Abstract
We have developed a method for bimodal control of neuronal membrane potential with subcellular resolution using optically independent two-photon uncaging of glutamate at 720 nm and GABA at 830 nm. Two-color, two-photon uncaging allowed action potentials to be fired and blocked with the two wavelengths of light and may enable optically independent control of many other symbiotic chemical messenger pairs.
Two-photon (2P) microscopy1 has recently revolutionized the biological sciences2. In particular, two features are proving to be very useful, when compared with normal excitation modalities, namely imaging fluorescent signals from deep within living tissue, and localized photochemical release of caged compounds. The non-linear nature of 2P excitation provides both of these benefits. Effective 2P uncaging is predicated upon several chemical properties of the photosensitive probe3. Nitroindolinyl-caged glutamates have been used by many laboratories for 2P uncaging experiments, implying such cages can be used with non-toxic photon fluxes. These probes are photolyzed in the 710–730 nm range such that the response at a spine head to the uncaged glutamate appears like single vesicle secretion4,5.
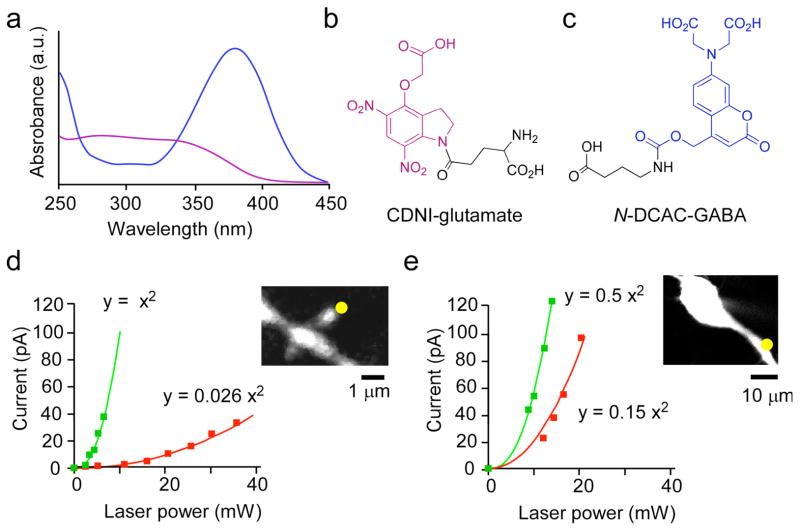
With such precise and effective control of glutamate concentrations by 2P photolysis of nitroindolinyl cages, we sought to develop a caged GABA that could be photolyzed independently, at a longer, complementary wavelength of light. To achieve this goal, we were attracted by the relative minimum at 280–330 nm and red-shifted absorption spectrum of the 7-aminocoumarin chromophore6–9 with respect to 4-carboxymethoxy-5,7-dinitroindolinyl [CDNI] caged glutamate10 (Fig. 1a, b). First, we chose to synthesize (Supplementary Methods) the 7-(dicarboxymethyl)-aminocoumarin [DCAC] caged GABA compound shown in Fig. 1c (we call this probe N-DCAC-GABA). Release of amines caged from carbamates have a half-time about 4 ms11 but such compounds have the advantage of being very stable at physiological pH and are uncaged with usable efficiency8. The synthesis of the analogous DCAC ester has been reported independently, with no biological tests12. A solution of N-DCAC-GABA at pH 7.4 was stable at room temperature for 2 months and was uncaged with a quantum yield of about 5%.
Figure 1.
Absorption spectra of chromophores for two-color photostimulation. (a) UV-visible absorption spectra of CDNI-Glu (b) and N-DCAC-GABA (c). (d) Power dependence of the evoked current from uncaging CDNI-Glu (5 mM) at 720 nm (green squares) and at 830 nm (red squares) near a spine head (inset) on a CA1 pyramidal neuron in an acutely isolated brain slices from the hippocampus of a 13-day old rat. (e) Power dependence of the evoked current from uncaging N-DCAC-GABA (4 mM) at 720 nm (green squares) and at 830 nm (red squares) at the proximal dendrites (inset). The lines drawn through the points were fitted to the equation y = k×x2 in (d) and (e). The ratio of constants (k) revealed that CDNI-Glu and N-DCAC-GABA were 38 and 3.3 times, respectively, more photosensitive at 720 nm compared with 830 nm.
