Abstract
Lactobacillus rhamnosus GG is a widely used probiotic, and the strain's salutary effects on the intestine have been extensively documented. We previously reported that strain GG can modulate inflammatory signaling, as well as epithelial migration and proliferation, by activating NADPH oxidase 1-catalyzed generation of reactive oxygen species (ROS). However, how strain GG induces these responses is unknown. Here, we report that strain GG's probiotic benefits are dependent on the bacterial-epithelial interaction mediated by the SpaC pilin subunit. By comparing strain GG to an isogenic mutant that lacks SpaC (strain GGΩspaC), we establish that SpaC is necessary for strain GG to adhere to gut mucosa, that SpaC contributes to strain GG-induced epithelial generation of ROS, and that SpaC plays a role in strain GG's capacity to stimulate extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) signaling in enterocytes. In addition, we show that SpaC is required for strain GG-mediated stimulation of cell proliferation and protection against radiologically inflicted intestinal injury. The identification of a critical surface protein required for strain GG to mediate its probiotic influence advances our understanding of the molecular basis for the symbiotic relationship between some commensal bacteria of the gut lumen and enterocytes. Further insights into this relationship are critical for the development of novel approaches to treat intestinal diseases.
INTRODUCTION
The mammalian intestinal microbiota is a complex and diverse community that normally thrives in a symbiotic relationship with the host. The intestinal lumen provides a temperature-controlled and nutrient-rich environment, while the resident microbial population contributes to host well-being by several means, including competitive exclusion of pathogens by occupying mucosal attachment sites, extraction of calories from indigestible complex carbohydrates, activation of epithelial transcriptional programs that stimulate mucosal development, stimulation of adaptive immune functions, and activation of cytoprotective pathways (1–3). Experiments in germfree animals have verified a compelling role for the microbiota in epithelial proliferation, migration, and wound recovery postinjury (4). Thus, an appropriate reciprocal dialog between host and the gut microbiota has been implicated in a wide variety of host functions. Conversely, a disturbed or “dysbiotic” relationship is thought to be central to the pathogenesis of inflammatory bowel disease (IBD), certain enteric infections, and likely a variety of systemic immune and metabolic disorders (5). In fact, therapeutic modification of the microbiota has been suggested as a possible solution to the abovementioned health conditions. Probiotics are viable microorganisms, generally representative members of the intestinal microbiota (often strains of Lactobacillus, Bifidobacterium, and Streptococcus), which exert beneficial effects on the health of the host, including enhancement of barrier function and suppression of inflammatory processes (2). While probiotic approaches have shown promise in numerous intestinal and systemic disorders, a plausible mode of action is largely unknown.
Research into the mechanisms by which bacteria influence host epithelial processes is in its infancy, but one clue has come from the study of bacterium-phagocyte interactions. The rapid generation of reactive oxygen species (ROS) is a cardinal feature of the phagocytic response to pathogenic and commensal bacteria, but evidence is accumulating that physiological levels of ROS are also elicited in other cell types in response to microbial signals (6, 7). Our laboratory has previously shown that mammalian intestinal epithelia generate physiological levels of ROS when contacted by certain strains of commensal bacteria (8, 9). This bacterium-induced ROS generation modulates numerous cellular functions, including transient oxidative inactivation of enzymes required for modulating cullin-dependent signaling (10, 11), inducing extracellular signal-regulated kinase (ERK)-specific signaling (12), and potentiating epithelial restitution via redox inactivation of focal adhesion kinase phosphatases (13). We also showed that specific taxa composing the microbiota, especially those within the genus Lactobacillus, can directly stimulate intestinal stem cell growth and accelerate epithelial growth during homeostasis (8) or wound healing by redox-dependent mechanisms involving NADPH oxidase 1 (14).
