Abstract
Gut microbiota mediated low-grade inflammation is involved in the onset of type 2 diabetes (T2DM). In this study, we used a high fat sucrose (HFS) diet-induced pre-insulin resistance and a low dose-STZ HFS rat models to study the effect and mechanism of Lactobacillus casei Zhang in protecting against T2DM onset. Hyperglycemia was favorably suppressed by L. casei Zhang treatment. Moreover, the hyperglycemia was connected with type 1 immune response, high plasma bile acids and urine chloride ion loss. This chloride ion loss was significantly prevented by L. casei via upregulating of chloride ion-dependent genes (ClC1-7, GlyRα1, SLC26A3, SLC26A6, GABAAα1, Bestrophin-3 and CFTR). A shift in the caecal microflora, particularly the reduction of bile acid 7α-dehydroxylating bacteria, and fecal bile acid profiles also occurred. These change coincided with organ chloride influx. Thus, we postulate that the prevention of T2DM onset by L. casei Zhang may be via a microbiota-based bile acid-chloride exchange mechanism.
Obesity-associated T2DM has drawn much scientific attention, as evident by the rapidly increasing number of published investigations. Data showed that the world population is facing a surge in T2DM as well as individuals with prediabetes due to rapid change in lifestyle1. Thus, both strategies for both the prevention and treatment of diabetes are needed, especially in the dietary aspect.
Diet is directly associated with intesinal microbiota. There is a growing interest in understanding the changes of gut microbiota in the context of diabetes. In recent years, metagenomics has opened a new era of microbial ecology that has allowed deeper understanding of microbiome associated hyperglycemia2,3. On the other hand, it is proposed that high-fat diet induces a low-grade inflammation through modifying microflora and thus increases lipopolysaccharides (LPS) and in turn triggers the development of metabolic diseases4. More interestingly, commensal microbiota and related bile acids profile could be rapidly reshaped by dietary alteration5, but how the pathogensis of T2DM relates with the interaction between bile acids and chloride ion is rarely studied. This aspect is of particular interest because both bile acids and choride ions can acted as regulating signaling molecules for metabolic homeostasis6,7.
Several studies have also shown that probiotic products could regulate the blood glucose level in diabetic human8,9. Moreover, L. casei Shirota has been reported to reduce blood glucose level through reducing lipopolysaccharide-binding protein10. One research showed that B. animalis 420 could prevent mice from obesity-induced T2DM through an improvement of bacterial translocation and overall inflammatory status11. Recently, the gut microbe, Akkermansia muciniphila, exhibited an insulin resistance-reducing effect and may have potential application in T2DM12.
Our previous research showed that L. casei Zhang could improve impaired glucose tolerance in rats due to altered microbiota composition which led to an upregulation of ostecalcin level13. The aims of the present study were to investigate whether probiotic L.casei Zhang supplementation could prevent the symptoms of rat model of T2DM and identify its mechanisms.
Methods
Animals and housing. The protocol was approved by the Animal Care and Use Committee at Inner Mongolia Agricultural University in Huhhot, China. All the methods were carried out in accordance with the approved guidelines. Male sprague-dawley (SD) rats, initial weight approximately 120 g (5 weeks old), were purchased from Vital River Laboratory Animal Co. Ltd. (Beijing, China) and housed free access to rodent diet and water under a standard 12-h light/dark cycle with controlled temperature (22°C ± 2°C) and humidity (55% ± 5%). All rats were acclimatized for 1 week before the experiment started.
Experimental design
Two separate but related rat experiments were performed to show the hypoglycemic effect of L. casei Zhang consumption. Firstly, the physiological change and the protective effect of L. casei Zhang was assessed by a short-term high fat-induced microbiota disturbance model (Fig. S1). Rats were randomly divided into high-fat-sucrose diet (HFS) group, HFS diet + L. casei Zhang group (PB, 4.0 × 109 CFU/rat·d) and normal control (NC, normal chow diet) group, with 8 rats per group. High-fat-sucrose diets consisted of 10% lard, 10% sucrose, 45% corn starch, 20% casein, 1% vitamin mix and 4% bone powder.
In a second set of experiment, a type-2-diabetic precomplication model was induced by HFS diet and challenged with low dose streptozotocin (STZ) (Fig. S1). In this experiment, rats were randomly assigned to A, M and P groups (9 rats per group). Rats in group A (n = 9) were fed a normal chow diet as previously described14. Group M rats (n = 9) were fed a high-fat-sucrose diet (HFS) which was described above. Group P rats (n = 9) consumed the same diet as group M and additionally were administrated 4.0 × 109 CFU/rat·d of L. casei Zhang. After ad libitum exposure to the HFS diet for 2 weeks, both M and P groups were given an intraperitoneal injection of STZ (40 mg/kg of body weight, Sigma, USA), which was dissolved in citrate buffer (pH 4.5). Group A rats received only the buffer.
Determination of blood glucose
Fasting (12 h) and postprandial 2 h blood glucose levels were checked weekly by a portable Bayer's Contour Blood Glucose Monitor (Contour® Meter, Bayer HealthCare LLC, USA). For oral glucose tolerance test (OGTT), rats were fasted for 12 h before being administered with an oral dose of glucose (2 g/kg of body weight). Blood glucose levels were measured at 0, 15, 30, 60, and 120 min after glucose administration.
Biochemical analysis
Blood was collected by cardiac puncture and rapidly transferred into anticoagulant tubes. Plasma was obtained by centrifuging at 3000 g for 15 min and stored at −80°C until use. Plasma cytokines including TNF-α, IFN-γ, IL-10 were respectively determined by ELISA kits (Cusabio, China), and fecal total bile acids levels were determined by a commercial kit (Randox, UK) with enzymatic colorimetric method. Plasma LPS level was detected by using a kit based on Limulus amoebocyte extract (Houshiji Company, Xiamen, China).
Rat urine collection
For urine collection (1 wk after STZ injection), animals were housed individually in metabolism cages for 12 h during the daytime, with free access to drinking water. Urine volume and pH were recorded, and samples were stored at −70°C until analysis. Urine NH4+ concentrations were determined by commercial kit (Nanjing Jiancheng Bioengineering Institute, China).