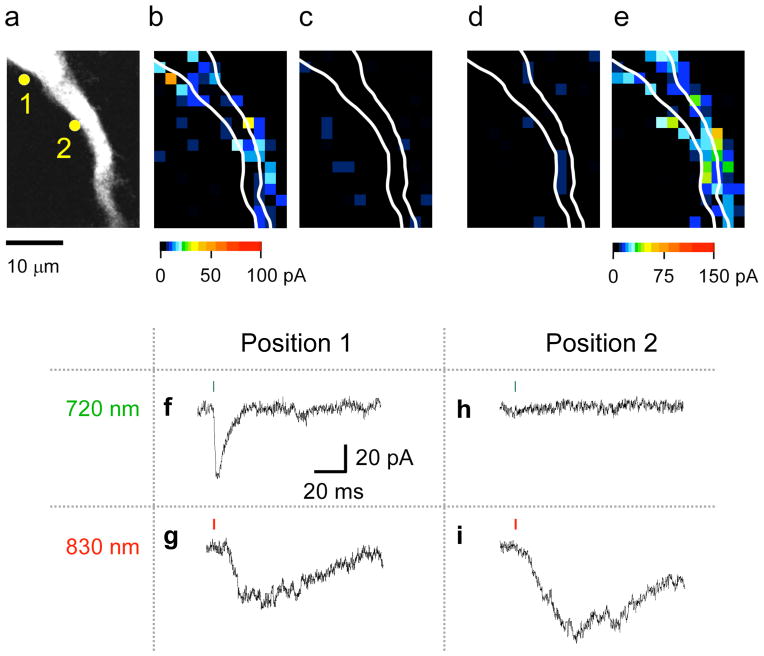
We found that CDNI-Glu was uncaged 38 times more times effectively at 720 nm than at 830 nm (relative 2P action cross-section [2PCS], Y1, is 0.026) (Fig. 1d). In contrast, N-DCAC-GABA absorbed relatively more light at 830 nm (Y2 = 0.3, Fig. 1e). Thus, we co-applied CDNI-Glu and N-DCAC-GABA to rat hippocampal brain slices at concentrations and powers that allowed independent, optically selective uncaging of glutamate and GABA (Fig. 2). For example, pixel 1 showed a 2P-evoked AMPA-R current (2pEPSC) at 720 nm (Fig. 2f, map, Fig. 2b) and a 2P-evoked GABA-A receptor current (2pIPSC) at 830 nm (Fig. 2g, map, Fig. 2e). However, neither wavelength of light photolyzed the other caged compound to any great extent (Fig. 2c, d). In pixel 2, irradiation at 830 nm evoked GABA-A receptor currents, whereas 720 nm light did not (compare Fig. 2i and Fig. 2h), implying true color separation of photo-evoked currents under the conditions used for this experiment. Further, irradiation at 720 nm did not evoke any AMPA receptor currents in pixel 2, suggesting receptor segregation along this portion of the dendrite. For the experiments shown in Fig. 2 and Fig. 3b the slower uncaging11 of N-DCAC-GABA works to our advantage, as the kinetics of the cage allows us to segregate responses at the two wavelengths temporally. If the selectivity ratio for wavelength is defined by the ratio between the amplitudes of 2pEPSC at 830 nm and 720 nm (or between those of 2pIPSC at 720 nm and 830 nm), it was less than the noise level, in line with our theory which predicts the cross-talk to be 0.086 (=Y1/Y2) (Supplementary text). In short, since two-photon excitation of CDNI-Glu at 720 nm tended to evoke much larger currents at lower power than DCAC-GABA (Fig. 1d, e), we selected concentrations of caged compounds such that irradiation at 720 nm cleanly stimulates AMPA receptors without GABA receptors. Since Y1=0.026, this allowed us to use higher power at 830 nm without effective uncaging of CDNI-Glu at this longer wavelength. Given Y2= 0.3, such power effectively uncaged N-DCAC-GABA at 830 nm.
