Abstract
Progress in the development of novel therapeutics to treat sepsis has come to virtual standstill. While enormous strides have been made in the understanding of basic molecular mechanisms that underlie the pathophysiology of sepsis, a distressingly long list of novel therapeutic agents have been tested in large clinical trials over the past 25 years without a single, specific, immunomodulating agent showing consistent benefit in sepsis trials. The only novel anti-sepsis agent to successfully complete a phase 3 sepsis trial, human recombinant activated protein C, was recently taken off the market after a follow up placebo-controlled trial (PROWESS SHOCK) failed to replicate the results of the initial registration trial (PROWESS) performed 10 yr earlier. We must critically re-examine our basic approach in the preclinical and clinical evaluation of new sepsis therapies. We propose 12 specific recommendations that if implemented could improve the outlook for developing new drugs for sepsis.
Keywords: Sepsis, severe sepsis, septic shock, clinical trial design, activated Protein C
“The definition of insanity is repeating the same experiment over and over again, expecting a different result” - Albert Einstein
Introduction
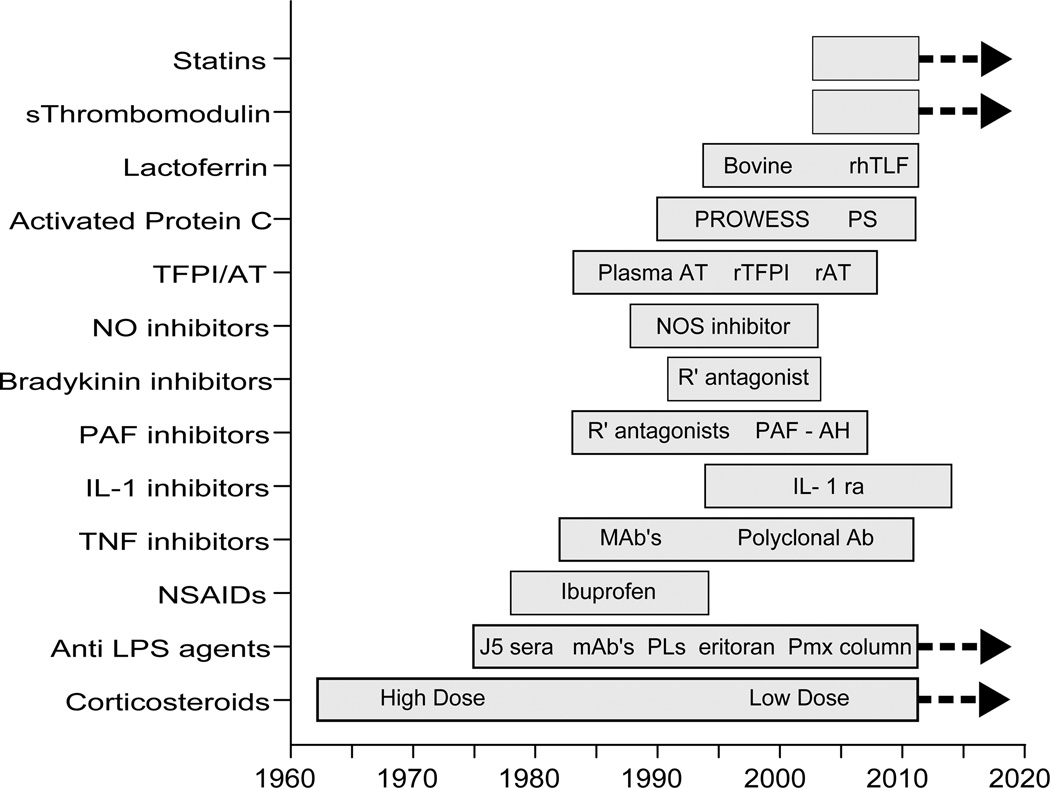
Over the past 30 years, the prognosis for patients with severe sepsis and septic shock has improved substantially, and yet this improvement is due almost exclusively to advances in the supportive care delivered to critically ill patients and expeditious use of antimicrobial agents (1–4). Hundreds of millions of dollars have been expended enrolling over 30,000 patients in clinical trials to test and develop new immunomodulating agents, anti-inflammatory agents, and anti-endotoxin agents (Figure 1). Yet, not a single agent has convincingly proven to be consistently efficacious in clinical trials. There are no new drugs on the market to show for all this effort.