Determination of chloride ion concentration
Tissues were homogenized in distilled water with chloride ion free Teflon-glass potter Homogenates were centrifuged, and the supernatants were collected. The chloride concentrations of the plasma, urine or tissue homogenates were detected by a colorimetric method15. Principally, chloride ions firstly reacted with mercuric thiocyanate and thereby released thiocyanate ions, which then bound to ferric ions and formed the brick red ferric thiocyanate complex, detectable at 460 nm.
Measurement of fecal bile acids by LC-MS
100 mg frozen feces were homogenized in methanol through ceramic beads-beating on a FastPrep ®-24 sample preparation system (MP Biomedicals, CA, USA). Cell-free supernatants were obtained after centrifugation and filtering of the homogenates through 0.22 μm membrane. Determination of bile acids contents including cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA) and lithocholic acid (LCA) was performed on an ACQUITY TQD UPLC/MS system (Waters, USA) by using negative mode. Standard solutions (1 mg/ml) were respectively prepared CA, CDCA, DCA and LCA (Sigma-Aldrich, China). The parameters of mass spectrometry were set as follows: capillary voltage, 3500 V; cone voltage, 30 V; ion source temperature, 120°C; desolvation temperature, 300°C; desolvation gas flow, 600 L/h; cone gas flow, 50 L/h. The column was C18 (Waters, 2.1 × 50 mm) and the mobile phase was acetonitrile −0.1% formic acid with a flow rate of 1 ml/min by gradient elution. The volume of sample injection is 3 μl.
Quantification of target intestinal bacteria
Quantitative PCR (qPCR) was performed to study the cecum microbial community as described before16. Total bacterial DNA was extracted from the ileocecum content of animals with the QIAamp DNA Stool Mini Kit (QIAGEN, Germany). Specific primers of Lactobacillus, Bifidobacterium, Eubacterium rectale–Clostridium coccoides cluster, Eubacterium rectale, Clostridium scindens, Clostridium sordellii and Clostridium cluster IV were listed in Table S1. All qPCR were performed with an ABI Detection System (Applied Biosystems, the Netherlands) using the qPCR SYBR Green kit in a 20 μl–30 μl reaction volume. Fluorescence intensities were detected during the last step of each cycle. The quantity of target bacteria was calculated according to the standard curve.
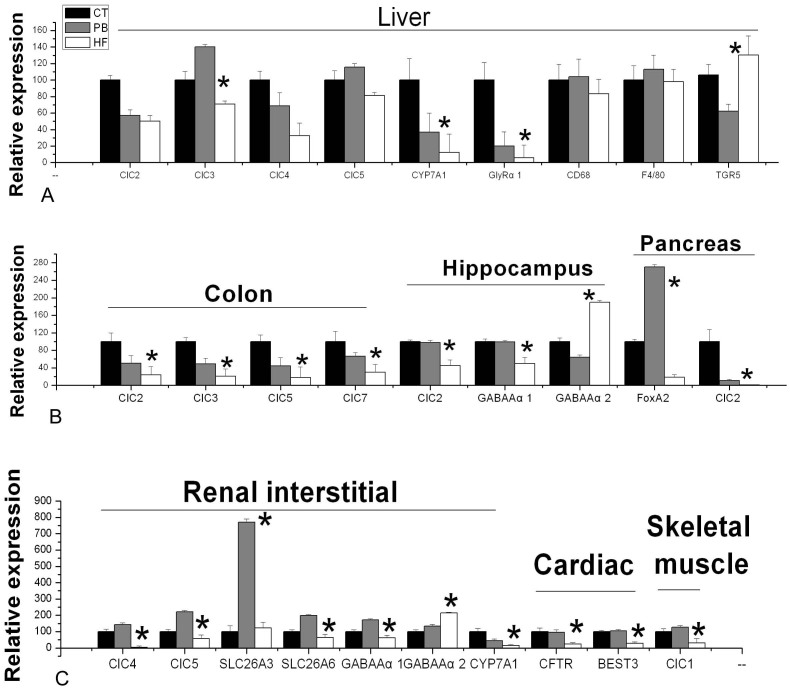
Figure 1. Effect of HFS diet and L. casei Zhang on relative expression of target genes in different organs, including (A) Liver, (B) Colon, Hippocampus and Pancreas and (C) Renal interstitial, Cardiac and Skeletal muscle.
Relative gene expression
To assess mRNA expression, total RNA was isolated from rat tissues by using Trizol reagents (TAKARA, Japan) on a FastPrep ®-24 sample preparation system at a setting of 6.0 M/s for 20 s. 500 ng of RNA was purified and reversely transcribed with PrimeScript® RT reagent kit (TAKARA, Japan) into cDNA. Real-time PCR was performed on an Applied Biosystems StepOne™ System with SYBR green reagent (TAKARA, Japan) according to the manufacturer's instructions. 18S rRNA was used as endogenous control to normalize the expression level of gene. Primer sequences for various genes were listed in Table S2. All reactions were done in a 20 μl or 30 μl reaction volume. A melt curve analysis was performed to verify the specificity of the amplification. The results were expressed as relative values after normalization to 18S mRNA. Data were calculated using the 2−ΔCT method.
Western blot analysis
Tissues were homogenized in a Teflon-glass potter filled with buffer and protease inhibitor cocktail (Cwbiotech, China). Samples were centrifuged at 12000 × g (15 min, 4°C) and protein levels were measured by BCA kit (Tiangen, China). Extracted proteins were denatured, electrophoresed, and transferred to PVDF membranes (Millipore, 0.45 μm). The membranes were then blocked in 5% nonfat milk at room temperature before incubating with rabbit anti-GlyRα1 antibody (1:1000,Abcam,UK), rabbit anti-ClC-2 antibody (1:200, Santa Cruz, USA), rabbit anti-ClC-3 antibody (1:200, Santa Cruz, USA), rabbit anti-FoxA2 antibody (1:1000, Cell Signaling, USA) or rabbit anti-beta actin (1:1000, Proteintech Group, China) antibody overnight (4°C). After washes in TBST, membranes were incubated (1 h, room temperature) in goat-anti-rabbit-HRP secondary antibody (1:2500, KPL Inc, USA). Results were detected by Pierce ECL Plus Western Blotting Substrate (Pierce, USA) on Kodak X- ray film.