Figure 2.
Optical independent two-color, 2P mapping of functional AMPA and GABA-A receptors on a CA1 pyramidal neuron. This experiment shows representative data from fice similar experiments in which CDNI-Glu (2 mM) and N-DCAC-GABA (4 mM) were co-applied to an acutely isolated brain slice from the rat hippocampus. The neuron was visualized by perfusion through a patch pipette with a CsCl-based solution containing Alexa-594. The proximal apical dendrite (a) was subjected to functional mapping by photolysis at 720 and 830 nm under voltage clamp by pseudo-random scanning of each pixel as shown in (b)–(e). For uncaging at 720 nm (b, c) 8.7 mW was applied for 1 ms (green bars in f and h), and at 830 nm (d, e) 20.6 mW for 2 ms (red bars in g and i). The evoked currents are represented on a pseudo-color scale: 0–100 pA for (b–d), and 0–150 pA for (e). Two time-windows for current integration [2–3 ms (b, d) and 30–35 ms (c, e)] after uncaging show the AMPA-receptor and GABA-A receptors are activated with optical independence at 720 nm and 830 nm, respectively. (f–i) Examples of current traces. The current trace from pixel 1 in (a) shows an AMPA current at 720 nm (f) but not 830 nm, and a GABA current at 830 nm but not 720 nm (g) whereas pixel 2 in (a) shows only a GABA current at 830 nm (i), and no current evoked by irradiation at 720 nm (h), showing only 830 nm light uncaged GABA. The membrane potential was held at −70 mV.
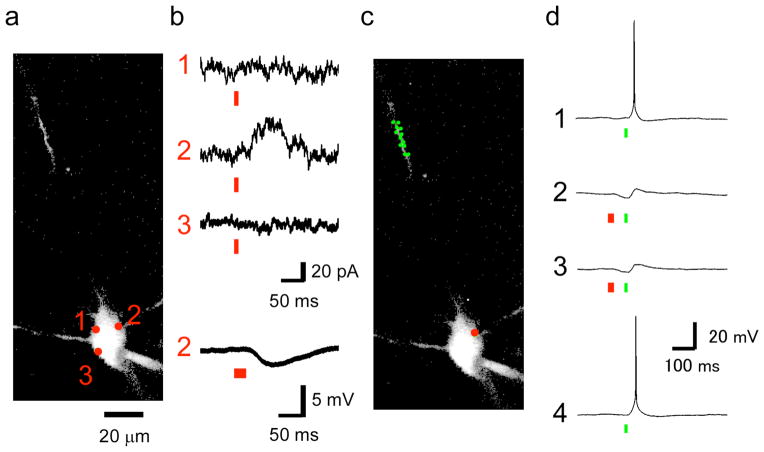
Figure 3.
Two-color, 2P uncaging of Glu and GABA fired and blocked action potentials. This figure shows representative data from seven similar experiments when CDNI-Glu (2 mM) and N-DCAC-GABA (4 mM) were co-applied to an acutely isolated brain slice from the rat hippocampus. (a) The neuron was visualized by perfusion through a patch pipette with a K-gluconate-based solution containing Alexa-594, and 2P imaging was performed at 900 nm. A two-dimensional projection of a three-dimensional z-stack is shown (the proximal dendrite was outside of the imaging volume). (b) Functional mapping of currents in voltage clamp of the soma at −20 mV. Two-photon photolysis at 830 nm and 55 mW for 9 ms (red bars) revealed a GABA-A receptor hotspot at position 2. The lowest trace indicates 2pIPSP induced by sequential uncaging of three points adjacent to the position 2 (red bars, 27 ms in total). (c) The somatic position of uncaging at 830 nm (red circle) and dendritic twelve positions (green circles) for uncaging at 720 nm are shown. (d) Four consecutive voltage traces where elicited with an interval of 5 s during the current clamp experiments in which membrane potential was held at −48 mV. Action potentials were elicited by sequential uncaging at 720 nm of the twelve positions in the dendrites (c) each with the laser power of 9 mW for 1 ms (green bars, 12 ms in total). 2pIPSCs were induced as shown in (b). Action potential was suppressed when 2pIPSP was induced.
