Abstract
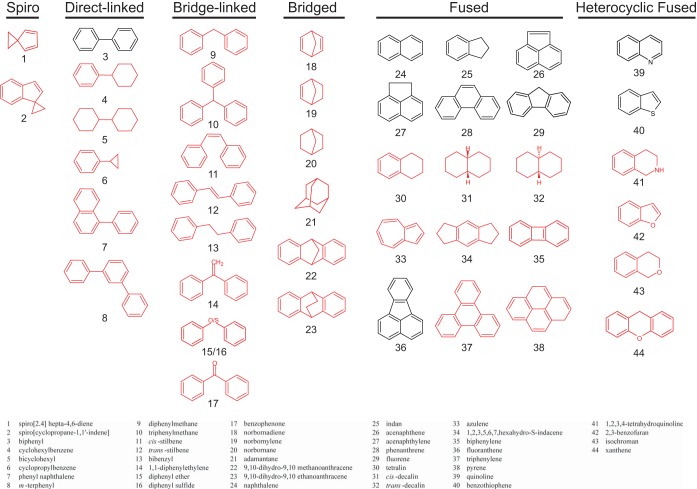
The most problematic hydrocarbons in hydraulic fracturing (fracking) wastewaters consist of fused, isolated, bridged, and spiro ring systems, and ring systems have been poorly studied with respect to biodegradation, prompting the testing here of six major ring structural subclasses using a well-characterized bacterium and a silica encapsulation system previously shown to enhance biodegradation. The direct biological oxygenation of spiro ring compounds was demonstrated here. These and other hydrocarbon ring compounds have previously been shown to be present in flow-back waters and waters produced from hydraulic fracturing operations. Pseudomonas sp. strain NCIB 9816-4, containing naphthalene dioxygenase, was selected for its broad substrate specificity, and it was demonstrated here to oxidize fundamental ring structures that are common in shale-derived waters but not previously investigated with this or related enzymes. Pseudomonas sp. NCIB 9816-4 was tested here in the presence of a silica encasement, a protocol that has previously been shown to protect bacteria against the extremes of salinity present in fracking wastewaters. These studies demonstrate the degradation of highly hydrophobic compounds by a silica-encapsulated model bacterium, demonstrate what it may not degrade, and contribute to knowledge of the full range of hydrocarbon ring compounds that can be oxidized using Pseudomonas sp. NCIB 9816-4.
INTRODUCTION
Many waters in natural and engineered systems contain multiple-ring aromatic hydrocarbons and heterocycles, and thus, bacteria able to degrade these compounds have been isolated and studied extensively. Among the most well-studied of these bacteria is Pseudomonas sp. strain NCIB 9816-4 containing naphthalene dioxygenase (NDO), which is known to initiate an attack on more than 100 compounds, most notably, fused-ring polycyclic aromatic hydrocarbons and heterocyclic ring compounds (1, 2). A list of the compounds, their structures, and the relevant literature citations are provided in Table S1 in the supplemental material.
The treatment and disposal of water used in hydraulic fracturing, or fracking, operations are controversial and have sparked interest in analytical chemistry for and biodegradation of chemicals within the waters. Recently, flow-back waters and waters produced from hydraulically fractured natural gas and liquid oil shales were analyzed for their chemical composition and shown to contain a significant number of ring hydrocarbons (3). From a human health perspective, the polycyclic aromatic hydrocarbons (PAHs) pose the greatest danger, given that this class includes numerous EPA priority pollutants and known carcinogens (4, 5). Indeed, most biodegradation studies on multiring aromatic compounds have been performed with fused-ring aromatic compounds, but the fracking (frac) waters analyzed also contained isolated and spiro ring compounds, classes much less well studied for biodegradation (6). Indeed, among the 123 compounds tested with Pseudomonas sp. NCIB 9816, there have been only 7 direct-linked isolated ring compounds tested, and no spiro ring, bridge-linked isolated ring, or bridged ring system compounds have previously been tested with NCIB 9186-4. The present study helps fill that gap.
Flow-back waters and waters produced from shales represent a problem for biodegradation because the high salinity of the waters could inhibit microbial activities, a problem that has been shown to be at least partly addressed by silica encapsulation to protect the microbes (3). In this context, the present study used silica-encapsulated Pseudomonas sp. NCIB 9816-4 and tested the cells against a large set of fused, isolated, bridged, and spiro ring compounds for their biodegradability (Fig. 1). The initial oxidation of multiring hydrocarbons by this bacterium has been attributed uniquely to the multicomponent enzyme system naphthalene dioxygenase, but detailed studies on products and reaction mechanisms will require further studies with purified enzyme components. In all, 44 compounds were tested, and 33 of these have not been tested with this organism previously. Of those 33 new compounds tested, spiro and bridge-linked compounds were observed to undergo extensive degradation, and several novel products that may be of value in subsequent mechanistic studies on the naphthalene dioxygenase enzyme system were determined. Moreover, we observed and report on the inability of the system to oxidize a number of compounds and thus better address the capabilities and limitations of this bacterium for biodegradation. The study also demonstrates that silica-encapsulated bacteria can degrade highly hydrophobic ring hydrocarbons, which, to our knowledge, has not been previously demonstrated.
FIG 1.
Classes of ring structures tested with Pseudomonas sp. NCIB 9816-4. Compounds not previously tested with NDO previously are drawn in red.
MATERIALS AND METHODS
Materials.
All chemicals were from Sigma-Aldrich, with the following exceptions: azulene, fluorene, and 2,2-diphenylethanol (Acros); cis-decalin, trans-decalin, diphenyl sulfide, and diphenyl ether (TCI America); fluoranthene (Research Organic Inorganic Chemical Corp.); biphenyl (Spectrum Chemical Mfg. Corp.); naphthalene (Matheson, Coleman and Bell); and tetralin, 1-phenyl naphthalene, cis-stilbene, isochroman, m-terphenyl, diphenylmethane, and 1,3-indanedione (Alfa Aesar).
Cell growth.
Saturated cultures of Pseudomonas sp. NCIB 9816-4 grown on LB at 30°C were used for inoculation of minimal medium at a starting optical density at 600 nm (OD600) of 0.01. Growth medium contained 1 g naphthalene per 300 ml minimal medium (MM). MM was made according to the methods of Turner et al. (7), with the following substitutions: 318 mg of Na2 EDTA·2H2O, 24 mg of CoSO4·7H2O, and 17.7 mg of Na2B4O7·10H2O. Cultures were grown in shake flasks (230 rpm) for 18 h at 25°C with bubbled air from a small fish tank pump. Cultures reached a final OD600 of 1.5 to 2.5. To remove the majority of unused naphthalene, cells were filtered through glass wool prior to harvesting by centrifugation at 5,000 × g for 5 min. Escherichia coli DH5α was grown in LB in shake flasks at 37°C.