Histological evaluation
Tissues of rats were fixed in 10% neutral formalin, followed by dehydrating in gradient alcohol (75%, 85%, 95% and 100%) and xylene (100%). The tissues were then embedded in paraffin and sectioned at 5 μm thickness. Sectioned tissues were stained with hematoxylin-eosin before microscopic assessment (Olympus, Japan).
Statistical analysis
All experimental data are shown as the mean ± S.E.M. Multiple groups were tested by one-way ANOVA followed by LSD test to determine which groups were significantly different from the control group. A p value < 0.05 was considered to be statistically significant. *, 0.01 < p < 0.05; **, 0.001 < p < 0.01; ***, p < 0.001; n, p > 0.05.
Results
Obesity-induced pre-insulin resistance rats
No significance difference was observed in the body weight, OGTT, plasma insulin, TBA, chloride ion, TNF-α and IL-10 levels among the three groups (Fig. S2, p > 0.05). Two-week high fat–sucrose intake with (PB rats) or without probiotic (HF rats) treatment induced a significantly higher plasma IL-6 level compared to CT group (Fig. S2H, p = 0.0342). There was no significant difference in plasma IL-6 between PB rats and HF rats (Fig. S2H, p = 0.169). Fig. S3 showed preliminary fatty liver morphology in HF and PB rats.
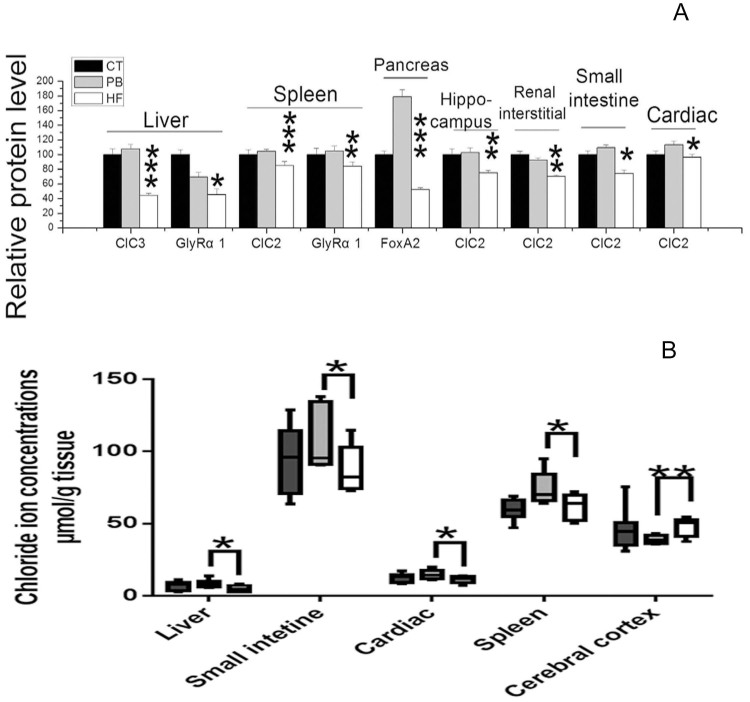
The liver GlyRα1,CYP7A1 and ClC3 mRNA levels in PB were greater than those in HF rats (Fig. 1A, p = 0.013, 0.010 and 0.0001), whereas TGR-5 mRNA levels in PB were much lower than HF (p = 0.020) and the ClC2, ClC4, ClC5, CD68 and F4/80 mRNA levels of PB rats were unaffected (Fig. 1A, p > 0.05). In addition, chloride ion concentrations of liver homogenates and GlyRα1 and ClC3 protein levels of PB group were higher than those in HF rats (Fig. 2A and Fig. S4, p = 0.027, 0.049 and 0.0001). As shown on Fig. 2A and Fig. 2B, splenic chloride ion levels, GlyRα1 and ClC2 protein expression were lower in CT and HF rats than those from the PB group (p = 0.043, 0.0019 and 0.0009).
Figure 2. Effect of HFS diet and L. casei Zhang on (A) target protein levels (see Fig. S4 for further information) and (B) chloride ion concentration in different organs.
The Fig. 1B revealed a substantial downregulation of colonic ClC2, ClC3, ClC5 and ClC7 mRNA levels in HF rats compared to CT, while PB rats displayed a 2- to 3-fold increase in the expression of those genes. In parallel, the chloride ion concentration and CIC2 protein of the small intestine in PB rats were higher than that in HF group (Fig. 2B, p = 0.035 and 0.023).
In comparison with PB rats, a 3–4 fold decrease in cardiac CFTR and BEST3 mRNA was observed (Fig. 1C). Moreover, there was a significant decrease in ClC2 protein expression of HF rats (p = 0.040), but without notable changes in CT rats (Fig. 2A and Fig. S4). There was also a significant difference in chloride ion concentrations between PB and HF rats (Fig. 2B, p = 0.026).
Statistical analysis revealed that there was an opposite effect on renal GABAAα1 and GABAAα2 mRNA between PB and HF. Renal ClC4, ClC5, CYP7A1, SLC26A3, SLC26A6 and GABAAα1 mRNA levels were elevated by more than 2-fold by dietary L. casei Zhang supplementation in the PB group compared to HF rats (Fig. 1C). In the muscle of HF rats, ClC1 mRNA level decreased by 3 to 5-fold compared with CT and PB rats (Fig. 1C).
The 2 week HF diet likewise induced a 5-fold reduction in the pancreatic FoxA2 mRNA level compared to CT, whereas the FoxA2 mRNA level of PB rats was significantly upregulated (Fig. 1A, p = 0.0001). As shown in Fig. 2A and Fig. S4, pancreatic ClC2 protein level was much lower in the PB and HF rats compared to CT (p = 0.0001 and 0.0055).
In hippocampus area of the brain, CT and PB rats showed more than 2 fold increased in GABAAα1 receptor and ClC2 mRNA compared to HF rats, while the GABAAα2 receptor mRNA had an opposite trend (Fig. 1B). In the prefrontal cortex area of the brain, the chloride ion concentration of PB rats was significant lower compared to HF rats (Fig. 2B, p = 0.007).