The combination of CDNI-Glu and N-DCAC-GABA has particular advantages for these two-color, 2P mapping experiments. Many caged glutamate compounds have been reported13, however only one (4-methoxy-7-nitroindolinyl-[or MNI]-Glu) has been widely used for 2P uncaging on brain slices. As a caged Glu, CDNI-Glu can be used at much lower concentrations than the widely used MNI-Glu probe and still allow effective 2P photolysis at subtoxic energy dosage. For this reason we selected this probe for the current work. A small variety of caged GABAs have been reported over the past 14 years8,14–17. Most of these probes14–16 absorb in the same wavelength region as CDNI so cannot be used for two-color uncaging. However, some offer longer wavelength absorption. RuBi-GABA probably cannot be used for 2P uncaging because it was reported toxic at concentrations greater than 20 μM16. Having two negative charges, N-DCAC-GABA is freely soluble in physiological buffer up to concentrations of at least 50 mM, whereas the simpler BC204 cage7 might not be soluble enough for application at concentrations required for effective 2P uncaging
The experiments shown in Fig. 2 can be performed by retuning a single uncaging laser between 720 nm and 830 nm, and even though modern lasers are computer controlled, the period for this switch is too long for rapid two-color photolysis with one uncaging laser. Thus, we developed a microscope with three Ti:sapphire lasers, which has two beams (at 720 and 830 nm) that were combined before entering the uncaging scan head (Supplementary Fig. 2). Using fast acousto-optical modulators, these two channels could be switched in about 10 μs. The third laser was used for imaging at 900 nm. Again, both CDNI-Glu and N-DCAC-GABA were applied simultaneously to a brain slice. Initially eight points around the soma of a CA1 pyramidal cell (Fig. 3a) were subjected to 2P uncaging at 830 nm to find a GABA receptor “hot spot” at the holding potential of −20 mV (importantly, no APMA receptor currents were detected here; Fig. 3b). Once such a density was located in voltage clamp, we switched to current clamp mode to induce 2pIPSP at 830 nm (Fig. 3b) and to trigger action potentials by uncaging at 12 dendritic locations at 720 nm (Fig. 3b). The firing was then blocked by prior uncaging of N-DCAC-GABA with 830 nm irradiation (Fig. 3d, 2,3), and resumed when the 830 nm beam was extinguished (Fig. 3d, 4). Thus, proper uncaging power levels and the concentrations of CDNI-Glu and N-DCAC-GABA enabled two-color, 2P uncaging of glutamate and GABA on the same neuron (Supplementary Text), with single synapse precision, and is the first example of multi-modal optical control of neuronal membrane potential by activation of native AMPA and GABA receptors.
Two other optical methods to control neuronal membrane potential have been developed18. One uses cis-trans isomerization of azobenzene chemical probes to block and unblock ion channels19,20. The second uses light-activated channels called channelrhodopsin-2 (ChR2) and halorhodopsin (NpHR) to control membrane potential21,22. The azobenzene approach can fire action potentials (illumination at 380 nm) and extinguish the evoked current by illumination at 500 nm20. The advantages of caged compounds and azobenzenes arise from their use of exogenous probes. These methods can be directed to activate native receptors and are complementary to channelrhodopsin. Importantly, caged compounds are highly 2P active, and because native receptors are often densely clustered, 2P photolysis can be used for selective stimulation of single synapses in neuronal circuits. Thus, uncaging is still the most useful method to address classical neurophysiological problems of synaptic function including dendritic integration, AND-OR gate systems, diversity of GABA input points, etc.
