-mentioned streptococcal groups. ComR is a transcriptional regulator of the Rgg family and ComS is the precursor of the secreted competence σX/ComX/SigX-inducing peptide (XIP) (19–21, 23, 24). The activation mechanism of the ComRS system was deeply investigated in Streptococcus thermophilus (19–21, 27) and Streptococcus mutans (22–24, 28). The extracellular pheromone XIP is not sensed by a phosphorelay system but is imported in the cytoplasm by the oligopeptide transporter Opp (Ami) (20, 24, 27), where it directly interacts with ComR (20). The XIP-ComR complex in turn directly activates the transcription of comX and comS by binding to the ComR box present in their promoter sequences (positive feedback loop) (20).
Besides the transcriptional control of comX by the ComRS signaling system, the activity of σX was also shown to be the target of a posttranslational negative control in one representative species of the pyogenic (25, 29), salivarius (30, 31), and mutans (32) groups. In these species, extensive in silico analyses did not allow the identification of a ComW homolog, suggesting that the regulatory system could be different from that in S. pneumoniae. We and others have shown that the adaptor protein MecA and the ClpC ATPase subunit act as negative regulators of competence development in both S. thermophilus (30, 31) and S. mutans (32). These studies revealed that the expression of late com genes and the abundance of σX were increased in MecA- and ClpC-depleted strains compared to the isogenic wild-type (WT) strains (30–32) In addition, bacterial two-hybrid (B2H) assays suggested that MecA could interact in vivo with both σX and ClpC (31, 32). By analogy to the posttranslational control of ComK in B. subtilis, we postulated that σX would be degraded by the MecA-ClpCP protease complex under nonpermissive conditions for competence development (31).
The aim of this study was to further investigate the role of the putative MecA-ClpCP complex in the posttranslational control of σX activity and to reveal its interplay with the ComRS system in the control of competence development in S. thermophilus. On one hand, we show that MecA directly interacts in vitro with both σX and ClpC and demonstrate that σX is specifically degraded by the MecA-ClpCP proteolytic machinery in the presence of ATP. On the other hand, we show that at low noninducing XIP concentrations, DNA transformation is significantly increased in the absence of a functional MecA-ClpCP complex. Altogether, these results support our model of posttranslational control of σX in ComRS-containing streptococci where the adaptor protein MecA selects σX for specific degradation by ClpCP to avoid competence development under unfavorable conditions.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table S1 in the supplemental material. Escherichia coli was grown with shaking at 37°C in lysogeny broth (LB) (33). S. thermophilus strains were grown anaerobically (BBL GasPak Systems, Becton, Dickinson, Franklin Lakes, NJ) at 37°C in M17 broth or Todd-Hewitt broth (THB) (Difco Laboratories Inc., Detroit, MI). Those media were supplemented with 1% (wt/vol) lactose or glucose (M17L or M17G and THBL or THBG, respectively). Solid agar plates were prepared by adding 2% (wt/vol) agar to the medium. When necessary, antibiotics were added to the media at the following concentrations: ampicillin at 250 μg ml−1 and kanamycin at 50 μg ml−1 for E. coli and erythromycin at 2.5 μg ml−1 for S. thermophilus.
Detection of absorbance and luminescence.
Growth (optical density at 600 nm [OD600]) and luciferase (Lux) activity (expressed in relative light units [RLU]) were monitored at 10-min intervals in a Varioskan Flash multimode reader (Thermo Fisher Scientific, Zellic, Belgium) as previously described (19).
Natural transformation experiments.
Overnight cultures of S. thermophilus grown in THBG or -L at 37°C were diluted 30-fold in fresh medium and further grown at 37°C. Small volumes (300 μl) of the cultures were then supplemented with ComS17-24 (purchased from Peptide 2.0, Chantilly, VA) at concentrations ranging from 0 to 40 nM. For kinetic transformation experiments, 1 μM ComS17-24 was added to the culture, and then 2 μg of plasmid pGIUD0855ery (19) was added 0, 0.5, 1, 2, 3, or 4 h later. Samples (100 μl of serial dilutions in medium) were spread on M17 plates for the viability count and on M17 plates containing erythromycin for the selection of transformants. The transformation frequency was calculated after 30 h of anaerobic incubation at 37°C as the number of antibiotic-resistant CFU ml−1 divided by the total number of viable CFU ml−1.
DNA techniques and electrotransformation.
For general molecular biology techniques, the instructions given by Sambrook et al. (33) were followed. Electrotransformation of E. coli was performed as described by Dower et al. (34). The primers used in this study were purchased from Eurogentec (Seraing, Belgium) and are listed in Table S2 in the supplemental material. PCRs were performed with Phusion high-fidelity DNA polymerase (Finnzymes, Espoo, Finland) in a GeneAmp PCR system 2400 (Applied Biosystems, Foster City, CA).
Construction of the pBADhisA-ComX, -MecA, -ClpC, -ClpE, and -ClpP expression vectors.
The open reading frame (ORF) of each gene was amplified by PCR from the chromosome of strain LMD-9 with primers listed in Table S2 in the supplemental material. The PCR product restricted by XbaI and KpnI and pBADhisA restricted by NheI and KpnI were ligated and then transformed in E. coli TG1.
Construction of the pBAD-ComX-Strep expression vector.
The comX::strep fusion (σX fused to the purification affinity tag StrepTagII at the C terminus) was amplified by PCR from the chromosome of strain CB0053 (31) with primers listed in Table S2 in the supplemental material. The PCR product and pBADhisA were restricted by NcoI and KpnI, ligated, and then transformed into E. coli TG1.
Bacterial two-hybrid assays and plasmid constructions.
The method used was described by Karimova et al. (35). In order to obtain pUT18C-MecAN1-103, pUT18C-MecA was digested by EcoRI and self-ligated. The partial coding sequences for the truncated versions of comX and mecA for all other constructs were amplified by PCR from S. thermophilus LMD-9 using primers reported in Table S2 in the supplemental material and inserted in plasmid pUT18, pUT18C, pKT25, or pKNT25. The complete list of recombinant plasmids used for B2H assays is reported in Table S3 in the supplemental material. B2H assays were performed as previously described (31). B2H assays were performed on MacConkey indicator plates supplemented with 1% maltose. Plates were incubated at 30°C for 36 h.
Purification of 6His-MecA, -ClpC, -ClpE, and -ClpP recombinant proteins.
Precultures (20 ml) of strain TG1 transformed with pBAD6His-MecA, -ClpC, -ClpE, and -ClpP were diluted to an OD600 of 0.05 in 1 liter prewarmed LB (42°C) containing ampicillin and incubated at 42°C with continuous shaking according to the “thermal shift” procedure described before (36). At an OD600 of ∼ 0.5, the culture was chilled on ice for 10 min with shaking every 2 min, and protein expression was then induced by adding 0.02% l-arabinose. After 4 h of induction at 28°C with continuous shaking, bacteria were centrifuged at 5,000 × g during 15 min. The pellet was washed once with cold 1× phosphate-buffered saline (PBS), frozen in liquid nitrogen, and kept at −80°C. The pellet was then resuspended in 40 ml lysis buffer (50 mM Tris-HCl [pH 8.0], 1 mM EDTA, 50 mM NaCl, 5% glycerol, 1 mM dithiothreitol [DTT], 100 μg ml−1 of lysozyme, 1 mM phenylmethylsulfonyl fluoride [PMSF]). Cells were sonicated at 4°C (Bioruptor; Diagenode, Liège, Belgium), and the soluble fraction was collected after centrifugation (13,000 × g for 20 min at 4°C). The lysate was loaded on ProBond nickel-chelating resin (Life Technologies, Ghent, Belgium) in a 10-ml column. After washing with 20 mM imidazole, 6His-tagged proteins were eluted with 250 mM imidazole. The concentrations and purities of the fractions were estimated by SDS-PAGE and on a NanoDrop instrument (Thermo Scientific, Wilmington, DE). Glycerol was added to the different elution fractions at a final concentration of 25% (vol/vol). The purest fractions were used for further experiments. ComR-Strep was purified as previously described (20).
Purification of 6His-σX and σX-Strep recombinant proteins from inclusion bodies.
For σX purifications, an overnight culture of E. coli TG1 cells carrying pBAD-ComX-Strep or pBADHisA-ComX was diluted 1:100 in 1 liter of LB containing ampicillin. Growth at 37°C with shaking was monitored until cells reached an OD600 of about 0.5 to 0.8. l-Arabinose was added to a final concentration of 0.02%. All further purification steps were carried out at 4°C unless otherwise specified. After an additional 4-h incubation at 28°C, the cells were harvested by centrifugation at 10,000 × g during 30 min and washed once with 1× PBS, and the pellet was frozen in liquid nitrogen and kept at −80°C. The pellet was then resuspended in 40 ml of lysis buffer (50 mM Tris-HCl [pH 8.0], 1 mM EDTA, 50 mM NaCl, 5% glycerol, 1 mM DTT, 100 μg ml−1 of lysozyme, 1 mM PMSF). Cell lysis was achieved by sonication for 10 min at 4°C (Bioruptor).
