More than 50 years ago, smooth muscle cells (SMC) of the carotid artery were shown to undergo “de-differentiation” upon ligation injury1. Since this classic study, scores of research groups have used a variety of in vivo and in vitro model systems as well as numerous clinical studies to demonstrate the conversion of normally contractile vascular SMC to a less differentiated state of proliferation, migration, and exuberant extracellular matrix secretion, a process generally referred to as phenotypic modulation or plasticity [reviewed in 2–6]. Such changes in vascular SMC phenotype are thought to underlie many vascular occlusive diseases. However, in a recent issue of Nature Communications, Tang et al.7 provide data from in vitro cell culture, flow cytometry, and lineage tracing experiments which they believe directly challenge this long-standing and widely accepted paradigm. Specifically, these authors contend that: (1) differentiated (SM myosin heavy chain, SM MHC+) vascular SMC are incapable of proliferation either in vivo in response to injury or in vitro in cell culture; (2) within the media of mature blood vessels there exists a small population (<10%) of undifferentiated (SM MHC-) cells that activate markers of mesenchymal stem cells, including Sox17, Sox10, and S100β, and proliferate to completely reconstitute medial cells in response to vascular injury; and (3) these so-called medial-derived multi-potential vascular stem cells (MVSC) also proliferate and express several mesenchymal stem cell markers when placed in cell culture, and can be induced to differentiate into neural, chondrogenic, and SMC lineages with appropriate culture methods. The authors’ bold conclusion that “MVSC activation and differentiation, instead of SMC de-differentiation, results in the proliferative and synthetic cells in the vascular wall”, if true, would have a huge impact on our understanding of the role of SMC in vascular injury-repair and disease. Since an implicit aspect of the authors’ claims is that previous studies have been conducted on MVSC, not SMC, it also potentially impacts conclusions from thousands of papers using cultured SMC as model systems. However, close examination of their results, which are in direct contradiction to a large body of published research, reveals a number of misinterpretations, overstatements, and technical deficiencies which seriously undermine most of their major conclusions as discussed further in this Perspective.
Although extensive studies have identified mechanisms that control the process of SMC phenotypic switching in cultured cells [reviewed in Owens et al. 2], there are still major ambiguities regarding the definitive identification of altered SMC phenotypes during vascular remodeling, including vascular injury-induced proliferation and atherosclerotic plaque progression since a key feature of this process is the loss of expression of SMC-selective gene products such as SM MHC and SM α-actin (SMαA) [reviewed in Gomez and Owens 8]. As stated by Tang et al.7, “a widely accepted explanation is that SMCs have phenotypic plasticity and that mature or contractile SMCs can de-differentiate into proliferative and synthetic SMCs. However, this de-differentiation process has not been directly demonstrated by tracking the fate of mature or contractile SMCs.” Indeed, we support this statement, i.e. that there is a critical need for definitive in vivo SMC lineage tracing studies, as emphasized in several recent reviews 6, 8.
Although not explicitly stated in the methods, the SMC lineage tracing model system utilized in the Tang et al.7 study was generated by crossing smMHCCre/eGFP mice, originally made by Michael Kotlikoff’s lab9 using a SM MHC promoter-enhancer provided by the Owens lab10 (which faithfully recapitulates expression of the endogenous SM MHC gene), with a Cre activatable indicator mouse strain, ROSA26-EGFP, both obtained from Jackson labs. The choice of a SM MHC based model system was a good one, since SM MHC is the most specific marker of differentiated SMC identified to date10. However, there is a major limitation of the model system of Tang et al.7 The model is not conditionally regulated and thus cannot rigorously lineage tag mature SMC at a given time point. Therefore, the model does not offer the opportunity to validate that the labeling is completely SMC-specific, and ultimately address to which cell type progeny belong. The lack of conditional SMC lineage tracing, along with deficiencies in experimental design and methods of data analyses, severely compromise the conclusions drawn by Tang et al.7. Indeed, a more compelling lineage tracing study of vascular SMC performed previously by Nemenoff et al. 11 provided clear evidence that differentiated SMC undergo phenotypic modulation in response to vascular injury using tamoxifen inducible SM MHC-CreERT2/Rosa26-floxStop/βGal mice, thus directly contradicting the findings of Tang et al.[see Figures 1A and 1C in Nemenoff et al.11].