Fecal CA and CDCA levels were similiar in the CT and HF groups (p > 0.05; Table 1). But these levels were significantly higher in the PB than HF group (p = 0.0114 and 0.0002). Compared with HF rats, the PB and CT rats exhibited lower fecal DCA and LCA levels (p = 0.0057 and 0.0003, HF vs PB; p = 0.0003 and 0.0001, HF vs CT). As a result, the PB rats showed significantly higher total bile acid level compared to HF rats (p = 0.0335), while the fecal bile acids level of CT rats was considerably lower.
Table 1. Fecal composition of bile acids in rats (n = 8 for each group). (1) + (2), (3) + (4) and (1) + (2) + (3) + (4) stand for primary, secondary and total bile acids, respectively.
| Bile acid type | CT | PB | HF |
|---|---|---|---|
| Cholic acid (1) | 0.30 ± 0.018 | 0.36 ± 0.012# | 0.24 ± 0.035 |
| Chenodeoxycholic acid (2) | 0.27 ± 0.015 | 1.33 ± 0.26*,# | 0.28 ± 0.042 |
| Deoxycholic acid (3) | 0.27 ± 0.019 | 0.36 ± 0.015# | 0.57 ± 0.080 |
| Lithocholic acid (4) | 0.079 ± 0.0023 | 0.094 ± 0.0024# | 0.30 ± 0.058 |
| (1) + (2) | 0.56 ± 0.031 | 1.69 ± 0.26*,# | 0.52 ± 0.083 |
| (3) + (4) | 0.35 ± 0.018 | 0.45 ± 0.0080# | 0.87 ± 0.093 |
| (1) + (2) + (3) + (4) | 0.91 ± 0.044 | 2.14 ± 0.27*,# | 1.39 ± 0.15 |
*represent significant difference from CT (p < 0.05) by Dunnett test.
#represent significant difference from HF (p < 0.05) by Dunnett test.
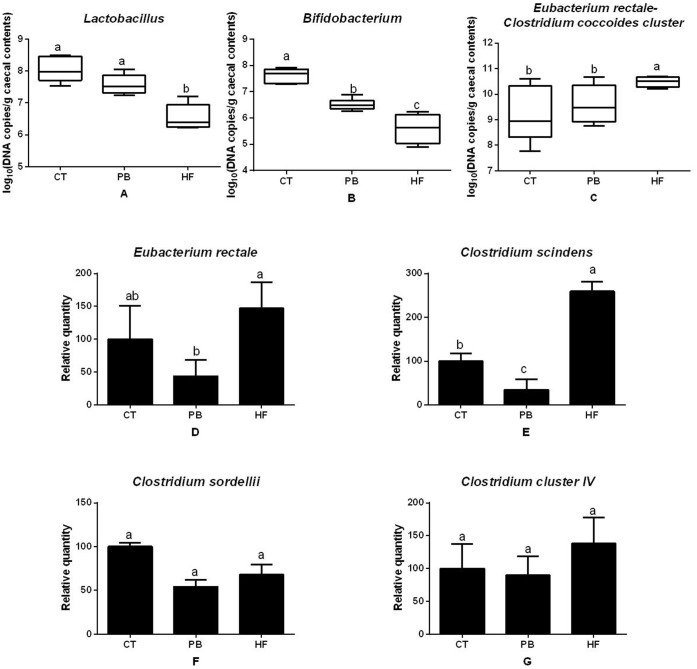
More caecal Bifidobacterium and Lactobacillus were found in the PB rats than the HF group (p = 0.0061 and 0.0001, Fig. 3A and Fig. 3B). In contrast, C. coccoides–E. rectale group and C. scindens members were much higher in HF than the PB and CT rats (p = 0.050 and 0.0001, Fig. 3C and Fig. 3D). Similar effects of E. rectale were observed between HF and PB rats (p = 0.0001, Fig. 3E) but not between HF and CT rats. No significant differences in the counts were found with Clostridium IV cluster and the genus of C. sordellii (p > 0.05, Fig. 3F and Fig. 3G).
Figure 3. Caecal bacterial content of (A) Lactobacillus, (B) Bifidobacterium, (C) Eubacterium rectale–Clostridium coccoides cluster, (D) Eubacterium rectale, (E) Clostridium scindens, (F) Clostridium sordellii, (G) Clostridium cluster IV. Bacterial quantities are expressed in gene copy number in A, B, C and relative units compared to CT group.
Short-term HFS fed rats challenged with low dose STZ
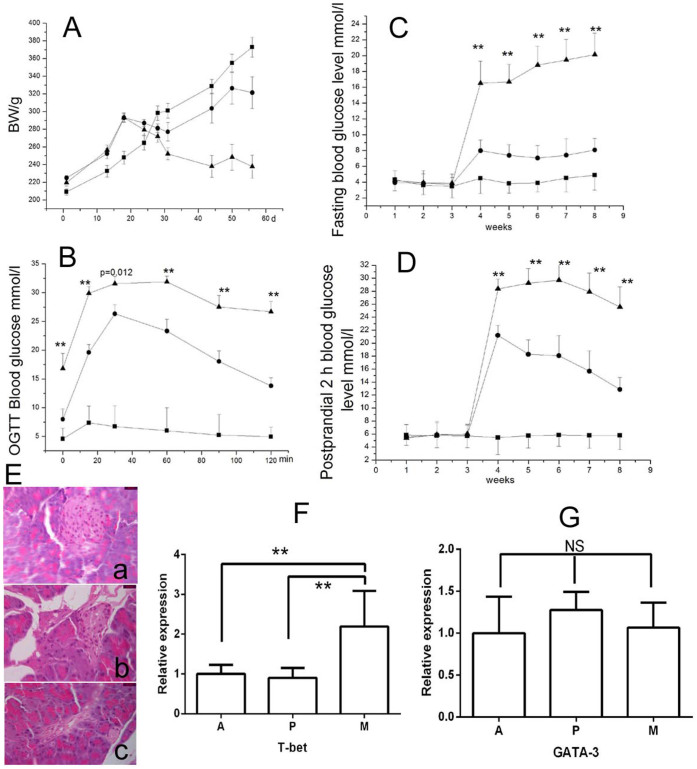
As shown in Fig. 4A, the body weight in the control rats without STZ injection (group A) continually increased during the whole experimental period, while the body weight of the rats in the treatment groups, P (probiotic plus STZ injection) and M (no probiotic but STZ injection), the body weight decreased after the STZ injection, and the body weight of P group rats started to increase after week 5. Fig. 4B showed the OGTT results of three groups. One week after STZ injection, group P rats exhibited a significantly lower blood glucose levels at all time points compared to group M rats (p = 0.010 (0 min), 0.006 (15 min), 0.012 (30 min), 0.004 (60 min), 0.002 (90 min) and 0.005 (120 min), respectively). Moreover, both fasting and postprandial 2 h blood glucose level were significantly lower in group P than group M 1 week after STZ injection (p = 0.010 (4 w), 0.002 (5 w), 0.001 (6 w), 0.001 (7 w) and 0.004 (8 w), Fig. 4C; p = 0.028 (4 w), 0.003 (5 w), 0.008 (6 w), 0.002 (7 w) and 0.001 (8 w), Fig. 4D). As shown in Fig. 4E, round integrated pancreatic islet and tightly arranged islet cells were found in healthy rats. Group M rats showed a severe necrosis of islets (Fig. 1Ec), group P rats exhibited a mild decrease of islets (Fig. 1Eb). Importantly, more than two fold changes of liver T-BET mRNA level was observed between A and M. L. casei significantly attenuated STZ-stimulated T-BET mRNA levels compared with M (Fig. 4F, p = 0.015). Changes of liver GATA-3 mRNA levels were not significant different among the three groups (Fig. 4G, p > 0.05).