Figure 1.
A summary of the recent history of experimental agents tested in clinical trials for the treatment of sepsis
S-soluble; rTLAF - human recombinant talactoferrin; PS - PROWESS/Shock; AT- antithrombin; TFPI - tissue factor pathway inhibitor; r-recombinant; NO-nitric oxide; NOS – nitric oxide synthase; PAF – platelet activating factor; R’- receptor; PAF-AH - PAF acetyl hydrolase ; IL-1ra- interleukin-1 receptor antagonist; mAb-monoclonal antibody; Ab-antibody; J5 sera - polyclonal antibodies in serum directed against the core glycolipid structure from the lipopolysaccharide of E. coli strain J5; PL’s – phospholipids to include phospholipid emulsions and high density lipoprotein; Pmx-polymyxin B hemoperfusion columns.
The bars indicate the approximate start dates and finish dates of each intervention in clinical trials. The bars with a thick black line on the right side of the bar indicate current ongoing studies.
Recent failed trials with activated Protein C (5,6), human recombinant lactoferrin (7) and the MD2- TLR4-LPS antagonist Eritoran (8) are further indicators that our basic underlying hypotheses and conventional assumptions are flawed, or the strategies employed to test these hypotheses in clinical trials are flawed. Justifiable skepticism now pervades the field of clinical sepsis research. Hopes for real progress in bringing novel therapeutics through the regulatory phase into the clinic has dimmed, and unless some new ideas are instituted in drug discovery and evaluation process, biotechnology investments and research programs will understandably redirect their resources and energies elsewhere. We must change course in the conduct of new drug evaluation for sepsis if we hope to succeed in the future. We propose some specific and readily achievable suggestions to redirect sepsis research toward a more hopeful future (table 1).
Table 1.
| Recommendation | Comments and advantages | |
|---|---|---|
| 1 | Preclinical animal studies are limited in value and need to be more realistic | Study aged animals using a salvage model after the septic insult has occurred |
| 2 | Develop biomarkers or response indicators to guide patient selection early in development | Biomarkers should fit the mechanism of action of the drug or device to select responsive patients and improve success |
| 3 | Phase 2 trials need several hundred patients with detailed mechanism of action studies, biomarker surveys, and dose finding | Phase 2 trial data should be large enough to assess accurately the chance for success in phase 3 studies |
| 4 | Improved biomarkers or surrogate endpoint measures are greatly needed to define optimal patient cohorts | Real time, dynamic genomics methods are needed to immunophenotype patients likely to benefit |
| 5 | Large phase 3 trials need to be adequately powered to find clinically meaningful improvements in outcome | Use conservative effect size calculations and low predicted mortality rates in control group |
| 6 | Consider study endpoints other than 28 day, all-cause mortality rates | Other patient centered outcomes (e.g. Ventilator-free days), or survival time analyses over longer periods (90 days) worth considering |
| 7 | Combinations of novel agents might be needed to show significant survival benefits | Combinations need to be mechanistically additive and safety needs to be assured |
| 8 | Adaptive trial design methodologies should be attempted in sepsis research | Early validation of a mechanistic pathway, supported by biomarkers, can identify a dose that affects the target pathway |
| 9 | Centers of excellence are optimal in phase 2; but phase 3 study sites with variable expertise in sepsis should assess the “real life” safety/efficacy of the experimental drug | Clinical coordinating centers can assist in phase 3 trials to limit variability and assure comparability across study centers |
| 10 | Study protocol entry criteria need to define a patient population at real risk for the study endpoint (e.g. death from sepsis) and some likelihood of responding to the study agent | Attributable risk for sepsis-related mortality is critical; Patients at high risk of death should not be excluded, they are most likely to benefit |
| 11 | Two large clinical trials, demonstrating reproducible efficacy in sepsis trials, will likely be necessary for drug or device registration | The costs are already prohibitive; an improved set of biomarkers, study design or addition of financial incentives will be needed to make these studies feasible |
| 12 | A central electronic repository of sepsis trial clinical information with standardized data collection needs to be established | Patient information derived from the past clinical trials, and a dedicated patient biobank of plasma and cells for genomic studies and biomarker studies are needed |
The short and long term consequences of sepsis already have an enormous impact on public health and these trends are only likely to worsen in the foreseeable future (10–12). The global expansion of vulnerable elderly patients, income disparities, and failure of the infrastructure capacity of developing nations to keep pace with the growing, increasing urbanized, human population make it likely that sepsis will increase in incidence for the next several decades. The widespread use of immunosuppressive medications for a wide variety of indications, rising use of implantable devices, and the spread of multiple, antibiotic-resistant, microbial pathogens will collectively conspire to keep infections high on the problem lists over the next 25 years (12). We re-examine the crucial elements in drug development for sepsis, highlight the barriers to further progress, and offer some potential solutions to move this field forward.
