Abstract
Candida tropicalis, a species closely related to Candida albicans, is an emerging fungal pathogen associated with high mortality rates of 40 to 70%. Like C. albicans and Candida dubliniensis, C. tropicalis is able to form germ tubes, pseudohyphae, and hyphae, but the genes involved in hyphal growth machinery and virulence remain unclear in C. tropicalis. Recently, echinocandin- and azole-resistant C. tropicalis isolates have frequently been isolated from various patients around the world, making treatment difficult. However, studies of the C. tropicalis genes involved in drug tolerance are limited. Here, we investigated the roles of calcineurin and its potential target, Crz1, for core stress responses and pathogenesis in C. tropicalis. We demonstrate that calcineurin and Crz1 are required for hyphal growth, micafungin tolerance, and virulence in a murine systemic infection model, while calcineurin but not Crz1 is essential for tolerance of azoles, caspofungin, anidulafungin, and cell wall-perturbing agents, suggesting that calcineurin has both Crz1-dependent and -independent functions in C. tropicalis. In addition, we found that calcineurin and Crz1 have opposite roles in controlling calcium tolerance. Calcineurin serves as a negative regulator, while Crz1 plays a positive role for calcium tolerance in C. tropicalis.
INTRODUCTION
Candida tropicalis is one of the most common Candida species that causes disease in humans, especially in tropical climates. C. tropicalis is responsible for 3 to 66% of cases of candidemia, depending on the geographic region (1–3). Non-albicans Candida species (NACS), including C. tropicalis, C. glabrata, C. krusei, C. dubliniensis, and C. parapsilosis, have increasingly been responsible for nosocomial bloodstream infections (4, 5) and account for almost 50% of nonsuperficial Candida infections (6). Mortality rates of 40 to 70% have been associated with the presence of C. tropicalis in the bloodstream, and these rates can be affected by other factors, such as leukemia, neutropenia, central venous catheters, parenteral nutrition, and extended time in intensive care units (7–9).
Within the last few years, C. tropicalis drug-tolerant or -resistant isolates have frequently been isolated from patients and environmental samples (10–14). For example, Garcia-Effron et al. showed that 7.5% (3/40) of clinical C. tropicalis isolates were caspofungin resistant owing to amino acid substitutions in beta-1,3-glucan synthase (Fks1p) that resulted in caspofungin-based therapy failures (10). An Asian national antifungal surveillance program found reduced susceptibility of C. tropicalis to fluconazole (12). Recently, Yang et al. reported that C. tropicalis strains isolated from environmental soil also showed reduced susceptibility to medical and agricultural azoles, advocating for the prudent use of azoles in agriculture (11). So far, few studies have focused on C. tropicalis drug resistance mechanisms. For example, Jensen et al. demonstrated that an S80P mutation of Fks1p leads to echinocandin resistance in C. tropicalis (15). Vandeputte et al. found that overexpression of C. tropicalis ERG11 (CtERG11), the gene encoding lanosterol 14α-demethylase, is associated with a missense mutation that might be responsible for the acquired azole resistance of a clinical C. tropicalis isolate (16). Eddouzi et al. showed that CtERG3 and CtERG11 mutations participate in azole resistance (17). Chen et al. demonstrated that the loss of heterozygosity of FCY2, a gene encoding purine-cytosine permease, enables C. tropicalis to develop flucytosine resistance (18). Thus, the mechanisms that C. tropicalis deploys for drug resistance still remain elusive and require further investigation.
The ability to undergo a morphogenic switch between yeast and hyphal growth is a major virulence factor for Candida albicans (19). For example, mutants locked in either the pseudohyphal (tup1/tup1) or yeast (cph1/cph1 efg1/efg1) form exhibit attenuated virulence in murine systemic infection models (20, 21). Although dimorphic transitions have been extensively studied in C. albicans, their studies in C. tropicalis are limited. For example, Porman et al. demonstrated that the overexpression of C. tropicalis WOR1 (CtWOR1), a master regulator of the white-opaque switch (22), promotes filamentous growth and biofilm formation of C. tropicalis (23). Thus, it will be of interest to study the C. tropicalis genes involved in dimorphic transitions and virulence.
Calcineurin, a potential drug target in fungi, is a calcium/calmodulin-dependent serine/threonine-specific protein phosphatase that is comprised of a catalytic subunit A (Cna1) and a regulatory B calcium binding subunit (Cnb1). Upon stimulation with calcium, calmodulin associates with the calcineurin A C-terminal domain, stimulating phosphatase activity by dislodging the autoinhibitory domain and converting signals to downstream targets, such as the transcription factor Crz1, by dephosphorylation. Dephosphorylated Crz1 migrates into the nucleus and regulates gene expression. Because active calcineurin is an AB heterodimer, the loss of the Cnb1 subunit often results in destabilization of the Cna1 catalytic subunit (24). Although the roles of calcineurin in hyphal growth of C. albicans, if any, remain unclear, calcineurin is required for hyphal growth in several fungal pathogens, including C. dubliniensis, Aspergillus fumigatus, and Magnaporthe oryzae (24).
In this study, we comprehensively studied the roles of calcineurin and Crz1 in hyphal growth in vitro, virulence, drug tolerance, and other stress responses in C. tropicalis. We demonstrated that C. tropicalis calcineurin and Crz1 are required for hyphal growth, micafungin tolerance, and virulence in a murine systemic infection model. Meanwhile, C. tropicalis calcineurin but not Crz1 was shown to govern azole tolerance and cell wall integrity. Our data suggest that calcineurin is a potential drug target and calcineurin inhibitors could be combined with current antifungal drugs for therapy.
MATERIALS AND METHODS
Yeast strains, media, and chemicals.
The C. tropicalis strains used in this study are listed in Table 1. The following media were used in this study: yeast extract-peptone-dextrose (YPD; 1% yeast extract, 2% peptone, 2% glucose) liquid medium and agar (2%), serum agar (50% serum, 2% agar), spider medium (10 g nutrient broth, 10 g mannitol, 4 g K2HPO4, 14 g Bacto agar in 1 liter double-distilled H2O [ddH2O], in which the pH was adjusted to 7.2 with H3PO4), synthetic low-ammonium dextrose [SLAD; 1.7 g yeast nitrogen base without amino acids and without ammonium sulfate, 20 g glucose, 5 ml of 10 mM (NH4)2SO4, 20 g Bacto agar in 1 liter ddH2O], and cornmeal agar (0.2% corn meal, 1.5% agar). YPD medium containing 100 μg/ml nourseothricin was used to select transformants. The following supplements were added to the media at the concentrations indicated below: FK506 (Astellas Pharma Inc.), sodium dodecyl sulfate (SDS; Fisher), calcofluor white (fluorescent brightener 28; Sigma), Congo red (Sigma), tunicamycin (Sigma), fetal bovine serum (Invitrogen), calcium chloride (Sigma), fluconazole (Bedford Laboratories), posaconazole (Merck), voriconazole (Sigma), caspofungin (Merck), micafungin (Astellas Pharma Inc.), and anidulafungin (Pfizer Inc.).
TABLE 1.
C. tropicalis strains used in this studyc
| Candida tropicalis strain | Genotype | Background |
|---|---|---|
| MYA3404 | Prototrophic wild type | Clinical isolate |
| YC130 | cnb1Δ::SAT1-FLP/CNB1 | MYA3404 |
| YC146 | cnb1Δ::FRT/CNB1 | YC130 |
| YC454a | cnb1Δ::FRT/cnb1Δ::SAT1-FLP | YC146 |
| YC132 | cnb1Δ::SAT1-FLP/CNB1 | MYA3404 |
| YC142 | cnb1Δ::FRT/CNB1 | YC132 |
| YC466a | cnb1Δ::FRT/cnb1Δ::SAT1-FLP | YC142 |
| YC173 | crz1Δ::SAT1-FLP/CRZ1 | MYA3404 |
| YC188 | crz1Δ::FRT/CRZ1 | YC173 |
| YC494b | crz1Δ::FRT/crz1Δ::SAT1-FLP | YC188 |
| YC176 | crz1Δ::SAT1-FLP/CRZ1 | MYA3404 |
| YC190 | crz1Δ::FRT/CRZ1 | YC176 |
| YC499b | crz1Δ::FRT/crz1Δ::SAT1-FLP | YC190 |
Two independent cnb1/cnb1 mutants.
Two independent crz1/crz1 mutants.
The source of all strains except the clinical isolate was this study.
Strain construction.