Silica gel formation.
Silica gels were formed using standard methods with activated alkoxide (tetramethoxylsilane [TMOS]) and preformed silica particles (Ludox TM-40) (8). Briefly, TMOS was activated by hydrolysis of a solution of TMOS, water, and 1 N HCl at a ratio of 1:1:0.001 (vol/vol/vol). Bacteria were encapsulated in gels by combining TM-40–phosphate-buffered saline (PBS)–polyethylene glycol 600 (2:2:1, vol/vol/vol) with the bacterial cells resuspended in PBS at 0.2 g (wet weight)/ml and the activated TMOS at a ratio of 2:2:1 (vol/vol/vol). The gels were hardened at room temperature for about 5 min before washing with 1 ml PBS.
Encapsulated cell activity assay.
Silica gel plugs (1 ml) were formed in the bottom of 125-ml serum bottles as described above. Water (3 ml) containing single compounds or mixtures of compounds was added to the gel. Bottles were crimp sealed with polytetrafluoroethylene-backed silicone seals. Stock solutions of each compound were made in methyl-t-butyl ether (MTBE) at 10 mg/ml for solid compounds and 12 μl/ml for liquid compounds. Due to limited solubility, stock solutions of 9,10-dihydro-9,10-methanoanthracene and triphenylene were made to 5 mg/ml and 3 mg/ml, respectively. Stocks were diluted 1,000-fold in distilled water immediately prior to incubation with encapsulated cells. For mixtures, compounds were assayed at 0.1 to 3 μg/ml. The composition of each of the three mixtures tested is given in Table S2 in the supplemental material. Each individual compound or mixture was assayed in triplicate. Bottles were shaken at 90 rpm and 23°C prior to extraction by injection of 1 ml MTBE at the times indicated below. Vortexed bottles were unsealed, and the MTBE was removed and placed into a new vial for analysis by gas chromatography (GC). Separation was achieved with an HP-1ms column (100% dimethylsiloxane capillary; 30 m by 250 μm by 0.25 μm), a helium flow rate of 1.75 ml/min, and a temperature of 250°C at the injection port. For samples containing norbornane, norbornylene, or norbornadiene, the GC oven was held at 40°C for 10 min, ramped to 60°C at 5°C/min, and then ramped to 320°C at 30°C/min. For samples containing cyclopropylbenzene, tetralin, diphenylmethane, isochroman, bibenzyl, cis-decalin, trans-decalin, bicyclohexyl, or cyclohexylbenzene, the GC oven was held at 60°C for 3 min and ramped to 320°C at 15°C/min. For all remaining compounds the GC oven was ramped from 100°C to 320°C at 30°C/min. All programs included a 5-min hold at 320°C. The sample was split at the column outlet between a flame ionization detector (FID; HP 7890A; Hewlett-Packard, Palo Alto, CA) and a mass spectrometer (MS; HP 5975C). Electron impact mass spectra were collected at 70 eV with positive polarity.
RESULTS
Frac water ring compounds tested.
A previous study analyzing flow-back waters and waters produced from shale gas and oil extraction found the presence of compounds containing multiple rings that were spiro, direct-linked, bridge-linked, bridged, fused, and heterocyclic compounds (3). To test the efficacy of Pseudomonas sp. NCIB 9816-4 with these compounds, as a variation on the resting cell assay, encapsulated cells were incubated with individual compounds or admixtures. The categories and structures of the compounds tested in this study are shown in Fig. 1, with compounds not previously tested with Pseudomonas sp. NCIB 9816-4 highlighted in red. Fused PAHs, previously known to be oxidized by this bacterium, served as positive controls for enzymatic activity. Negative controls were conducted with encapsulated E. coli cells, previously shown not to degrade aromatic hydrocarbons, to account for adsorption to cells or to silica gel material or other artifactual loss of the chemical tested. In addition, incubations were carried out in crimp-sealed serum bottles to prevent evaporation of volatile compounds. Starting material disappearance and product identification, when observed, were analyzed by GC/MS/FID. The results are presented in Table 1.
TABLE 1.
Disappearance of multiple-ring compounds caused by P. putida NCIB 9816-4 compared to that caused by the E. coli negative control according to the amount of compound remaining by GC/FID
| Ring classification and compound | GC/FID avg peak areaa (105) |
|
|---|---|---|
| E. coli | NCIB 9816-4 | |
| Spiro | ||
| Spiro[2.4]hepta-4,6-diene | 115 ± 23 | 8 ± 3 |
| Spiro[cyclopropane-1,1′-indene] | 9 ± 2 | Below detection limit |
| Direct linked | ||
| Cyclohexylbenzene | 41 ± 2 | 3.3 ± 0.5 |
| Cyclopropylbenzene | 92 ± 16 | 45 ± 6 |
| Phenyl naphthalene | 34 ± 15 | 13 ± 2 |
| m-Terphenyl | 75 ± 10 | 29 ± 6 |
| Bicyclohexyl | 37 ± 2 | 41 ± 4 |
| Bridge linked | ||
| Diphenylmethane | 32 ± 7 | 12 ± 1 |
| Triphenylmethane | 133 ± 11 | 66 ± 14 |
| cis-Stilbene | 1.0 ± 0.2 | Below detection limit |
| trans-Stilbene | 41 ± 8 | 5 ± 1 |
| Bibenzyl | 22 ± 2 | 1.1 ± 0.1 |
| 1,1-Diphenylethylene | 39 ± 11 | 4 ± 1 |
| Diphenyl ether | 61 ± 7 | 5 ± 2 |
| Benzophenone | 121 ± 5 | 53 ± 15 |
| Bridge fused | ||
| Norbornadiene | 67 ± 10 | 68 ± 8 |
| 9,10-Dihydro-9,10-ethanoanthracene | 36 ± 11 | 34 ± 2 |
| 9,10-Dihydro-9,10-methanoanthracene | 123 ± 30 | 108 ± 8 |
| Fused | ||
| Fluoranthene | 53 ± 11 | 22 ± 4 |
| Pyrene | 42 ± 10 | 20 ± 10 |
| Triphenylene | 23 ± 1 | 15 ± 4 |
| Tetralin | 103 ± 24 | Below detection limit |
| 1,2,3,5,6,7-Hexahydro-S-indacene | 4.0 ± 0.7 | Below detection limit |
| cis-Decalin | 38 ± 5 | 41 ± 4 |
| trans-Decalin | 35 ± 5 | 23 ± 6 |
| Heterocyclic fused | ||
| Xanthene | 20 ± 6 | 0.7 ± 0.3 |
| Isochroman | 119 ± 5 | Below detection limit |
| Benzofuran | 137 ± 22 | Below detection limit |
Measured after 24 h of incubation with silica-encapsulated cells. Data are means ± standard deviations for triplicate samples.