Preclinical Evaluation of New Sepsis Targets and interventions: What are we doing wrong?
A dichotomous view of the pathophysiology and outcome of sepsis is evolving in both animal models and in clinical studies. Classically, human sepsis has been characterized as a hyper-acute inflammatory syndrome manifest by the sudden systemic release of an array of inflammatory mediators. This presentation is mimicked in animal models of sepsis using overwhelming intoxication with endotoxin or high grade bacteremia. A clinical example of this presentation is the dramatic, hyper-acute clinical presentation seen in meningococcal septic shock or in the overwhelming post-splenectomy pneumococcal infection syndrome where a rapidly progressive syndrome runs its course over minutes to hours. However, such clinical presentations are only occasionally observed in hospitalized patients today.
More commonly, sepsis presents as a subacute illness with gradual development of multi-organ dysfunction that runs its course over days to weeks. Patients who survive this process are often left with impaired quality of life with cognitive defects and increased susceptibility to subsequent infections that persists for years (12–15). There is far less work done on the development of animal models that actually replicates this process in patient care. Measurable cognitive decline as seen in patients can be modeled in septic mice where survival from sepsis results in impaired memory function in mouse systems (Kevin Tracey, personal communication). Soluble mediators of cognitive decline are now under investigation. It is possible that the nuclear protein high mobility group box 1 may be one of the contributors to cognitive decline and a potential target for therapeutic intervention (14,15).
There are numerous examples in which pre-treatment models or models of systemic inflammation such as a lipopolysaccharide (LPS) challenge or intravenous bolus of bacteria does not predict clinical success (16). These models are essentially intoxication systems of acute severe inflammation and not true infection models. In some animal infection models, anti-cytokine therapy can actually worsen outcome (16,17). A recent transcriptome analysis comparing mouse models to the human host response to sepsis, trauma and burns revealed almost a complete lack of correlation in host response genes between mice and humans (18). Until we have validated models, we should be suspicious about their value and circumspect about our reliance on such models as guides to test efficacy in clinical drug development.
Animal models can still provide some, limited information in the development of new sepsis drugs. Pharmacokinetics, safety, potential toxicities and mechanisms of action can be investigated in animals and provide some margin of safety prior to exposing human volunteers to new therapies. Small animal models might be improved by the use of aged animals rather than young healthy animals, as is the current practice in most animal studies. Sepsis is primarily a disorder of older patients and animal models should be appropriately adjusted to account for this fact. Age-related changes in adaptive immune responses and increased susceptibility to apoptosis are well known immunologic events that likely impact the host response to sepsis (19). Preferably, such small animal studies should be followed by large animal models where careful hemodynamic and repeated physiologic measurements can be investigated. A more robust effort is needed in the further development of ex vivo systems and in silico models of the inflammatory response to determine if such systems can provide useful information.
The lack of precision sepsis definitions: What’s wrong with our terminology?