The insoluble fraction containing 6His-σX or σX-Strep was collected, washed, and then solubilized with Sarkosyl (N-lauroylsarcosine sodium salt solution, 30% aqueous solution; Sigma-Aldrich, Diegem, Belgium) as previously described (37). The centrifugation-clarified dialyzed sample was applied to a 10-ml Ni-nitrilotriacetic acid (NTA) agarose column in the case of 6His-σX and to a 1-ml Strep-Tactin Superflow column (IBA, Göttingen, Germany) in the case of the σX-Strep. 6His-σX was purified as described above. σX-Strep was purified according to the manufacturer's instructions for Strep-Tactin Superflow columns (IBA) using buffer W (100 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1 mM EDTA). The purest fractions containing σX (identified by SDS-PAGE) were then combined, dialyzed against storage buffer (50 mM Tris-HCl [pH 8.0], 50% glycerol, 250 mM NaCl, 0.1 mM EDTA, 1 mM DTT), and stored at −80°C.
SPR experiments.
Surface plasmon resonance (SPR) experiments were performed on a Biacore 3000 instrument using a research-grade CM5 sensor chip (GE Healthcare, Vélizy-Villacoublay, France). For all experiments, the temperature was set at 25°C and a continuous flow of running buffer (1× HBS-EP, consisting of 10 mM HEPES [pH 7.4], 150 mM NaCl, 3 mM EDTA, and 0.005% surfactant P20) was maintained over the sensor surface. 6His-MecA protein was immobilized on one flow cell of the sensor chip by amine coupling according to manufacturer's instructions (GE Healthcare). After activation of the carboxymethyl groups of the dextran surface with an N-hydroxysuccinimide (NHS)–1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) mixture, a 15 μg ml−1 MecA solution prepared in 10 mM sodium acetate buffer (pH 4.0) was injected during 1 min on the surface at 5 μl min−1. Finally, the surface was deactivated by an injection of ethanolamine (1 M). The level of covalently bound MecA protein was 1,150 resonance units (RU). Similarly, a reference flow cell was prepared (by activation and deactivation of the surface without injection of protein). All SPR analyses were performed at 20 μl min−1 by passing samples prepared in the running buffer for 120 s over the active and the reference flow cells. Return to baseline between each injection, i.e., removal of noncovalently bound analytes, was achieved by extended washing with the running buffer.
The sensorgrams, expressed as the difference between the active cell and the reference cell, were analyzed using BIAevaluation software version 4.0.1. Subtraction of a blank sensorgram, corresponding to a buffer injection at the beginning of each set of experiments, was performed in order to correct bulk effect and systematic artifacts. To determine the affinity of σX for MecA, a dose-response curve (steady-state response versus σX concentration) was constructed and fitted using the BIAevaluation software, considering a 1:1 stoichiometry. The equilibrium dissociation constant (KD) for the MecA-σX complex was calculated with the following equation: KD = [MecA][σX]/[MecA − σX].
In vitro degradation assay.
Purified σX-Strep or ComR-Strep (0.6 μM) was incubated at 37°C in KTME buffer (100 mM KCl, 25 mM Tris-HCl [pH 8], 5 mM MgCl2, 0.1 mM EDTA) with equimolar concentrations of 6His-MecA, -ClpC (or -ClpE), -ClpP, and ATP (5 mM) in a final volume of 40 μl. An ATP regeneration system consisting of pyruvate kinase (PK) (1.17 μM) and phosphoenolpyruvate (PEP) (2 mM) (Sigma-Aldrich) was added to ensure that ATP was not limiting the ATPase activity of ClpC or ClpE. At 30 min after addition of ATP, 10 μl of Laemmli buffer (5×) was added to each sample and heated for 5 min at 96°C. SDS-PAGE, electrotransfer onto nitrocellulose membranes, and Western blot analyses were performed as previously described (31). Whole protein content was analyzed by SDS-PAGE with Coomassie blue staining. σX-Strep and the negative control ComR-Strep were detected using monoclonal antibody against StrepTagII (StrepMAB-Classic) as the primary antibody and a horseradish peroxidase-conjugated rabbit anti-mouse polyclonal antibody as a secondary antibody according to the manufacturer's instructions (IBA). Quantifications were performed by densitometric analyses (Kodak 1DV.3.5.3 software) of the intensities of the different bands.
RESULTS
MecA interacts in vitro with both σX and ClpC.
Previous in vivo bacterial two-hybrid (B2H) assays strongly suggested that MecA from S. thermophilus interacts with both ClpC and σX (31). To validate the MecA-σX and MecA-ClpC interactions in this species, real-time interactions were tested in vitro using surface plasmon resonance (SPR). For this purpose, His-tagged MecA, ClpC, and σX (N-terminal fusions) were purified to homogeneity. His-tagged ClpE, an alternative Clp ATPase subunit (47% of identity with ClpC), was also used as control in MecA interaction experiments since it has a molecular weight similar to that of ClpC. The SPR sensor chip was functionalized with 6His-MecA.
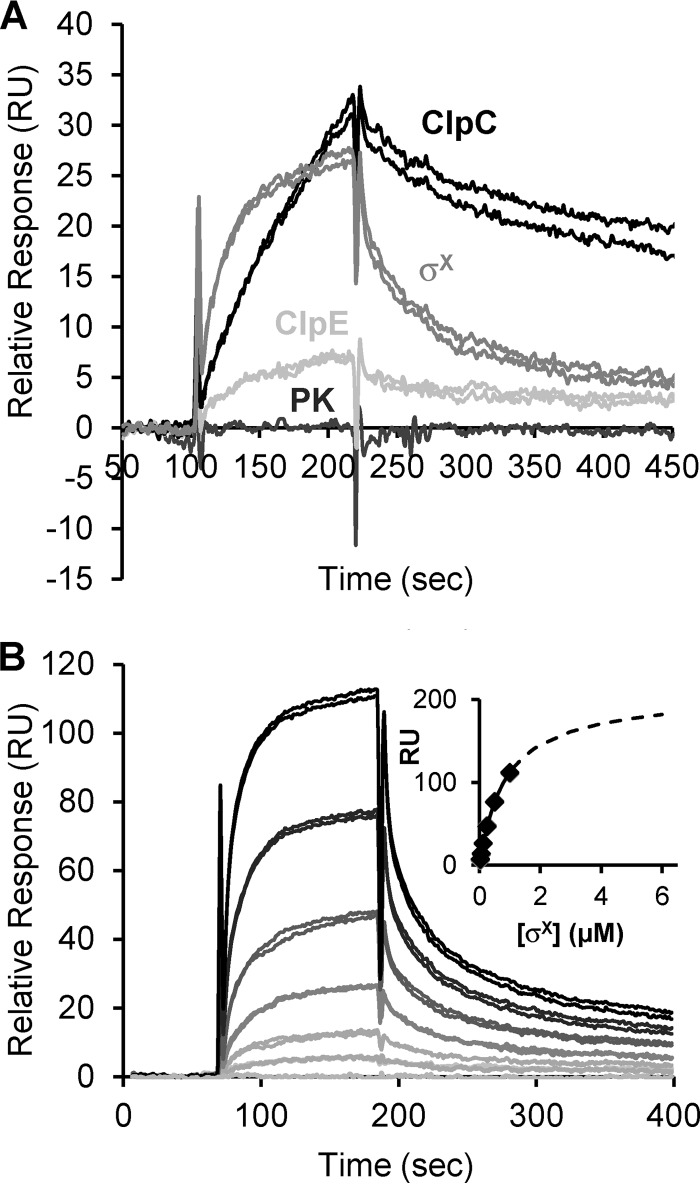
In a first set of experiments, the specificity of the MecA-ClpC interaction was investigated by injecting (in duplicates) 0.1 μM pyruvate kinase (PK) (negative control), 6His-CplC, or 6His-ClpE on the sensor chip (Fig. 1A). As expected, no increase in the response was observed during PK injection on the surface (Fig. 1A). Regarding Clp ATPases, the response obtained at the end of the association phase for 6His-ClpC was ∼4-fold higher than that for 6His-ClpE, indicating that ClpC interacts more strongly with MecA than ClpE.
FIG 1.
Interactions of MecA with σX, ClpC, or ClpE, evaluated by SPR. (A) Sensorgrams depicting the in vitro interactions of pyruvate kinase (PK) and 6His-ClpE, -ClpC, and -σX (in duplicates) with 6His-MecA immobilized on the sensor chip. (B) Sensorgrams showing the real-time interaction of 6His-σX with 6His-MecA. The response curve values, in resonance units (RU), were recorded for 6His-σX solutions injected in duplicates at the following concentrations: 0.03, 0.06, 0.12, 0.25, 0.5, and 1 μM. RU signals were then normalized as described in Materials and Methods. The inset shows the normalized RU response as a function of σX concentration (binding isotherm) as calculated at the end of the injection. Diamonds represent experimental points, and the dotted line represent the extrapolated curve.