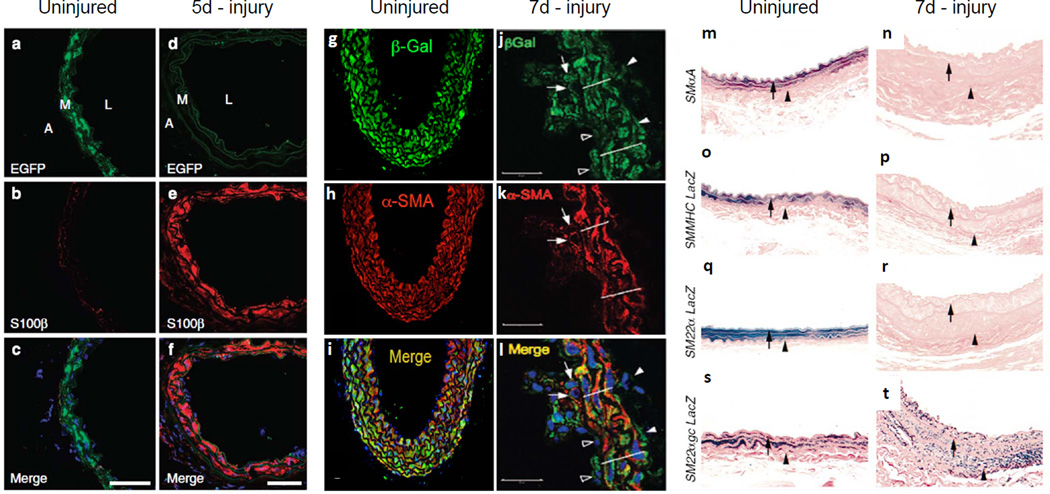
Since the lineage tracing systems differ in the Tang et al.7 and Nemenoff et al.11 studies, it is necessary to further scrutinize the discrepancies between these published results [represented in Figure 1 of this commentary]. Tang et al.7 show a notable lack of GFP staining 5 days post-wire injury that they interpreted as loss of mature SMC, though no formal demonstration of this implied conclusion was presented (e.g., TUNEL staining). Instead, the authors suggest total re-cellularization of the vascular wall by MVSC (Fig. 1a-1f herein which is adapted from Fig. 7a-f in Tang et al.7). In contrast, Nemenoff et al.11 showed that βGal+ SMC down-regulate SMαA and contribute to neointima formation at 7 days post-femoral artery wire injury (Fig. 1g-1l). Nemenoff et al.11 also demonstrated that a fraction of β-Gal+SMC are BrdU+ within the intima and media 3 weeks post injury, consistent with the prevailing dogma wherein mature SMC undergo injury-induced SMC phenotypic switching with onset of cell proliferation. Tang et al.7 proposed that the discrepancies in their results and those of Nemenoff et al.11 were due to the acquisition of βGal expression in MVSC after prolonged vascular remodeling. However, this is highly unlikely, because the conditionally regulated lineage tracing system used by Nemenoff et al.11 should not activate βGal in MVSC following vascular injury since tamoxifen was not present. Consistent with this, the tamoxifen serum half-life in mice is 12 hours12 and Nemenoff et al.11 completed their tamoxifen injections one week prior to injury. As such, we conclude that proliferating MVSC could not acquire βGal expression and that, in fact, mature SMC participate in vascular remodeling after injury. One possible explanation is that the extent of wire injury in the Tang et al.7 studies may have been very severe, leaving behind no resident GFP+ SMC in the media (compare Fig. 1a versus 1d), thus precluding their ability to analyze the role of SMC phenotypic modulation in vascular remodeling and neointima formation. Indeed, the failure to perform SM-MHC imaging and characterization of the mechanisms responsible for loss of medial SMC at early time points following injury is a major weakness of the Tang et al.7 studies. Moreover, based on the methods section, it appears that Tang et al.7 may not have properly fixed carotid arteries prior to OCT embedding, which is required to prevent soluble GFP from leaking 13, 14. As such, it is also possible that improper fixation methods used by Tang et al.7 resulted in markedly reduced sensitivity to detect GFP+ SMC, including those that proliferate or contribute to long term repair of vascular injury.
Figure 1.