Sepsis research is hampered by the lack of accurate diagnostic methodologies or readily available, predictive biomarkers to direct clinical trial design and define patient populations likely to respond to specific anti-sepsis interventions. The diagnostic criteria for sepsis and severe sepsis are vague and readily confused with other medical and surgical diseases. Sepsis is not a discreet nosologic entity. Rather, it is a syndrome consisting of a constellation of signs and symptoms and laboratory features that together suggest a systemic inflammatory response related to infection accompanied by new onset organ dysfunction. Attempts to improve the diagnostic validity of the term sepsis and septic shock have been conducted since the early days when Bone and colleagues first attempted to codify the clinical terminology (11,20). In a recent epidemiologic investigation, modest changes in the interpretation of infection and SIRS criteria resulted in markedly different patient populations and major differences in overall mortality rate (21). This lack of precision in definitions of sepsis results in variation in defining septic patients across different centers and different clinical trials.
Patient heterogeneity has been and continues to be a hallmark of sepsis populations within clinical trials. This problem remains a major impediment to defining a responsive patient population for a specific intervention, and this issue remains a major unmet medical need in clinical trial design in sepsis studies. The use of sepsis biomarkers might help but no specific single or set of proposed biomarkers has been shown to be sufficiently discriminatory to help limit heterogeneity and improve the diagnostic utility of the term sepsis. Hopefully efforts in computational biology and personalized medicine will define a responsive patient population for sepsis trials rather than SIRS criteria for clinical trials (22).
Two basic strategies have been proposed to deal with the heterogeneity problem in sepsis trials: 1) use a pathogen-specific, mediator-based or an organ site-specific clinical trial design that is focused on a more homogenous patient population; or 2) conduct large, simple trial designs (mega trials) involving tens of thousands of patients to detect small but significant overall trends in large sepsis populations. There are arguments in favor and against both approaches. Pathogen-specific diseases that would require specific microbiologic diagnosis, which would likely require rapid, preferably non-culture, nucleic acid based methods, and still may not define a very specific patient population. Consider a pathogen such as Staphylococcus aureus that causes a much different illness and markedly varied outcome if the infection is staphylococcal meningitis, endocarditis, or a soft tissue infection. Additionally, there is a concern that single organism studies would lack external validity and could not be readily extrapolated and replicated in serious infections by other microorganisms. Identifying microbial or host derived, target mediators before study entry is another approach to identify a potentially responsive patient population for future sepsis studies.
Conversely, “mega trials” where tens of thousands of patients are pooled together in a large population studies would have greatly enhanced statistical power to find treatment benefits over current phase 3 trial sample size. However, large simple trial designs in sepsis could lose validity in sepsis trials if only a subset of patients were benefited while another subgroup of patients were worsened by the same treatment intervention. This would be difficult to decipher in a large, simple trial design where details of individual patients might not be collected. It may also obscure pathophysiological mechanisms accounting for these differences in outcome.
Can adaptive trial design improve the success rate in sepsis trials?
Adaptive trial design has been proposed as an improved method to design and implement sepsis trials rather than traditional “frequentist” statistical designs. Adaptive design is a Bayesian methodology in which multiple arms can be run simultaneously and the trial assumptions can adapt to the early findings during the enrollment process using predefined analytic rules (23). As enrollment proceeds, patients can be preferentially randomized to the most effective arms in a multi-dose study, thereby reducing or eliminating the number of patients who are assigned the less effective arms. This design offers the opportunity in some instances to provide an answer about efficacy or lack of efficacy in a shorter period of time then would be possible using the standard frequentist approach. Basically, an adaptive design allows for reassessment of ongoing trial assumptions of sample size, enrollment criteria, surrogate endpoint validity, etc. using a priori planned boundaries based on early information gleaned from the early incoming data.
There are a number of potential advantages to this process including the capacity to detect early and significant differences in time-related or biomarker-related outcomes. Adaptive trial design allows for seamless transition from a phase II to a phase III clinical trial and the possibility of a continuous trial design where new drugs can be added or rejected, if deemed appropriate, to an ongoing clinical trial with an expanding placebo group data base. Another major advantage of this technique is the capacity to set up trial simulations and run repeated sample trials on computer software. Adaptive designs can potentially make greater use of pre-trial simulations, and generate mathematical predictions of the likelihood of success or failure in intervention trials in sepsis. The final benefit of adaptive trial design is the capacity to recommend early discontinuation of non-effective drugs thereby sparing time, money and human resources on nonproductive drug doses or drug choices.