In summary, we have now advanced two-photon (2P) uncaging microscopy2 so that we can control localized signaling of two chemical messengers with rapid, subcellular precision using two wavelengths of light. With our three-laser, 2P microscope, we achieved optically independent, bidirectional control of neuronal membrane potential effecting neuronal excitation at 720 nm (Glu uncaging) and inhibition at 830 nm (GABA uncaging), during cell observation at longer wavelengths (850–1050 nm). Two-color, 2P uncaging is a significant addition to the optical toolbox that is available to biologists, as it offers direct photocontrol of symbiotic chemical messengers with subcellular resolution deep within biological tissue. Because uncaging works with the widest possible range of signaling systems4, two-color 2P uncaging offers the possibility of uncaging other pairs of chemical signaling partners beyond the ones we report here.
Supplementary Material
Acknowledgments
This work was supported by the NIH (USA) grant GM53395 (to GCRE-D) and by Grants-in-Aids for Specially Promoted Area No. 2000009 (to HK), for Scientific Research on Priority Areas No. 20021008 (to MM) and for Young Scientist (A) No. 19680020 (to MM) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan, and PRESTO and CREST, Japan Science and Technology Agency, Japan (to MM).
Footnotes
Author contributions
GCRE-D and SK made and characterized the caged compounds. HK designed the three-beam, 2P microscope and theoretical treatment of two-color uncaging. MM and YK did the biological experiments and analyzed the data. GCRE-D and HK wrote the paper. All authors commented upon the manuscript.
References
- 1.Denk W, Strickler JH, Webb WW. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 2.Svoboda K, Yasuda R. Neuron. 2006;50:823–39. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Ellis-Davies GCR. Nat Methods. 2007;4:619–28. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuzaki M, et al. Nat Neurosci. 2001;4:1086–92. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez VA, Sabatini BL. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 6.Hagen V, et al. Angew Chem Int Ed Engl. 2001;40:1045–1048. doi: 10.1002/1521-3773(20010316)40:6<1045::aid-anie10450>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 7.Shembekar VR, Chen Y, Carpenter BK, Hess GP. Biochemistry. 2005;44:7107–14. doi: 10.1021/bi047665o. [DOI] [PubMed] [Google Scholar]
- 8.Rothman SM, Perry G, Yang XF, Hyrc K, Schmidt BF. Epilepsy Res. 2007;74:201–9. doi: 10.1016/j.eplepsyres.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan LJ, Lewis RW, Hess GP, Ganem B. Bioorg Med Chem Lett. 2009;19:3932–3933. doi: 10.1016/j.bmcl.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 10.Ellis-Davies GCR, Matsuzaki M, Paukert M, Kasai H, Bergles DE. J Neurosci. 2007;27:6601–4. doi: 10.1523/JNEUROSCI.1519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papageorgiou G, Corrie JE. Tetrahedron. 1997;53:3917–3932. [Google Scholar]
- 12.Senda N, Momotake A, Arai T. Bull Chem Soc Jpn. 2007;80:2384–2388. [Google Scholar]
- 13.Ellis-Davies GCR. New Encyclopedia of Neuroscience. 2008:e876. [Google Scholar]
- 14.Gee KR, Wieboldt R, Hess GP. J Am Chem Soc. 1994;116:8366–8367. [Google Scholar]
- 15.Canepari M, Nelson L, Papageorgiou G, Corrie JE, Ogden DJ. Neurosci Meth. 2001;112:29–42. doi: 10.1016/s0165-0270(01)00451-4. [DOI] [PubMed] [Google Scholar]
- 16.Alvina K, Walter JT, Kohn A, Ellis-Davies G, Khodakhah K. Nat Neurosci. 2008;11:1256–8. doi: 10.1038/nn.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rial Verde EM, Zayat L, Etchenique R, Yuste R. Front Neu Cir. 2008;2:2. doi: 10.3389/neuro.04.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorostiza P, Isacoff EY. Science. 2008;322:395–9. doi: 10.1126/science.1166022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Nat Neurosci. 2004;7:1381–6. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volgraf M, et al. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han X, Boyden ES. PLoS ONE. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang F, et al. Nature. 2007;446:633–9. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.