Synthetic smooth muscles contribute to vascular remodeling following wire injury. (a-f) Lineage-tracing data at 5 days post-carotid wire injury using a non-inducible SM-MHC-Cre/eGFP floxed-stop Rosa GFP mouse, adapted from Tang et al.7. Following wire injury, no SMC can be detected in the media (d), precluding the ability to analyze the role of SMC phenotypic modulation. The media is replaced by cells expressing S100b (e). (g-l) Lineage-tracing data at 7 days post-femoral artery wire injury using a tamoxifen-inducible SM MHC-CreERT2/Rosa26-floxStop βGal mouse, adapted from Nemenoff et al. 11. Following wire injury, intimal bGal+SMaA+ SMC (arrows) and bGal+SMaA-phenotypically modulated SMC (open arrowheads) are observed, including BrdU+ LacZ+ proliferating cells (see Fig. 1c of Nemenoff et al. supporting the role of synthetic SMC in vascular remodeling and neointima formation. Lines delineate the arterial media. (m-t) Lineage tracing data at 7 days post-carotid wire injury using SM-MHC-LacZ, SM22a-LacZ, or SM22agc-LacZ (mutated G/C repressor) reporter mice, adapted from Regan et al.17. Following wire injury, SMC de-differentiate as evidenced by no positive staining in SM-MHC-LacZ mice SM22a-LacZ. Medial and intimal phenotypically modulated SMC are identified by LacZ+ staining post-injury in the SM22agc-LacZ (mutated G/C repressor) reporter mice, further supporting the role of synthetic SMC in vascular remodeling and neointima formation. Arrows denote internal elastic lamina and arrowheads denote external elastic lamina.
We agree with Tang et al.7 that “…the fate of mature or contractile SMCs” requires direct in vivo lineage tracing. Although Nemenoff et al.11 did not provide data elucidating the ultimate fate of phenotypically modulated SMC, neither did Tang et al., despite their claims. Surprisingly, Tang et al.7 only presented data at a single 5-day time point post-wire injury in their smMHCCre/eGFP Rosa26-EGFP mice [Fig. 7D and Supplemental Figure 14 in Tang et al.7], and then, for reasons that are not clear, they switch to use of non-lineage tracing rats for all subsequent time points. Since the authors do not analyze cell fates beyond 5-days of injury, and since their lineage tracing mouse is non-inducible such that any cell that transiently expresses SM MHC will activate their lineage tracing gene and be labeled, they cannot draw any conclusions as to whether or not resident differentiated medial SMC contributed to vascular remodeling after wire injury. In addition, it is unclear whether Tang et al. performed high resolution confocal analyses with Z-stack imaging as is necessary for rigorous lineage tracing [reviewed in Hoofnagle et al.15, 16]. Moreover, many of the images are of low magnification and provide poor resolution of individual cells as required for definitive lineage tracing, and/or immunofluorescence tissue staining is inconsistent across samples. For example, the staining for SMαA (compare Fig. S1 with Fig. 6e), and EGFP (compare Fig. 3a with Fig. 7a), varies drastically between figures. Finally, despite the major focus of the paper being on analysis of SM MHC-MVSCs, many of the key figures fail to show staining for this critical marker.
There are also numerous other previous studies that contradict the key findings of Tang et al. 7 using independent experimental approaches and animal models. Due to space constraints we can only describe a few examples of these reports, and we apologize to the many groups whose excellent work we are unable to cite. For example, Owens and co-workers17, 18 performed lineage tracing studies using a novel transgenic mouse harboring a SM22α-LacZ reporter gene containing a mutated G/C repressor element17 which faithfully recapitulates expression of the endogenous SM22α gene throughout development and maturation. However, this reporter is almost completely resistant to down-regulation during SMC phenotypic switching in response to wire injury17 (Fig. 1t), or during development of experimental atherosclerosis18. Thus, phenotypically modulated SMC can be detected by LacZ expression when endogenous SMC marker genes are silenced. Using this transgenic mouse, Regan et al.17 observed LacZ+ phenotypically-modulated SMC in the media and neointima 7 days after carotid wire injury [see Fig. 4n in reference 17 as well as Fig. 1t herein]. Most importantly, these LacZ+ cells were not the result of transient activation of the transgene in other cell types since positive staining was not observed at 7 days post-injury in either a SMαA LacZ, SM MHC LacZ, or SM22α LacZ mice [Figs. 4b,4f,4j in reference 17 and Figs. 1n,1p,1r herein]. Similarly, Wamhoff 18 showed a high frequency of proliferating intimal SMC within lesions of Western diet fed ApoE-/- mice using the SM22α G/C repressor lineage tracing mouse model. Consistent with the preceding findings that SMC undergo phenotypic transitions, SM22α Cre dependent lineage tracing has provided evidence that SMC give rise to osteochrondrogenic cells within calcified arterial media19, as well as atherosclerotic lesions20. These latter studies in more pathophysiologically relevant atherosclerosis models, as compared to wire injury, provide additional compelling evidence for SMC phenotypic transitions.