Assuming that regulatory agencies support the process, adaptive trial designs should be tested in future sepsis trials, in parallel to traditional methods, to determine if adaptive methods can accurately advance research findings is sepsis research. Adaptive trial design studies are already underway in a number of ongoing clinical trials in other fields of medicine. Risks of adaptive design methods include the relative lack of experience (and suspicion) of some statisticians and many clinical investigators in the use of this methodology, the problem of distinguishing type I and type II errors, and the possible difficulties if predicted association between pre-specified biomarkers and surrogate markers for primary endpoint (mortality) is not highly correlated, as anticipated at the beginning of the trial.
Do clinical coordinating centers help or hinder interventional sepsis trials?
The capacity to generate reproducible results in clinical trials is predicated upon the ability to define and repeatedly capture similar patient populations with which to test a novel intervention in multiple clinical trials. Sepsis trials are particularly challenging as the heterogeneity of the patient population, changes in baseline standard of care and the lack of precision in defining specific populations from one study to another. The entry criteria written into the clinical trial protocol are supposed to define the optimal patient population, but variations in interpretation of the entry criteria by investigators continue to plague trial execution in sepsis studies.
One potential strategy to limit variability of the study population expected is to employ a central clinical coordinating center to screen potential study participants. These centers have to be available around the clock, and center personnel need to skilled clinical investigators who are very familiar with the protocol and its nuances. This is not a simple process of simply checking the boxes for entry and exclusionary criteria on an enrollment form. Clinical decisions need to be made expeditiously by knowledgeable investigators and the research team as to whether a patient is eligible or not.
There is ample evidence of a “learning curve” where the first and second patients enrolled at each site in any clinical trial where more protocol violations occur and lack of full understanding of the patient population to be studied is evident. One role of the clinical coordinating center is to shorten or eliminate the duration of this learning curve in appropriate enrollment for sepsis trials (24).
An important aspect is the center selection. We should only use “Centers for Excellence” who are familiar with the problems associated with clinical trials in sepsis. They see sufficiently large numbers of patients to maintain a high level of proficiency and interest in the study to assure high quality enrollment of patients for sepsis trials. What constitutes a center for excellence has not been clearly defined. Additionally, it is not clear that there are sufficient numbers of centers for excellence to generate the large number of patients in a reasonable time period to complete large phase III trials. Moreover, there is a question of external validity of the data if it is just done in a few high volume centers. How well does this correlate with the field conditions within smaller ICUs scattered around the globe?
Who should own the data?
A simmering tension exists between clinical investigators and industry sponsors who often fund phase 3 sepsis trials over ownership of the data and clinical samples and specimens. The study sponsors have paid large sums of money to see the study completed and justifiably feel that ownership of the patient samples and data bases are theirs alone. Intellectual property issues are understandably paramount in the eyes of study sponsors and are of little concern to most academic investigators. Clinical investigators are much more interested in interrogating large data bases in septic patients to test new hypotheses. Ideally this would allow the capacity to run new biomarker panels or a series of other diagnostic and prognostic markers on plasma and cell banks derived from existing clinical trial material. Attempts to generate a central data base and form a repository for at least those patients enrolled in placebo group in sepsis trials has been argued for many years (16). This could be open to academicians and industry alike to test new ideas and new hypotheses. The logistics are complicated but a repository of clinical, laboratory and research data, while honoring patient confidentiality, should be a joint goal for the future as a shared data base, including hemodynamic parameters, plasma banks and cell banks. The development of such “knowledge networks” that function in an open and collaborative spirit is a laudable effort and should be vigorously pursued in sepsis and acute inflammation research.
Why have so many phase 2 sepsis trials look promising but fail in phase 3 trials?