Both alleles of the C. tropicalis CNB1 and CRZ1 genes were disrupted with the SAT1 flipper (25). For CNB1 gene disruption, approximately 1-kb 5′ (amplified with primers JC182/JC183; see Table S1 in the supplemental material) and 3′ (amplified with primers JC184/JC185) noncoding regions (NCRs) of the CNB1 open reading frame (ORF) (CNB1NCR) were PCR amplified from genomic DNA of genome-sequenced reference strain MYA3404 (26). The 4.2-kb SAT1 flipper sequence was amplified from plasmid pSFS2A (25) with primers JC17/JC18. The three PCR products were treated with ExoSAP-IT (USB Corp.) to remove contaminating primers and deoxynucleoside triphosphates and then combined in a 1:3:1 molar ratio (5′ CNB1NCR, SAT1 flipper, and 3′ CNB1NCR) to generate the disruption allele by overlap PCR using flanking primers JC186/JC187 (which are ∼100 bp closer to the CNB1 ORF than JC182/JC185, respectively, with primers JC182/JC185 being reserved for use for further integration confirmation), resulting in an ∼6-kb 5′ CNB1NCR-SAT1 flipper-3′ CNB1NCR CNB1 disruption allele.
The first allele of the CNB1 gene was disrupted in wild-type strain MYA3404 by transformation with 0.2 to 1 μg of gel-purified disruption DNA using a Frozen-EZ yeast transformation kit (Zymo Research). Two independent heterozygous nourseothricin-resistant mutants (YC130 and YC132; Table 1) were obtained from two separate transformations. Liquid YPM (1% yeast extract, 2% peptone, 2% maltose) medium was used to drive expression of the FLP recombinase under the control of the C. albicans MAL2 promoter. The SAT1 flipper was then excised, which left an FLP recombination target (FRT) sequence and resulted in nourseothricin-sensitive CNB1/cnb1 mutant strains (YC146 and YC142).
Despite multiple attempts, the second allele of the CNB1 gene could not be disrupted with the same overlap PCR disruption allele. We thus amplified 5′ CNB1NCR with JC182/JC402 and 3′ CNB1NCR with JC400/JC185 from the second wild-type allele and mixed these with the SAT1 flipper to produce an overlap PCR CNB1 disruption allele specific for the second allele of the CNB1 gene. After transformation, two independent nourseothricin-resistant homozygous cnb1/cnb1 mutants (YC454 and YC466) derived from two separate transformations were obtained (Table 1).
A similar approach was employed to disrupt the CRZ1 gene, with ∼1-kb 5′ and 3′ noncoding regions being used for homologous recombination. To generate the ∼6.0-kb CRZ1 disruption allele, the overlap PCR DNA products 5′ CRZ1NCR (amplified with primers JC215/JC216), SAT1 flipper (amplified with primers JC17/JC18), and 3′ CRZ1NCR (amplified with primers JC217/JC218) were mixed in a 1:3:1 molar ratio and amplified with primers JC219/JC220 (which are ∼100 bp closer to the CRZ1 ORF than JC215/JC218, respectively). A similar approach was used to disrupt the second allele of the CRZ1 gene. We amplified the 5′ CRZ1NCR with JC215/JC405 and 3′ CRZ1 NCR with JC406/JC218 from the second CRZ1 allele. Two independent nourseothricin-resistant crz1/crz1 mutants (YC494 and YC499; Table 1) derived from two separate transformations were obtained. These mutants were confirmed by PCR and validated by Southern blotting (data not shown).
Serial dilution growth assays.
Cells were grown overnight at 30°C and washed twice with distilled H2O (dH2O), and the optical density at 600 nm (OD600) was measured. Cells were resuspended in an appropriate volume of dH2O to achieve 1 OD unit/ml. Three microliters of 5-fold serial dilutions of each strain was spotted onto solid medium with a multichannel pipette. The plates were then incubated at the temperatures indicated below for 48 h and photographed.
Growth curve and doubling time measurement.
To determine whether the loss of calcineurin and Crz1 affects cell growth at 37°C, we measured the growth curves and doubling times of the strains. For growth curves, cells were grown overnight at 30°C, washed twice with dH2O, diluted to 0.1 OD600 unit/ml in fresh YPD medium, and incubated at 37°C with shaking at 200 rpm. The OD600 of the cultures was measured at 0, 3, 6, 9, 12, 24, 48, 72, and 96 h via microplate spectrophotometer readings (Spectra MAX 190; Molecular Devices). The experiments were performed in triplicate, and the data were plotted using Prism (version 5.03) software.
The doubling time was calculated by using the formula T·ln2/(ln(ODT/ODT0) where ODT and ODT0 represent the OD600 at time T and the initial time (time zero), respectively. The log-phase time points from 0 to 6 h were chosen.
Murine systemic infection model.
Five- to 6-week-old male CD1 mice from The Jackson Laboratory (n = 10 for each group, except n = 9 for the wild type) were used in this study. This was a single experiment because 10 mice per group provided sufficient power to obtain statistically significant P values. C. tropicalis strains were grown in 5 ml YPD overnight at 30°C with shaking at 250 rpm. Cultures were washed twice with 10 ml of phosphate-buffered saline (PBS), and the cells were then resuspended in 2 ml of PBS. Cells were counted with a hemocytometer and resuspended in an appropriate amount of PBS to obtain an infection inoculum of 2.5 × 107 cells/ml. Two hundred microliters (5 × 106 cells) was used to infect mice by lateral tail vein injection. The course of infection was monitored for up to 42 days. The survival of mice was monitored twice daily, and moribund mice (mice that were unable to eat or drink, whose body weight was reduced by >30%, or that were hunched) were euthanized with CO2. All experimental procedures were carried out according to NIH guidelines and Duke IACUC protocols for the ethical treatment of animals. Appropriate dilutions of the cells were plated onto YPD and incubated at 30°C for 48 h to confirm the numbers of CFU and viability.
To determine fungal burden, both kidneys and the spleen of C. tropicalis-infected mice (n = 5 for each strain) were dissected at day 10 postinfection. Half-organ portions were weighed, transferred to a 15-ml Falcon tube filled with 5 ml PBS, and homogenized for 10 s at 13,600 rpm/min (Power Gen 500; Fisher Scientific). Tissue homogenates were serially diluted, and 100 μl was plated onto a YPD plate. The plates were incubated at 30°C for 48 h to determine the number of CFU per gram of kidney or spleen. The identity of the colonies recovered from the organs was confirmed by PCR and by growth or no growth on YPD medium containing 0.01% SDS (for cnb1/cnb1 mutants) or 0.4 M CaCl2 (for crz1/crz1 mutants). The significance of differences in fungal burden was determined using one-way analysis of variance (ANOVA) and Dunnett's multiple-comparison test. For histopathological analysis, half-organ samples of kidney and spleen were fixed in 10% phosphate-buffered formalin (Fisher), and Gomori methenamine silver (GMS) and hematoxylin-eosin (H&E) stainings were performed by the Department of Pathology at Duke University. After slide preparation, each sample was examined by microscopy for analysis of Candida colonization (GMS) and tissue necrosis (H&E). Images were captured using an Olympus Vanox microscope (PhotoPath; Duke University Medical Center).
Scanning electron microscopy.
The cultures were excised from the agar and fixed in 3% glutaraldehyde in 0.1 M Na cacodylate buffer (pH 6.8) for 2 days at 4°C. They were then rinsed in three 30-min changes of cold 0.1 M Na cacodylate buffer (pH 6.8), followed by a graded dehydration series of 2-h changes in cold 30% and 50% ethanol (EtOH), and held overnight in 70% EtOH. Dehydration was completed with 1-h changes of cold 95% and 100% EtOH at 4°C and then warmed to room temperature in 100% EtOH. Two additional 1-h changes of room temperature 100% EtOH completed the dehydration series. The samples were then critical point dried in liquid CO2 (Samdri-795; Tousimis Research Corp., Rockville, MD) for 15 min at the critical point. The agar pieces were mounted on stubs with double-stick tape, pressed down completely around the edge, and then sealed with silver paint to ensure good conductivity. The samples were then sputter coated with 50-Å Au/Pd (Hummer, version 6.2; Anatech USA, Hayward, CA). Samples were stored in a vacuum desiccator until they were viewed under a JEOL JSM 5900LV scanning electron microscope at 15 kV.
Murine ocular infection model.
The C. tropicalis wild type (MYA3404), cnb1/cnb1 mutants (YC454, YC466), and crz1/crz1 mutants (YC494, YC499) and C. albicans strain SC5314 were grown overnight in YPD broth at 25°C. Ten milliliters of broth culture was pelleted by centrifugation at 3,000 rpm for 10 min and then washed three times with PBS (pH 7.4). Cells were resuspended in PBS and then diluted to a concentration equal to 108 CFU/5 μl. The concentration of Candida cells was determined by using the spectrophotometer optical density reading at a wavelength of 600 nm and multiplying it by a conversion factor in which 1 OD600 unit is equivalent to 3 × 107 cells/ml. The numbers of CFU and cell viability were verified by plating cells onto YPD agar plates with incubation for 48 h at 25°C.