In a second set of SPR experiments, the MecA-σX interaction was investigated by injecting 0.1 μM 6His-σX (in duplicates). The σX response obtained at the end of the association phase (27 RU) reflected a strong and direct interaction (Fig. 1A). Increasing concentrations of σX (from 0.003 to 1 μM) were next injected on the biochip, and an extrapolated dose-response curve was constructed to determine the affinity constants as described in Materials and Methods (38). As expected, the SPR signal was proportional to the 6His-σX concentration (from 0.003 to 1 μM) (Fig. 1B). The MecA-σX stoichiometry was set to 1:1 as proposed for the MecA-ComK complex (9). An equilibrium dissociation constant (KD) equal to 0.87 ± 0.2 μM was calculated for the MecA-σX complex.
Altogether, these results validate the specificity of the interaction of MecA with both ClpC and σX, reinforcing the hypothesis that these three proteins from a ternary complex in vivo.
MecA interacts with a predicted surface-exposed region of the N-terminal domain of σX.
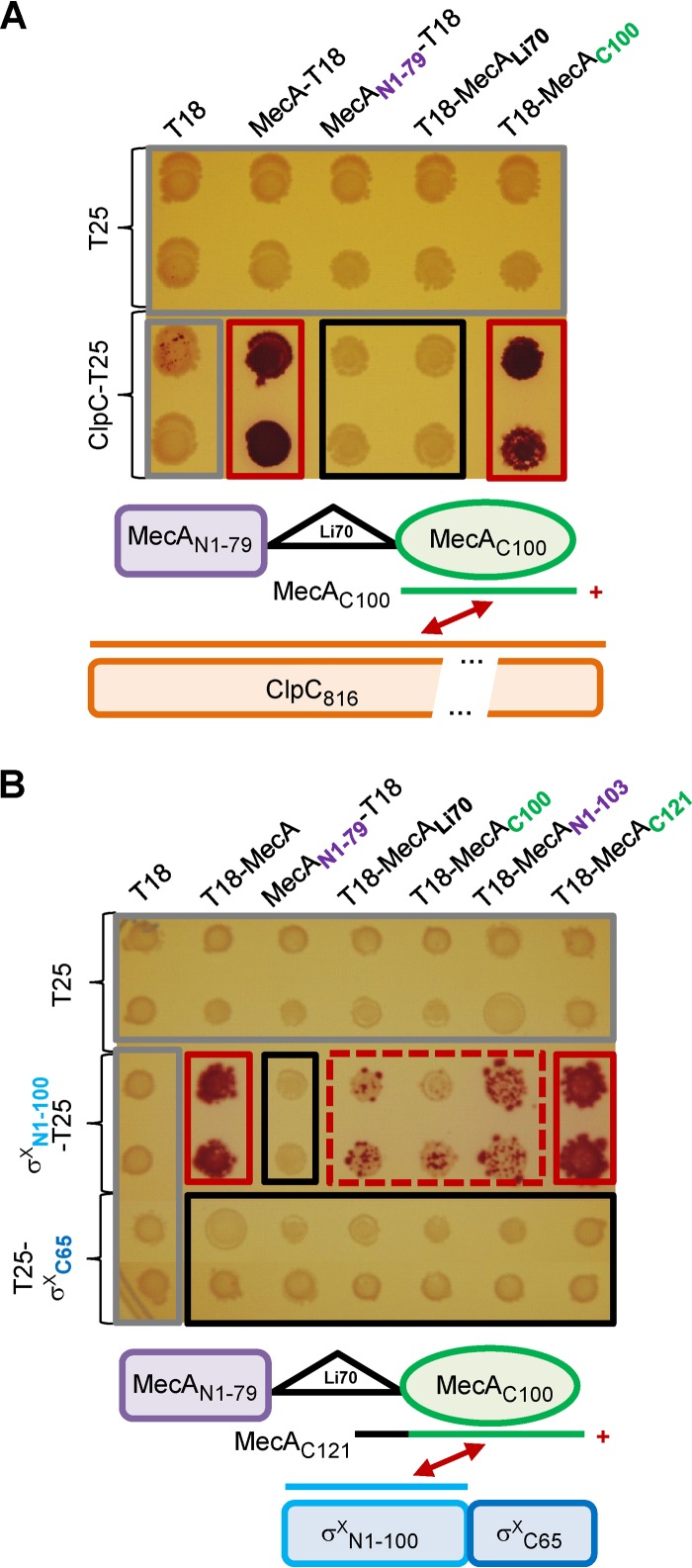
The B2H assay was used to further map the different MecA subdomains interacting with ClpC and σX. In B2H, interacting T18 and T25 fusion proteins lead to functional α-complementation of the adenylate cyclase activity and thus enable maltose utilization (colonies display a red coloration on MacConkey agar-based medium supplemented with 1% maltose) (35). Although less quantitative, this method seems reliable to study MecA interactants. Indeed, interactions observed using SPR were in good agreement with those deduced from our previous B2H results (31). Regions of the mecA and comX or clpC ORFs were fused to the T25- and T18-encoding fragments of cyaA, respectively, and the resulting plasmids were cotransformed in the appropriate E. coli strain. In most cases, hybrid proteins were designed so that the native orientation of the protein domains was respected. The T18-X or T25-Y and X-T18 or Y-T25 nomenclatures indicate that protein X (or a truncated domain thereof) is fused to the C terminus and N terminus of T18 or T25, respectively.
MecA from B. subtilis is organized in three macrodomains (8). Multiple-sequence alignment with streptococcal orthologs (31) (see Fig. S1 in the supplemental material) was used to design three T18 fusion proteins for our B2H assays, i.e., MecAN1-79-T18, T18-MecAC100, and MecALi70-T18, which correspond to the first 79 amino acids (aa) (N), to the last 100 aa (C), and to a 70-aa linker region (Li) between domains N and C of MecA, respectively (Fig. 2A; see Fig. S1 in the supplemental material). It is noteworthy that the MecA Li region is much less conserved among orthologs than domains N and C (31) (see Fig. S1 in the supplemental material). First, we showed that the full-length ClpC interacts solely with the C domain of MecA (Fig. 2A). Then, we investigated whether specific domains of MecA and σX were interacting. Based on the three-dimensional (3D) structural prediction for σX (see Fig. S2A in the supplemental material), the sigma factor was divided into two macrodomains (see Fig. S2B in the supplemental material): the first 100 aa (N, σXN1-100-T25), which corresponds to region 2 of σ70 (Pfam domain 04542), and the last 65 aa (C, T25-σXC65), corresponding to a domain of unknown function (see Fig. S2A in the supplemental material). The B2H results presented in Fig. 2B suggest that the domain N of σX (first 100 aa) is able to interact with full-length MecA but that it is not able to react or reacts extremely weakly with either of its macrodomains. Two additional MecA-T18 derivative fusions, T18-MecAN1-103 and T18-MecAC121, were thus constructed to expand domains N and C, respectively, in order to include part of the linker domain (see Fig. S1 in the supplemental material). Interaction between domain N of σX and the extended domain C of MecA (T18-MecAC121) was found to be similar to that between domain N of σX and full-length MecA (T18-MecA) (Fig. 2B). Based on all these B2H assays, we propose that both domains Li and C of MecA are important for the interaction with the domain N of σX.
FIG 2.
Subdomain interactions of MecA with σX or ClpC, evaluated by B2H assays. (A) Top, matrices of B2H interactions between MecA domains and full-length ClpC on MacConkey plates. Bottom, MecA domain organization and positive (+) MecAC100-ClpC interaction (red arrow). (B) Top, matrices of B2H interactions between MecA domains and σX domains on MacConkey plates. Bottom, organization of MecA and σX domains and positive (+) MecAC121-σXN1-100 interaction (red arrow). In both panels, controls are surrounded by a gray rectangle, and T25 and T18 correspond to the empty vectors pKT25 and pUT18, respectively. Red, red dashed, and black rectangles highlight positive, weak, and negative interactions, respectively.
