Abstract
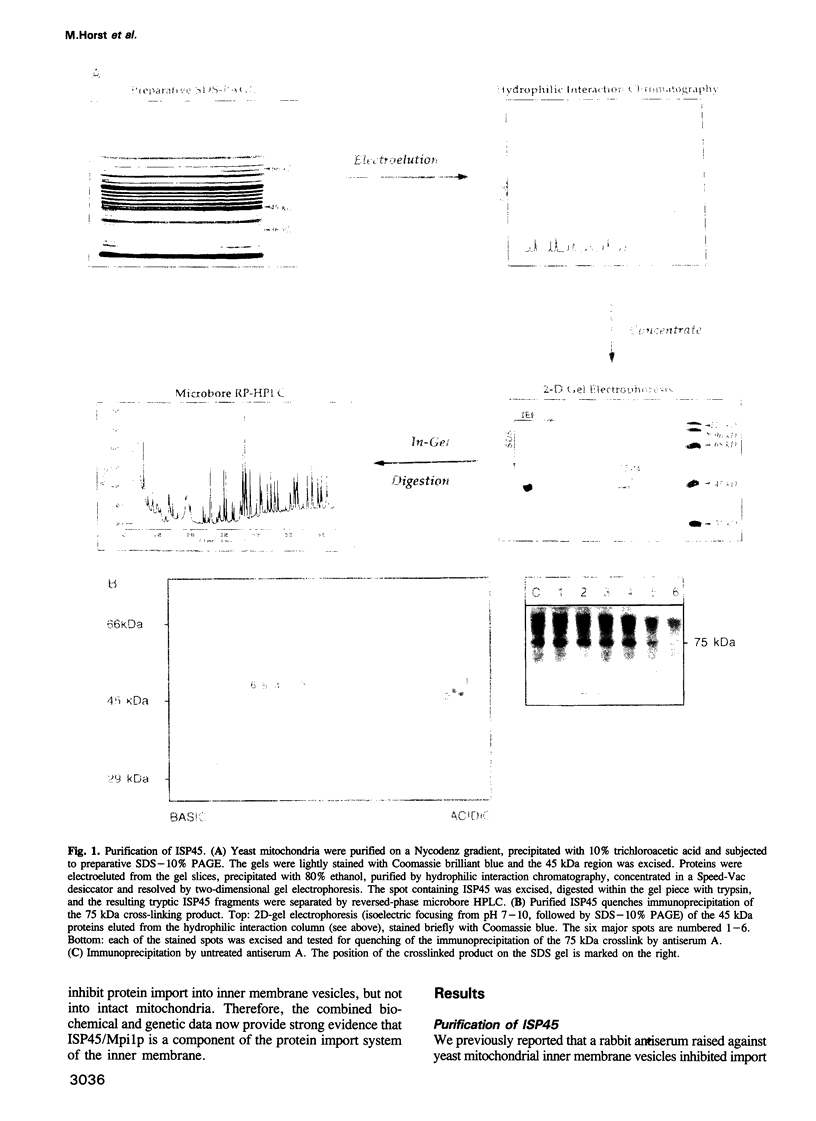
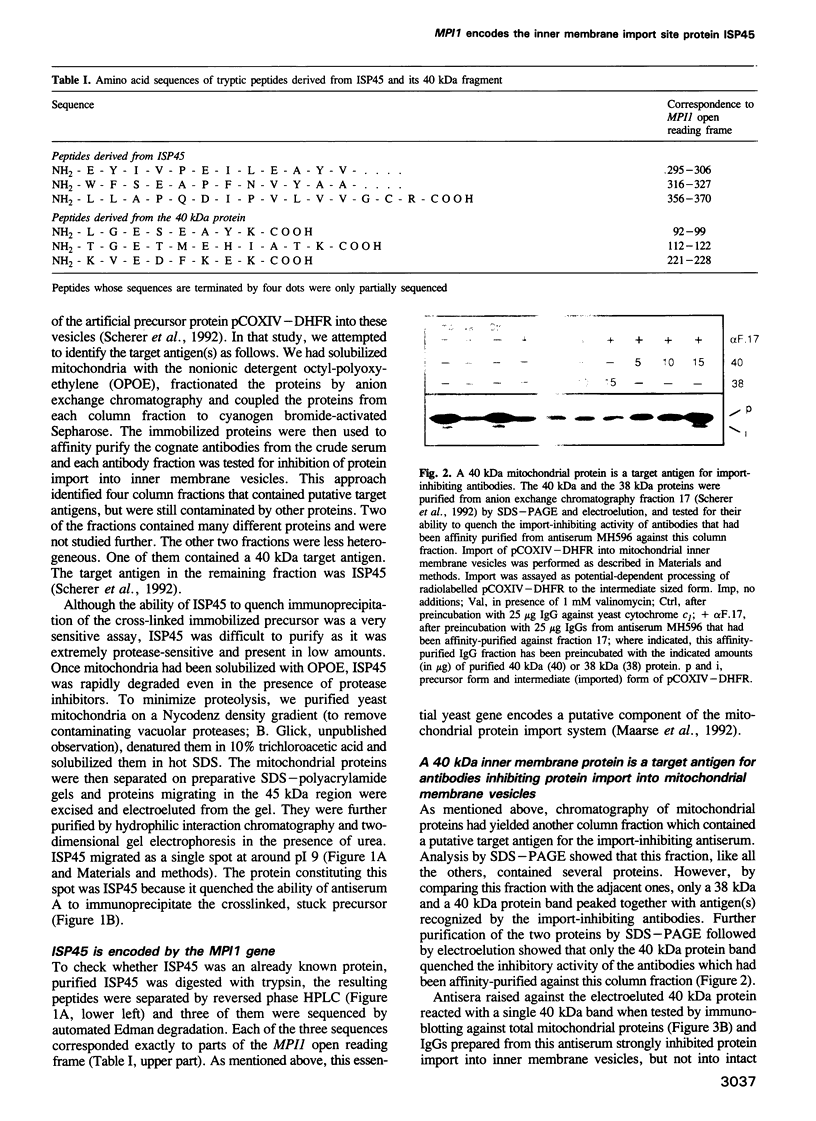
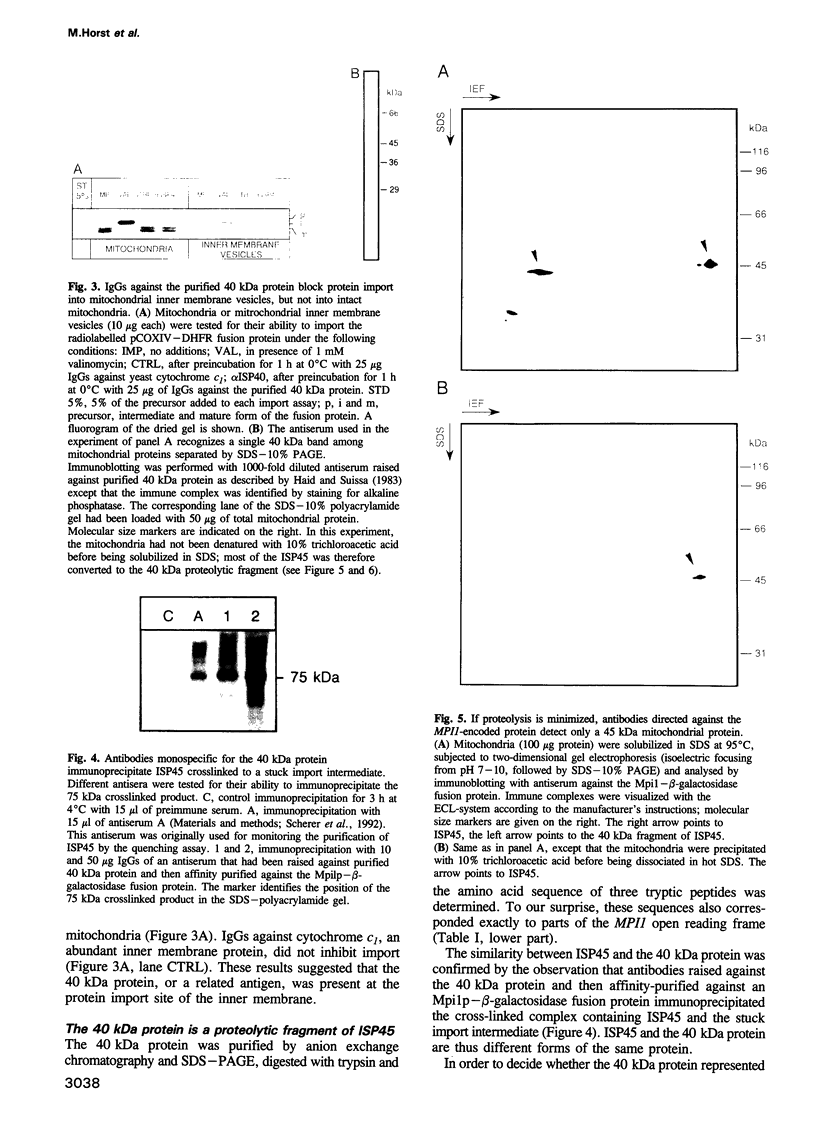
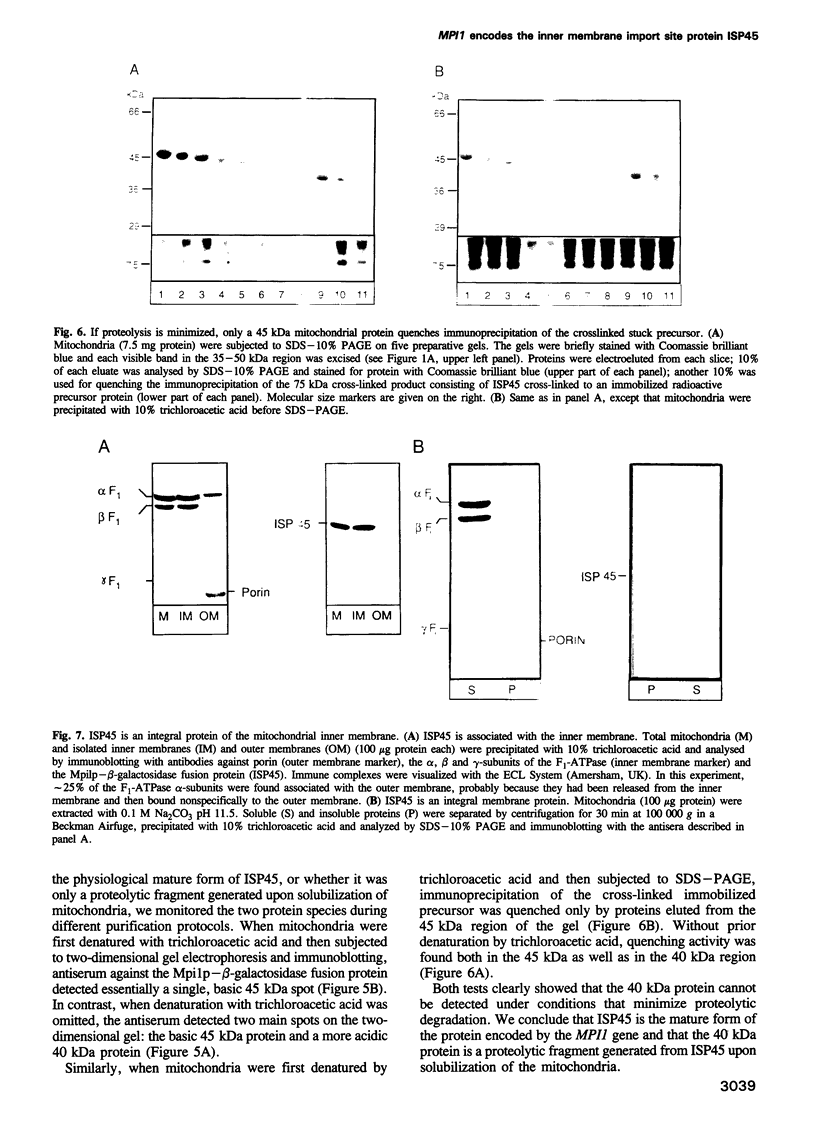
Protein import across both mitochondrial membranes is mediated by the cooperation of two distinct protein transport systems, one in the outer and the other in the inner membrane. Previously we described a 45 kDa yeast mitochondrial inner membrane protein (ISP45) that can be cross-linked to a partially translocated precursor protein (Scherer et al., 1992). We have now purified ISP45 to homogeneity and identified it as the product of the nuclear MPI1 gene. Identity of ISP45 with the MPI1 gene product was shown by microsequencing of three tryptic ISP45 peptides and by demonstrating that an antibody against an Mpi1p-beta-galactosidase fusion protein specifically recognizes ISP45. Antibodies monospecific for ISP45 inhibited protein import into right-side-out mitochondrial inner membrane vesicles, but not into intact mitochondria. On solubilizing mitochondria, ISP45 was rapidly converted to a 40 kDa proteolytic fragment unless mitochondria were first denatured with trichloroacetic acid. The combined genetic and biochemical evidence identifies ISP45/Mpi1p as a component of the protein import system of the yeast mitochondrial inner membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert A. J. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J Chromatogr. 1990 Jan 19;499:177–196. doi: 10.1016/s0021-9673(00)96972-3. [DOI] [PubMed] [Google Scholar]