In our investigations, we have used the extensively characterized commensal bacterium Lactobacillus rhamnosus GG as our probiotic model and found that this strain is especially potent in stimulating ROS generation in contacted mammalian cells (8). The mechanism for this specific property is unknown, and characterization would provide a paradigm for future identification and characterization of candidate probiotics. One possibility is that commensal bacteria that interact with the host have enhanced binding to the intestinal epithelium. Recent studies have identified surface proteins of strain GG that are necessary for binding to intestinal mucus. Specifically, a genetic island within the strain GG genome contained genes for three secreted LPxTG-like pilins (spaCBA). Of these gene products, SpaC was shown to bind mucus ex vivo and was required for strain GG adherence to cultured intestinal epithelial monolayers (15–17). Here, we advance these findings by showing that strain GG-induced probiotic effects are SpaC dependent both in vitro and in vivo. We found that an isogenic strain GG spaC mutant (GGΩspaC) exhibited qualitatively lower adherence to both cultured cells and murine intestinal mucosa than did wild-type bacteria. The isogenic spaC mutant also induced the generation of markedly lower levels of ROS, induced weaker phosphorylation of ERK, and stimulated less cell proliferation than did wild-type strain GG. In addition, strain GG's ability to protect against intestinal damage following radiological insult (18) was abolished in mice treated with the spaC mutant. Together, these data are evidence that the SpaC pilin subunit is necessary to maintain intimate contact of strain GG with the host intestinal epithelia and that bacterium-host contact is necessary for strain GG to elicit beneficial cellular responses.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains used in this study include Lactobacillus rhamnosus GG (ATCC 53103), an isogenic strain GG-spaC mutant (ΩspaC::Eryr, CMPG10102) (15), and a laboratory strain of Escherichia coli K-12 (DH5). For routine culture, lactobacilli were grown in de Man-Rogosa-Sharpe (MRS) or lactobacillus selection (LBS) medium (Difco) at 37°C under static, microaerophilic conditions, while E. coli was grown in Luria-Bertani (LB) medium at 37°C with aeration. Green fluorescent protein (GFP)-expressing strains GG and GGΩspaC were created by transformation with plasmid pJM09, which harbors the GFPmut3* (19) gene inserted at the EcoRI site of plasmid pGK13 (a kind gift from J. Kok; unpublished data). Plasmids pCM (chloramphenicol resistant) and pEM (erythromycin resistant) were employed for in vivo experiments requiring antibiotic selection. Chloramphenicol or erythromycin was added to medium as needed at 10 μg/ml and 2.5 μg/ml, respectively. Prior to use, all bacterial cultures were centrifuged at 6,000 × g for 30 s, and the resulting pellets were resuspended in Hanks' balanced salt solution (HBSS) or phosphate-buffered saline (PBS). This process was repeated twice. Then, the bacterial cultures were diluted to the appropriate concentration.
Cell culture.
The human intestinal epithelial cell lines Caco-2 and T84 were grown in the presence of high-glucose (4.5 g/liter) Dulbecco's modified Eagle's medium (Sigma-Aldrich, St. Louis, MO; catalog no. D6429) supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 4% CO2 atmosphere.
Mice.
Six- to 10-week-old C57BL/6 (wild-type) mice used for all experiments were purchased from The Jackson Laboratory (Bar Harbor, ME) or bred in the small-animal facility at Emory University. After experimental procedures, mice were anesthetized with CO2 and euthanized by cervical dislocation. All murine experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee at Emory University and were performed according to the Emory guidelines for the ethical treatment of animals.
Bacterial adhesion assay.
