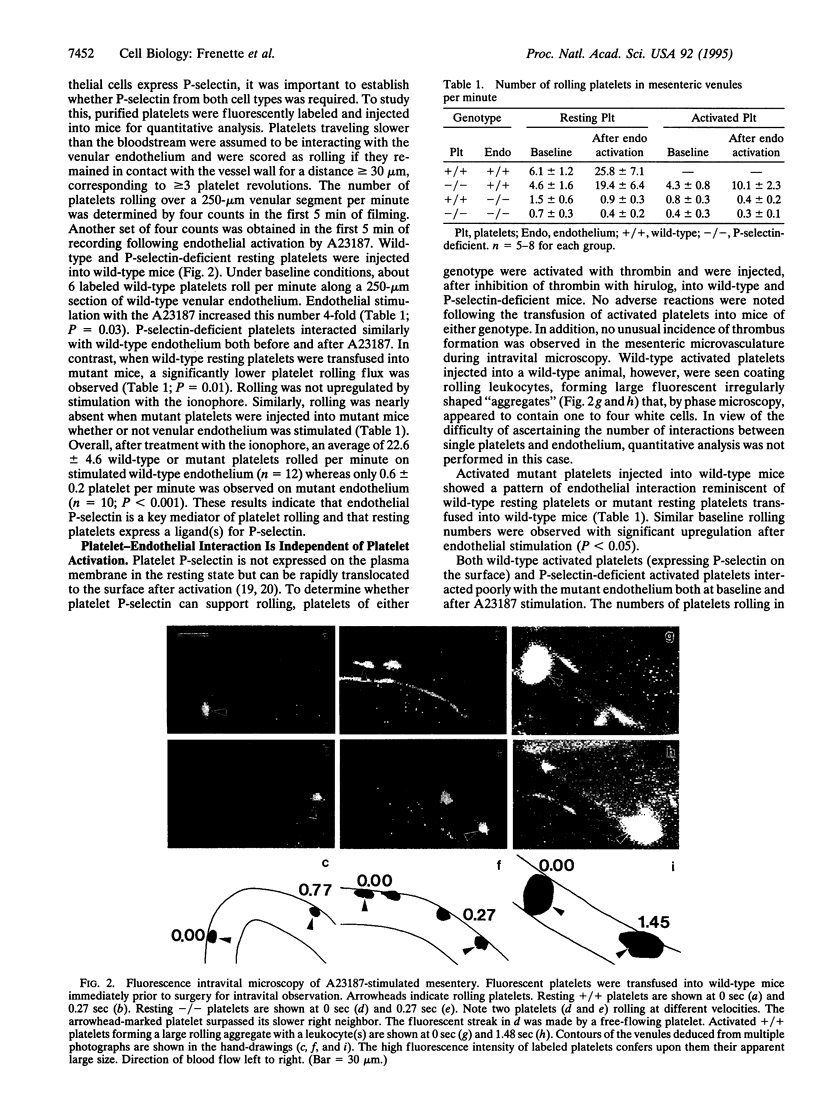
Abstract
P-selectin, found in storage granules of platelets and endothelial cells, can be rapidly expressed upon stimulation. Mice lacking this membrane receptor exhibit a severe impairment of leukocyte rolling. We observed that, in addition to leukocytes, platelets were rolling in mesenteric venules of wild-type mice. To investigate the role of P-selectin in this process, resting or activated platelets from wild-type or P-selectin-deficient mice were fluorescently labeled and transfused into recipients of either genotype. Platelet-endothelial interactions were monitored by intravital microscopy. We observed rolling of either wild-type or P-selectin-deficient resting platelets on wild-type endothelium. Endothelial stimulation with the calcium ionophore A23187 increased the number of platelets rolling 4-fold. Activated P-selectin-deficient platelets behaved similarly, whereas activated wild-type platelets bound to leukocytes and were seen rolling together. Platelets of either genotype, resting or activated, interacted minimally with mutant endothelium even after A23187 treatment. The velocity of platelet rolling was 6- to 9-fold greater than that of leukocytes. Our results demonstrate that (i) platelets roll on endothelium in vivo, (ii) this interaction requires endothelial but not platelet P-selectin, and (iii) platelet rolling appears to be independent of platelet activation, indicating constitutive expression of a P-selectin ligand(s) on platelets. We have therefore observed an interesting parallel between platelets and leukocytes in that both of these blood cell types roll on stimulated vessel wall and that this process is dependent on the expression of endothelial P-selectin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alon R., Rossiter H., Wang X., Springer T. A., Kupper T. S. Distinct cell surface ligands mediate T lymphocyte attachment and rolling on P and E selectin under physiological flow. J Cell Biol. 1994 Dec;127(5):1485–1495. doi: 10.1083/jcb.127.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton A., Born G. V. Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol. 1972 Apr;222(2):447–474. doi: 10.1113/jphysiol.1972.sp009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman C. L., Yeo E. L., Wencel-Drake J. D., Furie B. C., Ginsberg M. H., Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest. 1986 Jul;78(1):130–137. doi: 10.1172/JCI112542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti R., Furie B. C., Furie B., Wagner D. D. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989 Apr;73(5):1109–1112. [PubMed] [Google Scholar]
- Bout D., Joseph M., Pontet M., Vorng H., Deslée D., Capron A. Rat resistance to schistosomiasis: platelet-mediated cytotoxicity induced by C-reactive protein. Science. 1986 Jan 10;231(4734):153–156. doi: 10.1126/science.3079916. [DOI] [PubMed] [Google Scholar]
- Burgers J. A., Schweizer R. C., Koenderman L., Bruijnzeel P. L., Akkerman J. W. Human platelets secrete chemotactic activity for eosinophils. Blood. 1993 Jan 1;81(1):49–55. [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Leukocyte-endothelial adhesion molecules. Blood. 1994 Oct 1;84(7):2068–2101. [PubMed] [Google Scholar]