- Baker K. P., Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991 Jan 17;349(6306):205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- Bressan G. M., Stanley K. K. pUEX, a bacterial expression vector related to pEX with universal host specificity. Nucleic Acids Res. 1987 Dec 10;15(23):10056–10056. doi: 10.1093/nar/15.23.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Schatz G. Identification of cytochrome c oxidase subunits in nuclear yeast mutants lacking the functional enzyme. J Biol Chem. 1978 Jun 25;253(12):4396–4401. [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B., Wachter C., Schatz G. Protein import into mitochondria: two systems acting in tandem? Trends Cell Biol. 1991 Oct;1(4):99–103. doi: 10.1016/0962-8924(91)90037-a. [DOI] [PubMed] [Google Scholar]
- Haid A., Suissa M. Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Horst M., Kronidou N. G., Schatz G. Protein translocation: through the mitochondrial inner membrane. Curr Biol. 1993 Mar;3(3):175–177. doi: 10.1016/0960-9822(93)90265-p. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The amino-terminal region of an imported mitochondrial precursor polypeptide can direct cytoplasmic dihydrofolate reductase into the mitochondrial matrix. EMBO J. 1984 Dec 20;3(13):3149–3156. doi: 10.1002/j.1460-2075.1984.tb02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. T., Wachter C., Schatz G. Protein import into the yeast mitochondrial matrix. A new translocation intermediate between the two mitochondrial membranes. J Biol Chem. 1991 Nov 5;266(31):21083–21089. [PubMed] [Google Scholar]