All categories of ring compounds showed extensive or complete biodegradation of individual parent compounds, except for the bridged ring compounds, such as norbornadiene (compound 18). The heterocyclic compounds were extensively degraded, as were most of the bridge-linked compounds. It was particularly interesting that spiro ring parent compounds were eliminated completely or nearly so.
Spiro ring compounds.
The previously untested spiro ring compounds, spiro[2.4]hepta-4,6-diene (compound 1) and spiro[cyclopropane,1,1′-indene] (compound 2), were shown here to be substrates for Pseudomonas sp. NCIB 9816-4, and the E. coli negative control did not show significant substrate disappearance. The extent of disappearance suggested that the compounds were good substrates. Moreover, only very minor product peaks could be discerned by GC/MS/FID (Fig. 2; see also Table S3 in the supplemental material). Two products were observed from compound 1. The mass spectrum of one product shown in Fig. 2A is consistent with dioxygenation at a double bond in the cyclopentadiene ring system. Product 2 from compound 1 (Fig. 2B) is consistent with a keto hydroxy compound that would arise if the dihydrodiol product 1 were acted on by dihydrodiol dehydrogenase. Note that diol dehydrogenases have previously been shown to act as alcohol dehydrogenases to produce keto alcohols, which isomerize with rearomation to a catechol in the case of keto alcohols of cyclohexadienes (9). With an oxidation series for cyclopentadiene ring compounds similar to that found in compound 1, a keto alcohol would not isomerize, and so this would be the stable structure. For the product shown in Fig. 2B, the positions of the ketone and hydroxy group are not known with certainty, as no standard compounds are available for this class of compounds.
FIG 2.
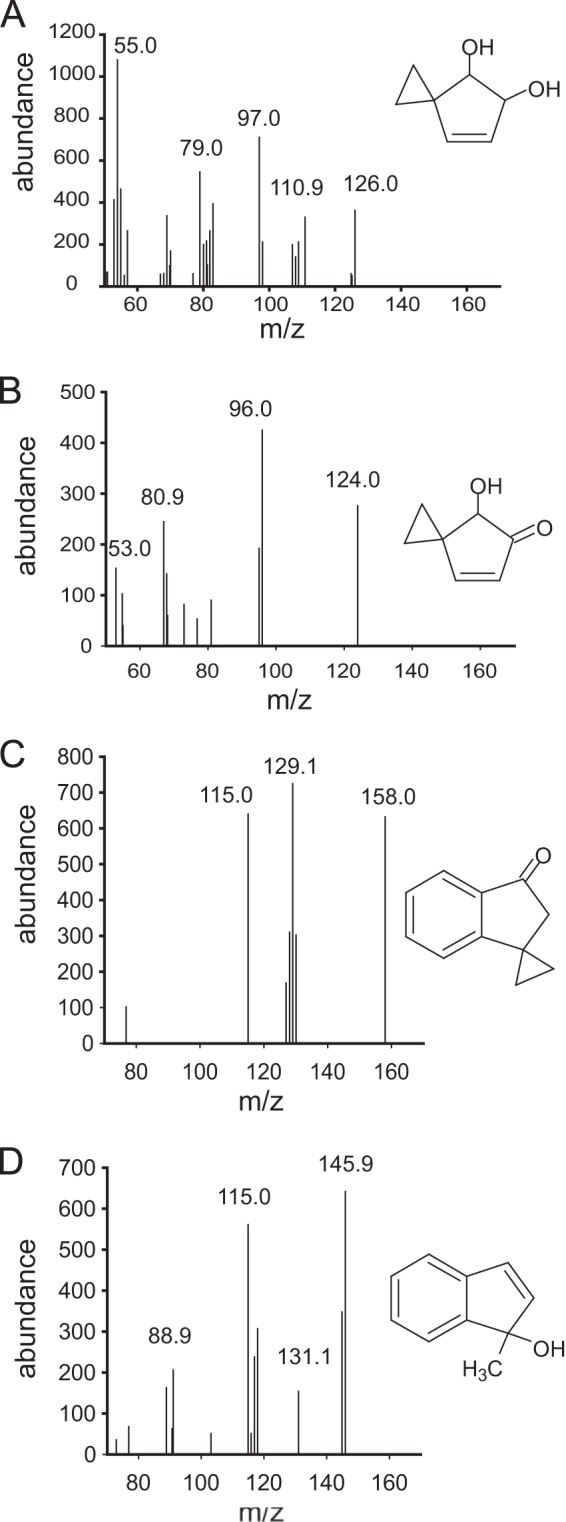
Mass spectra of products from spiro compounds and putative structural identifications. (A) Spiro[2.4]hept-6-ene-4,5-diol; (B) spiro[2.4]hept-6-ene-4-ol-5-one; (C) spiro[cyclopropane-1,1′-[1H]inden]-3′(2′H)-one; (D) 1-methylinden-1-ol.
The first oxidation product observed with spiro compound 2 as the substrate showed a mass spectrum consistent with a monooxygenation reaction at the cyclopentene ring (Fig. 2C), consistent with previous observations with indan, in which 1-indanone is a major product (10). The second observed product had a mass spectrum consistent with its identity as 1-methylinden-1-ol (Fig. 2D). Product identification as either 2-methylindanone, 1,2-indanedione, or 1,3-indanedione, each of which has the same molecular mass as the observed product, was ruled out on the basis of comparison to authentic standards. The oxidation of the spiro carbon, while somewhat unexpected, is consistent with previous observations of 1-indenol as a product of NDO oxidation of indene (10). The loss of a cyclopropyl carbon was unexpected, but cyclopropyl rings are known to undergo unusual ring-opening reactions, and hence, this could be an interesting substrate for future studies on the mechanism of naphthalene dioxygenase.
Direct- and bridge-linked isolated rings.