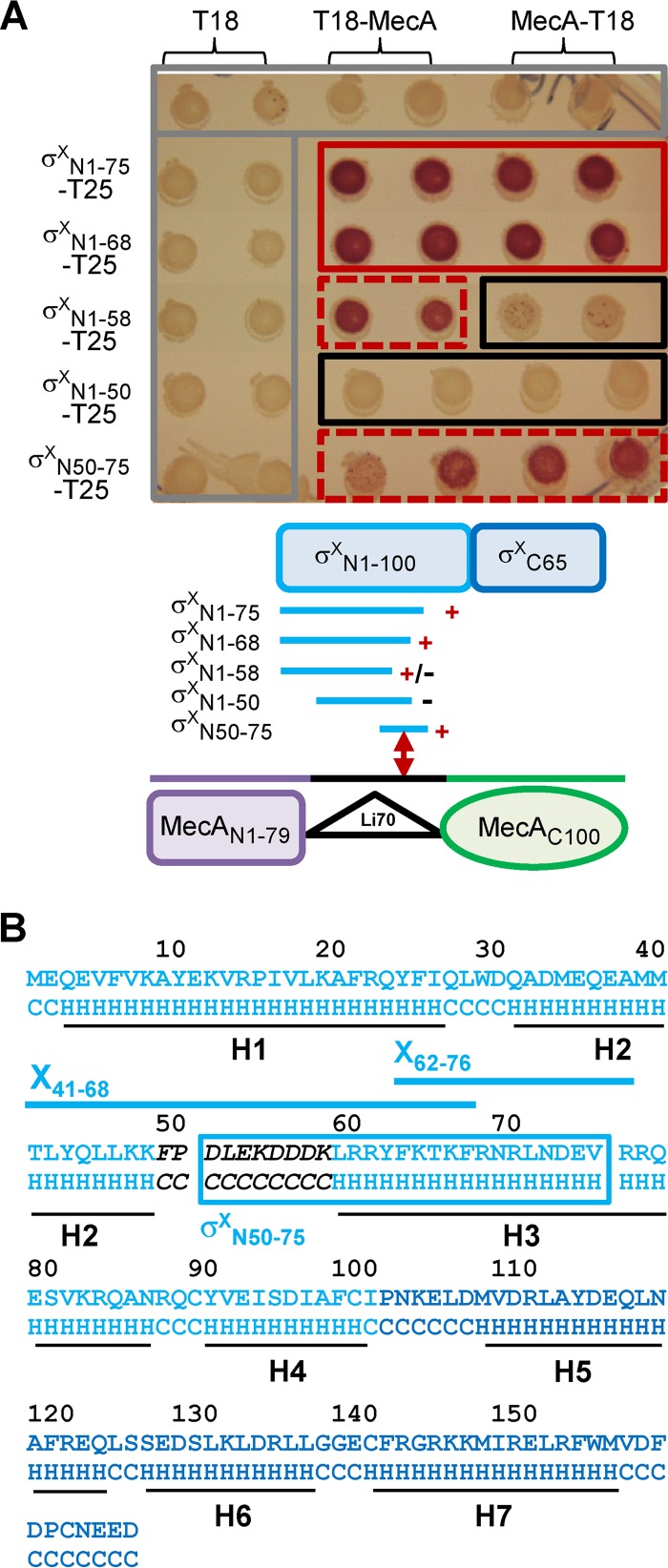
To further delimit a region in domain N of σX necessary for MecA interaction, truncated derivatives of the σXN1-100-T25 fusion, encompassing either the first 75 (σXN1-75-T25) or 50 (σXN1-50-T25) residues of σX, were tested. Only σXN1-75-T25 still interacted with MecA (both T18-MecA and MecA-T18) (Fig. 3A), indicating that the 25-aa region between positions 50 and 75 of σX is involved in this interaction. Interestingly, fragment σX50-75 was sufficient to complement the adenylate cyclase activity (fusion σXN50-75-T25 in Fig. 3A). This region is predicted to include a surface-exposed loop (F49 to K58) which is located between α-helices H2 and H3 of the N domain (Fig. 3B; see Fig. S2A in the supplemental material) and could therefore constitute a preferential domain of interaction with MecA. However, B2H results indicate that residues located downstream of this predicted loop, i.e., downstream of residue 58, were also involved in σX-MecA interaction, since fusion σXN1-58-T25 was affected in its ability to interact with both T18-MecA fusions, in contrast to σXN1-68-T25, which behaved similarly to the σXN1-75-T25 fusion (Fig. 3A). Altogether, these results suggest that a short region of the domain N of σX, located between residues 50 and 68 and encompassing a putative surface-exposed loop, has a major contribution in the formation of the σX-MecA complex (Fig. 3B).
FIG 3.
Interactions between MecA and truncated N-domain variants of σX, evaluated by B2H assays. (A) Top, matrices of B2H interactions between full-length MecA and truncated N-domain variants of σX on MacConkey plates. The color code for rectangles is the same as in Fig. 2. Bottom, organization of MecA and σX domains and summary of interactions. +, +/−, and − indicate positive, weak, and negative interactions, respectively. (B) σX sequence and predicted secondary structure (LOMETS server [http://zhanglab.ccmb.med.umich.edu/LOMETS/]) with α-helixes H1 to H7 (black lines). The predicted secondary structure is indicated below the protein sequence. H, α-helix; C, coil. Light and dark blue sequences correspond to the N and C domains of σX, respectively. X41-68 and X62-76 peptides and their position are mapped with blue lines. σXN50-75, which interacts with MecA in B2H assays, is indicated by a blue box. The sequence of the predicted surface-exposed loop is in black italic.
σX is degraded in vitro by the MecA-ClpCP complex.
Previous studies have shown that MecA of B. subtilis can either sequester a regulatory protein (i.e., Spo0A) (39) or address it to the ClpCP proteolytic machinery (i.e., ComK, ComS) (9, 10). From our previous in vivo results, we have proposed that MecA not only sequesters σX but is responsible for its degradation by ClpCP (31). To test this hypothesis, an in vitro degradation assay was set up.
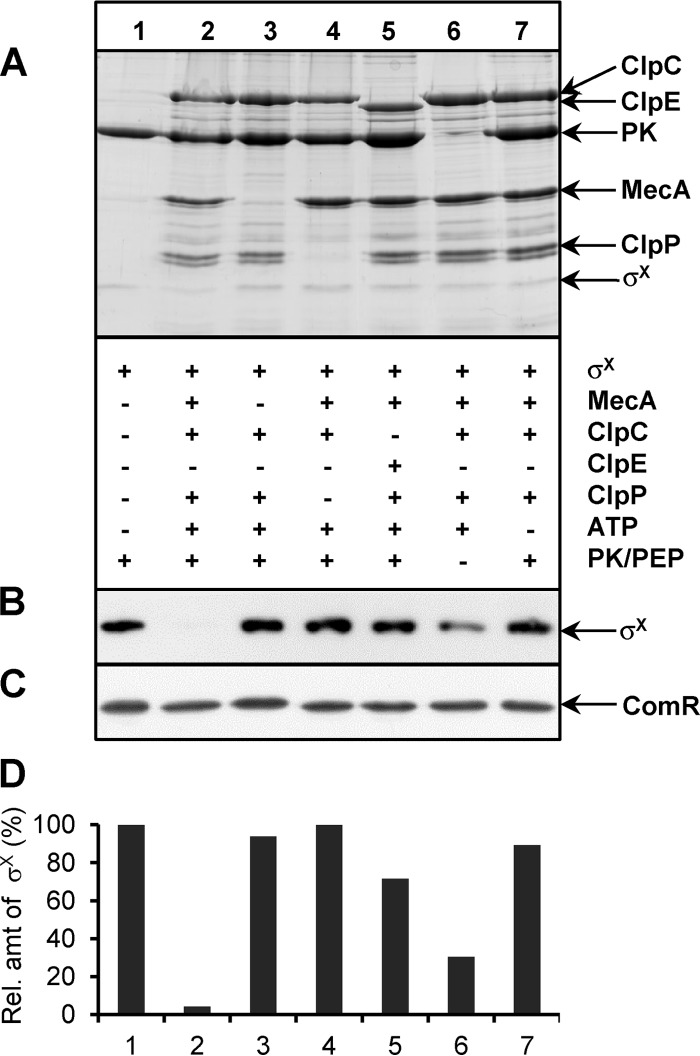
Purified samples of σX fused to a C-terminal StrepII tag (σX-Strep) were incubated for 30 min at 37°C in absence or presence of equimolar preparations of purified 6His-MecA, -ClpC, and/or -ClpP. The assay was started by adding ATP and/or phosphoenolpyruvate (PEP)/PK in order to ensure energy regeneration in the reaction mixture (the activity of Clp proteases is ATP dependent) (Fig. 4A). At the end of the assay, Western blotting using a monoclonal anti-StrepTagII antibody was performed (Fig. 4B), and relative amounts of σX-Strep were determined by densitometry (using lane 1, i.e., σX alone in the reaction, as the 100% value) (Fig. 4D). The results indicate that σX-Strep degradation is strictly dependent on 6His-MecA, -ClpC, and -ClpP and ATP, with a higher degradation observed in the presence of the ATP regeneration system (Fig. 4, compare lanes 2 and 6). Importantly, Clp-dependent degradation is specific to σX-Strep, since ComR-Strep, which was used instead of σX-Strep in parallel reactions, was stable under all conditions tested (Fig. 4C). In addition, 6His-ClpE was found to be ∼ 20-fold less efficient than 6His-ClpC in these in vitro assays (compare conditions 2 and 5), which corroborates the SPR results where weak MecA-ClpE interactions were detected (Fig. 1A). Interestingly, the abundance of 6His-MecA was also found to decline in the presence of ClpCP, although more slowly than that of σX, which may indicate a sequential degradation of σX and MecA by the ClpCP protease complex (Fig. 4A; see Fig. S3 in the supplemental material). These in vitro experiments strengthen a model where σX is degraded in vivo in a MecA- and ATP-dependent manner by the protease complex ClpCP.