For in vivo adhesion assays, bacteria were labeled with either CellTrace carboxyfluorescein succinimidyl ester (CFSE) or CellTrace Far Red 7-hydroxy-9H(1,3-dichloro-9,9-dimethylacridin-2-one)–succinimidyl ester (DDAO-SE) fluorescent dye (Life Technologies, Grand Island, NY; catalog numbers 34554 and 34553, respectively) per the manufacturer's instructions. Briefly, bacteria were incubated in 10 μM dye at 37°C for 10 min, the reaction was quenched on ice, and the fluorescently labeled bacteria were washed three times prior to being diluted to the appropriate concentration. For quantitation of in vitro bacterial adhesion, 200 μl of the stained GG, GGΩspaC, or E. coli strain at 1 × 108 CFU/ml was applied to confluent Caco-2 monolayers in an 8-well microscopic chamber slide. Then, after 1 h of incubation at 37°C in a 4% CO2 atmosphere, the cells were gently washed 4 times with 400 μl of HBSS and fixed with 4% p-formaldehyde (PFA). Cellular actin was stained with Alexa Fluor 633-phalloidin (Life Technologies; catalog no. A12379) or Alexa Fluor 488-phalloidin (Life Technologies; catalog no. A22284). Cell-attached bacteria were counted by confocal microscopy (Zeiss LSM 510) at an ×40 magnification in 20 random fields and averaged. For visualization and quantification of in vivo bacterial adhesion, mice were given a single 200-μl dose of strain GG/pJM09 (expressing GFP and conferring erythromycin resistance), strain GGΩspaC/pJM02 (expressing GFP and conferring chloramphenicol resistance), or E. coli/pJM02 (expressing GFP and conferring chloramphenicol resistance) at 1 × 1011 CFU/ml by oral gavage. At least 4 mice were used for each experimental condition. After 1 h, mice were sacrificed and a 2-cm section of the proximal jejunum was removed, opened longitudinally, and washed twice with 0.6 ml of 0.2% Triton X-100 in PBS. For bacterial visualization, washed sections were frozen in OCT medium, cut into 7-μm sections, mounted, fixed with 4% PFA, stained with Alexa Fluor 546-phalloidin and lectin Helix pomatia agglutinin (HPA) Alexa Fluor 647 (Life Technologies; catalog numbers A22283 and L32454, respectively), and visualized at 40× by confocal microscopy. For bacterial quantitation, washed sections were homogenized and plated on MRS or LB medium containing either erythromycin (5 μg/ml) or chloramphenicol (10 μg/ml).
FISH probes.
Oligonucleotide probes were designed to match the specifications of previously validated fluorescence in situ hybridization (FISH) probes. The Eub338 (5′-GCTGCCTCCCGTAGGAGT-3′) probe binds the bacterial 16S rRNA region of a broad range of bacteria genera (20), and the Lcas467 probe (5′-CCGTCACGCCGACAACAG-3′) binds the 16S region of a few select Lactobacillus species, including L. rhamnosus, with high specificity (21). A negative-control probe consisting of 5′-ACATCCTACGGGAGGC-3′ was used to control for nonspecific hybridization. All probes were labeled at the N terminus with fluorescein isothiocyanate (FITC). Eub338 and the negative-control probe were synthesized by Sigma-Aldrich (St. Louis, MO), and Lcas467 was synthesized by Eurofins MWG Operon (Ebersberg, Germany).
Fluorescence in situ hybridization.
Female wild-type mice were orally gavaged with 200 μl of PBS or the GG, GGΩspaC, or E. coli strain at 1 × 1010 CFU/ml daily for 3 days, from day 0 through day 2. On day 3, 24 h after the last feeding, mice were euthanized and segments of the jejunum and colon were snap-frozen in OCT. Three mice were used for each experimental condition. The tissue was cut into 5-μm sections and mounted on slides. Prepared slides were fixed in 10% formalin for 15 min, rinsed in PBS, and stored at −20°C until use. Before hybridization, tissue sections were incubated with lysozyme buffer (1 mg/ml lysozyme, 5 mM EDTA, 1 M Tris-HCl, pH 7.5) for 10 min at room temperature to permeabilize the cell wall of slide-mounted bacteria. To prevent nonspecific binding of the oligonucleotide probes, tissue sections were then incubated with 1% bovine serum albumin (BSA) in PBS for 30 min. A hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl, 0.01% SDS, 35 to 40% formamide) was prepared with 35% formamide for the Eub338 and negative-control probes and 40% formamide for the Lcas467 probe. The probes were added to the buffer at a final concentration of 2.5 ng/μl for the Eub338 and negative-control probes and 5 ng/μl for the Lcas467 probe. Tissue sections were incubated with each probe in buffer at 46°C for 3 h. Slides were then washed thoroughly to remove excess probe, and the nuclear counterstain TO-PRO-3 iodide (Life Technologies; catalog no. T3605) was applied. FISH samples were preserved with Vectashield mounting medium (Vector Laboratories, Burlingame, CA; catalog no. H1000) and imaged at 40× using confocal fluorescence microscopy.
In vitro measurements of ROS generation.