Significant issues remain surrounding the translation of clinical results from phase 2 studies to phase 3 trials. This has been a major stumbling block in a large number of sepsis trials dating back to the initial trials with human recombinant interleukin-1 receptor antagonist (IL-1ra) phase II trial performed 20 years ago (25). This small, open label clinical trial demonstrated the remarkable improvement in 28 day all cause survival with an over 50% reduction in relative risk of mortality by IL-1ra. Unfortunately, this seemingly dramatic benefit was not replicated in a phase III trial where IL-1ra did not demonstrate any significant improvement in outcome in two subsequent phase III trials (26,27). Similar studies with other anti-sepsis agents appeared to be favorable in phase II but no significant survival benefits were confirmed in subsequent phase 3 trials. The review of the data suggests that larger phase 2 trials are probably indicated to reduce the risk of studying inactive drugs in phase III studies. Large phase 2 trials assure that there is adequate statistical power in calculating the effect size in a phase 3 clinical trial.
The decision to “go” or “no go” into a phase 3 trial based upon a phase 2 research program is difficult at best. This decision is often made on an emotional basis hoping to see trends in some subgroup and largely ignoring disturbing evidence of chance observations in small studies. Phase II trials in sepsis are generally underpowered to show a survival benefit and therefore the effect size calculations attributable to the test agent is often based upon mechanism of action studies and biologic surrogates for disease activity. The lack of reproducibility of clinical trial results in sepsis patients is the major impediment to progress in developing new drugs for sepsis. A dispassionate, realistic review of the data with due consideration for confidence intervals needs to drive the decision to go forward or to halt further clinical development of an experimental agent.
The PROWESS versus the PROWESS SHOCK study of activated protein C: What happened?
After all the controversy that has arisen about the efficacy of recombinant human activated protein C (rhAPC) in severe sepsis after the publication of the original PROWESS trial (5), a second, phase 3, placebo- controlled, confirmatory trial was undertaken, the PROWESS SHOCK study (6). Theoretically the PROWESS SHOCK study was expected to enroll more severely ill patients with a higher mortality rate than in the original PROWESS trial. Patients were required to be in fluid, non-responsive, vasopressor-dependent, (>4 hours) septic shock with sepsis-induced organ failure. Nonetheless, a significantly lower mortality rate was observed in PROWESS SHOCK (n=1680) than in the original PROWESS (n=1290) placebo-treated groups (24.2% [PROWESS SHOCK] versus 30.8% [PROWESS]). Regrettably, the mortality rate in the rhAPC-treated group did not change substantially from 24.7% in PROWESS to 26.4% in PROWESS SHOCK. The end result was that the highly statistically significant improvement in 28-day outcome with rhAPC over placebo in the original study (P=0.006) was not confirmed in the PROWESS SHOCK study (p=0.31). For this reason, rhAPC has now been taken off the market.
What accounts for the differences in these two studies? The two studies are separated by approximately 10 years and improvements in supportive care have occurred during this period of time. The standard of care has improved for septic patients worldwide through the introduction of the Surviving Sepsis Campaign Guidelines (9). This may account for the improved placebo event rate but it does not explain the lack of similar decline in mortality rate that might have been expected in the rhAPC treated group. Another potential explanation is the fact that the confirmatory PROWESS SHOCK study was not repeated in many of the same institutions that participated in PROWESS. In fact, many institutions where rhAPC was regularly used did not participate in the PROWESS SHOCK study. It is difficult to consider clinical equipoise between treatment and placebo in a situation in which rhAPC was an approved drug that had been shown to save lives in severely septic patients based on the PROWESS trial results. The PROWESS SHOCK investigators were chosen because they often were not convinced that the survival advantage of PROWESS and thought the efficacy of the drug was still in question. The net effect of this study issue was the removal a number of centers of excellence that would have participated in the PROWESS study and were experienced in its use. The impact of this change in study sites selected for PROWESS SHOCK is unknown but is worth serious consideration. The learning curve observed in the first phase III trial (24) had to recur in each of the new investigative sites for the second phase 3 trial. A detailed analysis of the failure of the PROWESS SHOCK trial to replicate the survival advantage with rhAPC in the original trial needs to be undertaken.