Six- to 8-week-old outbred ICR mice (22 to 30 g) were purchased from the Research Institute for Tropical Medicine (RITM), Alabang, Philippines. Animals were handled in accordance with the ARVO statement for the use of animals in ophthalmic and vision research. The murine keratomycosis induction protocol described previously for C. dubliniensis (27) was performed with minor modification and was approved by the University of Perpetual Help Institutional Review Board. Briefly, mice were immunocompromised by intraperitoneal administration of cyclophosphamide (180 to 200 mg/kg of body weight) on days 5, 3, and 1 prior to inoculation of the test strains. Before applying the inoculum, the mice were placed under general anesthesia by intramuscular injection of tiletamine hypochloride-zolazepam hypochloride (10 to 15 mg/kg of body weight; Zoletil 50; Virac, Australia), followed by topical application of proparacaine hydrochloride ophthalmic solution (Alcaine; Alcon-Couvreur, Belgium) in the right eyes until the blink sensation was lost. Excess solution in the eye was removed with a sterile cotton swab. Eyes were superficially scarified before applying the inoculum. An inoculum with 108 CFU per 5-μl dose was distributed uniformly by rubbing the eye for a few seconds with the eyelid. Sterile PBS was applied in negative controls. Clinical scoring of disease severity of fungal keratitis was assessed for 8 days as described previously (27). The visual scoring system (28) evaluates three physical features of the eyes, namely, (i) the area of opacity, (ii) the density of opacity, and (iii) surface irregularity. A grade of 0 to 4 was assigned for each of these parameters to yield a maximum score of 12. At 4 and 8 days postinfection (p.i.), three mice were sacrificed by cervical dislocation. For mouse groups showing low infection rates (<6 mice), only 1 or 2 eyes were evaluated for histological evaluation after 8 days. Eyes were removed and fixed in neutral formalin solution (10% formaldehyde in PBS) before being submitted for histological staining and examination. Two-group comparisons were analyzed using Student's t test. A P value of <0.05 was considered statistically significant.
RESULTS
Identification of calcineurin and Crz1 orthologs in C. tropicalis.
The C. tropicalis orthologs of the C. albicans and Saccharomyces cerevisiae calcineurin regulatory subunit (CNB1) and the calcineurin target CRZ1 genes were identified by reciprocal BLAST searches between the two species and in all cases identified a reciprocal best BLAST hit ortholog as the C. tropicalis CNB1 (CTRG_06124) and CRZ1 (CTRG_02450) genes (26). C. tropicalis Cnb1 shares 91% and 61% identity over the full-length protein with its corresponding C. albicans and S. cerevisiae orthologs, respectively (see Fig. S1A in the supplemental material), while Crz1 shares 54% and 21% identity (see Fig. S2A in the supplemental material) over the full-length protein with its corresponding C. albicans and S. cerevisiae orthologs, respectively. C. tropicalis Cnb1 has four helix E-loop-helix F (EF) hand Ca2+ binding motifs (see Fig. S1B in the supplemental material), while Crz1 shares two C2H2 zinc finger domains with the respective orthologs in C. albicans and S. cerevisiae (see Fig. S2B in the supplemental material).
Calcineurin is required for hyphal growth.
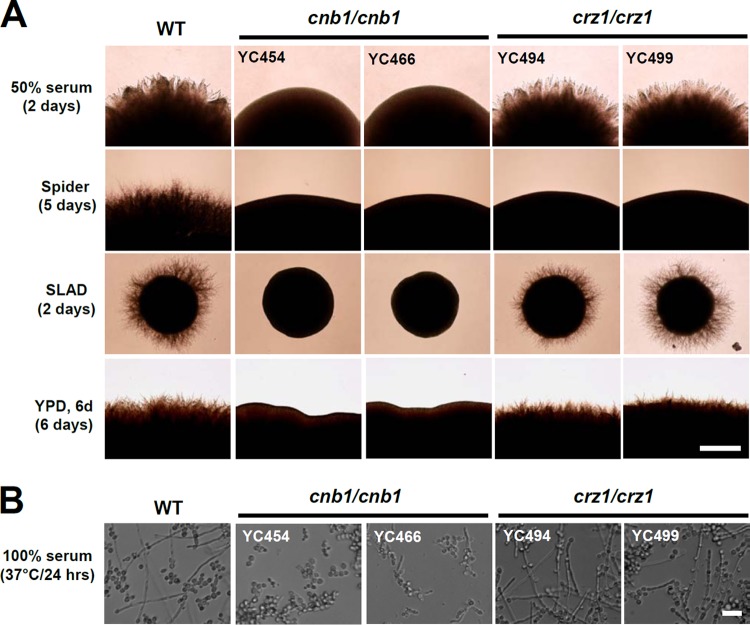
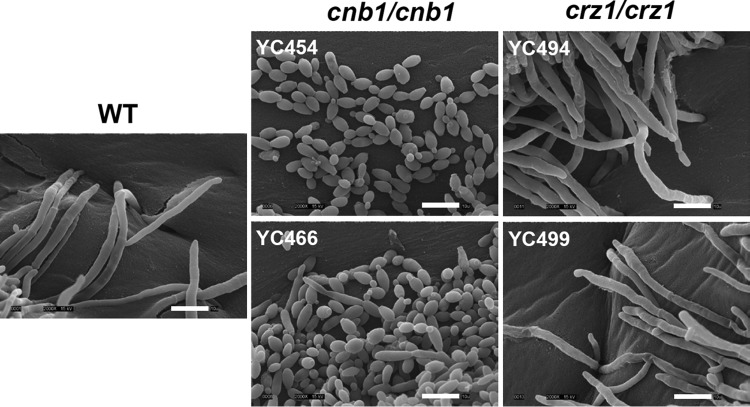
The C. tropicalis genes involved in the dimorphic transition, an important virulence factor, have not been identified. Based on previous studies on the roles of calcineurin in plant and human fungal pathogens (24), we hypothesized that calcineurin signaling might be required for the dimorphic transition. To test this hypothesis, we disrupted the calcineurin (CNB1) and CRZ1 genes in the genome-sequenced C. tropicalis MYA3404 isolate (26). Here, we investigated the roles of calcineurin and Crz1 in hyphal growth. We demonstrated that calcineurin and Crz1 are required for hyphal growth in filament-inducing spider medium (carbon source starvation), while calcineurin but not Crz1 controls hyphal growth in another filament-inducing medium (50% serum or SLAD [nitrogen source starvation]) or nutrient-rich YPD medium (Fig. 1A). In liquid 100% serum, calcineurin mutants also exhibited attenuated hyphal growth, while crz1/crz1 mutants showed wild-type hyphal growth (Fig. 1B). Under a high-resolution scanning electron microscope, we found that calcineurin mutants exhibited mainly yeast and a few pseudohyphal forms, while crz1/crz1 mutants exhibited wild-type hyphae and invasive growth in solid 50% serum agar medium (Fig. 2).
FIG 1.
Calcineurin is required for hyphal growth in C. tropicalis. (A) Hyphal growth of C. tropicalis wild-type (WT) and mutant strains on filament-inducing agar plates. Cells were grown overnight, washed twice with dH2O, and serially diluted to 103 cells/ml (based on an OD600 of 1 being equal to ∼4 × 107 cells/ml). One hundred microliters containing ∼100 cells was spread on a variety of filament-inducing media and incubated at 37°C for the number of days indicated. The experiments were repeated at least three times, and one representative image is shown. Bar = 0.1 mm. (B) Hyphal growth of C. tropicalis wild-type and mutant strains in liquid bovine calf serum (100%). Cell preparations were as described above with minor modifications. Two microliters of cells at an OD600 of 1/ml were added to microtiter wells prefilled with 98 μl of 100% bovine calf serum, resulting in an OD600 of 0.00004 (∼1.6 × 103 cells) in each well. Cultures in the 96-well polystyrene plates were incubated at 37°C without shaking for 24 h. Bar = 40 μm.
FIG 2.
Scanning electron microscopy images of C. tropicalis on filament-inducing media. Calcineurin mutants (cnb1/cnb1) display yeast or pseudohyphal growth, while wild-type and crz1/crz1 mutants exhibit hyphal growth. Cells grown on 50% serum agar medium for 48 h at 37°C were processed for scanning electron microscopy and imaged (see Materials and Methods). Magnification = ×2,000. Bars = 10 μm.
Deletion of calcineurin and Crz1 attenuates virulence in mice.
It has been demonstrated that C. tropicalis exhibits greater or reduced virulence in animal infection models than several C. albicans strains (29–31). However, in general, C. tropicalis is considered to be the second most virulent Candida species in mice, after C. albicans. Previous studies showed that C. tropicalis is able to colonize and form hyphae in murine kidneys (29). Here, we found that C. tropicalis calcineurin mutants (YC454 and YC466) exhibited significantly attenuated virulence, based on survival curves, compared with the wild type (P < 0.0001) (Fig. 3A). C. tropicalis wild-type strain MYA3404 caused 100% mortality of mice by day 10, while independent calcineurin mutants resulted in only 10% mortality, even after 42 days (Fig. 3A). Interestingly, the crz1/crz1 mutants (YC494 and YC499) exhibited intermediate virulence between the wild-type and calcineurin mutants (P < 0.001 compared to the wild type; P < 0.005 compared to calcineurin mutants). This suggests that calcineurin control of pathogenesis in mice is in part mediated by Crz1 in C. tropicalis (Fig. 3A).
FIG 3.