Figure 4.
The body weight (A), OGTT (B), fasting (C) and postprandial 2 h blood glucose level (D) of three groups of rats. Black triangles = M group; black circles = P group; black squares = A group. (**P < 0.01 represents significant difference between groups P and M); (E)(a), (b) and (c) are representative pancreas tissue section respectively from group A, P and M rats (×1000). (F) Liver T-bet mRNA level; (G) Liver GATA-3 mRNA level. **, p < 0.01; ns, p > 0.05.
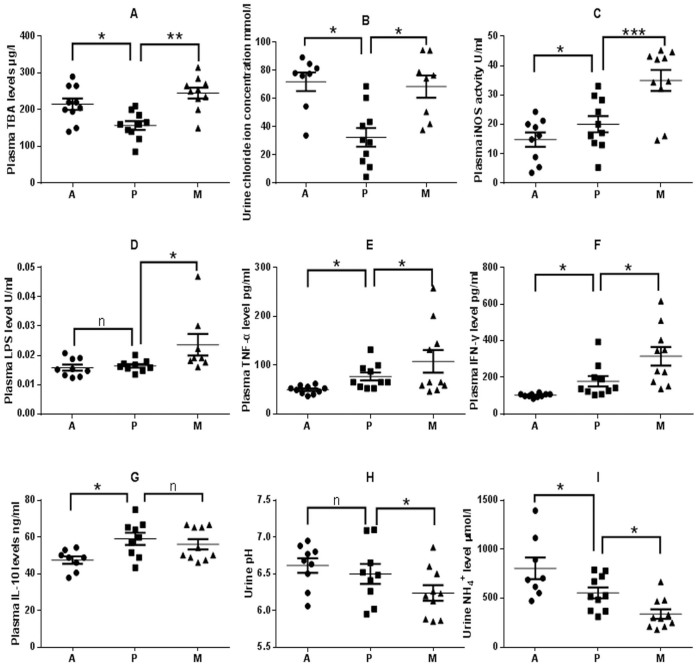
The plasma TBA levels of L. casei Zhang-treated rats (group P) were markedly lower than groups A and M (Fig. 5A, p = 0.015 and p = 0.006, respectively). And 12 h urine chloride ion level was also significantly lower in group P than that in groups A and M (Fig. 5B, p = 0.048). The iNOS activity of STZ-injection rats significantly increased compared with normal rats (p = 0.0008), and the probiotic-treated rats (group P) had a markedly lower level of iNOS activity than the M group rats (p = 0.001) (Fig. 5C). Fig. 5D showed that the STZ-injection induced a significant increase in plasma LPS level (p = 0.047), while it is maintained at a healthy level in the group P rats (p = 0.880).
Figure 5. Comparison of biomarkers of control (Group A), and STZ-injected rats (Groups P, M) 1 week post injection of STZ.
(A) Plasma TBA levels. (B) Urine chloride ion levels. (C) Plasma iNOS actvities.(D) Plasma LPS levels. (E) Plasma IFN-γ levels. (F) Plasma TNF-α levels. (G) Plasma IL-10 levels. (H) pH of 12 h urine.(I) Urine NH4+ concentrations. Samples were taken 1 week post injection of STZ. *, p < 0.05; **, p < 0.01; ***, p < 0.001; n, p > 0.05.
Proinflammatory cytokines (IFN-γ and TNF-α) were significantly elevated in STZ-injection groups compared with normal rats, but it was significantly lowered in the probiotic-treated rats (p = 0.030, Fig. 5E; p = 0.017, Fig. 5F). There was no significant difference in IL-10 between P and M groups (p = 0.723) (Fig. 5G). The pH of the collected urine was significantly lower in group M compared to that of groups A and P (Fig. 5H, p = 0.048). Urine NH4+ concentration in group P was significantly lower than that of group A but higher than that of group M (Fig. 5I, p = 0.027 and 0.024).
Discussion
The primary findings of the present study are that L. casei Zhang ingestion markedly prevents rats from the onset and development of glycemia in both fasting and postprandial 2 h blood glucose levels, as well as OGTT levels. These findings are consistent with a previous study, which showed that STZ-diabetic rats pretreated 2-week with L. johnsonii La1 had a significant lower blood glucose level16. It is well-established that chronic inflammation induced by gut derived endotoxin plays a key role in the onset and development of T2DM4. Our result indicated that L. casei Zhang reduced the endotoxin LPS production induced by STZ injection and downregulated iNOS level.
The immunomodulatory effect of probiotics is well-established and they can regulate the balance of Th1 and Th2 responses through the production of different cytokines17. In this study, L. casei Zhang administration significantly inhibited the Th1 associated pro-inflammatory cytokines (IFN-γ and TNF-α) as well as Th1 immune response related to T-bet gene mRNA level and thus remarkably inhibited the development of T2DM in rats. We further exmined the mechanisms participate in the Th1 immunomodulatory effect of L. casei Zhang.