Several classic studies completed more than 30 years ago also directly refute the major conclusions of Tang et al. 7. First, innovative experiments by Wilbur Thomas and co-workers21, conducted in hyperlipidemic pig models of atherosclerosis, used 3H-thymidine pulse labeling experiments followed by complete serial sectioning of the thoracic aortas and construction of ancestor tables based on grain counting. Their experiments relied on the simple principle that when a cell undergoes sequential rounds of cell division in vivo, there is sequential halving of grain counts. Remarkably, the results of Thomas et a.l21 provided compelling evidence showing that large numbers of medial SMC undergo multiple rounds of cell division during development of experimental atherosclerosis. Second, the comprehensive studies of Alexander Clowes, Monika Clowes, and co-workers22, 23, as well as the seminal work of Stemerman and colleagues24 provided clear evidence that a large number of medial SMC are capable of proliferation following vascular injury. Briefly, Clowes et al.22, 23 performed 3H-thymidine labeling studies following balloon catheter induced denudation of the rat carotid artery, and then determined growth fractions (i.e. the fraction of initial cells that enter the cell cycle) at various time points post injury. Of major relevance to the focus of this commentary, 46% of medial SMC entered the cell cycle within 48 hours post injury22. Further, experiments by Miano et al.25 showed rapid induction of a number of early growth response genes in the medial layer of the rat aorta following balloon injury; immunolocalization studies confirmed such rapid activation in clusters of SMC (much more than 10%) subjacent to the intima where platelet- and serum-derived factors first emerge following injury (Figure 4D in25). Taken together, these studies cast serious doubt over the concept advanced by Tang et al.7 that differentiated SMC are post-mitotic, and that the “proliferating” population of cells following vascular injury, are derived solely from a minor stem cell population.
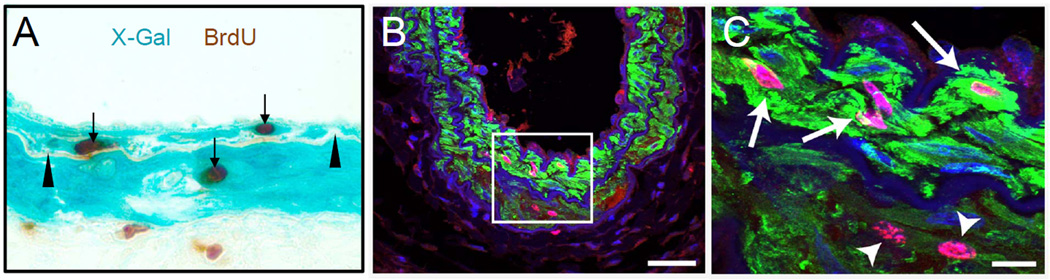
Another deficiency in Tang et al.7 is a general failure to provide rigorous quantitation of their data. For example, insufficient evidence is presented to substantiate their claim that 10% of medial cells are SM MHC-, such as performing flow cytometric assays, and/or showing high resolution confocal images of histological sections clearly illustrating this purported cell population along with graphical data with sufficiently high replicates to make statistical inferences. Moreover, their broad conclusion that there is a lack of proliferating SM MHC+ medial cells is based on showing one cell in vitro (Fig. 1c in Tang et al.7). Of greatest concern, they fail to show a single image with co-staining for SM MHC and the proliferation marker Ki67 in their in vivo model, which is arguably the most important experiment for testing their stated hypothesis. For example, the data shown in Fig. 6g, 6h, and 6i would have been far more informative had they stained for SM MHC rather than SMα, and also included Ki67 staining. Indeed, the results shown in Figure 2 herein, based on unpublished studies from the laboratories of Drs. Weiser-Evans, and Miano, clearly show that SM MHC+ SMC do, in fact, enter the cell cycle 7 days following distinct modes of murine arterial injury thus directly refuting one of the major conclusions of the Tang et al.7 study. Importantly the results shown in Figure 2 were derived from two separate laboratories using independent but complementary methodologies. Finally, there is a growing body of evidence from studies of SM MHC promoter-dependent conditional knockout mice that are highly inconsistent with the conclusions of Tang et al.7 that mature SM MHC+ medial SMC are incapable of phenotypic transitions. Just two, of many examples, include the studies of Nemenoff et al.11 and Offermanns et al.26, showing that SMC-specific conditional knockout of PTEN, or the α-subunits of Gq-G11 (or G12-G13), respectively, resulted in profound changes in SMC phenotype.