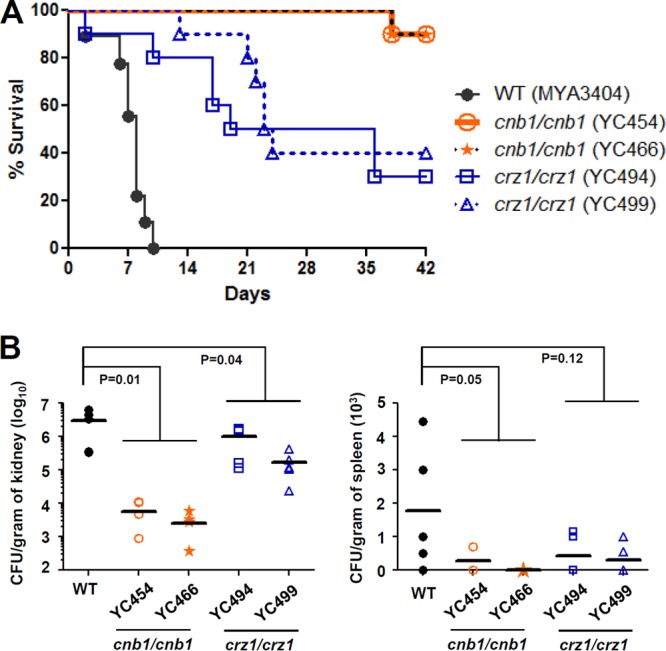
C. tropicalis calcineurin and crz1/crz1 mutants are compromised for virulence in a murine systemic infection model. (A) The survival of mice following intravenous challenge with 5 × 106 C. tropicalis yeast cells was monitored for up to 42 days. Ten mice per strain were used for all strains, except 9 mice were used for the wild type (1 animal died during the experimental procedures). (B) The fungal burden in the kidneys and spleens was determined on day 10 after C. tropicalis infection. Five mice per strain were used for all strains.
To determine colonization ability, we performed fungal burden analyses in the kidneys and spleens of mice infected with the wild-type and mutant strains. In contrast to C. glabrata, but similar to C. albicans and C. dubliniensis, the C. tropicalis wild type preferentially colonized the kidneys rather than the spleen (Fig. 3B). The calcineurin mutants exhibited a 736-fold and 13-fold reduced fungal burden in the kidneys (P = 0.01) and spleens (P = 0.05), respectively, compared with the wild type (Fig. 3B). Meanwhile, the crz1/crz1 mutants (YC494 and YC499) exhibited a 5.2- and 4.8-fold reduced fungal burden in the kidneys (P = 0.04) and spleens (P = 0.12), respectively, compared with the wild type (Fig. 3B). Taken together, mice infected with calcineurin and crz1/crz1 mutants exhibited a reduced fungal burden in the kidneys and a marginally reduced fungal burden in the spleen (Fig. 3B).
In histopathological analyses, similar to published studies for C. albicans and C. dubliniensis (27, 32), GMS-stained kidney tissues revealed that the C. tropicalis wild-type strain readily forms hyphae and proliferates extensively (Fig. 4, left). Here, we demonstrated that C. tropicalis calcineurin mutants had an impaired ability to colonize kidney tissues, while crz1/crz1 mutants continued to form hyphae in the kidneys (Fig. 4; left). Colonization by the C. tropicalis wild type and mutants was not observed in the spleen (data not shown). In the H&E staining, tissue damage or necrosis was observed only in mice infected with the wild type or crz1/crz1 mutants and not in those infected with the calcineurin mutants (Fig. 4, right).
FIG 4.
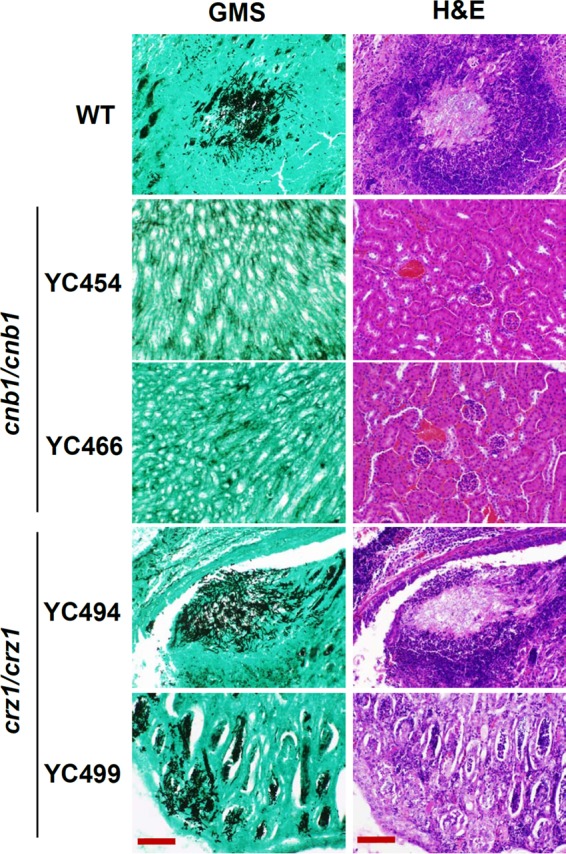
Histopathological sections of kidneys dissected from mice infected with wild-type or calcineurin or crz1/crz1 mutant strains. Mice were infected with 106 yeast cells and sacrificed at day 10. GMS and H&E stains were used to observe C. tropicalis colonization and tissue necrosis, respectively. Bars = 100 μm.
Calcineurin controls ocular infection in a murine keratitis model.
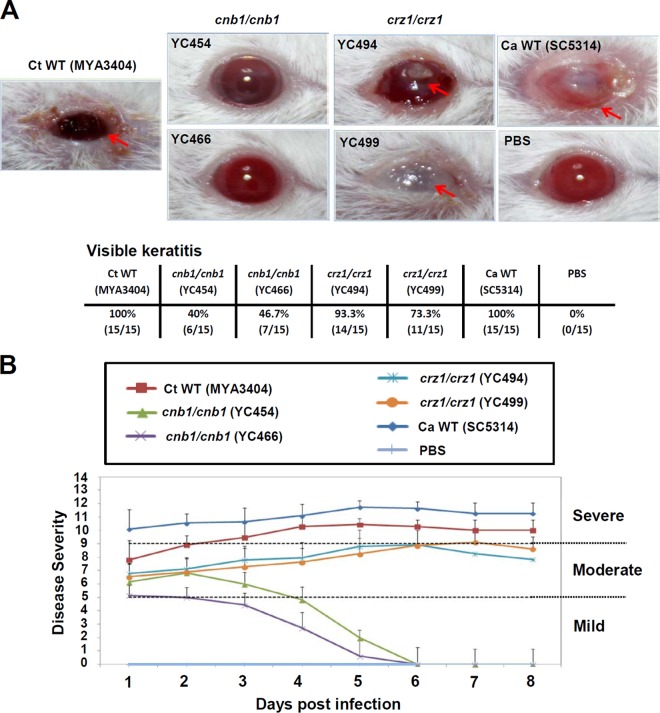
C. tropicalis and other Candida species are frequently isolated worldwide in ocular regions of patients with candidemia or endophthalmitis (33–37). However, the mechanisms and genes that operate during C. tropicalis ocular infections are largely unknown. Here, we investigated the roles of calcineurin for C. tropicalis in a murine keratitis model. The corneal virulence of the C. tropicalis wild type, calcineurin mutants, and crz1/crz1 mutants was evaluated in an immunocompromised mouse model using a slightly modified version of the previously described experimental keratomycosis protocol (27). The C. tropicalis wild type resulted in visible and persistent keratitis in immunocompromised mice (15/15, 100%) (Fig. 5A). The disease scores pooled from 15 mice infected with the C. tropicalis wild type showing keratomycosis were initially moderate (day 1 = 7.8 ± 1.6), and then by the 2nd day the mice developed a severe infection (disease score = 9.3 ± 1.3) which persisted until the end of the 8-day observation period. We also evaluated the clinical manifestations of keratitis, such as corneal opacity, inflammation, and ulcerations (28, 38). Calcineurin mutants showed attenuated virulence in mice, as shown by lower visible keratitis and mean keratitis scores (Fig. 5A and B). Independent calcineurin mutants caused an average of ∼43% of the wild-type level of keratitis in mice (Fig. 5A). The mean keratitis scores observed for either of the calcineurin mutants were significantly lower than those for the wild type (P < 0.001, Student's t test), indicating an important role of calcineurin in the corneal virulence of C. tropicalis. However, the two independent C. tropicalis crz1/crz1 mutants caused an average of 83% of the wild-type level of keratitis, with a mean keratitis score of 8.3, and thus exhibited corneal virulence similar to that of the wild type (Fig. 5).
FIG 5.