Surprisingly, naphthalene dioxygenase from Pseudomonas sp. NCIB 9816-4 had not been reported to oxidize many isolated ring structures. All known substrates in this ring class, biphenyl, two flavones, and biphenyl-like compounds with heteroatoms, are direct-linked ring compounds (11–13). No bridge-linked isolated ring substrates have been identified previously. The present study tested an additional 14 compounds in this class. All except bicyclohexyl (compound 5) were observed here to be substrates. The new direct-linked ring substrates demonstrated here were cyclohexylbenzene (compound 4), cyclopropylbenzene (compound 6), phenyl naphthalene (compound 7), and m-terphenyl (compound 8). Additionally, nine new bridge-linked isolated ring compounds were shown to be substrates: diphenylmethane (compound 9), triphenylmethane (compound 10), cis-stilbene (compound 11), trans-stilbene (compound 12), bibenzyl (compound 13), 1,1-diphenylethylene (compound 14), diphenyl ether (compound 15), diphenyl sulfide (compound 16), and benzophenone (compound 17).
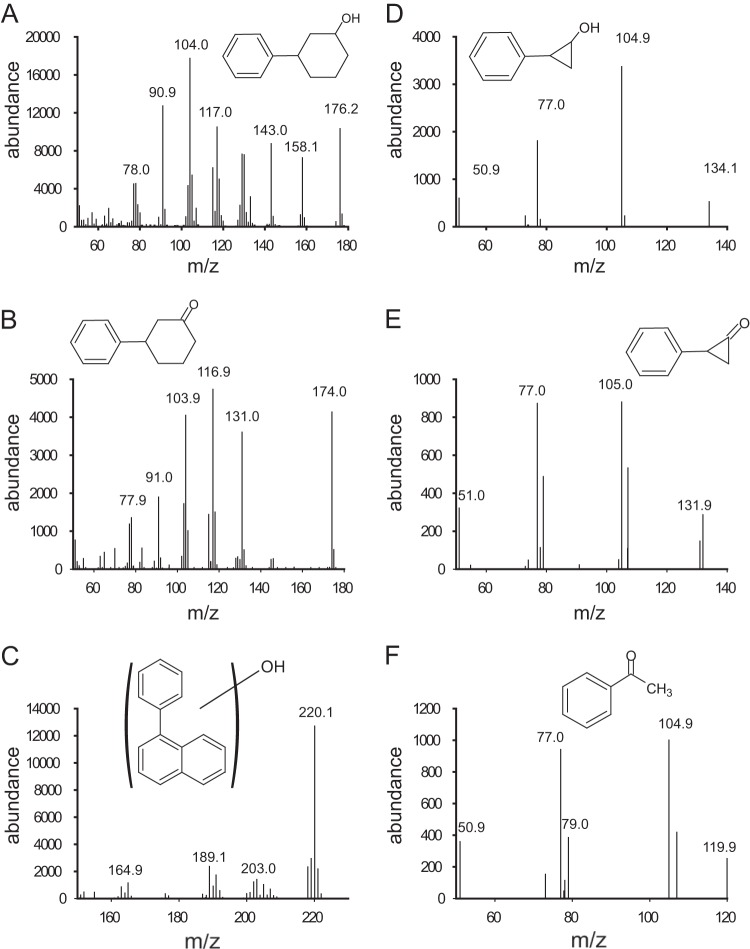
There were both anticipated and unexpected products from some of the compounds in this class. For example, diphenylmethane (compound 9) was oxidized to diphenylmethanol, an expected product given the known propensity for NDO to carry out benzylic monooxygenation (see Fig. S1; see also Table S3 in the supplemental material) (10, 14, 15). With cyclohexylbenzene (compound 4), the major product was 3-phenyl cyclohexanol (Fig. 3A; see also Table S3 in the supplemental material). Note that the mass spectrometry library contained mass spectra for 2-, 3-, and 4-phenyl cyclohexanols, and 3-phenyl cyclohexanol was the strongest match. The ketone (Fig. 3B) likely derives from a dehydrogenase-catalyzed oxidation of the alcohol, similar to the products observed from indan with Pseudomonas sp. NCIB 9816-4 (10). With phenyl naphthalene (compound 7), three phenolic products were obtained. All three had similar mass spectra; one is presented in Fig. 3C. These products likely result from a dioxygenation and subsequent elimination of water. Since the wild-type Pseudomonas sp. NCIB 9816-4 was used in this experiment, it is likely that the diol dehydrogenase could not work on the initial dihydrodiol products that gave rise to the observed phenols. Indeed, the accumulated products account for the majority of the starting material, as judged by relative GC/FID peak areas (see Table S3 in the supplemental material). The exact position of the hydroxyl group giving rise to the observed products was not determined. Likewise, with diphenyl ether (compound 15), a phenoxy-phenol was observed (see Fig. S2 and Table S3 in the supplemental material). Among the most interesting of the product profiles, one was observed with cyclopropylbenzene (compound 6) as the substrate (Fig. 3D to F; see also Table S3 in the supplemental material). Of those, the most unexpected product was acetophenone (Fig. 3F). This product identification was confirmed by both the GC retention time and the mass spectrum in comparison with those of an authentic standard. We considered possible contaminants, acetophenone or ethylbenzene; the latter could be oxidized to yield acetophenone. However, a careful examination of the substrates and solutions used in these experiments did not uncover significant levels of either compound. It is presently unclear what oxidation reaction(s) could produce this product from cyclopropylbenzene, but this appears to be another example of a cyclopropyl ring-opening reaction during substrate oxidation by NDO.
FIG 3.
Mass spectra of products from direct-linked compounds and putative structural identifications. (A) 3-Phenyl cyclohexanol; (B) 3-phenyl cyclohexanone; (C) hydroxy-1-phenyl napthalenol; (D) 2-phenyl cyclopropanol; (E) 2-phenyl cyclopropanone; (F) acetophenone.
Other unexpected products were observed when 1,1-diphenylethylene (compound 14) was incubated with Pseudomonas sp. NCIB 9816-4 (Fig. 4; see also Table S3 in the supplemental material). Diphenyl acetaldehyde (Fig. 4A) could be generated by either dioxygenation of the ethylene group of 1,1-diphenyl ethylene followed by dehydration or epoxidation and oxirane ring isomerization to yield the observed aldehyde.
FIG 4.