- Hwang S., Jascur T., Vestweber D., Pon L., Schatz G. Disrupted yeast mitochondria can import precursor proteins directly through their inner membrane. J Cell Biol. 1989 Aug;109(2):487–493. doi: 10.1083/jcb.109.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jascur T., Goldenberg D. P., Vestweber D., Schatz G. Sequential translocation of an artificial precursor protein across the two mitochondrial membranes. J Biol Chem. 1992 Jul 5;267(19):13636–13641. [PubMed] [Google Scholar]
- Lewin A. S., Hines V., Small G. M. Citrate synthase encoded by the CIT2 gene of Saccharomyces cerevisiae is peroxisomal. Mol Cell Biol. 1990 Apr;10(4):1399–1405. doi: 10.1128/mcb.10.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarse A. C., Blom J., Grivell L. A., Meijer M. MPI1, an essential gene encoding a mitochondrial membrane protein, is possibly involved in protein import into yeast mitochondria. EMBO J. 1992 Oct;11(10):3619–3628. doi: 10.1002/j.1460-2075.1992.tb05446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Krieg U. C., Scherer P. E., Schatz G. Sequential action of mitochondrial chaperones in protein import into the matrix. EMBO J. 1991 Nov;10(11):3273–3280. doi: 10.1002/j.1460-2075.1991.tb04891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ohba M., Schatz G. Disruption of the outer membrane restores protein import to trypsin-treated yeast mitochondria. EMBO J. 1987 Jul;6(7):2117–2122. doi: 10.1002/j.1460-2075.1987.tb02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Rassow J., van der Klei I. J., Neupert W. A dynamic model of the mitochondrial protein import machinery. Cell. 1992 Mar 20;68(6):999–1002. doi: 10.1016/0092-8674(92)90069-o. [DOI] [PubMed] [Google Scholar]
- Pon L., Moll T., Vestweber D., Marshallsay B., Schatz G. Protein import into mitochondria: ATP-dependent protein translocation activity in a submitochondrial fraction enriched in membrane contact sites and specific proteins. J Cell Biol. 1989 Dec;109(6 Pt 1):2603–2616. doi: 10.1083/jcb.109.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. The protein import machinery of mitochondria. Protein Sci. 1993 Feb;2(2):141–146. doi: 10.1002/pro.5560020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer P. E., Manning-Krieg U. C., Jenö P., Schatz G., Horst M. Identification of a 45-kDa protein at the protein import site of the yeast mitochondrial inner membrane. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11930–11934. doi: 10.1073/pnas.89.24.11930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segui-Real B., Kispal G., Lill R., Neupert W. Functional independence of the protein translocation machineries in mitochondrial outer and inner membranes: passage of preproteins through the intermembrane space. EMBO J. 1993 May;12(5):2211–2218. doi: 10.1002/j.1460-2075.1993.tb05869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. A chimeric mitochondrial precursor protein with internal disulfide bridges blocks import of authentic precursors into mitochondria and allows quantitation of import sites. J Cell Biol. 1988 Dec;107(6 Pt 1):2037–2043. doi: 10.1083/jcb.107.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]