Figure 2.
SM MHC+ SMC replicate following arterial injury. (A) Section of femoral artery of SM MHC-CreERT2/Rosa26-floxStop-βGal reporter mouse 7 days following wire injury [data provided by the laboratory of Dr. Mary C. Weiser-Evans] [see Nemenoff et al.11 for additional methodological details]. Arrow heads denote internal elastic lamina and arrows indicate proliferating BrdU+ SMC. (B, C) 10 μm thick reconstructed Z-stacked confocal microscopy of twenty 0.5 μm images from a wildtype mouse carotid artery 7 days following ligation injury [unpublished data provided by the laboratory of Dr. Joseph M. Miano]. Most medial cells are SM MHC positive (green) with many exhibiting clear Ki67 staining (magenta). Nuclei are stained with DAPI. The bar in panel B is 50 μm. Boxed region in panel B is shown at higher magnification in panel C with arrows pointing to obvious SM MHC+/Ki67+ medial SMC and arrowheads indicating weakly stained SM MHC+/Ki67+ cells. The bar in panel C is 10 μm.
Taken together, prior studies clearly refute the major conclusions of the Tang et al. 7 paper, and clearly establish that in vivo SMC can exhibit a modulated phenotype after vascular injury or in experimental atherosclerosis that includes de-differentiation, rapid induction of numerous early growth response genes, proliferation, and neointima migration as well as other phenotypic transitions. In light of this body of evidence, what conclusions, if any, can be drawn from the in vitro data of Tang et al.7? In their attempt to establish primary SMC cell lines using both enzymatic digestion and tissue explant methods, they serendipitously established MVSC cell lines that were capable of differentiating into ectodermal and mesodermal lineages. Tang et al.7 conclude that the proliferative-synthetic SMC studied by most laboratories until now are actually derived from these MVSC. Although it is plausible that current cell lines of synthetic SMC may be derived from, or may contain a proportion of MVSC, there is a much simpler explanation for the results of Tang et al.7 that may render them of questionable significance due to several critical deficiencies in their SMC isolation and culture methods long appreciated by most SMC labs. First, although the author’s claim that MVSC do not arise from the adventitia, it is virtually impossible to effectively remove the adventitia from large vessels without a pre-digestion step followed by careful micro-dissection which allows one to peel off the adventitial layer like a sock. Tang et al.7 apparently failed to use this pre-digestion step to remove the adventitia, which especially with small rodent vessels, can result in cultures highly contaminated with adventitial fibroblasts and adventitial Sca1+ stem cells since these cells show much higher plating efficiencies and growth rates as compared to SMC. Although the MVSC described by Tang et al.7 are Sca1-, it is known that expression of this gene is rapidly down-regulated once cells are placed in culture27. The preceding problems, particularly if compounded with use of non-optimized methods for isolating and culturing SMC, result in derivation of cultured cells virtually devoid of SMC. Although the data presented in Fig. 3b indicate that the cell isolates derived by Tang et al.7 are primarily GFP+ at time zero, the cells that actually adhere to the culture dish and give rise to cultures 24 hours later are weakly stained for SMα (Fig. 6a; described as SMα “low” by authors), which is in marked contrast to the results of experienced SMC laboratories where >90% of plated medial cells continue to express SMC markers for many days in culture5. Indeed, Blank et al.28 showed that although enzymatically dissociated SMC rapidly suppress synthesis of SMC marker genes, SMαA and SM MHC are long-lived proteins so that they remain detectable well after cells have undergone phenotypic switching and initiated proliferation in culture. Second, it is well documented that it takes over one week for SMC to adhere, proliferate, and migrate using the tissue explant method 29–31, which was used extensively in the Tang et al.7 study. Thus, the authors cannot exclude the possibility that after 3 days of culture, adventitial cells, not a novel medial MVSC cell population, accounted for the SM-MHC-GFP-cells. Third, whereas we do not doubt that Tang et al.7 have isolated and identified a population of progenitor/stem cells capable of differentiating into multiple cell types in vitro, further controls and validation studies are needed to determine whether they are truly derived from the media, and/or are distinct from the multi-potential adventitial/perivascular stem cells previously described by several groups 27, 32–36. However, based on the marker expression patterns, they appear to be distinct from the stem cells identified by Peault and co-workers33, 37, but similar to those characterized by Klein et al.32 which were also CD44+CD29+CD31-CD34-CD146-, and of adventitial origin. Definitive resolution of this issue will require: 1) use of a conditionally-regulated SMC lineage tracing system such as that employed by Nemenoff et al.11 combined with high resolution confocal analyses of the subsequent fate of mature SMC at multiple time points following vascular injury, or induction of disease, as well as determination of the fate and properties of mature SMC in culture using techniques optimized for survival of the SMC in vitro; and 2) development of methods to directly lineage tag the putative MVSC population in vivo followed by rigorous quantitative fate mapping studies both in vivo and in vitro.