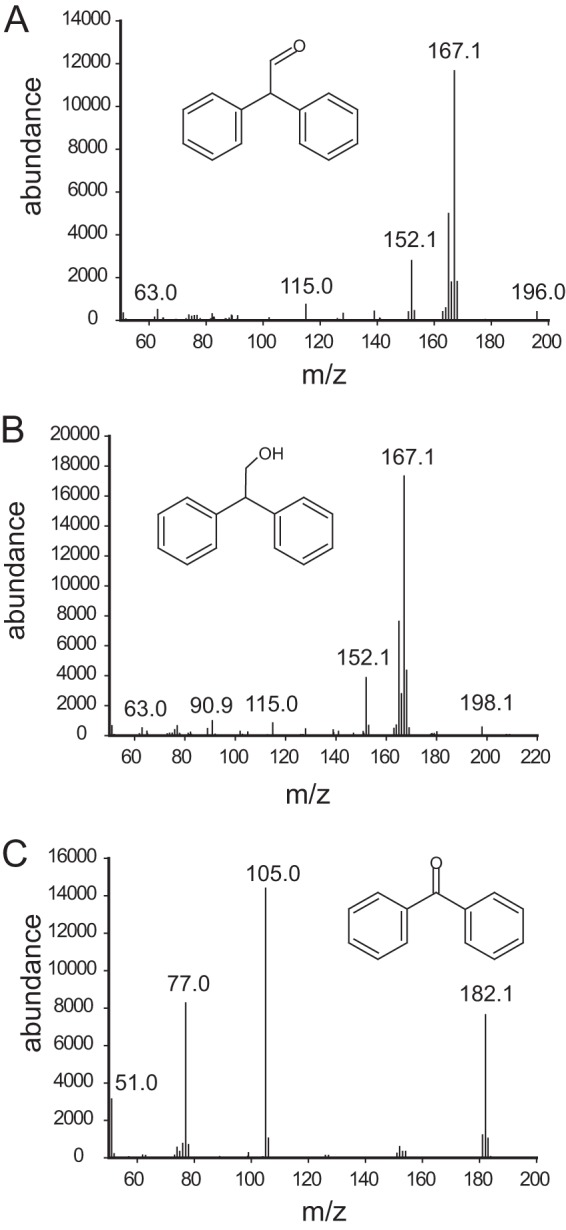
Mass spectra and putative structure identification of products from 1,1-diphenyl ethylene. (A) 2,2-Diphenylacetaldehyde; (B) 2,2-diphenylethanol; (C) benzophenone.
The alcohol could be derived from a reduction of the aldehyde (Fig. 4B). Benzophenone was also identified as a product and confirmed by comparison to the authentic standard (Fig. 4C). This could arise from dioxygenation of the ethylene group, followed by oxidative cleavage of the diol C—C bond.
Bridged and planar fused-ring systems.
None of the bridged compounds (compounds 18 to 23) that were tested were determined to be substrates. This is in contrast to the results obtained with planar fused-ring systems, which are the largest known class of NDO substrates. It is likely that the three-dimensional structures of the bridged compounds, shaped like a prolate spheroid, precluded their entry into or proper fit in the active site of naphthalene dioxygenase.
Several fused-ring compounds that had not previously been reported, to our knowledge, for this specific organism or enzyme system were shown here to be substrates: tetralin (compound 30), trans-decalin (compound 32), azulene (compound 33), 1,2,3,5,6,7-hexahydro-S-indacene (compound 34), biphenylene (compound 35), triphenylene (compound 37), and pyrene (compound 38). Tetralin was shown to produce at least one accumulating intermediate putatively identified as tetrahydronaphthalene-dihydrodiol (see Fig. S3 and Table S3 in the supplemental material).
Interestingly, trans-decalin, but not cis-decalin, was determined to be a substrate, as a small but significant decrease in starting material was observed. Its functioning as a substrate was supported by the observation of two product peaks in reaction mixtures. The mass spectra are consistent with identification of the products as decahydronapthenol and octahydronapthenone, though the position of oxygenation was not determined, as authentic standards were not available (see Fig. S4 and Table S3 in the supplemental material). No evidence for contamination of the starting material with the products was found.
Heterocyclic ring systems.
In general, heterocyclic ring compounds that resemble naphthalene or anthracene were previously known to be substrates for NDO (see Table S1 in the supplemental material). Several additional substrates were demonstrated here in experiments where they were provided as individual compounds or in mixtures with other compounds. Those newly identified substrates are 1,2,3,4-tetrahydroquinoline (compound 41), 2,3-benzofuran (compound 42), isochroman (compound 43), and xanthene (compound 44).
Simulated frac water ring-type mixture incubations.
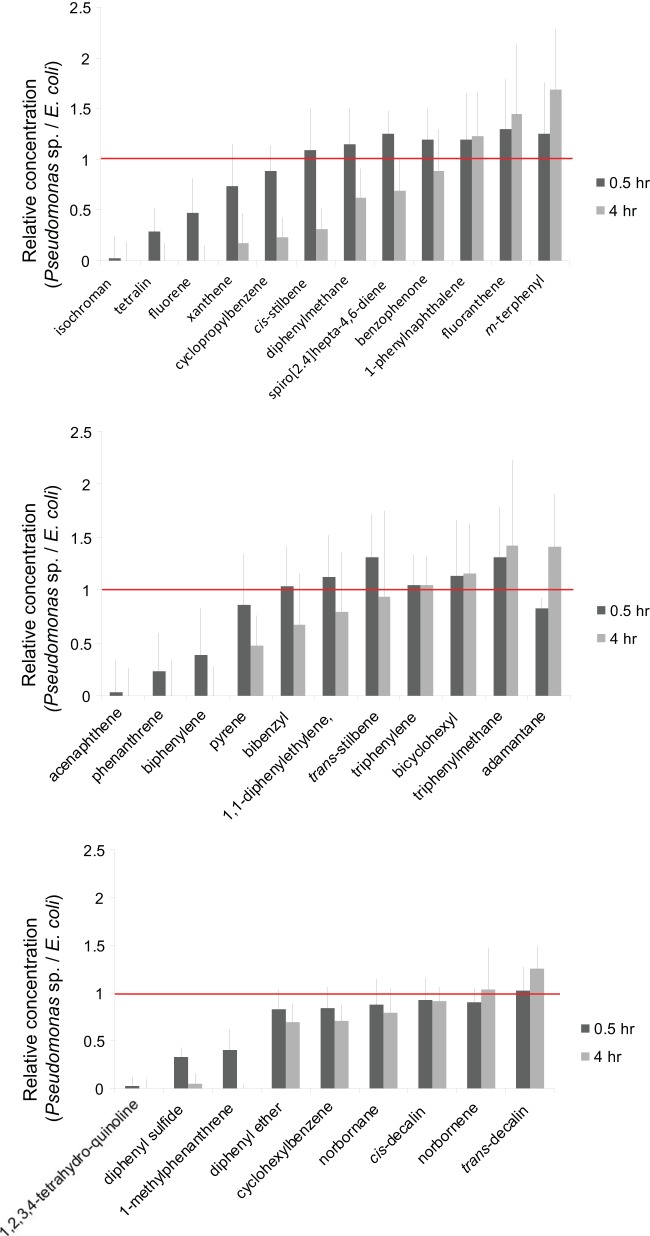
Waters derived from hydraulic fracturing contain mixtures of substrates with the ring types investigated in the present study. In that context, experiments were conducted with Pseudomonas sp. NCIB 9816-4 encapsulated into silica and incubated with a simulated multiring hydrocarbon fraction of frac waters. Mixtures typically contained one dozen compounds representing ring types found in frac waters near the solubility limit of the mixture in water (Fig. 5; see also Table S2 in the supplemental material). Experiments were carried out in crimp-sealed vials to decrease the loss of volatile compounds. Data were collected over the course of 4 h before oxygen levels became limiting.