FIG 4.
In vitro degradation of σX. (A) Top, SDS-PAGE with Coomassie blue staining of various combinations of σX-Strep, 6His-MecA, 6His-ClpC, 6His-ClpE, 6His-ClpP, ATP, and the pyruvate kinase/phosphoenolpyruvate (PK/PEP) ATP regeneration system. Bottom, presence (+) or absence (−) of a specific compound in the reaction mixture. (B) σX-Strep detection by Western blotting. (C) ComR-Strep detection by Western blotting. ComR-Strep was used as negative control and substitute for σX-Strep in a parallel experiment performed under the same conditions. (D) Quantifications by densitometry of the relative amount of σX-Strep detected by Western blotting, using control lane 1 as 100%. The specific in vitro degradation of σX in the presence of MecA-ClpCP and ATP has been reproduced with 4 independent batches of purified proteins. The presented experiment with all the controls on one gel was performed in duplicates, which showed similar results.
σX degradation is inhibited by competition with a peptide encompassing the surface-exposed region of its N-terminal domain.
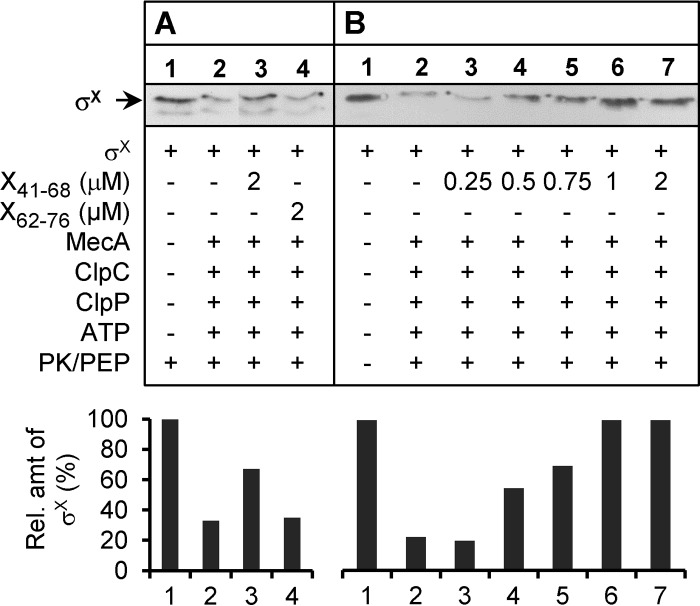
An interesting result of the B2H assays reported above is the identification of a short region (18 aa) encompassing a putative surface-exposed loop in domain N of σX as required for the interaction with MecA (Fig. 3A and B). To evaluate the ability of this region to interfere in vitro with σX degradation, two artificial peptides, X41-68 and X62-76,were synthesized and tested in the in vitro degradation assay (Fig. 3B). Peptide X41-68 was designed such as to cover the surface-exposed loop with adjacent residues of α-helices H2 and H3. In contrast, peptide X62-76 excludes the predicted loop and corresponds to the N-terminal part of α-helix H3 (Fig. 3A). In a first set of experiments, an ∼3-fold molar excess of X41-68 or X62-76 (2 μM) was mixed with equimolar concentrations of the other partners, σX-Strep, 6His-MecA, -ClpC, and -ClpP (0.6 μM), and incubated in the presence of ATP and phosphoenolpyruvate (PEP)/PK (30 min at 37°C). Interestingly, σX-Strep degradation, as deduced from its quantification after Western blotting, was ∼3-fold less efficient in the presence than in the absence of X41-68 (Fig. 5A, compare lanes 2 and 3). In contrast, X62-76 had no inhibitory effect (Fig. 5A, compare lanes 2 and 4). In a second set of experiments, increasing concentrations of X41-68 (from 0 to 2 μM) were mixed with σX-Strep under the same incubation conditions (Fig. 5B). Notably, the amount of remaining σX-Strep was proportional to the concentration of X41-68 (Fig. 5B, compare lanes 2 to 6), with a complete relief of σX-Strep degradation observed at a 2:1 molar ratio of X41-68 and σX-Strep. These data strongly suggests that residues 41 to 68 of σX interact with an essential σX-binding region in MecA.
FIG 5.
Competition of synthetic peptides for in vitro σX degradation. (A) Addition of synthetic peptides X41-68 and X62-76 (2 μM) to the degradation assay mixture. (B) Addition of X41-68 at increasing concentrations (0 to 2 μM) to the degradation assay mixture. In both experiments, σX-Strep (0.6 μM) was incubated with 6His-MecA, -ClpC, and -ClpP, ATP, and the pyruvate kinase/phosphoenolpyruvate (PK/PEP) ATP regeneration system. + or −, presence or absence of a specific compound in the reaction mixture, respectively. σX-Strep was detected by Western blotting. Quantifications by densitometry of the relative amount of σX-Strep using control lane 1 as 100% are shown under the respective Western blots.
σX degradation is a locking mechanism that prevents natural transformation at low ComS concentrations.
In S. thermophilus LMD-9, MecA and/or ClpC depletion activates the expression of late com genes under nonpermissive competence conditions (i.e., complex THBG medium) but not under permissive conditions (i.e., CDM medium) (31). However, we were not able to show any difference in transformation efficiency between MecA- or ClpC-depleted and WT strains (31). Since production of XIP is probably the limiting factor in THB, in contrast to CDM, we hypothesized that intermediate XIP concentrations could be required to observe differences in transformation efficiencies.
We thus compared the effects of low nonsaturating ComS17-24 (synthetic XIP) concentrations (from 0 to 40 nM) on competence development of WT and MecA-depleted strains under THBG growth conditions. Both natural transformation frequencies and expression of the late com gene comGA (PcomGA::luxAB transcriptional fusion) were monitored. The results presented in Fig. 6 show that MecA depletion improved the transformation efficiency at all low ComS17-24 concentrations tested (Fig. 6A) and increased PcomGA activity compared to that of the WT strain (Fig. 6B). However, differences were attenuated as the ComS17-24 concentration increased. Indeed, the ratios of transformation frequencies between the strains were ∼10 to 15-fold from 5 to 20 nM ComS17-24 but were reduced to only ∼1.5-fold at 40 nM (Fig. 6B). In accordance, the ratios between maximal luciferase activities driven by PcomGA also became smaller with increasing ComS17-24 concentrations (from ∼100 to ∼5 between 0 and 40 nM ComS). At saturating ComS17-24 concentrations (1 μM), both strains were similarly competent (data not shown). This strongly suggests that (i) MecA plays a negative-control role in natural transformation at limiting concentrations of ComS17-24 and (ii) above a certain threshold of ComS17-24 concentration in the medium, activation of the ComRS system overcomes the locking mechanism mediated by MecA-ClpCP.
FIG 6.