Conclusions and recommendations
Sepsis and septic shock are not going away; in fact the evidence is quite the opposite. A recent report from the CDC suggests the incidence continues to climb in the USA (28). As the aggregate age of the population increases, the incidence of sepsis will likely increase. Similar demographics and trends are found worldwide and the public health consequences of sepsis are only beginning to be fully recognized. Sepsis survivors spend significantly longer in acute care hospitals and longer length of stays in ICUs than patients admitted with other diagnoses. Septic patients are also more likely to need extended care in other acute or long-term, health care facilities upon discharge than patients admitted with other diagnoses (36 vs.14%). An estimated 14.6 billion dollars were spent in the US in 2008 in direct patient care of sepsis/septicemia (30). The cost to society in terms of loss of life, productivity and loss of quality of life from physical and cognitive impairment due to sepsis is staggering (28,29).
Coming up with improved therapies for sepsis will be not be easy for this complex, heterogeneous, and rapidly evolving clinical syndrome (30–32). Our current developmental paradigm is not working and demands we change our direction. We suggest that the following recommendations be considered in changing the current skepticism into finding new drugs to treat sepsis with a reasonable chance of success (see table 1). Our patients need better treatments. We should rethink what we are doing but definitely not give up.
Acknowledgments
Copyright form disclosures: Dr. Opal served as board member for Kenta biotech (advice on monoclonal antibody targets for bacterial infections) and Arsanis (Advice on anti-LPS antibody targets), consulted for Medimmune (Monoclonal antibody strategies), received royalties from Elsevier (Book royalties for an Infectious Disease textbook), and disclosed DSMB for Tetraohase, DSMB for Achaogen (data review committee). His institution received grant support from Asahi Kasei, Sirtris, and GSK and has patents with the University of Maryland (patent on a novel vaccine for endotoxin) and Prothera biologics (patent on a novel therapy for anthrax). Dr. Masur received support for article research from NIH. Dr. Angus consulted for Idaho Technology, Pfizer, Elsai, and Medimmune, LLC and disclosed other: Eli Lilly (member). His institution received grant support from Elsai Inc. (grant to University of Pittsburgh Medical Center. Dr. Angus served as PI from 2006–2010 and served as co-investigator from 2010–2012).
Footnotes
Presented in part at the 10th Stephen F. Lowry ISF Colloquium, Bethesda, MD, May 4–5, 2012.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Steven M. Opal, The Alpert Medical School of Brown University, Providence, RI
R. Phillip Dellinger, Robert Wood Johnson Medical School, Camden, New Jersey
Jean-Louis Vincent, Erasme University Hospital, Brussels, Belgium.
Henry Masur, National Institutes of Health, Bethesda, Maryland
Derek C. Angus, University of Pittsburgh School of Medicine, Pittsburgh, PA.
References
- 1.Mullard A. Drug withdrawal sends critical care specialists back to basics. Lancet. 2011;378:1769. doi: 10.1016/s0140-6736(11)61761-3. [DOI] [PubMed] [Google Scholar]
- 2.Xigris [Drotrecogin alpha (activated)]; Market withdrawal – failure to show survival benefit. http://www.FDA.gov/safety/medwatch/safetyinformation/safetyalertforhumanmedicalproducts/ucm277143.htm. Post October 25, 2011. [Google Scholar]
- 3.Cohen J, Calandra T, Opal SM. Sepsis studies need new direction. Lancet Infectious Diseases. 2012;12:503–505. doi: 10.1016/S1473-3099(12)70136-6. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC. The search for effective therapy for sepsis – back to the drawing board? JAMA. 2011;306(23):2614–2615. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 5.Bernard GR, Vincent JL, Laterre PF, et al. Recombinant human protein C worldwide evaluation in severe sepsis (PROWESS) study group. Efficacy and safety of recombinant activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 6.Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) for adults with septic shock. N Engl J Med. 2012;366(22):2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 7.Opal SM, McCulloh R. Human recombinant lactoferrin for adult sepsis: Too good to be true? Critical Care Med. 2013 doi: 10.1097/CCM.0b013e3182770fd6. (In press) [DOI] [PubMed] [Google Scholar]