C. tropicalis calcineurin mutants are attenuated in a murine ocular infection model. (A) Clinical photographs of corneas of immunosuppressed (cyclophosphamide-treated) ICR mice 8 days after inoculation with 108 yeast cells. Fungal keratitis (red arrows) was observed only in animals infected with the C. tropicalis (Ct) wild type, crz1/crz1 mutants, and C. albicans (Ca) type strain SC5314 and not in animals infected with C. tropicalis calcineurin mutants. (B) Each cornea of an immunosuppressed mouse was inoculated with 108 yeast cells of each strain, and the disease severity was scored for 8 days. C. albicans type strain SC5314 served as a reference control. Mice infected with C. tropicalis calcineurin mutants or the PBS control exhibited normal corneas. The disease scores of mice infected with the C. tropicalis wild type or crz1/crz1 mutants and C. albicans strain SC5314 exhibiting visible signs of keratitis were plotted.
Calcineurin is required for cell wall integrity and drug tolerance in C. tropicalis.
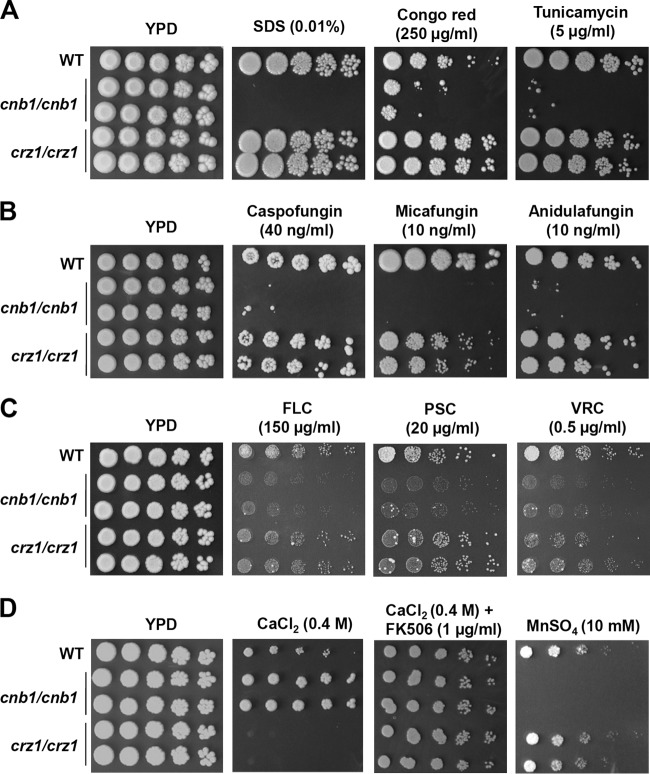
A straightforward explanation for the attenuated virulence of C. tropicalis calcineurin mutants is their hyphal growth defects (Fig. 1 and 2). However, it is possible that other mechanisms are required for calcineurin to establish and maintain infections. The maintenance of cell wall integrity is important for virulence in multiple fungal pathogens (24, 39–41). Nevertheless, the roles of the C. tropicalis genes involved in cell wall integrity remain unclear. Here, we demonstrated that calcineurin is essential for cell wall integrity based on the sensitivity of calcineurin mutants to SDS (which compromises cell wall integrity), Congo red (which intercalates between glucan polymers), or tunicamycin (which blocks the synthesis of N-linked glycoproteins) (Fig. 6A). However, the crz1/crz1 mutants did not exhibit sensitivity to these cell wall-perturbing agents (Fig. 6A), suggesting that either calcineurin control of cell wall integrity is Crz1 independent or Crz1 is redundant with other factors or pathways (Fig. 7).
FIG 6.
Calcineurin is required for cell wall integrity, drug tolerance, and cation homeostasis in C. tropicalis. (A) Calcineurin mutants are sensitive to cell wall integrity-damaging agents (SDS and Congo red) and an endoplasmic reticulum stress-inducing chemical (tunicamycin). Cells were grown overnight in YPD at 30°C, 5-fold serially diluted, spotted onto YPD medium containing SDS, Congo red, or tunicamycin at the concentrations indicated, and then incubated at 30°C for 48 h and photographed. (B) Calcineurin mutants are sensitive to echinocandins. Cells were grown overnight in YPD at 30°C, 5-fold serially diluted, and spotted onto YPD medium containing caspofungin, micafungin, or anidulafungin at the concentrations indicated, and then incubated at 30°C for 48 h and photographed. (C) Calcineurin mutants did not exhibit sensitivity to triazoles. Cells were grown overnight in YPD at 30°C, 5-fold serially diluted, and spotted onto YPD medium containing fluconazole (FLC), posaconazole (PSC), or voriconazole (VRC) at the concentrations indicated, and then incubated at 30°C for 48 h and photographed. (D) Roles of calcineurin and Crz1 in controlling cation homeostasis in C. tropicalis. Cells were grown overnight in YPD at 30°C, 5-fold serially diluted, and spotted onto YPD medium containing CaCl2 (with or without FK506), MnSO4, LiCl, or NaCl at the concentrations indicated. The plates were incubated at 30°C for 36 h.
FIG 7.
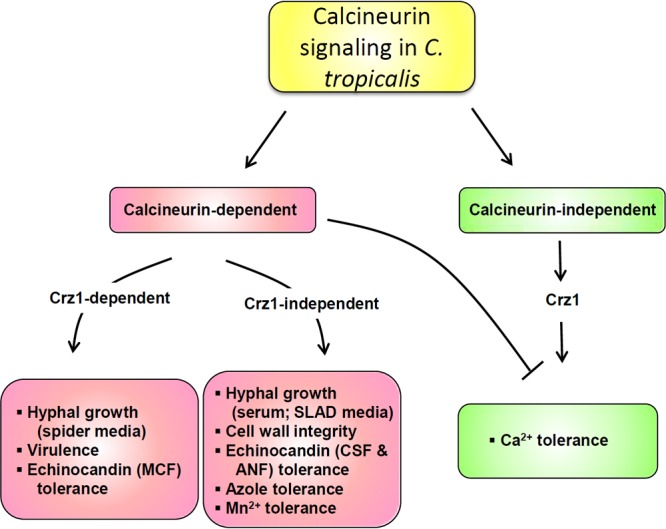
Proposed roles of calcineurin and Crz1 in hyphal growth, virulence, and drug tolerance in C. tropicalis. C. tropicalis calcineurin controls hyphal growth (in response to carbon source starvation), virulence, and micafungin tolerance in a Crz1-dependent fashion. Meanwhile, C. tropicalis calcineurin governs Crz1-independent functions, such as hyphal growth induced by serum or SLAD medium, cell wall integrity, caspofungin/anidulafungin tolerance, azole tolerance, or Mn2+ tolerance. Interestingly, Crz1 plays a positive role for Ca2+ tolerance functions, while calcineurin serves a negative role. MCF, micafungin; CSF, caspofungin; ANF, anidulafungin.
Antifungal drug-resistant C. tropicalis isolates are frequently isolated from patients with candidemia or leukemia and can pose treatment challenges (10, 12, 15, 42, 43). Calcineurin is a potential drug target on the basis of its requirement for drug tolerance and virulence in multiple fungal pathogens (24, 44–46). Information on the roles of the C. tropicalis genes involved in drug tolerance is limited. Here, we demonstrated that calcineurin is required for echinocandin tolerance because calcineurin mutants were found to be susceptible to echinocandins, such as caspofungin, micafungin, or anidulafungin (Fig. 6B and Table 2). Interestingly, the crz1/crz1 mutants exhibited an susceptibility phenotype intermediate between that of the wild-type and calcineurin mutants in response to micafungin but were not more susceptible than the wild type to caspofungin or anidulafungin (Fig. 6B). In response to azoles, calcineurin but not crz1/crz1 mutants exhibited susceptibility to fluconazole and voriconazole on the basis of spot dilution plating assays and Etest analyses (Fig. 6C and Table 2).
TABLE 2.
Calcineurin is required for drug resistance in C. tropicalis
| Strain | MIC or MIC range (μg/ml)a |
||||
|---|---|---|---|---|---|
| Caspofungin | Ketoconazole | Voriconazole | Fluconazole | Amphotericin B | |
| MYA3404 (wild type) | 0.032 | 0.064 | 0.125 | 2.0 | 0.38–0.5 |
| YC454 cnb1/cnb1 | 0.016 | 0.016 | 0.047 | 1.0 | 0.38–0.5 |
| YC466 cnb1/cnb1 | 0.016 | 0.016 | 0.047 | 1.0 | 0.38–0.5 |
| YC494 crz1/crz1 | 0.064 | 0.094 | 0.19–0.25 | 3.0 | 0.25 |
| YC499 crz1/crz1 | 0.064 | 0.094 | 0.19–0.25 | 2.0–3.0 | 0.25 |
Cells were grown overnight at 30°C and washed twice with ddH2O. Then, cells at an OD of 0.5 (in 500 μl) were spread on RPMI 1640 medium (R04067; Remel). After 20 min, the Etest strips (bioMérieux Corp.) were transferred to the surface of the medium. The MIC was read after 24 h of incubation at 35°C according to the manufacturer's instructions.
Roles of calcineurin and Crz1 in cation homeostasis.