ROS generation in cultured cells was measured with the cell-membrane-permeant hydrocyanine-3 dye (hydro-Cy3), which was kindly provided by Niren Murthy (Georgia Institute of Technology, Atlanta, GA, USA). Briefly, 100 μM hydro-Cy3 in cell-line-specific medium was preloaded into Caco-2 cells grown in black-sided 96-well plates by incubation at 37°C in a 4% CO2 atmosphere for 1 h under low-light conditions followed by washing with HBSS. Washed bacteria (1 × 108 CFU) were then applied apically, and cellular ROS generation was measured at various time points by a fluorescence microplate reader (SpectraMax M2; Molecular Devices, Sunnyvale, CA, USA) using excitation and emission wavelengths of 544 nm and 574 nm, respectively.
Confocal microscopy for in vitro and in vivo ROS generation.
For the detection of ROS generation by laser scanning confocal microscopy in in vitro cell culture, Caco-2 cells were grown in multichamber slides, hydro-Cy3 was preloaded, and bacteria were applied as described above. After a predetermined incubation period, cells were washed with HBSS and a coverslip was applied. Fluorescence images were then immediately captured at 40× by laser scanning confocal microscopy using a helium-neon excitation laser at 543 nm and a 505- to 530-nm band-pass filter set. Measurements of in vitro ROS generation are the averages of at least 3 independent experiments, and representative confocal images are shown. For the visualization of in vivo ROS generation, mice were fasted for 16 h and then given 200 μl of 100 μM hydro-Cy3 by intraperitoneal (i.p.) injection 15 min before the administration of either 200 μl of HBSS or a bacterial suspension at 1 × 1010 CFU/ml by oral gavage. Mice were sacrificed 1 h postgavage, and the proximal jejunum was prepared by a whole-mount technique, as previously described (12). Fluorescent images were immediately captured at 40× by laser scanning confocal microscopy, as described above. For quantitation of ROS generation from intestinal epithelia, fluorescence was measured at 5 random positions on each slide and processed using the ImageJ software package (http://rsb.info.nih.gov/ij) from the National Institutes of Health. Five mice were used in each treatment group. Representative confocal images are shown.
Immunoblot analysis for in vitro and in vivo ERK phosphorylation.
ERK phosphorylation in cultured epithelial cells in response to bacterial contact was assessed by Western blot analysis. Briefly, 1 × 108 CFU of strain GG or GGΩspaC was applied to polarized epithelial monolayers, incubated for a predetermined period of time, and washed once with HBSS, and then the cells were lysed in SDS-PAGE loading buffer. For the detection of ERK phosphorylation in the murine colon, mice were fasted for 2 to 4 h and then 100 μl of PBS or strain GG, the GGΩspaC mutant, or E. coli at 1 × 107 CFU/ml was administered intrarectally. After 7 min, the colon was removed and opened along the mesenteric border, and epithelial cells were removed from the most distal 5 cm of the colon by scraping and lysed in radioimmunoprecipitation assay (RIPA) buffer at 100 mg tissue/ml. Samples were then sonicated and centrifuged at 21,130 × g for 20 min at 4°C. At least four mice were used in each treatment group. Cell lysates from both in vitro and in vivo experiments were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with a primary antibody specific for phospho-ERK (Cell Signaling, Danvers, MA; catalog no. 4370S) or β-actin (Sigma-Aldrich; catalog no. A5441). Protein-specific bands were detected using horseradish peroxidase (HRP)-conjugated secondary antibodies (GE Healthcare; catalog numbers NA934V and NA931V) together with SuperSignal West Pico chemiluminescent substrate (Thermo Scientific; catalog no. 34080).
EdU assay for in vitro cellular proliferation.
Caco-2 cells were assayed for cellular proliferation by 5-ethynyl-2′-deoxyuridine (EdU) incorporation. Semiconfluent Caco-2 monolayers were grown in a chamber slide format and coincubated with 1 × 108 CFU strain GG or strain GGΩspaC. After 4 h, EdU (Life Technologies; catalog no. C10337), which is incorporated during active DNA synthesis, was added to the culture medium and incubated for an additional 30 min. Cells were then fixed, stained for DNA, washed, and visualized at 20× by laser scanning confocal microscopy. The percentage of proliferating cells was calculated as a ratio of EdU-positive cells to all cells in a given visual field.
PHH3 assay for in vivo cellular proliferation.