FIG 5.
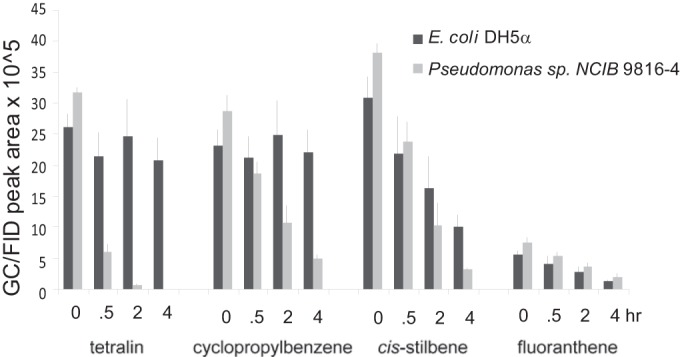
GC/FID peak areas of representative compounds from a water mixture sampled in triplicate over 4 h. Gels contained either Pseudomonas sp. NCIB 9816-4 or E. coli.
Negative-control incubations containing silica-encapsulated E. coli DH5α were run in parallel in all experiments to determine if the sequestration of compounds by silica and/or cells contributes significantly to the disappearance of any compound. These controls showed that in some cases where the compound is strongly hydrophobic (fluoranthene), there was a significant decrease in recovery in both control and Pseudomonas sp. NCIB 9816-4 incubations. Note that fluoranthene had been shown in previous work to be a substrate for naphthalene dioxygenase, and so further efforts to overcome this problem were not made and disappearance was merely corrected for by the use of any decreases observed with E. coli cells.
With these background corrections, we readily observed significant differences in substrate preference in three separate experiments with different mixtures of compounds at two time points (Fig. 6). The bars represent the amount of material remaining, so compounds on the left side of the graphs represent parent compounds that were very rapidly cleared in the incubations. The following compounds were not detected in mixtures (GC/FID peak areas were the below detection limit after 0.5 h of incubation with Pseudomonas NCIB 9816-4): 2,3-benzofuran, benzothiophene, quinoline, biphenyl, acenaphthylene, indan, azulene, and 1,2-dimethylnaphthalene.
FIG 6.
Three separate experiments containing mixtures of chemicals incubated with silica-encapsulated Pseudomonas sp. NCIB 9816-4. The GC/FID peak area (triplicate) was corrected for absorption by silica or cells by normalization to that for silica-encapsulated E. coli as a negative control.
Note that the compounds that reacted the most rapidly are fused-ring hydrocarbons and heterocycles that most resemble naphthalene structurally. In general, the next class that reacted was the bridge-linked isolated ring compounds. Compounds poorly or not degraded were the larger ring system compounds, such as phenyl naphthalene, and the bridged compounds that were shown not to be substrates in separate incubations. The degradation profile did not change when mixtures were incubated with free cells.
DISCUSSION
The presence of ring compounds distinct from the well-studied polycyclic fused-ring substrates in hydraulic fracturing waters gave impetus to the present study (3). It was of particular interest to determine the reactivity of spiro, bridged, and isolated ring compounds with Pseudomonas sp. NCIB 9816-4. The reactivity of Pseudomonas sp. NCIB 9816-4 with PAHs is attributed in all known cases to the naphthalene dioxygenase (NDO) enzyme system, and this has been supported by studies with the purified enzyme system, which has shown a broad specificity with compounds containing fused rings (15–18).
The present study has significantly extended the known substrate specificity of Pseudomonas sp. NCIB 9816-4 and NDO. Ninety-four percent of previously known substrates of NDO consisted of fused- or single-ring structures (Fig. 7). The experiments here focused specifically on the types of compounds present in hydraulic fracturing waters and ring configurations not previously examined with NDO. This study extended the known NDO substrate range to include a variety of isolated ring systems and spiro compounds. Neither bridge-linked nor spiro compounds had previously been tested with Pseudomonas sp. NCIB 9816-4.
FIG 7.
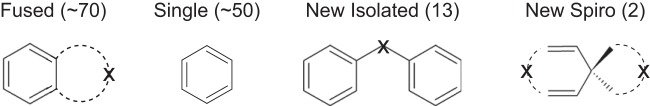
Generalized representation of substrate ring types acted on by Pseudomonas sp. NCIB 9816-4 and NDO, with the number of compounds in each class shown in parentheses.
The incubations with mixed substrates that simulated the PAH mixtures found in hydraulic fracturing waters also served as the substrate competition experiments. The data in Fig. 5 and 6 illustrate that fused-ring substrates are generally preferred by NDO, except when the ring system gets too large; for example, fluoranthene is relatively poorly degraded (Table 1 and Fig. 5). This is consistent with the findings of previous studies, in which two and three fused-ring substrates were largely used in NDO studies (15, 17–30). Of the direct-linked and bridge-linked substrates, biphenyl was oxidized the most rapidly. The preference for linked rings generally decreased as the size of the bridge increased and if one or both rings were saturated. In the latter case, cyclohexylbenzene was a substrate and bicyclohexyl was not.
Many substrates were oxidized without the appearance of products, while others accumulated one or more products, some of which may have significance for studies on the reaction mechanism of NDO. In general, direct-linked and bridge-linked substrates were incompletely metabolized. For example, biphenyl is rapidly oxidized to form a yellow compound, 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate, which accumulates (data not shown). The appearance of hydroxylated and ketone products with other substrates (cyclopropylbenzene and cyclohexylbenzene) suggests that direct-linked rings with one saturated ring are hydroxylated on the saturated ring and can undergo subsequent dehydrogenation to the respective ketone. It is unclear at this time if these products are further metabolized or if other metabolic routes may give more complete degradation of the rings.