- Curwen K. D., Gimbrone M. A., Jr, Handin R. I. In vitro studies of thromboresistance: the role of prostacyclin (PGI2) in platelet adhesion to cultured normal and virally transformed human vascular endothelial cells. Lab Invest. 1980 Mar;42(3):366–374. [PubMed] [Google Scholar]
- Czervionke R. L., Hoak J. C., Fry G. L. Effect of aspirin on thrombin-induced adherence of platelets to cultured cells from the blood vessel wall. J Clin Invest. 1978 Oct;62(4):847–856. doi: 10.1172/JCI109197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Chang D., Griffin G. L., Heinrikson R. L., Kaiser E. T. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J. G., Bevilacqua M. P., Moore K. L., McIntyre T. M., Prescott S. M., Kim J. M., Bliss G. A., Zimmerman G. A., McEver R. P. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990 Feb 22;343(6260):757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G. Comparative bactericidal activities of blood serum and plasma serum. J Exp Med. 1960 Jul 1;112:15–22. doi: 10.1084/jem.112.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoak J. C., Czervionke R. L., Fry G. L., Smith J. B. Interaction of thrombin and platelets with the vascular endothelium. Fed Proc. 1980 Jul;39(9):2606–2609. [PubMed] [Google Scholar]
- Hsu-Lin S., Berman C. L., Furie B. C., August D., Furie B. A platelet membrane protein expressed during platelet activation and secretion. Studies using a monoclonal antibody specific for thrombin-activated platelets. J Biol Chem. 1984 Jul 25;259(14):9121–9126. [PubMed] [Google Scholar]
- Hynes R. O. The complexity of platelet adhesion to extracellular matrices. Thromb Haemost. 1991 Jul 12;66(1):40–43. [PubMed] [Google Scholar]
- Ibele G. M., Kay N. E., Johnson G. J., Jacob H. S. Human platelets exert cytotoxic effects on tumor cells. Blood. 1985 May;65(5):1252–1255. [PubMed] [Google Scholar]
- Joseph M., Auriault C., Capron A., Vorng H., Viens P. A new function for platelets: IgE-dependent killing of schistosomes. Nature. 1983 Jun 30;303(5920):810–812. doi: 10.1038/303810a0. [DOI] [PubMed] [Google Scholar]
- Kaplan J. E., Moon D. G., Weston L. K., Minnear F. L., Del Vecchio P. J., Shepard J. M., Fenton J. W., 2nd Platelets adhere to thrombin-treated endothelial cells in vitro. Am J Physiol. 1989 Aug;257(2 Pt 2):H423–H433. doi: 10.1152/ajpheart.1989.257.2.H423. [DOI] [PubMed] [Google Scholar]
- Larsen E., Celi A., Gilbert G. E., Furie B. C., Erban J. K., Bonfanti R., Wagner D. D., Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989 Oct 20;59(2):305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Ley K., Gaehtgens P., Fennie C., Singer M. S., Lasky L. A., Rosen S. D. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991 Jun 15;77(12):2553–2555. [PubMed] [Google Scholar]
- Marcus A. J. Pathways of oxygen utilization by stimulated platelets and leukocytes. Semin Hematol. 1979 Jul;16(3):188–195. [PubMed] [Google Scholar]
- Mayadas T. N., Johnson R. C., Rayburn H., Hynes R. O., Wagner D. D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993 Aug 13;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- McCaffery P. J., Berridge M. V. Expression of the leukocyte functional molecule (LFA-1) on mouse platelets. Blood. 1986 Jun;67(6):1757–1764. [PubMed] [Google Scholar]