- 8.Opal SM, Laterre P-F, Francois B, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis. JAMA. 2013;309(11):1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 9.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: Results of an international guideline – base performance improvement program targeting severe sepsis. Intensive Care Med. 2010;336:222–231. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar G, Kumar N, Taneja A, et al. Nationwide trends in severe sepsis in the 21st Century (2000–2007) Chest. 2011;140:1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 11.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 12.Angus DC. The lingering consequences of sepsis: the hidden public health disaster? JAMA. 2010;304(16):1833–1834. doi: 10.1001/jama.2010.1546. [DOI] [PubMed] [Google Scholar]
- 13.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Longterm cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Lundback P, Ottosson L, et al. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box 1 (HMGB-1) Molecular Med. 2012;18(3):258–259. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Quartin AA, Schein RM, Kett DH, Peduzzi PN. Department of Veteran’s Affairs Sepsis Cooperative Studies Group. Magnitude and duration of effect of sepsis on survival. JAMA. 1997;277(13):1058–1063. [PubMed] [Google Scholar]
- 16.Eidelman LA, Sprung CL. Why have new effective therapies for sepsis not been developed? Crit Care Med. 1994;22(8):1330–1334. doi: 10.1097/00003246-199408000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Lorente JA, Marshall JC. Neutralization of tumor necrosis factor in preclinical models of sepsis. Shock. 2005;24(S1):107–119. doi: 10.1097/01.shk.0000191343.21228.78. [DOI] [PubMed] [Google Scholar]
- 18.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1222878110. ( www.pnas.org/cdi/doi/10.1074/pnas.1222878110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Opal SM. Immunotherapy for sepsis: A new approach against an ancient foe. N Engl J Med. 2010;363(1):87–89. doi: 10.1056/NEJMcibr1004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bone RC, Balk RA, Cerra FB, et al. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 21.Klein Klouwenberg PMC, Ong DSY, Bonten MJM, Cremer OL. Classification of sepsis, severe sepsis and septic shock: The impact and minor variations in data capture and the definition of SIRS criteria. Intens Care Med. 2012;38(50):811–819. doi: 10.1007/s00134-012-2549-5. [DOI] [PubMed] [Google Scholar]
- 22.Vincent J-L, Opal SM, Marshall JC, Tracey KL. Sepsis definitions: time for change. Lancet. 2013;381:774–775. doi: 10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang T. Adaptive trial design: could we use this approach to improve clinical trials in the field of global health? Am J Trop Med Hyg. 2011;85(6):967–970. doi: 10.4269/ajtmh.2011.11-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macias WL, Vallet B, Bernard GR, et al. Sources of variation on the estimate of treatment effect in the PROWESS trial: Implications for the design and conduct of future studies in severe sepsis. Crit Care Med. 2004;32(12):2385–2391. doi: 10.1097/01.ccm.0000147440.71142.ac. [DOI] [PubMed] [Google Scholar]
- 25.Fisher CJ, Slotman G, Opal SM, et al. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome. Crit Care Med. 1994;22:12–21. doi: 10.1097/00003246-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Fisher CJ, Dhainaut J-F, Opal SM, et al. Human recombinant interleukin-1 receptor antagonist reduces mortality in sepsis syndrome as a function of disease severity: A randomized, double-blind, placebo-controlled trial. JAMA. 1994;271:1836–1846. [PubMed] [Google Scholar]
- 27.Opal SM, Fisher CJ, Pribble JP, et al. The confirmatory interleukin-1 receptor antagonist trial in sepsis: a phase III randomized, double-blind, placebo-controlled, multicenter trial. Crit Care Med. 1997;25:1115–1123. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Hall MJ, Williams SN, DeFrances CJ, Golosinsky A. Inpatient care of septicemia or sepsis: A challenge for patients and hospitals. National Center for Health Statistics. 2011;62:1–8. [PubMed] [Google Scholar]
- 29.Agency for Healthcare Research and Quality. HCUP facts and figures: Statistics on hospital-based care in the United States. 2008 ( http//www.hcupus.ahrq.gov/reports/factsandfigures/2008/TOC_2008.jsp)
- 30.Xiao W, Mindrinros MN, Seok J, et al. A genomic storm in critical injured humans. J Exp Med. 2011 doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boomer JS, To K, Chang KC, et al. immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosas-Ballina M, Olofsson PS, Ochani M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]