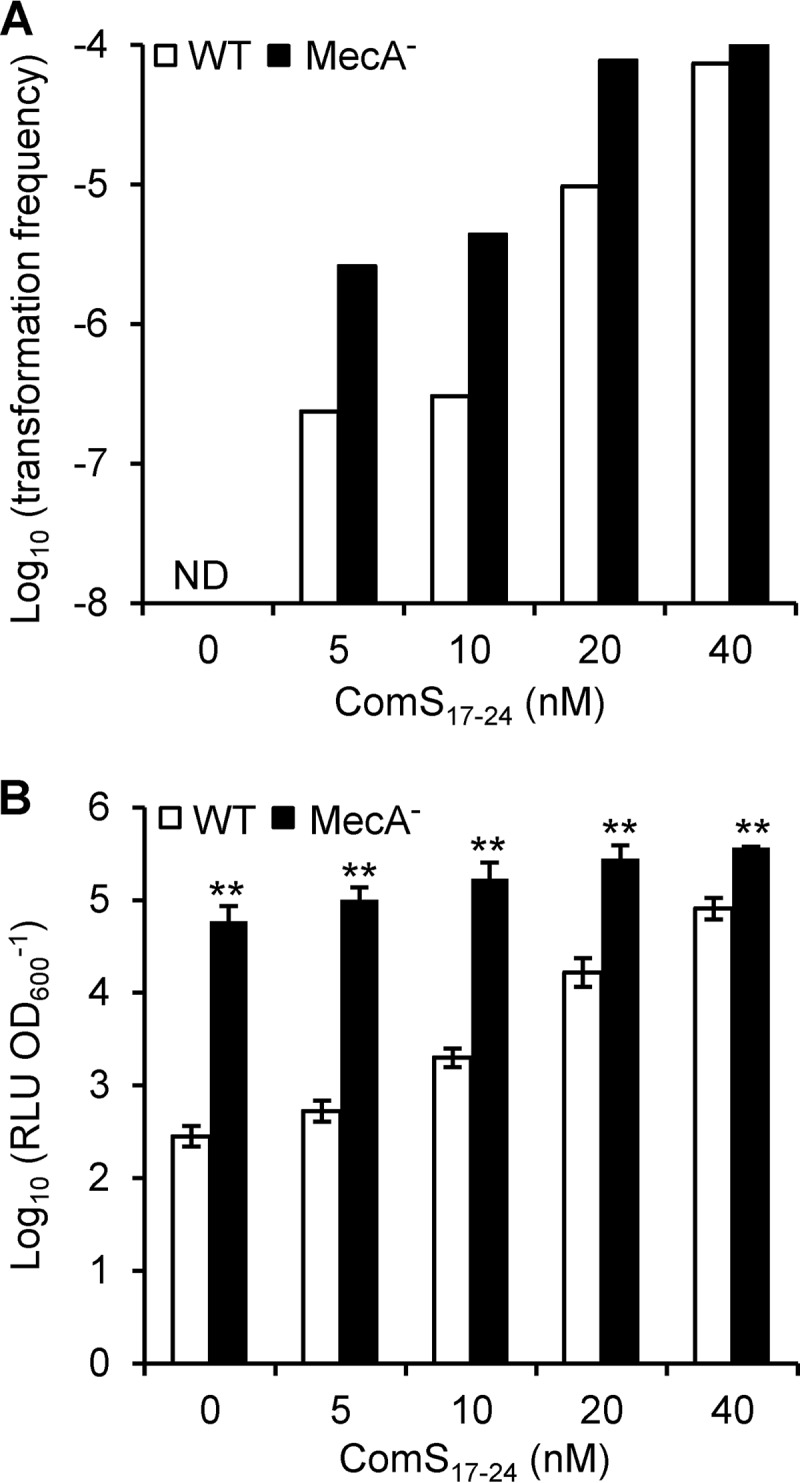
Effect of MecA depletion on DNA transformation frequencies and PcomGA activity. (A) Transformation frequencies (expressed in log10) of strains CB007 (WT, white bars) and CB0072 (MecA−, black bars). Cultures in THBG medium (OD600 = 0.05) were supplemented with ComS17-24 at concentrations ranging from 0 to 40 nM. ND, below the detection limit of 10−8. (B) Maximum specific luciferase activities (RLU OD600−1) of PcomGA-luxAB fusions of strains CB007 (WT, white bars) and CB0072 (MecA−, black bars) at each ComS17-24 concentration reported in panel A. The presented results are from one representative experiment from two independent experiments showing similar results. Each experiment was performed with triplicate cultures of each strain. The transformation frequency was calculated from one randomly selected culture. Specific luciferase activities are expressed as geometric means ± standard deviations from triplicate cultures (expressed in log10). Significant difference between WT and MecA− is based on Student's t test performed on log10-transformed data; **, P < 0.01.
DISCUSSION
The importance of the MecA-ClpCP complex in the negative posttranslational control of the central competence regulator σX has recently been highlighted in two ComRS-containing streptococci, S. thermophilus (30, 31) and S. mutans (32). Based on genetic evidence and B2H assays, it was proposed that the adaptor protein MecA interacts with both σX and ClpC (31, 32). By analogy with the posttranslational control of ComK by the MecA-ClpCP complex in B. subtilis, it was hypothesized that MecA directly targets σX for degradation by the protease complex ClpCP (31, 32). In this work, we strengthened this model through complementary in vitro experiments. First, MecA-σX and MecA-ClpC interactions were confirmed by SPR experiments with purified proteins (Fig. 1). Interestingly, the KD values for MecASth-σX and MecABsu-ComK complexes extrapolated from SPR experiments are in the same range (∼1 μM), suggesting that the adaptor protein MecA interacts with similar strengths with both regulators. Second, we were able to show that the in vitro degradation of σX is fully dependent on the presence of MecA and requires ATP (Fig. 4). In addition, MecASth is also degraded by ClpCP, as reported for MecA from B. subtilis (see Fig. S3 in the supplemental material) (11, 40). Intriguingly, the ClpESth protein was able to partially substitute for ClpCSth in the in vitro assays (Fig. 4), while we previously reported an absence of interaction between MecA and ClpE using B2H assays and no σX accumulation in a ClpE-deficient strain (31). Interestingly, independent results obtained with another S. thermophilus strain (LMG18311) grown under different conditions also suggested that ClpE could participate in the posttranslational control of σX, but with a lower contribution than ClpC (30). Thus, it remains unclear if ClpE could be a partner of MecA for σX degradation in vivo.
During this work, we also explored the MecA-σX and MecA-ClpC complexes in order to identify subdomain interactions. We found that domain C of MecASth was responsible for the MecA-ClpC interaction (Fig. 2A). This is fully consistent with data obtained for B. subtilis, where two regions of domain C of MecABsu, around E184 and E198, were shown to be important for interaction with ClpCBsu (41, 42). Interestingly, these two glutamate residues are conserved in all MecA proteins from streptococci (see Fig. S1 in the supplemental material and data not shown). This suggests that evolution has maintained a similar MecA-ClpC interaction to selectively address specific target proteins to degradation by the serine protease ClpP. More interesting is the dissection of the MecA-σX interaction, which shows that the interacting domains are different from those between MecA and ComK (Fig. 7). Concerning MecASth, it appears that domain N is not involved, while it is essential for the MecA-ComK interaction (8). Conversely, the C-terminal region (last 121 aa) encompassing domain C and part of the interdomain linker seems to interact with σX. In B. subtilis, MecA was shown to be flexible in terms of interactions with different partners. For instance, it could sequester the Spo0A regulator of B. subtilis based on interactions with both domains N and C while keeping its ability to bind ComK (39). Concerning σX, B2H and competitive degradation assays point toward a short region (18 aa) encompassing a predicted surface-exposed loop (F49 to K58) of its domain N as playing a preponderant role in its interaction with MecA (Fig. 3 and 5). This contrasts with the major interacting region of ComK, which consists of a hexameric peptide localized close to the C terminus (9). The remarkable fact from all these results is that MecA has maintained an adaptor role between bacilli and ComRS-containing streptococci to address the central competence regulator to ClpCP degradation by interacting differently with proteins that do not display any primary sequence identity but perform similar functions.
FIG 7.
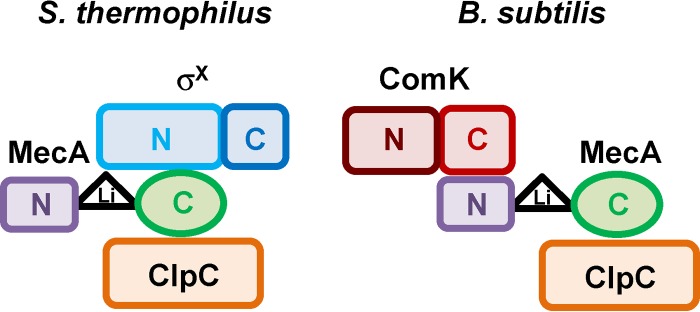
Model of subdomain interactions in the putative σX-MecA-ClpC ternary complex of S. thermophilus (left) compared to the B. subtilis ComK-MecA-ClpC complex (right). N, C, and Li correspond to domain N, domain C, and the linker region, respectively.
An important question is the role of MecA in posttranslational control of competence development in other streptococci, containing either the ComRS or the ComCDE signaling system. Notably, the MecA protein is ubiquitous among streptococci and could be found in the complete genomes of all species sequenced so far (data not shown). Concerning ComRS-containing streptococci, the role of the MecA-ClpCP complex could easily be extrapolated to S. mutans based on previous results (32) and eventually to S. pyogenes based on the central role played by ClpP in σX degradation and the absence of a ComW protein (25, 29). Concerning ComCDE-containing streptococci, with S. pneumoniae as a model species, the situation is less clear, since σX is preferentially degraded by ClpEP and the ComW protein, which stabilized and activated σX, is degraded by ClpCP (17, 18). The possibility that MecA from S. pneumoniae could interact with its cognate σX protein was quickly evaluated by B2H assays. In addition, we also included cognate pairs of S. thermophilus and S. mutans to check cross-interactions between MecA and σX (see Fig. S4 in the supplemental material). From these assays, it appears that the three MecA proteins are flexible to recognize σX from S. thermophilus and S. mutans but do not or very weakly recognize σX from S. pneumoniae (see Fig. S4 in the supplemental material). This may indicate that σX from S. pneumoniae has diverged during evolution in such a way that it lacks recognition by MecA. Interestingly, the predicted loop proposed to be a key determinant in the MecA-σX interaction in S. thermophilus is poorly conserved in S. pneumoniae. While S. thermophilus and S. mutants have 7 out of 10 similar residues, S. pneumoniae shares only 3 similar residues with the loop of S. thermophilus (see Fig. S2B in the supplemental material). Corroborating these B2H assays, the inactivation of MecA in S. pneumoniae has no major impact on the in vivo degradation of σX but dramatically stabilizes ComW (43). This makes sense since ClpCP degrades ComW and ClpC has been considered until now a required partner of MecA (7, 42). Notably, this shows again a high flexibility of the adaptor protein MecA to potentially recognize an important player in competence activation that displays no sequence similarity with either σX or ComK. However, the role of this specific degradation of ComW by MecA-ClpCP in the posttranslational control of competence in S. pneumoniae remains to be investigated.