Several studies have reported that the blood lipid-reducing effect of probiotics (including L. casei Zhang) was mainly due to fecal bile acid elimination18,19. Our data showed that plasma bile acids level lowered by L. casei Zhang administration exhibited a notable reduction of glycemia risk in a rat model established by HFS-diet accompanied with low dose STZ-injection. Thus, it is suggested that the plasma bile acids level does not only associate with dyslipidemia but may also be related to the risk of glycemia. Our finding is supported by a previous metabolomic study showing the close correlation between the change of plasma bile acids and OGTT20. Moreover, endogenous bile acids alteration or exogenous bile acid (derivative) administration has therapeutic potential for treating metabolic diseases6. Thus, the manipulation of endogenous bile acids is a potential target for diabetes prevention.
Fecal bacteria with bile acid 7α-DH activities are mostly members of the genera Eubacterium and Clostridium21,22. Moreover, C. scindens, C. hiranonis and C. hylemonae were found to have high 7α-DH activity while C. sordellii, C. leptum and C. bifermentans were of low 7α-DH activity23,24. In this study, a decrease in the fecal 7α-DH bacteria by L. casei Zhang administration appear to restrict the conversion of primary bile acids and reduce secondary bile acids production in the intestine. Consequently, liver and renal 7 alpha-dehydroxylating activity (CYP7A1) genes were significantly upregulated due to the excessive production of primary bile acids. This is consistent with other probiotic researches with high CYP7A1 expression25.
Previous research showed that a “bile acid–chloride exchanger” exists, as confirmed by the discovery of TGR5 signaling pathway which directly contributes to both bile acid uptake and chloride secretion26. Our data also showed the reduction of bile acid level by L. casei Zhang could cause a significant decrease in urinary Cl− excretion in the T2DM rats, as well as a tissue chloride influx and down-regulation of TGR5. Our observation supports the “bile acid–chloride exchanger” hypothesis.
Principally, the administration of L. casei Zhang prevents the loss of pancreatic ClC-2 and FoxA2 expression in high-fat-sucrose fed rats. Low pancreatic FoxA2 expression level is proven to be positively correlated with insulin resistance and the risk of T2DM27. Interestingly, the liver of FoxA2-deficient mice had shown high bile acid accumulation28. In addition, it has been proposed that a high intracellular Cl– in the β-cell of pancreas is essential to electrical activity of β-cell membrane and insulin release29.Considering all these, we presumed that probiotic pretreatment protects the pancreas in high-fat-sucrose fed rats by enhancing pancreatic ClC-2 expression and eliminating bile acids in feces through a bile acid–chloride exchanging mechanism.
Furthermore, we speculate that L. casei Zhang exerts protective effect on STZ challenge and reduced the release of LPS into blood via a liver GlyRs upregulation mechanism. Incidentally, liver kupffer cells contain a glycine gated chloride channel and glycine could decrease LPS induced inflammatory TNF-alpha release from kupffer cells30,31. Thus, kupffer cells may play an important role in the chloride influx induced protective effect on T2DM. Additionally, it has been suggested that liver ClC-3 channel activation may also participate in the protective effect of L. casei Zhang since ClC-3 channel is closely associated with the inflammatory nuclear factor (NF)-κB signaling32. In spleen, splenic macrophages also contain a glycine gated chloride channel and may participate in beneficial effect of L. casei Zhang33.
In the small intestine and colon, L. casei Zhang may participate in the maintenance of Cl− secretion and chloride channel protein expression. This effect may maintain the normal function of epithelial tight junction barrier34. Especially, M cells containing ClC-2-ClC-7 channels might play a role in acting as epithelial barrier35. Likewise, probiotics such as S. boulardii and B. breve C50 also act as Cl− secretion regulator in the intestine36,37.
In the skeletal muscle, ClC-1 has been reported to be responsible for muscle electric excitability which is closely related to human myotonic disorders7. Typically, most myotonic dystrophy patients tend to comorbid with insulin resistance38. Therefore, it is worth testing if dietary L. casei Zhang has any potential to improve muscle excitability and prevention of myotonia.
Clinical studies have shown that cystic fibrosis (CF) is closely related to diabetes39. Interestingly, our data indicate that CF and diabetes shared the same pathogenic mechanism via the downregulation of chloride dependent genes expression. L. casei Zhang was shown to enhanced cardiac CFTR expression with the potential to prevent CF. Additionally, Bestrophin-3 (Best3) upregulation might improve microvascular perfusion and vasomotion for diabetes7.
Patients with T2D are characterized by low urine pH, significantly lower bicarbonate level and higher NH4+ concentration40. What is more, SLC26A3 deficient mice tend to display a chloride-losing diarrhea41. Thus, elevated SLC26A3 expression by L. casei Zhang was possibly involved in preventing STZ injected rats from lowing urine pH and electrolyte imbalance, which in turn alleviate diarrhea. Our result was also supported by Raheja et al who demonstrated Lactobacillus acidophilus could upregulate SLC26A3 expression42.
Previous investigators have confirmed that urolithiasis and kidney stones are common complicating diseases of T2D and SLC26A6 played a major constitutive role in limiting absorption of oxalate43,44. Thus, stimulation of the SLC26A6 expression by L. casei Zhang may reduce the risk of calcium oxalate formation.
Hypertension is known to be a T2D complication. GABA receptor agonists are antihypertensive through inhibiting renal sympathetic nerve activity mediated by sympathoadrenal axis45. Thus, the GABA receptor regulatory role of L. casei Zhang in the kidney might protect the host from hypertension. Similar studies conducted in rats showed that L. johnsonii La1 could reduce renal sympathetic nerve activity and enhance parasympathetic nerve activity and thereby decreased blood pressure and glucose levels14,46.