In summary, there are serious deficiencies that undermine most of the major conclusions of Tang et al.7. Contrary to their statements, there is compelling evidence that mature SMC are not terminally differentiated, and are capable of transitions in phenotype including cell proliferation and loss of differentiation markers. However, the Tang et al.7 studies do re-emphasize the importance of definitive characterization of the origins of SMC cultures, and the urgent need for more rigorous and complete SMC lineage tracing studies in vivo. Indeed, at present there is a scarcity of studies defining the ultimate fate of mature SMC in different models of vascular remodeling (repair) and disease, and further investigations are needed in this important area [reviewed by Gomez and Owens8]. Finally, although several groups have identified a perivascular origin of mesenchymal stem cells 33–36, it remains to be determined if synthetic SMC represent a reservoir of stem cells capable of trans-differentiation in vivo in different models of injury-repair. Indeed, should this prove to be true, it would have major therapeutic implications, including identification of potential approaches to augment the stem cell-like properties of SMC for purposes such as plaque stabilization, augmentation of vascular remodeling, stabilization of the tumor vasculature, and promoting overall tissue regeneration and repair.
Footnotes
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
Disclosures
None
References
- 1.Buck RC. Intimal Thickening After Ligature of Arteries An Electron-Microscopic Study. Circ Res. 1961 Mar 1;9(2):418–426. [Google Scholar]
- 2.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004 Jul;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 3.Alexander MR, Owens GK. Epigenetic Control of Smooth Muscle Cell Differentiation and Phenotypic Switchingin Vascular Development and Disease. Annu Rev Physiol. 2012 Feb 15;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 4.Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell. Science. 1973;180:1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- 5.Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979;59:1–6. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular Smooth Muscle Progenitor Cells: Building and Repairing Blood Vessels. Circ Res. 2011 Feb 4;108(3):365–377. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA, Li S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012 Mar 8; doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xin HB, Deng KY, Rishniw M, Ji G, Kotlikoff MI. Smooth muscle expression of Cre recombinase and eGFP in transgenic mice. Physiol Genomics. 2002 Sep 3;10(3):211–215. doi: 10.1152/physiolgenomics.00054.2002. [DOI] [PubMed] [Google Scholar]
- 10.Madsen CS, Regan CP, Hungerford JE, White SL, Manabe I, Owens GK. Smooth muscle-specific expression of the smooth muscle myosin heavy chain gene in transgenic mice requires 5"-flanking and first intronic DNA sequence. Circ Res. 1998;82:908–917. doi: 10.1161/01.res.82.8.908. [DOI] [PubMed] [Google Scholar]
- 11.Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, VanPutten V, Crossno J, Offermanns S, Weiser-Evans MC. SDF-1alpha induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2011 Jun;31(6):1300–1308. doi: 10.1161/ATVBAHA.111.223701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson SP, Langan-Fahey SM, Johnson DA, Jordan VC. Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab Dispos. 1991 Jan;19(1):36–43. [PubMed] [Google Scholar]
- 13.Jockusch H, Voigt S, Eberhard D. Localization of GFP in frozen sections from unfixed mouse tissues: immobilization of a highly soluble marker protein by formaldehyde vapor. J Histochem Cytochem. 2003 Mar;51(3):401–404. doi: 10.1177/002215540305100315. [DOI] [PubMed] [Google Scholar]
- 14.Kusser KL, Randall TD. Simultaneous detection of EGFP and cell surface markers by fluorescence microscopy in lymphoid tissues. J Histochem Cytochem. 2003 Jan;51(1):5–14. doi: 10.1177/002215540305100102. [DOI] [PubMed] [Google Scholar]