Both spiro[cyclopropane-1,1′-indene] and cyclopropylbenzene yield unexpected products that must arise from removal of a carbon atom from the cyclopropane ring systems. It cannot be determined if these product derive exclusively from a reaction(s) catalyzed by NDO. Regardless, this suggests an interesting mechanism and warrants further study with purified enzyme. Note that Chakrabarty and coworkers showed, using norcarane as a substrate, that NDO generates substrate carbon radical species that undergo carbon-carbon cleavage reactions (31), and this may relate to the observations made here.
The possible structures of substituted ring hydrocarbons and heterocycles are enormous (32). Estimates of the number of compounds derived from frac water and other hydrocarbon and heterocyclic ring mixtures derived from shale or petroleum number in the tens of thousands, and many of those compounds are not commercially available in purified form (33–38). With the knowledge that more than 150 fused, direct-linked, bridge-linked, heterocyclic, and spiro ring compounds are oxidized, there is now a solid experimental baseline for calibrating computational studies using the high-resolution X-ray structure of NDO (17, 39) to predict the ability of the enzyme and, hence, Pseudomonas sp. NCIB 9816-4 to oxidize ring compounds in complex mixtures.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by funding from the National Science Foundation Partnerships for Innovation Building Innovation Capacity (PFI: BIC) subprogram grant no. 1237754 to L.P.W. and A.A. and by MnDRIVE Postdoctoral Fellowship to K.G.A.
We thank Jack Richman for helpful mass spectra analysis, Adi Radian, Jonathan Sakos, and Diego Escalante for helpful discussion, and Trevor Polivka for compiling NDO substrates.
Footnotes
Published ahead of print 6 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01100-14.
REFERENCES
- 1.Davies JI, Evans WC. 1964. Oxidative metabolism of naphthalene by soil pseudomonads. The ring-fission mechanism. Biochem. J. 91:251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Chen RF, Shiaris MP. 1994. Metabolism of naphthalene, fluorene, and phenanthrene: preliminary characterization of a cloned gene cluster from Pseudomonas putida NCIB 9816. J. Bacteriol. 176:2158–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strong L, Gould T, Kasinkas L, Sadowsky M, Aksan A, Wackett L. 2013. Biodegradation in waters from hydraulic fracturing: chemistry, microbiology, and engineering. J. Environ. Eng. 140:B4013001. 10.1061/(ASCE)EE.1943-7870.0000792 [DOI] [Google Scholar]
- 4.Bingham E, Trosset RP, Warshawsky D. 1979. Carcinogenic potential of petroleum hydrocarbons: a critical review of the literature. J. Environ. Pathol. Toxicol. 3:483–563 [PubMed] [Google Scholar]
- 5.Pashin YV, Bakhitova LM. 1979. Mutagenic and carcinogenic properties of polycyclic aromatic hydrocarbons. Environ. Health Perspect. 30:185–189. 10.1289/ehp.7930185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sukul P, Zuhlke S, Lamshoft M, Rosales-Conrado N, Spiteller M. 2010. Dissipation and metabolism of (14)C-spiroxamine in soil under laboratory condition. Environ. Pollut. 158:1542–1550. 10.1016/j.envpol.2009.12.025 [DOI] [PubMed] [Google Scholar]
- 7.Turner K, Xu S, Pasini P, Deo S, Bachas L, Daunert S. 2007. Hydroxylated polychlorinated biphenyl detection based on a genetically engineered bioluminescent whole-cell sensing system. Anal. Chem. 79:5740–5745. 10.1021/ac0705162 [DOI] [PubMed] [Google Scholar]
- 8.Reátegui E, Reynolds E, Kasinkas L, Aggarwal A, Sadowsky MJ, Aksan A, Wackett LP. 2012. Silica gel-encapsulated AtzA biocatalyst for atrazine biodegradation. Appl. Microbiol. Biotechnol. 96:231–240. 10.1007/s00253-011-3821-2 [DOI] [PubMed] [Google Scholar]
- 9.Morawski B, Casy G, Illaszewicz C, Griengl H, Ribbons DW. 1997. Stereochemical course of two arene-cis-diol dehydrogenases specifically induced in Pseudomonas putida. J. Bacteriol. 179:4023–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson DT, Resnick SM, Lee K, Brand JM, Torok DS, Wackett LP, Schocken MJ, Haigler BE. 1995. Desaturation, dioxygenation, and monooxygenation reactions catalyzed by naphthalene dioxygenase from Pseudomonas sp. strain 9816-4. J. Bacteriol. 177:2615–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd D, Sharma N, Coen G, Hempenstall F, Ljubez V, Malone J, Allen C, Hamilton J. 2008. Regioselectivity and stereoselectivity of dioxygenase catalysed cis-dihydroxylation of mono- and tri-cyclic azaarene substrates. Org. Biomol. Chem. 6:3957–3966. 10.1039/b810235j [DOI] [PubMed] [Google Scholar]
- 12.Seo J, Kang S, Ryu J, Lee Y, Park K, Kim M, Won D, Park H, Ahn J, Chong Y, Kanaly R, Han J, Hur H. 2010. Location of flavone B-ring controls regioselectivity and stereoselectivity of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4. Appl. Microbiol. Biotechnol. 86:1451–1462. 10.1007/s00253-009-2389-6 [DOI] [PubMed] [Google Scholar]
- 13.Thawng C, Ryu J, Han J, Cha C, Hur H. 2011. Biotransformation of N-heterocyclic compounds 1-phenylpyrazole and 1-phenylpyrrole by Escherichia coli (pDTG141) expressing naphthalene dioxygenase of Pseudomonas sp. strain NCIB 9816-4. J. Korean Soc. Appl. Biol. Chem. 54:229–236. 10.3839/jksabc.2011.037 [DOI] [Google Scholar]
- 14.Lee K, Gibson DT. 1996. Toluene and ethylbenzene oxidation by purified naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4. Appl. Environ. Microbiol. 62:3101–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resnick SM, Lee K, Gibson DT. 1996. Diverse reactions catalyzed by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J. Ind. Microbiol. 17:438–457. 10.1007/BF01574775 [DOI] [Google Scholar]