Several animal studies suggest that an altered GI flora affects the gut–brain axis, but the exact mechanisms are unclear47. In our study, GI microflora possessing 7α-DH activity varied in response to L. casei Zhang administration and had led to an opposite chloride distribution in the prefrontal cortex and hippocampus, as compared to HF rats. This is consistent with similar study on L. rhamnosus (JB-1) showing that the provision of JB-1 could induce opposite changes of GABA(A) and GABA(B) mRNA in the prefrontal cortex and hippocampus48. Accumulating evidence suggests that T2D is associated with an increased risk of Alzheimer's disease (AD)49. Moreover, AD brains have low levels of GABA and a loss of functional GABA(A) receptors50. Thus, L. casei Zhang may act as a potential modulator of chloride ionotropic GABA-A receptors in hippocampus of rats, and aid in the prevention from AD-like symptoms. The downregulation of GABAAα 1 and increase of GABAAα 2 were previously observed in hippocampus of AD50.
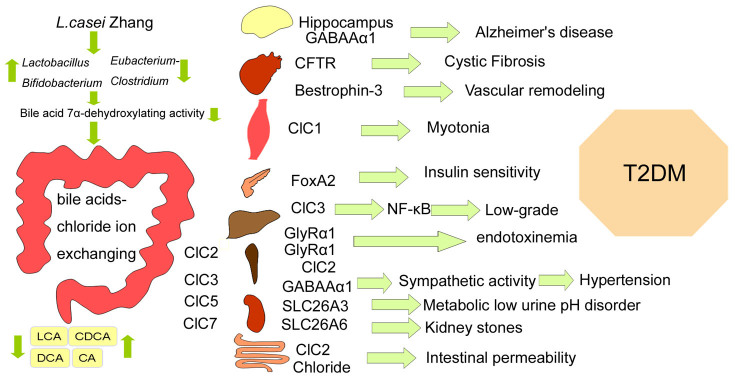
In summary, the results reveal that there seemingly exist some connections between the T2DM and its comorbid medical conditions/diseases characterized by mild organ chloride loss (compared to CT group, Fig. 2B), and illustrate the potential mechanisms of L. casei Zhang in preventing from T2DM onset and development. Interestingly, a mild organ chloride loss was consistent with a mild microflora change in a large-scale T2DM clinic research3. The preventive effect of L. casei Zhang on T2DM may be related to the reduced number of 7α-DH activity possessing bacteria, change in fecal bile acids composition, bile acids-chloride ion exchange, expression of various chloride-dependent genes and thus reduction of inflammatory response.
Conclusion
In conclusion, L. casei Zhang may be able to improve the onset and development of glycemia by rapidly altering gut microbiota. The short-term response to probiotic-altered microbiota may result in a chloride ion influx in multiple organs before chronic inflammation occurs. Moreover, tissue chloride ion loss and related genes suppression linked T2DM pathogenesis to its comorbid medical conditions/diseases (Fig. 6). Future studies will need to address the precise mechanisms involved in the bile acids-chloride ion exchange.
Figure 6. Proposed mechanism of L. casei Zhang-driven change in gut microflora and whole body chloride ion influx, and their probably relation with T2DM and its comorbid medical conditions/diseases.
Author Contributions
Y.Z., X.G., J.G., H.Q., Y.S. and L.H. contributed to experiment. X.G. analyzed the data. H.Z. designed the experiments and reviewed/edited the manuscript extensively. Y.Z. wrote the manuscript. Y.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary Material
Supplementary Info
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 31025019), the Innovation Team Development of the Ministry of Education of 386 China (Grant No. IRT0967), and China Agriculture Research System (Grant No. CARS-37).
References
- Holman R. R. Type 2 diabetes mellitus in 2012: Optimal management of T2DM remains elusive. Nat. Rev. Endocrinol. 9, 67–68 (2013). [DOI] [PubMed] [Google Scholar]
- Larsen N. et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5, e9085 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012). [DOI] [PubMed] [Google Scholar]
- Everard A. & Cani P. D. Diabetes, obesity and gut microbiota. Best. Pract. Res. Clin. Gastroenterol. 27, 73–83 (2013). [DOI] [PubMed] [Google Scholar]
- David L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Pellicciari R., Pruzanski M., Auwerx J. & Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug. Discov. 7, 678–693 (2008). [DOI] [PubMed] [Google Scholar]
- Verkman A. S. & Galietta L. J. Chloride channels as drug targets. Nat. Rev. Drug Discov. 8, 153–171 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejtahed H. S., Mohtadi-Nia J. & Homayouni-Rad A. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 28, 539–543 (2012). [DOI] [PubMed] [Google Scholar]
- Moroti C., Souza Magri L. F., de Rezende Costa M., Cavallini D. C. & Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 11, 29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E. et al. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J.Appl. Microbiol. 110, 650–657 (2011). [DOI] [PubMed] [Google Scholar]
- Amar J., Chabo C. & Waget A. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol. Med. 3, 559–572 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U S A, in press (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. Probiotic Lactobacillus casei Zhang ameliorates high-fructose induced impaired glucose tolerance in hyperinsulinemia rats. Eur. J. Nutr. in press (2013). [DOI] [PubMed] [Google Scholar]
- Sehoenfeld B. G. & Lewellen C. J. A colorimetrie method for determination of serum chloride. Clin. Chem. 10, 533 (1964). [PubMed] [Google Scholar]
- Neyrinck A. M., Possemiers S., Verstraete W., De Backer F., Cani P. D. & Delzenne N. M. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J. Nutr. Biochem. 23, 51–59 (2012). [DOI] [PubMed] [Google Scholar]
- Yamano T. et al. Effects of the probiotic strain Lactobacillus johnsonii strain La1 on autonomic nerves and blood glucose in rats. Life Sci. 79, 1963–1967 (2006). [DOI] [PubMed] [Google Scholar]
- Delcenserie V., Martel D., Lamoureux M., Amiot J., Boutin Y. & Roy D. Immunomodulatory effects of probiotics in the intestinal tract. Curr. Issues Mol. Biol. 10, 37–54 (2008). [PubMed] [Google Scholar]
- Ooi L. G. & Liong M. T. Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int. J. Mol. Sci. 11, 2499–2522 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Du R., He Q., Li H. & Zhang H. Beneficial effects of L. casei Zhang on liver lipids of diet-induced hypercholesterolemia rats. Scientia Agricultura Sinica 45, 943–950 (2012) (in Chinese). [Google Scholar]