The maintenance of cation homeostasis is an essential mechanism for living organisms to survive in biological niches. In several human or plant fungal pathogens, including C. albicans, C. dubliniensis, C. neoformans, C. gattii, A. fumigatus, and Magnaporthe oryzae, calcineurin is required for Ca2+ homeostasis (27, 46–50). However, in S. cerevisiae, C. glabrata, and C. lusitaniae, Crz1 plays an even more significant role than calcineurin in controlling Ca2+ homeostasis (51–55), indicating divergent roles of calcineurin and Crz1 in different fungal pathogens. Here, we investigated the roles of C. tropicalis calcineurin and Crz1 in controlling cation homeostasis and found that C. tropicalis calcineurin and Crz1 play opposite functions in controlling Ca2+ homeostasis. Calcineurin mutants exhibited tolerance to Ca2+ stress, while crz1/crz1 mutants showed sensitivity but the wild type did not (Fig. 6D). In response to other cations, we demonstrated that calcineurin, but not Crz1, is required for Mn2+ homeostasis, while neither calcineurin nor Crz1 appeared to be involved in Na+ or Li+ homeostasis (Fig. 6D and data not shown).
DISCUSSION
Roles of calcineurin and Crz1 in hyphal growth of C. tropicalis.
Whether C. tropicalis forms pseudohyphae, hyphae, or both is thought to be isolate and medium dependent. Our data suggest that C. tropicalis is able to form hyphae (see Fig. S3 in the supplemental material) and pseudohyphae (data not shown) on cornmeal solid agar medium. However, so far, the genes involved in hyphal growth, a potential phenotype linked to the virulence of C. tropicalis, are unclear. Calcineurin is required for hyphal growth in C. dubliniensis (27), but any role in C. albicans hyphal growth is unclear because two groups, including our own, were unable to find a role for calcineurin in hyphal growth (45, 56), while another group reported that calcineurin mutants exhibited hyphal growth defects on filament-inducing solid medium (46). Our data suggest that calcineurin is critical for hyphal growth of C. tropicalis (Fig. 1 and 2) and, hence, plays a role similar to its role in hyphal growth of the related species C. dubliniensis. However, the mechanisms via which calcineurin controls the dimorphic transition of C. tropicalis remain to be clarified. It is possible that calcineurin regulates downstream targets important for hyphal growth. One target is the transcription factor Crz1, which serves as a calcineurin target in both S. cerevisiae and C. albicans (57, 58). The roles of Crz1 in hyphal growth of C. albicans remain elusive because Karababa et al. (57) reported that Crz1 is required for hyphal growth, but Noble et al. (59) demonstrated that Crz1 is not critical for hyphal growth in a systematic screen. However, similar to C. dubliniensis (27, 57), we demonstrated that C. tropicalis Crz1 is critical for hyphal growth on spider medium (carbon source starvation), suggesting that the hyphal growth machinery involves Crz1-dependent calcineurin signaling (Fig. 7) and is conserved in the two related species C. dubliniensis and C. tropicalis.
Roles of calcineurin and Crz1 in virulence of C. tropicalis.
Previous studies of the C. tropicalis genes involved in virulence are limited (2). Our data provide evidence that calcineurin control of C. tropicalis virulence in a murine systemic infection model is Crz1 dependent because crz1/crz1 mutants exhibit virulence intermediate between that of the wild type and calcineurin mutants (Fig. 3A). The straightforward explanation for the reduced virulence of C. tropicalis calcineurin and crz1/crz1 mutants observed in the murine systemic infection model is that these mutants exhibit hyphal growth defects. In addition, the virulence defects may be attributable in part to cell wall integrity defects of calcineurin mutants (Fig. 6). It is also possible that the loss of calcineurin or Crz1 results in reduced growth at 37°C and, hence, affects the virulence properties of strains. However, we eliminated this possibility on the basis of the similar growth curves and doubling times at 37°C (see Fig. S5 in the supplemental material). Our findings on the roles of calcineurin and Crz1 in the virulence of C. tropicalis are similar to those on the roles of calcineurin and Crz1 in the virulence of C. dubliniensis (27, 57), suggesting conserved functions for calcineurin and Crz1 in the virulence of the closely related species C. tropicalis and C. dubliniensis in a murine systemic infection model. In contrast, in the murine ocular infection model, C. tropicalis calcineurin but not Crz1 is critical for virulence, similar to findings in C. dubliniensis (27). Thus, calcineurin is, in general, required for the virulence of C. tropicalis and C. dubliniensis in both murine systemic and ocular infection models, while Crz1 is required for the virulence of C. tropicalis and C. dubliniensis only in a murine systemic infection model and not in an ocular infection model, suggesting a specific niche requirement (bloodstream versus ocular surface) of Crz1 in both C. tropicalis and C. dubliniensis (27).
In addition to being frequently isolated from patients (11, 17, 60–62), C. tropicalis has also been isolated from the mouse intestine (where it constitutes up to 65% of the overall fungal component) (63) and environmental compost and soil (11, 64). Previous studies suggest that C. tropicalis can be transferred by hand-to-hand contact (65), indicating a potential route for human-human transmission. Further studies to assess if C. tropicalis strains isolated from patients originate from an environmental source and whether calcineurin is critical for the growth of C. tropicalis isolated from patients and the environment will be important.
Mouse Toll-like receptor 4 (TLR4) is a pattern recognition receptor that recognizes lipopolysaccharides from Gram-negative bacteria and initiates innate immunity. TLR4 has been demonstrated to play a role against Aspergillus fumigatus infection in a murine keratitis model (66), while it may play a role in defending C. albicans, depending upon the strains used (67). In previous studies, we demonstrated that mouse TLR4 is not critical for defense against C. glabrata infection in a murine urinary tract infection model (52). Using C3H/HeJ mice with a TLR4 mutation, which we compared to C3H/HeOuJ mice with wild-type TLR4, we found that mouse TLR4 is not required for defense against C. tropicalis in a murine systemic infection model (see Fig. S4 in the supplemental material).
Roles of calcineurin and Crz1 in drug tolerance of C. tropicalis.
C. tropicalis calcineurin mutants are sensitive to cell wall-perturbing agents, such as SDS, Congo red, and tunicamycin (Fig. 6A), indicating that these mutants might be susceptible to antifungal drugs that target the cell wall. Indeed, these calcineurin mutants are susceptible to echinocandins, such as caspofungin, micafungin, and anidulafungin (Fig. 6B and Table 2). Meanwhile, C. tropicalis calcineurin mutants were found to exhibit susceptibility to azoles in Etest strip analyses and spot dilution assays on solid medium (Table 2 and Fig. 6C). Interestingly, we found that the difference between the wild type and calcineurin mutants based on the results of the spot dilution assays could be seen only at extremely high concentrations of azoles (i.e., 150 μg/ml of fluconazole), which is in contrast to the 1 μg/ml of fluconazole that allowed us to observe a difference between the wild type and calcineurin mutants in C. albicans and C. dubliniensis (27). The difference might be due to the fact that (i) C. tropicalis is intrinsically more tolerant to azoles than C. albicans and C. dubliniensis and/or (ii) C. tropicalis exhibited a higher growth/metabolism rate than C. albicans and C. dubliniensis. In summary, we demonstrate that calcineurin controls hyphal growth (in response to carbon source starvation), virulence (in a murine systemic infection model), and drug tolerance (micafungin) and that these functions are in part dependent upon Crz1 in C. tropicalis (Fig. 7). Meanwhile, C. tropicalis calcineurin has Crz1-independent functions (or the Crz1 function is redundant with other factors) for hyphal growth induced by serum or SLAD medium (nitrogen source starvation), cell wall integrity, and tolerance to caspofungin, anidulafungin, or Mn2+ (Fig. 7). Interestingly, Crz1 plays a positive role in Ca2+ tolerance functions, while calcineurin serves as a negative regulator of Ca2+ tolerance functions (Fig. 7). The requirement for calcineurin in virulence and drug tolerance supports calcineurin as a potential drug target in C. tropicalis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Valerie Lapham (North Carolina State University) for assistance with scanning electron microscopy, Joachim Morschhäuser for the SAT1 cassette, and the Broad Institute Candida Database website (http://www.broadinstitute.org/annotation/genome/candida_group/MultiHome.html) for DNA and protein sequences of C. tropicalis. We appreciate members of the Y.-L. Chen, J. Heitman, and M. E. Cardenas laboratories for many helpful discussions. We thank Marilou Nicolas of the National Institutes of Health, University of the Philippines—Manila, and Fedelino Malbas, Jr., of the Histopathology Department of the Regional Institute for Tropical Medicine, Philippines, for technical assistance with the murine ocular infection models. We thank Ted White and June Kwon-Chung for advice.
This work was supported in part by the National Science Council in Taiwan (NSC 102-2320-B-002-041-MY2 to Y.-L.C.) and the Duke University Center for AIDS Research (CFAR), a National Institutes of Health (NIH)-funded program (2P30 AI064518-06 to Y.-L.C.) and NIH/National Institute of Allergy and Infectious Diseases R01 grant 5R01-AI-050438-10 (to J.H.). This work was also supported by pilot funds from Astellas Pharma Inc. and Merck & Co. Inc. (to J.H. and Y.-L.C.).