- McEver R. P., Beckstead J. H., Moore K. L., Marshall-Carlson L., Bainton D. F. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989 Jul;84(1):92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P., Martin M. N. A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem. 1984 Aug 10;259(15):9799–9804. [PubMed] [Google Scholar]
- Moore K. L., Stults N. L., Diaz S., Smith D. F., Cummings R. D., Varki A., McEver R. P. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992 Jul;118(2):445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J., Berndt M. C., Gorski J., White G. C., 2nd, Lyman S., Paddock C., Muller W. A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990 Mar 9;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Roth G. J. Platelets and blood vessels: the adhesion event. Immunol Today. 1992 Mar;13(3):100–105. doi: 10.1016/0167-5699(92)90150-6. [DOI] [PubMed] [Google Scholar]
- Sagawa T., Kodama T., Tominaga A., Okada M. Human platelets effectively kill K-562 cells, a chronic myelogenic leukemia cell line, in vitro. Jpn J Cancer Res. 1990 May;81(5):449–453. doi: 10.1111/j.1349-7006.1990.tb02590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako D., Chang X. J., Barone K. M., Vachino G., White H. M., Shaw G., Veldman G. M., Bean K. M., Ahern T. J., Furie B. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993 Dec 17;75(6):1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- Sporn L. A., Marder V. J., Wagner D. D. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986 Jul 18;46(2):185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stenberg P. E., McEver R. P., Shuman M. A., Jacques Y. V., Bainton D. F. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985 Sep;101(3):880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Selectin ligands. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini C. M., Fenton J. W., 2nd, Minnear F. L., Kaplan J. E. Rat platelets adhere to human thrombin-treated rat lungs under flow conditions. Thromb Haemost. 1989 Nov 24;62(3):1006–1010. [PubMed] [Google Scholar]
- Venturini C. M., Weston L. K., Kaplan J. E. Platelet cGMP, but not cAMP, inhibits thrombin-induced platelet adhesion to pulmonary vascular endothelium. Am J Physiol. 1992 Aug;263(2 Pt 2):H606–H612. doi: 10.1152/ajpheart.1992.263.2.H606. [DOI] [PubMed] [Google Scholar]
- Wagner D. D. The Weibel-Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb Haemost. 1993 Jul 1;70(1):105–110. [PubMed] [Google Scholar]
- Yeo E. L., Sheppard J. A., Feuerstein I. A. Role of P-selectin and leukocyte activation in polymorphonuclear cell adhesion to surface adherent activated platelets under physiologic shear conditions (an injury vessel wall model). Blood. 1994 May 1;83(9):2498–2507. [PubMed] [Google Scholar]
- Yong E. C., Chi E. Y., Fritsche T. R., Henderson W. R., Jr Human platelet-mediated cytotoxicity against Toxoplasma gondii: role of thromboxane. J Exp Med. 1991 Jan 1;173(1):65–78. doi: 10.1084/jem.173.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]