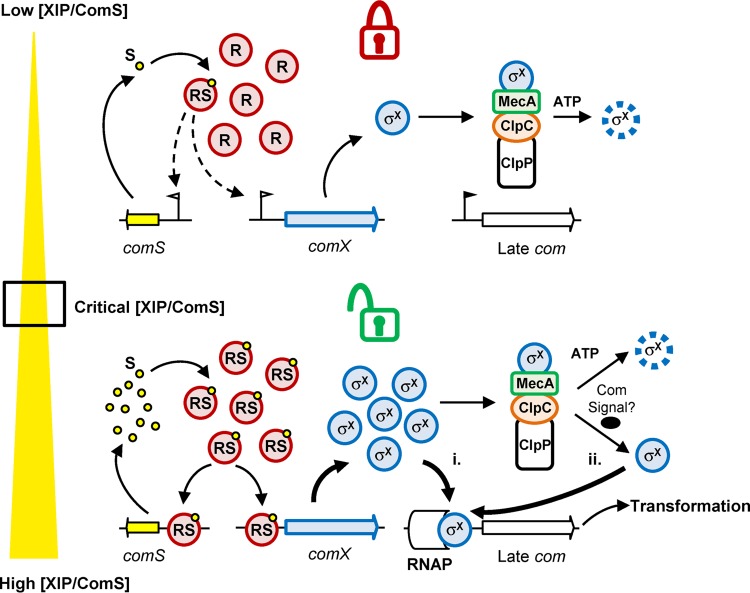
The next essential question is the importance of the posttranslational control of σX for competence development in ComRS-containing streptococci. In B. subtilis, the posttranslational control of ComK exerted by the MecA-ClpCP machinery is of key importance, since the release of a small amount of ComK dramatically affects its abundance by activating the autoamplification loop (5). The inactivation of MecA in this species resulted in DNA transformation which became independent of growth conditions (44). In ComRS-containing streptococci, previous results showed that the inactivation of one or more members of the MecA-ClpCP complex has an impact on the activation of the late competence phase but not on DNA transformation efficiency (25, 29, 31, 32), except for the inactivation of ClpC in S. thermophilus LMG18311, where a moderate improvement of DNA transformation was observed in a very specific growth medium (30). In addition, the competence state in MecA- and ClpC-deficient strains of S. mutans was recently shown to be prolonged in rich medium supplemented with XIP, suggesting a role of the posttranslational control in competence shutoff (32). However, our investigations showed that the kinetics of the activation of late genes and DNA transformation in S. thermophilus is unaffected by MecA inactivation under similar growth conditions, indicating that this role could not be extended to S. thermophilus (reference 31 and data not shown). By using subinducing concentrations of XIP, we show here that the MecA-ClpCP degradation machinery not only restricts the transcriptional activation of the late phase but also abolishes DNA transformation under unfavorable conditions in S. thermophilus (Fig. 6). Conversely to the case for ComK of B. subtilis, σX production is not autoregulated in streptococci, and the key control checkpoint of competence development is the signaling system where the positive feedback loop takes place (1). Thus, the MecA-ClpCP degradation machinery acts downstream of ComRS regulation in S. thermophilus as a complementary locking device to tightly control competence activation when XIP availability is too low. Moreover, in the absence of the degradation machinery, activation of late genes seems much less responsive to increasing XIP concentrations than the classical nonlinear dose response observed in the wild type (Fig. 6B). This suggests that σX degradation by MecA-ClpCP participates in the dynamics of the response performed by the ComRS system on the activation of the late phase. A model of the interplay between ComRS and MecA-ClpCP to control competence in S. thermophilus is proposed in Fig. 8. Below a critical XIP concentration, σX that is present at a basal level would be degraded by MecA-ClpCP, and the cell remains in a noncompetent state. When a critical concentration of XIP is reached, the positive feedback loop is activated, resulting in XIP autoproduction, which in turn leads to an increase of σX and possibly a not-yet-identified interfering early gene product. Two nonexclusive scenarios then could be envisaged: (i) the increase of σX alone is sufficient to overcome the MecA-ClpCP degradation machinery, which would be the limiting factor, and free σX could then induce competence, and/or (ii) an interfering early gene product that would act as anti-anti-sigma factor might release σX from the complex, similarly to ComS of B. subtilis, which compete with ComK for MecA binding (9, 10). Future work will aim to investigate these two scenarios in order to unravel the unlocking mechanism.
FIG 8.
Model of the interplay between the ComRS and MecA-ClpCP systems for competence regulation in S. thermophilus. (Top) Locked competence. At very low XIP/ComS (S) concentrations, free ComR (R) or a small amount of ComRS (RS) complexes is present, which results in a low level of σX production. Under these conditions, adaptor protein MecA represses the onset of competence by selecting σX for degradation by the protease complex MecA-ClpCP. (Bottom) Unlocked competence. Above a critical XIP/ComS concentration, ComRS complexes are formed in large amounts, which results in a high production of σX. Either the high and abrupt σX production saturates the proteolytic machinery (i) or a so-far-unidentified competence signal (Com signal) releases σX from the MecA-ClpCP degradation complex (ii). In both scenarios, free σX molecules interact as an alternative sigma factor with the RNA polymerase (RNAP) and induce late com genes and DNA transformation. Degradation of σX is indicated by a dashed line.
Supplementary Material
ACKNOWLEDGMENTS
We thank D. Morrison for sharing unpublished results and critically reading the manuscript, Y.-H. Li for fruitful discussions on MecA-σX cross-interactions, and the Institut de Médecine Prédictive et de Recherche Thérapeutique (IFR 114, Lille, France) for its technical support with SPR experiments.
This work was supported by the Interuniversity Attraction Poles Programme initiated by the Belgian Science Policy Office and FNRS. A.W., L.F., and P.H. are a postdoctoral fellow, a postdoctoral researcher, and a senior research associate, respectively, at FNRS.
Footnotes
Published ahead of print 16 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01758-14.