The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 17 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00302-13.
REFERENCES
- 1.Arendrup MC, Bruun B, Christensen JJ, Fuursted K, Johansen HK, Kjaeldgaard P, Knudsen JD, Kristensen L, Moller J, Nielsen L, Rosenvinge FS, Roder B, Schonheyder HC, Thomsen MK, Truberg K. 2011. National surveillance of fungemia in Denmark (2004 to 2009). J. Clin. Microbiol. 49:325–334. 10.1128/JCM.01811-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chai LY, Denning DW, Warn P. 2010. Candida tropicalis in human disease. Crit. Rev. Microbiol. 36:282–298. 10.3109/1040841X.2010.489506 [DOI] [PubMed] [Google Scholar]
- 3.Arendrup MC, Fuursted K, Gahrn-Hansen B, Schonheyder HC, Knudsen JD, Jensen IM, Bruun B, Christensen JJ, Johansen HK. 2008. Semi-national surveillance of fungaemia in Denmark 2004-2006: increasing incidence of fungaemia and numbers of isolates with reduced azole susceptibility. Clin. Microbiol. Infect. 14:487–494. 10.1111/j.1469-0691.2008.01954.x [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133–163. 10.1128/CMR.00029-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, Sobel JD, Pappas PG, Kullberg BJ, Mycoses Study Group 2012. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin. Infect. Dis. 54:1110–1122. 10.1093/cid/cis021 [DOI] [PubMed] [Google Scholar]
- 6.Sobel JD. 2006. The emergence of non-albicans Candida species as causes of invasive candidiasis and candidemia. Curr. Infect. Dis. Rep. 8:427–433. 10.1007/s11908-006-0016-6 [DOI] [PubMed] [Google Scholar]
- 7.Gottfredsson M, Vredenburgh JJ, Xu J, Schell WA, Perfect JR. 2003. Candidemia in women with breast carcinoma treated with high-dose chemotherapy and autologous bone marrow transplantation. Cancer 98:24–30. 10.1002/cncr.11470 [DOI] [PubMed] [Google Scholar]
- 8.Leung AY, Chim CS, Ho PL, Cheng VC, Yuen KY, Lie AK, Au WY, Liang R, Kwong YL. 2002. Candida tropicalis fungaemia in adult patients with haematological malignancies: clinical features and risk factors. J. Hosp. Infect. 50:316–319. 10.1053/jhin.2002.1194 [DOI] [PubMed] [Google Scholar]
- 9.Wingard JR. 1995. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin. Infect. Dis. 20:115–125. 10.1093/clinids/20.1.115 [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Effron G, Kontoyiannis DP, Lewis RE, Perlin DS. 2008. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob. Agents Chemother. 52:4181–4183. 10.1128/AAC.00802-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang YL, Lin CC, Chang TP, Lauderdale TL, Chen HT, Lee CF, Hsieh CW, Chen PC, Lo HJ. 2012. Comparison of human and soil Candida tropicalis isolates with reduced susceptibility to fluconazole. PLoS One 7:e34609. 10.1371/journal.pone.0034609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang YL, Wang AH, Wang CW, Cheng WT, Li SY, Lo HJ. 2008. Susceptibilities to amphotericin B and fluconazole of Candida species in Taiwan Surveillance of Antimicrobial Resistance of Yeasts 2006. Diagn. Microbiol. Infect. Dis. 61:175–180. 10.1016/j.diagmicrobio.2008.01.011 [DOI] [PubMed] [Google Scholar]
- 13.Yang YL, Ho YA, Cheng HH, Ho M, Lo HJ. 2004. Susceptibilities of Candida species to amphotericin B and fluconazole: the emergence of fluconazole resistance in Candida tropicalis. Infect. Control Hosp. Epidemiol. 25:60–64. 10.1086/502294 [DOI] [PubMed] [Google Scholar]
- 14.Kothavade RJ, Kura MM, Valand AG, Panthaki MH. 2010. Candida tropicalis: its prevalence, pathogenicity and increasing resistance to fluconazole. J. Med. Microbiol. 59:873–880. 10.1099/jmm.0.013227-0 [DOI] [PubMed] [Google Scholar]
- 15.Jensen RH, Johansen HK, Arendrup MC. 2013. Stepwise development of a homozygous S80P substitution in Fks1p, conferring echinocandin resistance in Candida tropicalis. Antimicrob. Agents Chemother. 57:614–617. 10.1128/AAC.01193-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandeputte P, Larcher G, Berges T, Renier G, Chabasse D, Bouchara JP. 2005. Mechanisms of azole resistance in a clinical isolate of Candida tropicalis. Antimicrob. Agents Chemother. 49:4608–4615. 10.1128/AAC.49.11.4608-4615.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eddouzi J, Parker JE, Vale-Silva LA, Coste A, Ischer F, Kelly S, Manai M, Sanglard D. 2013. Molecular mechanisms of drug resistance in clinical Candida species isolated from Tunisian hospitals. Antimicrob. Agents Chemother. 57:3182–3193. 10.1128/AAC.00555-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen YN, Lo HJ, Wu CC, Ko HC, Chang TP, Yang YL. 2011. Loss of heterozygosity of FCY2 leading to the development of flucytosine resistance in Candida tropicalis. Antimicrob. Agents Chemother. 55:2506–2514. 10.1128/AAC.01777-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen ID, Wilson D, Wachtler B, Brunke S, Naglik JR, Hube B. 2012. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti Infect. Ther. 10:85–93. 10.1586/eri.11.152 [DOI] [PubMed] [Google Scholar]
- 20.Braun BR, Johnson AD. 1997. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 277:105–109. 10.1126/science.277.5322.105 [DOI] [PubMed] [Google Scholar]
- 21.Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939–949. 10.1016/S0092-8674(00)80358-X [DOI] [PubMed] [Google Scholar]
- 22.Porman AM, Alby K, Hirakawa MP, Bennett RJ. 2011. Discovery of a phenotypic switch regulating sexual mating in the opportunistic fungal pathogen Candida tropicalis. Proc. Natl. Acad. Sci. U. S. A. 108:21158–21163. 10.1073/pnas.1112076109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porman AM, Hirakawa MP, Jones SK, Wang N, Bennett RJ. 2013. MTL-independent phenotypic switching in Candida tropicalis and a dual role for Wor1 in regulating switching and filamentation. PLoS Genet. 9:e1003369. 10.1371/journal.pgen.1003369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen YL, Kozubowski L, Cardenas ME, Heitman J. 2010. On the roles of calcineurin in fungal growth and pathogenesis. Curr. Fungal Infect. Rep. 4:244–255. 10.1007/s12281-010-0027-5 [DOI] [Google Scholar]
- 25.Reuss O, Vik A, Kolter R, Morschhauser J. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119–127. 10.1016/j.gene.2004.06.021 [DOI] [PubMed] [Google Scholar]
- 26.Butler G, Rasmussen MD, Lin MF, Santos MA, Sakthikumar S, Munro CA, Rheinbay E, Grabherr M, Forche A, Reedy JL, Agrafioti I, Arnaud MB, Bates S, Brown AJ, Brunke S, Costanzo MC, Fitzpatrick DA, de Groot PW, Harris D, Hoyer LL, Hube B, Klis FM, Kodira C, Lennard N, Logue ME, Martin R, Neiman AM, Nikolaou E, Quail MA, Quinn J, Santos MC, Schmitzberger FF, Sherlock G, Shah P, Silverstein KA, Skrzypek MS, Soll D, Staggs R, Stansfield I, Stumpf MP, Sudbery PE, Srikantha T, Zeng Q, Berman J, Berriman M, Heitman J, Gow NA, Lorenz MC, Birren BW, Kellis M, Cuomo CA. 2009. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459:657–662. 10.1038/nature08064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YL, Brand A, Morrison EL, Silao FG, Bigol UG, Malbas FF, Jr, Nett JE, Andes DR, Solis NV, Filler SG, Averette A, Heitman J. 2011. Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis. Eukaryot. Cell 10:803–819. 10.1128/EC.00310-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu TG, Wilhelmus KR, Mitchell BM. 2003. Experimental keratomycosis in a mouse model. Invest. Ophthalmol. Vis. Sci. 44:210–216. 10.1167/iovs.02-0446 [DOI] [PubMed] [Google Scholar]
- 29.Hurley R, Winner HI. 1962. The pathogenicity of Candida tropicalis. J. Pathol. Bacteriol. 84:33–38. 10.1002/path.1700840104 [DOI] [PubMed] [Google Scholar]
- 30.Hasenclever HF, Mitchell WO. 1961. Pathogenicity of C. albicans and C. tropicalis. Sabouraudia 1:16–21 [PubMed] [Google Scholar]
- 31.de Repentigny L, Phaneuf M, Mathieu LG. 1992. Gastrointestinal colonization and systemic dissemination by Candida albicans and Candida tropicalis in intact and immunocompromised mice. Infect. Immun. 60:4907–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YL, Montedonico AE, Kauffman S, Dunlap JR, Menn FM, Reynolds TB. 2010. Phosphatidylserine synthase and phosphatidylserine decarboxylase are essential for cell wall integrity and virulence in Candida albicans. Mol. Microbiol. 75:1112–1132. 10.1111/j.1365-2958.2009.07018.x [DOI] [PubMed] [Google Scholar]