- 15.Hoofnagle MH, Wamhoff BR, Owens GK. Lost in transdifferentiation. J Clin Invest. 2004 May;113(9):1249–1251. doi: 10.1172/JCI21761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoofnagle MH, Thomas JA, Wamhoff BR, Owens GK. Origin of Neointimal Smooth Muscle: We've Come Full Circle. Arterioscler Thromb VAsc Biol. 2006 Dec;26(12):2579–2581. doi: 10.1161/01.ATV.0000249623.79871.bc. [DOI] [PubMed] [Google Scholar]
- 17.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000 Nov;106(9):1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C Element Mediates Repression of the SM22alpha Promoter Within Phenotypically Modulated Smooth Muscle Cells in Experimental Atherosclerosis. Circ Res. 2004 Nov 12;95(10):981–988. doi: 10.1161/01.RES.0000147961.09840.fb. [DOI] [PubMed] [Google Scholar]
- 19.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009 Mar 27;104(6):733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naik V, Leaf EM, Hu JH, Yang HY, Nguyen NB, Giachelli CM, Speer MY. Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study. Cardiovasc Res. 2012 Jun 1;94(3):545–554. doi: 10.1093/cvr/cvs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas WA, Florentin RA, Reiner JM, Lee WM, Lee RT. Alterations in population dynamics of arterial smooth muscle cells during atherogenesis: IV Evidence for a polyclonal origin of hypercholesteremic diet-induced atherosclerotic lesions in young swine. Exp Mol Pathol. 1976;24:244–260. doi: 10.1016/0014-4800(76)90009-5. [DOI] [PubMed] [Google Scholar]
- 22.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 23.Clowes AW, Schwartz SM. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985;56:139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg ID, Stemerman MB, Schnipper LE, Ransil BJ, Crooks GW, Fuhro RL. Vascular smooth muscle cell kinetics: a new assay for studying patterns of cellular proliferation in vivo. Science. 1979 Aug 31;205(4409):920–922. doi: 10.1126/science.472713. [DOI] [PubMed] [Google Scholar]
- 25.Miano JM, Vlasic N, Tota RR, Stemerman MB. Localization of Fos and Jun proteins in rat aortic smooth muscle cells after vascular injury. Am J Pathol. 1993;142:715–724. [PMC free article] [PubMed] [Google Scholar]
- 26.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind S, Offermanns S. G(12)-G(13)-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008 Jan;14(1):64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 27.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci U S A. 2008 Jul 8;105(27):9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blank RS, Thompson MM, Owens GK. Cell cycle versus density dependence of smooth muscle alpha actin expression in cultured rat aortic smooth muscle cells. J Cell Biol. 1988;107:299–306. doi: 10.1083/jcb.107.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koba S, Pakala R, Watanabe T, Katagiri T, Benedict CR. Vascular smooth muscle proliferation: synergistic interaction between serotonin and low density lipoproteins. J Am Coll Cardiol. 1999 Nov 1;34(5):1644–1651. doi: 10.1016/s0735-1097(99)00349-6. [DOI] [PubMed] [Google Scholar]
- 30.Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen D, Haudenschild CC. Activation of smooth muscle cell outgrowth from BB/Wor rat aortas. Diabetes. 1988 Oct;37(10):1380–1385. doi: 10.2337/diab.37.10.1380. [DOI] [PubMed] [Google Scholar]
- 32.Klein D, Weisshardt P, Kleff V, Jastrow H, Jakob HG, Ergun S. Vascular wall-resident CD44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation. PLoS One. 2011;6(5):e20540. doi: 10.1371/journal.pone.0020540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008 Sep 11;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007 Mar;9(3):255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 35.Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 36.Paul G, Ozen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E, Jansson K, Dannaeus K, Henriques-Oliveira C, Roybon L, Anisimov SV, Renstrom E, Svensson M, Haegerstrand A, Brundin P. The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS One. 2012;7(4):e35577. doi: 10.1371/journal.pone.0035577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012 May 20;21(8):1299–1308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]