- 16.Parales RE, Emig MD, Lynch NA, Gibson DT. 1998. Substrate specificities of hybrid naphthalene and 2,1-dinitrotoluene dioxygenase enzyme systems. J. Bacteriol. 180:2337–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parales RE, Resnick SM, Yu CL, Boyd DR, Sharma ND, Gibson DT. 2000. Regioselectivity and enantioselectivity of naphthalene dioxygenase during arene cis-dihydroxylation: control by phenylalanine 352 in the alpha subunit. J. Bacteriol. 182:5495–5504. 10.1128/JB.182.19.5495-5504.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferraro D, Okerlund A, Mowers J, Ramaswamy S. 2006. Structural basis for regioselectivity and stereoselectivity of product formation by naphthalene 1,2-dioxygenase. J. Bacteriol. 188:6986–6994. 10.1128/JB.00707-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd D, Sharma N, McMurray B, Haughey S, Allen C, Hamilton J, McRoberts W, O'Ferrall R, Nikodinovic-Runic J, Coulombel L, O'Connor K. 2012. Bacterial dioxygenase- and monooxygenase-catalysed sulfoxidation of benzothiophenes. Org. Biomol. Chem. 10:782–790. 10.1039/c1ob06678a [DOI] [PubMed] [Google Scholar]
- 20.Barnsley EA. 1975. The bacterial degradation of fluoranthene and benzo[a]pyrene. Can. J. Microbiol. 21:1004–1008. 10.1139/m75-148 [DOI] [PubMed] [Google Scholar]
- 21.Jeffrey AM, Yeh HJC, Jerina DM, Patel TR, Davey JF, Gibson DT. 1975. Initial reactions in oxidation of naphthalene by Pseudomonas putida. Biochemistry 14:575–584. 10.1021/bi00674a018 [DOI] [PubMed] [Google Scholar]
- 22.Jerina DM, Selander H, Yagi H, Wells MC, Davey JF, Mahadevan V, Gibson DT. 1976. Dihydrodiols from anthracene and phenanthrene. J. Am. Chem. Soc. 98:5988–5996. 10.1021/ja00435a035 [DOI] [PubMed] [Google Scholar]
- 23.Ensley BD, Ratzkin BJ, Osslund TD, Simon MJ, Wackett LP, Gibson DT. 1983. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science 222:167–169. 10.1126/science.6353574 [DOI] [PubMed] [Google Scholar]
- 24.Resnick SM, Gibson DT. 1996. Regio- and stereospecific oxidation of 9,10-dihydroanthracene and 9,10-dihydrophenanthrene by naphthalene dioxygenase: structure and absolute stereochemistry of metabolites. Appl. Environ. Microbiol. 62:3355–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Resnick SM, Gibson DT. 1996. Regio- and stereospecific oxidation of fluorene, dibenzofuran, and dibenzothiophene by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4. Appl. Environ. Microbiol. 62:4073–4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selifonov S, Grifoll M, Eaton R, Chapman P. 1996. Oxidation of naphthenoaromatic and methyl-substituted aromatic compounds by naphthalene 1,2-dioxygenase. Appl. Environ. Microbiol. 62:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee K, Resnick SM, Gibson DT. 1997. Stereospecific oxidation of (R)- and (S)-1-indanol by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4. Appl. Environ. Microbiol. 63:2067–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyd D, Sheldrake G. 1998. The dioxygenase-catalysed formation of vicinal cis-diols. Nat. Prod. Rep. 15:309–324. 10.1039/A815309Y [DOI] [Google Scholar]
- 29.Bowers NI, Boyd DR, Sharma ND, Kennedy MA, Sheldrake GN, Dalton H. 1998. Stereoselective cis-dihydroxylation of azulene and related non-aromatic polyenes. Tetrahedron Asymmetry 9:1831–1834. 10.1016/S0957-4166(98)00161-X [DOI] [Google Scholar]
- 30.Chopard C, Bertho G, Prangé T. 2012. Naphthalene-dioxygenase catalysed cis-dihydroxylation of bicyclic azaarenes. RSC Adv. 2:605–615. 10.1039/c1ra00706h [DOI] [Google Scholar]
- 31.Chakrabarty S, Austin RN, Deng DY, Groves JT, Lipscomb JD. 2007. Radical intermediates in monooxygenase reactions of Rieske dioxygenases. J. Am. Chem. Soc. 129:3514–3515. 10.1021/ja068188v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reymond J, Ruddigkeit L, Blum L, van Deursen R. 2012. The enumeration of chemical space. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2:717–733. 10.1002/wcms.1104 [DOI] [Google Scholar]
- 33.Hurtubise RJ, Schabron JF, Feaster JD, Therkildsen DH, Poulson RE. 1977. Fluorescence characterization and identification of polynuclear aromatic hydrocarbons in shale oil. Anal. Chim. Acta 89:377–382. 10.1016/S0003-2670(01)84735-8 [DOI] [Google Scholar]
- 34.Mackenzie AS, Leythaeuser D, Schaefer RG, Bjorøy M. 1983. Expulsion of petroleum hydrocarbons from shale source rocks. Nature 301:506–509. 10.1038/301506a0 [DOI] [Google Scholar]
- 35.Akaegbobi IM, Nwachukwu JI, Schmitt M. 2000. Aromatic hydrocarbon distribution and calculation of oil and gas volumes in post-Santonian shale and coal, Anambra Basin, Nigeria, p 233–245 In AAPG memoir, vol 73 American Association of Petroleum Geologists, Tulsa, OK [Google Scholar]
- 36.Zeigler C, MacNamara K, Wang Z, Robbat A., Jr 2008. Total alkylated polycyclic aromatic hydrocarbon characterization and quantitative comparison of selected ion monitoring versus full scan gas chromatography/mass spectrometry based on spectral deconvolution. J. Chromatogr. A 1205:109–116. 10.1016/j.chroma.2008.07.086 [DOI] [PubMed] [Google Scholar]
- 37.Lemkau KL, Peacock EE, Nelson RK, Ventura GT, Kovecses JL, Reddy CM. 2010. The M/V Cosco Busan spill: source identification and short-term fate. Mar. Pollut. Bull. 60:2123–2129. 10.1016/j.marpolbul.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 38.Mello MR, Katz BJ. 2000. Petroleum systems of South Atlantic margins, p 1–13 In AAPG memoir, vol 73 American Association of Petroleum Geologists, Tulsa, OK [Google Scholar]
- 39.Kauppi B, Lee K, Carredano E, Parales RE, Gibson DT, Eklund H, Ramaswamy S. 1998. Structure of an aromatic-ring-hydroxylating dioxygenase-naphthalene 1,2-dioxygenase. Structure 6:571–586. 10.1016/S0969-2126(98)00059-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.