REFERENCES
- 1.Johnston C, Martin B, Fichant G, Polard P, Claverys JP. 2014. Bacterial transformation: distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 12:181–196. 10.1038/nrmicro3199 [DOI] [PubMed] [Google Scholar]
- 2.Magnuson R, Solomon J, Grossman AD. 1994. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell 77:207–216. 10.1016/0092-8674(94)90313-1 [DOI] [PubMed] [Google Scholar]
- 3.D'Souza C, Nakano MM, Zuber P. 1994. Identification of comS, a gene of the srfA operon that regulates the establishment of genetic competence in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 91:9397–9401. 10.1073/pnas.91.20.9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamoen LW, Eshuis H, Jongbloed J, Venema G, SDvan 1995. A small gene, designated comS, located within the coding region of the fourth amino acid-activation domain of srfA, is required for competence development in Bacillus subtilis. Mol. Microbiol. 15:55–63. 10.1111/j.1365-2958.1995.tb02220.x [DOI] [PubMed] [Google Scholar]
- 5.van Sinderen D, Venema G. 1994. comK acts as an autoregulatory control switch in the signal transduction route to competence in Bacillus subtilis. J. Bacteriol. 176:5762–5770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamoen LW, Venema G, Kuipers OP. 2003. Controlling competence in Bacillus subtilis: shared use of regulators. Microbiology 149:9–17. 10.1099/mic.0.26003-0 [DOI] [PubMed] [Google Scholar]
- 7.Kirstein J, Schlothauer T, Dougan DA, Lilie H, Tischendorf G, Mogk A, Bukau B, Turgay K. 2006. Adaptor protein controlled oligomerization activates the AAA+ protein ClpC. EMBO J. 25:1481–1491. 10.1038/sj.emboj.7601042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persuh M, Turgay K, Mandic-Mulec I, Dubnau D. 1999. The N- and C-terminal domains of MecA recognize different partners in the competence molecular switch. Mol. Microbiol. 33:886–894. 10.1046/j.1365-2958.1999.01544.x [DOI] [PubMed] [Google Scholar]
- 9.Prepiak P, Dubnau D. 2007. A peptide signal for adapter protein-mediated degradation by the AAA+ protease ClpCP. Mol. Cell 26:639–647. 10.1016/j.molcel.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turgay K, Hamoen LW, Venema G, Dubnau D. 1997. Biochemical characterization of a molecular switch involving the heat shock protein ClpC, which controls the activity of ComK, the competence transcription factor of Bacillus subtilis. Genes Dev. 11:119–128. 10.1101/gad.11.1.119 [DOI] [PubMed] [Google Scholar]
- 11.Turgay K, Hahn J, Burghoorn J, Dubnau D. 1998. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 17:6730–6738. 10.1093/emboj/17.22.6730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogura M, Liu L, Lacelle M, Nakano MM, Zuber P. 1999. Mutational analysis of ComS: evidence for the interaction of ComS and MecA in the regulation of competence development in Bacillus subtilis. Mol. Microbiol. 32:799–812. 10.1046/j.1365-2958.1999.01399.x [DOI] [PubMed] [Google Scholar]
- 13.Havarstein LS. 2010. Increasing competence in the genus Streptococcus. Mol. Microbiol. 78:541–544. 10.1111/j.1365-2958.2010.07380.x [DOI] [PubMed] [Google Scholar]
- 14.Johnsborg O, Eldholm V, Havarstein LS. 2007. Natural genetic transformation: prevalence, mechanisms and function. Res. Microbiol. 158:767–778. 10.1016/j.resmic.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 15.Martin B, Soulet AL, Mirouze N, Prudhomme M, Mortier-Barriere I, Granadel C, Noirot-Gros MF, Noirot P, Polard P, Claverys JP. 2013. ComE/ComE∼P interplay dictates activation or extinction status of pneumococcal X-state (competence). Mol. Microbiol. 87:394–411. 10.1111/mmi.12104 [DOI] [PubMed] [Google Scholar]
- 16.Luo P, Li H, Morrison DA. 2004. Identification of ComW as a new component in the regulation of genetic transformation in Streptococcus pneumoniae. Mol. Microbiol. 54:172–183. 10.1111/j.1365-2958.2004.04254.x [DOI] [PubMed] [Google Scholar]
- 17.Sung CK, Morrison DA. 2005. Two distinct functions of ComW in stabilization and activation of the alternative sigma factor ComX in Streptococcus pneumoniae. J. Bacteriol. 187:3052–3061. 10.1128/JB.187.9.3052-3061.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piotrowski A, Luo P, Morrison DA. 2009. Competence for genetic transformation in Streptococcus pneumoniae: termination of activity of the alternative sigma factor ComX is independent of proteolysis of ComX and ComW. J. Bacteriol. 191:3359–3366. 10.1128/JB.01750-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontaine L, Boutry C, de Frahan MH, Delplace B, Fremaux C, Horvath P, Boyaval P, Hols P. 2010. A novel pheromone quorum-sensing system controls the development of natural competence in Streptococcus thermophilus and Streptococcus salivarius. J. Bacteriol. 192:1444–1454. 10.1128/JB.01251-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontaine L, Goffin P, Dubout H, Delplace B, Baulard A, Lecat-Guillet N, Chambellon E, Gardan R, Hols P. 2013. Mechanism of competence activation by the ComRS signalling system in streptococci. Mol. Microbiol. 87:1113–1132. 10.1111/mmi.12157 [DOI] [PubMed] [Google Scholar]
- 21.Gardan R, Besset C, Gitton C, Guillot A, Fontaine L, Hols P, Monnet V. 2013. Extracellular life cycle of ComS, the competence-stimulating peptide of Streptococcus thermophilus. J. Bacteriol. 195:1845–1855. 10.1128/JB.02196-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai K, Mashburn-Warren L, Federle MJ, Morrison DA. 2012. Development of competence for genetic transformation of Streptococcus mutans in a chemically defined medium. J. Bacteriol. 194:3774–3780. 10.1128/JB.00337-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan R, Rukke HV, Ricomini Filho AP, Fimland G, Arntzen MO, Thiede B, Petersen FC. 2012. Extracellular identification of a processed type II ComR/ComS pheromone of Streptococcus mutans. J. Bacteriol. 194:3781–3788. 10.1128/JB.00624-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mashburn-Warren L, Morrison DA, Federle MJ. 2010. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol. Microbiol. 78:589–606. 10.1111/j.1365-2958.2010.07361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mashburn-Warren L, Morrison DA, Federle MJ. 2012. The cryptic competence pathway in Streptococcus pyogenes is controlled by a peptide pheromone. J. Bacteriol. 194:4589–4600. 10.1128/JB.00830-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison DA, Guedon E, Renault P. 2013. Competence for natural genetic transformation in the Streptococcus bovis group streptococci S. infantarius and S. macedonicus. J. Bacteriol. 195:2612–2620. 10.1128/JB.00230-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardan R, Besset C, Guillot A, Gitton C, Monnet V. 2009. The oligopeptide transport system is essential for the development of natural competence in Streptococcus thermophilus strain LMD-9. J. Bacteriol. 191:4647–4655. 10.1128/JB.00257-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Q, Ahn SJ, Kaspar J, Zhou X, Burne RA. 2014. Growth phase and pH influence peptide signaling for competence development in Streptococcus mutans. J. Bacteriol. 196:227–236. 10.1128/JB.00995-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opdyke JA, Scott JR, Moran CP., Jr 2003. Expression of the secondary sigma factor sigmaX in Streptococcus pyogenes is restricted at two levels. J. Bacteriol. 185:4291–4297. 10.1128/JB.185.15.4291-4297.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biornstad TJ, Havarstein LS. 2011. ClpC acts as a negative regulator of competence in Streptococcus thermophilus. Microbiology 157:1676–1684. 10.1099/mic.0.046425-0 [DOI] [PubMed] [Google Scholar]
- 31.Boutry C, Wahl A, Delplace B, Clippe A, Fontaine L, Hols P. 2012. Adaptor protein MecA is a negative regulator of the expression of late competence genes in Streptococcus thermophilus. J. Bacteriol. 194:1777–1788. 10.1128/JB.06800-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian XL, Dong G, Liu T, Gomez ZA, Wahl A, Hols P, Li YH. 2013. MecA protein acts as a negative regulator of genetic competence in Streptococcus mutans. J. Bacteriol. 195:5196–5206. 10.1128/JB.00821-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 34.Dower WJ, Miller JF, Ragsdale CW. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127–6145. 10.1093/nar/16.13.6127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karimova G, Ullmann A, Ladant D. 2001. Protein-protein interaction between Bacillus stearothermophilus tyrosyl-tRNA synthetase subdomains revealed by a bacterial two-hybrid system. J. Mol. Microbiol. Biotechnol. 3:73–82 [PubMed] [Google Scholar]
- 36.Lambin M, Nicolas E, Oger CA, Nguyen N, Prozzi D, Hallet B. 2012. Separate structural and functional domains of Tn4430 transposase contribute to target immunity. Mol. Microbiol. 83:805–820. 10.1111/j.1365-2958.2012.07967.x [DOI] [PubMed] [Google Scholar]
- 37.Luo P, Morrison DA. 2003. Transient association of an alternative sigma factor, ComX, with RNA polymerase during the period of competence for genetic transformation in Streptococcus pneumoniae. J. Bacteriol. 185:349–358. 10.1128/JB.185.1.349-358.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rich RL, Myszka DG. 2009. Extracting affinity constants from biosensors binding responses, p 48–84 In Cooper MA. (ed), Label-free biosensors: techniques and applications. Cambridge University Press, New York, NY [Google Scholar]
- 39.Prepiak P, Defrancesco M, Spadavecchia S, Mirouze N, Albano M, Persuh M, Fujita M, Dubnau D. 2011. MecA dampens transitions to spore, biofilm exopolysaccharide and competence expression by two different mechanisms. Mol. Microbiol. 80:1014–1030. 10.1111/j.1365-2958.2011.07627.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mei Z, Wang F, Qi Y, Zhou Z, Hu Q, Li H, Wu J, Shi Y. 2009. Molecular determinants of MecA as a degradation tag for the ClpCP protease. J. Biol. Chem. 284:34366–34375. 10.1074/jbc.M109.053017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, Mei Z, Qi Y, Yan C, Xiang S, Zhou Z, Hu Q, Wang J, Shi Y. 2009. Crystal structure of the MecA degradation tag. J. Biol. Chem. 284:34376–34381. 10.1074/jbc.M109.053033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F, Mei Z, Qi Y, Yan C, Hu Q, Wang J, Shi Y. 2011. Structure and mechanism of the hexameric MecA-ClpC molecular machine. Nature 471:331–335. 10.1038/nature09780 [DOI] [PubMed] [Google Scholar]
- 43.Ahlawat S. 2010. PhD thesis. University of Illinois at Chicago, Chicago, IL [Google Scholar]
- 44.Dubnau D, Roggiani M. 1990. Growth medium-independent genetic competence mutants of Bacillus subtilis. J. Bacteriol. 172:4048–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.