- Zhao X. et al. Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am. J. Physiol. Endocrinol. Metab. 296, 384–393 (2009). [DOI] [PubMed] [Google Scholar]
- Doerner K. C., Takamine F., LaVoie C. P., Mallonee D. H. & Hylemon P. B. Assessment of fecal bacteria with bile acid 7 alpha-dehydroxylating activity for the presence of bai-like genes. Appl. Environ. Microbiol. 63, 1185–1188 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara M., Takamine F., Imamura T. & Benno Y. Assignment of Eubacterium sp. VPI 12708 and related strains with high bile acid 7alpha-dehydroxylating activity to Clostridium scindens and proposal of Clostridium hylemonae sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 50, 971–978 (2000). [DOI] [PubMed] [Google Scholar]
- Prabha V. & Ohri M. Review: Bacterial transformations of bile acids. World J. Microbiol. Biotechnol. 22, 191–196 (2006). [Google Scholar]
- Wells J. E., Williams K. B., Whitehead T. R., Heuman D. M. & Hylemon P. B. Development and application of a polymerase chain reaction assay for the detection and enumeration of bile acid 7alpha-dehydroxylating bacteria in human feces. Clin. Chim. Acta 331, 127–134 (2003). [DOI] [PubMed] [Google Scholar]
- Watanabe S. et al. Effect of Lactobacillus brevis 119-2 isolated from Tsuda kabu red turnips on cholesterol levels in cholesterol-administered rats. J. Biosci. Bioeng. 116, 45–51 (2013). [DOI] [PubMed] [Google Scholar]
- Keitel V., Cupisti K., Ullmer C., Knoefel W. T., Kubitz R. & Häussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 50, 861–870 (2009). [DOI] [PubMed] [Google Scholar]
- Ohtsubo K., Chen M. Z., Olefsky J. M. & Marth J. D. Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat. Med. 17, 1067–1075 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkis I. M., Rubins N. E., White P., Furth E. E., Friedman J. R. & Kaestner K. H. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat. Med. 14, 828–836 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best L. Glucose-induced electrical activity in rat pancreatic β-cells: dependence on intracellular chloride concentration. J. Physiol. 568, 137–144 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikejima K., Qu W., Stachlewitz R. F. & Thurman R. G. Kupffer cells contain a glycine-gated chloride channel. Am. J. Physiol. 272, 1581–1586 (1997). [DOI] [PubMed] [Google Scholar]
- Yin M. et al. Glycine accelerates recovery from alcohol-induced liver injury. J. Pharmacol. Exp. Ther. 286, 1014–1019 (1998). [PubMed] [Google Scholar]
- Yang H. et al. Decrease of intracellular chloride concentration promotes endothelial cell inflammation by activating nuclear factor-κB pathway. Hypertension 60, 1287–1293 (2012). [DOI] [PubMed] [Google Scholar]
- Froh M., Thurman R. G. & Wheeler M. D. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 283, 856–863 (2002). [DOI] [PubMed] [Google Scholar]
- Nighot P. K. & Blikslager A. T. Chloride channel ClC-2 modulates tight junction barrier function via intracellular trafficking of occludin. Am. J. Physiol. Cell Physiol. 302, 178–187 (2012). [DOI] [PubMed] [Google Scholar]
- Kulka M., Schwingshackl A. & Befus A. D. Mast cells express chloride channels of the ClC family. Inflamm. Res. 51, 451–456 (2002). [DOI] [PubMed] [Google Scholar]
- Girard P., Pansart Y., Coppe M. C. & Gillardin J. M. Saccharomyces boulardii inhibits water and electrolytes changes induced by castor oil in the rat colon. Dig.Dis. Sci. 50, 2183–2190 (2005). [DOI] [PubMed] [Google Scholar]
- Heuvelin E., Lebreton C., Bichara M., Cerf-Bensussan N. & Heyman M. A Bifidobacterium probiotic strain and its soluble factors alleviate chloride secretion by human intestinal epithelial cells. J. Nutr. 140, 7–11 (2010). [DOI] [PubMed] [Google Scholar]
- Fernández-Real J. M. et al. Tumor necrosis factor system activity is associated with insulin resistance and dyslipidemia in myotonic dystrophy. Diabetes 48, 1108–1112 (1999). [DOI] [PubMed] [Google Scholar]
- Bridges N. Diabetes in Cystic Fibrosis. Paediatr. Respir. Rev. in Press (2013). [DOI] [PubMed] [Google Scholar]
- Maalouf N. M., Cameron M. A., Moe O. W. & Sakhaee K. Metabolic basis for low urine pH in type 2 diabetes. Clin. J. Am. Soc. Nephrol. 5, 1277–1281 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinfest C. W. et al. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J. Biol. Chem. 281, 37962–37971 (2006). [DOI] [PubMed] [Google Scholar]
- Raheja G. et al. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 298, 395–401 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. N., Stampfer M. J. & Curhan G. C. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 68, 1230–1235 (2005). [DOI] [PubMed] [Google Scholar]
- Jiang Z. et al. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat. Genet. 38, 474–478 (2006). [DOI] [PubMed] [Google Scholar]
- Unger T. et al. Antihypertensive effect of the GABA receptor agonist muscimol in spontaneously hypertensive rats. Role of the sympathoadrenal axis. Circ. Res. 54, 30–37 (1984). [DOI] [PubMed] [Google Scholar]
- Tanida M., Yamano T., Maeda K., Okumura N., Fukushima Y. & Nagai K. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci. Lett. 389, 109–114 (2005). [DOI] [PubMed] [Google Scholar]
- Foster J. A. & McVey Neufeld K. A. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. in Press (2013). [DOI] [PubMed] [Google Scholar]
- Bravo J. A. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci.U S A 108, 16050–16055 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer R. A., Karter A. J., Yaffe K., Quesenberry C. P. & Selby J. V. Hypoglycemic Episodes and Risk of Dementia in Older Patients with Type 2 Diabetes Mellitus. JAMA 301, 1565–1572 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limon A., Reyes-Ruiz J. M. & Miledi R. Loss of functional GABA(A) receptors in the Alzheimer diseased brain. Proc. Natl. Acad. Sci. U S A 109, 10071–10076 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Info