- 33.Huynh N, Chang HY, Borboli-Gerogiannis S. 2012. Ocular involvement in hospitalized patients with candidemia: analysis at a Boston tertiary care center. Ocul. Immunol. Inflamm. 20:100–103. 10.3109/09273948.2011.646383 [DOI] [PubMed] [Google Scholar]
- 34.Dozier CC, Tarantola RM, Jiramongkolchai K, Donahue SP. 2011. Fungal eye disease at a tertiary care center: the utility of routine inpatient consultation. Ophthalmology 118:1671–1676. 10.1016/j.ophtha.2011.01.038 [DOI] [PubMed] [Google Scholar]
- 35.Yang YS. 2009. Results of extensive surgical treatment of seven consecutive cases of postoperative fungal endophthalmitis. Korean J. Ophthalmol. 23:159–163. 10.3341/kjo.2009.23.3.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakrabarti A, Shivaprakash MR, Singh R, Tarai B, George VK, Fomda BA, Gupta A. 2008. Fungal endophthalmitis: fourteen years' experience from a center in India. Retina 28:1400–1407. 10.1097/IAE.0b013e318185e943 [DOI] [PubMed] [Google Scholar]
- 37.Feman SS, Nichols JC, Chung SM, Theobald TA. 2002. Endophthalmitis in patients with disseminated fungal disease. Trans. Am. Ophthalmol. Soc. 100:67–70 [PMC free article] [PubMed] [Google Scholar]
- 38.Shukla PK, Kumar M, Keshava GB. 2008. Mycotic keratitis: an overview of diagnosis and therapy. Mycoses 51:183–199. 10.1111/j.1439-0507.2007.01480.x [DOI] [PubMed] [Google Scholar]
- 39.Bates S, Hughes HB, Munro CA, Thomas WP, MacCallum DM, Bertram G, Atrih A, Ferguson MA, Brown AJ, Odds FC, Gow NA. 2006. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J. Biol. Chem. 281:90–98. 10.1074/jbc.M510360200 [DOI] [PubMed] [Google Scholar]
- 40.Baker LG, Specht CA, Lodge JK. 2011. Cell wall chitosan is necessary for virulence in the opportunistic pathogen Cryptococcus neoformans. Eukaryot. Cell 10:1264–1268. 10.1128/EC.05138-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puttikamonkul S, Willger SD, Grahl N, Perfect JR, Movahed N, Bothner B, Park S, Paderu P, Perlin DS, Cramer RA., Jr 2010. Trehalose 6-phosphate phosphatase is required for cell wall integrity and fungal virulence but not trehalose biosynthesis in the human fungal pathogen Aspergillus fumigatus. Mol. Microbiol. 77:891–911. 10.1111/j.1365-2958.2010.07254.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang C, Dong D, Yu B, Cai G, Wang X, Ji Y, Peng Y. 2013. Mechanisms of azole resistance in 52 clinical isolates of Candida tropicalis in China. J. Antimicrob. Chemother. 68:778–785. 10.1093/jac/dks481 [DOI] [PubMed] [Google Scholar]
- 43.Pasquale T, Tomada JR, Ghannoun M, Dipersio J, Bonilla H. 2008. Emergence of Candida tropicalis resistant to caspofungin. J. Antimicrob. Chemother. 61:219. 10.1093/jac/dkm453 [DOI] [PubMed] [Google Scholar]
- 44.Steinbach WJ, Reedy JL, Cramer RA, Jr, Perfect JR, Heitman J. 2007. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 5:418–430. 10.1038/nrmicro1680 [DOI] [PubMed] [Google Scholar]
- 45.Cruz MC, Goldstein AL, Blankenship JR, Del Poeta M, Davis D, Cardenas ME, Perfect JR, McCusker JH, Heitman J. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546–559. 10.1093/emboj/21.4.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48:959–976. 10.1046/j.1365-2958.2003.03495.x [DOI] [PubMed] [Google Scholar]
- 47.Chen YL, Lehman VN, Lewit Y, Averette AF, Heitman J. 2013. Calcineurin governs thermotolerance and virulence of Cryptococcus gattii. G3 (Bethesda) 3:527–539. 10.1534/g3.112.004242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi JH, Kim Y, Lee YH. 2009. Functional analysis of MCNA, a gene encoding a catalytic subunit of calcineurin, in the rice blast fungus Magnaporthe oryzae. J. Microbiol. Biotechnol. 19:11–16 [PubMed] [Google Scholar]
- 49.Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16:2576–2589. 10.1093/emboj/16.10.2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinbach WJ, Cramer RA, Jr, Perfect BZ, Asfaw YG, Sauer TC, Najvar LK, Kirkpatrick WR, Patterson TF, Benjamin DK, Jr, Heitman J, Perfect JR. 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:1091–1103. 10.1128/EC.00139-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen YL, Konieczka JH, Springer DJ, Bowen SE, Zhang J, Silao FG, Bungay AA, Bigol UG, Nicolas MG, Abraham SN, Thompson DA, Regev A, Heitman J. 2012. Convergent evolution of calcineurin pathway roles in thermotolerance and virulence in Candida glabrata. G3 (Bethesda) 2:675–691. 10.1534/g3.112.002279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cunningham KW, Fink GR. 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 124:351–363. 10.1083/jcb.124.3.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Withee JL, Sen R, Cyert MS. 1998. Ion tolerance of Saccharomyces cerevisiae lacking the Ca2+/CaM-dependent phosphatase (calcineurin) is improved by mutations in URE2 or PMA1. Genetics 149:865–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Silao FG, Bigol UG, Bungay AA, Nicolas MG, Heitman J, Chen YL. 2012. Calcineurin is required for pseudohyphal growth, virulence, and drug resistance in Candida lusitaniae. PLoS One 7:e44192. 10.1371/journal.pone.0044192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bader T, Schroppel K, Bentink S, Agabian N, Kohler G, Morschhauser J. 2006. Role of calcineurin in stress resistance, morphogenesis, and virulence of a Candida albicans wild-type strain. Infect. Immun. 74:4366–4369. 10.1128/IAI.00142-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karababa M, Valentino E, Pardini G, Coste AT, Bille J, Sanglard D. 2006. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 59:1429–1451. 10.1111/j.1365-2958.2005.05037.x [DOI] [PubMed] [Google Scholar]
- 58.Stathopoulos AM, Cyert MS. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432–3444. 10.1101/gad.11.24.3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noble SM, French S, Kohn LA, Chen V, Johnson AD. 2010. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 42:590–598. 10.1038/ng.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galan-Ladero MA, Blanco-Blanco MT, Hurtado C, Perez-Giraldo C, Blanco MT, Gomez-Garcia AC. 2013. Determination of biofilm production by Candida tropicalis isolated from hospitalized patients and its relation with cellular surface hydrophobicity, plastic adherence and filamentation ability. Yeast 30:331–339. 10.1002/yea.2965 [DOI] [PubMed] [Google Scholar]
- 61.Chang TP, Ho MW, Yang YL, Lo PC, Lin PS, Wang AH, Lo HJ. 2013. Distribution and drug susceptibilities of Candida species causing candidemia from a medical center in central Taiwan. J. Infect. Chemother. 19:1065–1071. 10.1007/s10156-013-0623-8 [DOI] [PubMed] [Google Scholar]
- 62.Yang YL, Chen HT, Lin CC, Chu WL, Lo HJ, TSARY Hospitals. 2013. Species distribution and drug susceptibilities of Candida isolates in TSARY 2010. Diagn. Microbiol. Infect. Dis. 76:182–186. 10.1016/j.diagmicrobio.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 63.Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, Rotter JI, Wang HL, McGovern DP, Brown GD, Underhill DM. 2012. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 336:1314–1317. 10.1126/science.1221789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheon SA, Jung KW, Chen YL, Heitman J, Bahn YS, Kang HA. 2011. Unique evolution of the UPR pathway with a novel bZIP transcription factor, Hxl1, for controlling pathogenicity of Cryptococcus neoformans. PLoS Pathog. 7:e1002177. 10.1371/journal.ppat.1002177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rangel-Frausto MS, Houston AK, Bale MJ, Fu C, Wenzel RP. 1994. An experimental model for study of Candida survival and transmission in human volunteers. Eur. J. Clin. Microbiol. Infect. Dis. 13:590–595. 10.1007/BF01971311 [DOI] [PubMed] [Google Scholar]
- 66.Leal SM, Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. 2010. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 6:e1000976. 10.1371/journal.ppat.1000976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Netea MG, Gow NA, Joosten LA, Verschueren I, van der Meer JW, Kullberg BJ. 2010. Variable recognition of Candida albicans strains by TLR4 and lectin recognition receptors. Med. Mycol. 48:897–903. 10.3109/13693781003621575 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.