Mice were gavaged orally with 200 μl of PBS or the GG, GGΩspaC, or E. coli strain at 1 × 1010 CFU/ml. Two to 4 h after feeding, a 3-cm section of the proximal jejunum was removed, snap-frozen in OCT, cut into 6-μm sections, mounted on a glass slide, fixed, stained, and visualized at 20× by confocal laser scanning microscopy. The number of phospho-histone H3 (PHH3)-positive cells per crypt was counted for 5 random fields per mouse and averaged. At least four mice were used for each treatment group.
Irradiation protection assay in vivo.
Female C57/B6 mice were orally gavaged with 200 μl of PBS or the GG, GGΩspaC, or E. coli strain at 1 × 1010 CFU/ml daily for 4 days, beginning at day 0 through day 3. After feeding on day 3, mice were subjected to 12-Gy whole-body irradiation, or 0.631 Gy/min for 19.01 min, in a γ Cell 40 137Cs irradiator. Six hours after irradiation, mice were euthanized via cervical dislocation and dissected. The small bowel was opened along the mesenteric border, prepared as a Swiss roll, and fixed in 10% formalin while shaking overnight. The tissue was processed and embedded in paraffin before being cut for histological analysis. Slides were stained for terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) using the Apoptag Plus peroxidase in situ apoptosis detection kit (Millipore, Billerica, MA; catalog no. S7101) according to the manufacturer's instructions, with the exception of using hematoxylin as a counterstain. The number of apoptosis-positive cells per jejunal crypt was determined in 50 crypts per mouse at ×40 magnification and averaged. Four mice were used for each treatment group.
Statistical analyses and data presentation.
Differences between groups of at least P ≤ 0.05 by a standard two-tailed Student t test were considered significant. Error bars represent the standard errors of the means (SEM). GraphPad Prism 6 was used for all graphing and statistical analyses.
RESULTS
SpaC is necessary for L. rhamnosus GG adhesion to cultured cells and to the murine intestinal epithelia.
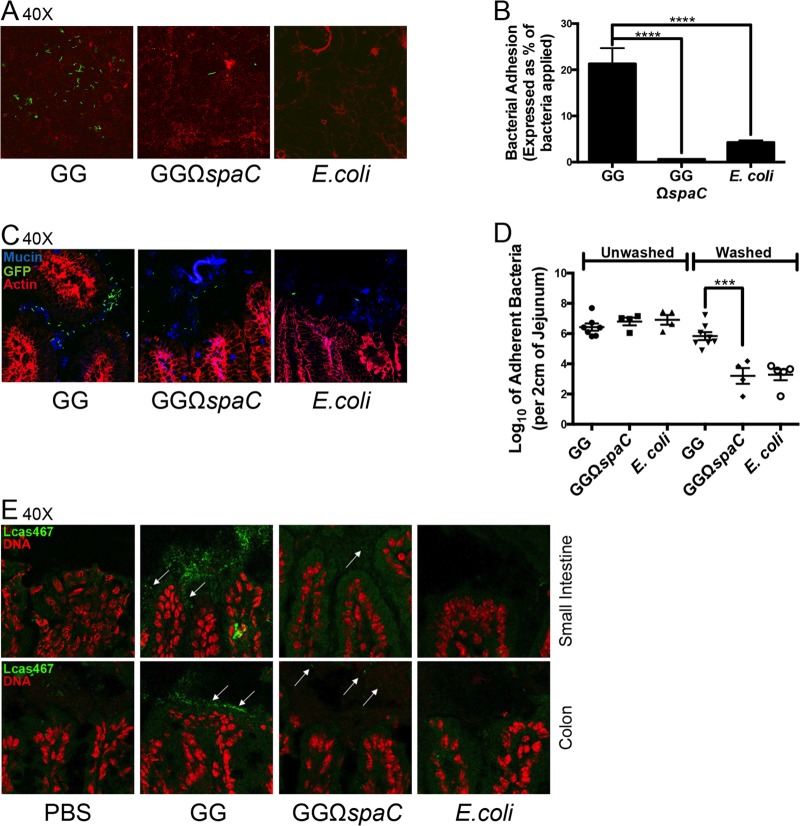
Previous studies investigating strain GG adherence employed an ex vivo approach where mucus was isolated from the human intestine and binding was assessed in vitro (15, 16). As an improvement to this approach, we investigated strain GG adherence by direct visualization of fluorescent bacteria both in vitro and in vivo. In addition, in vivo adherence was assessed by plate count. Bacteria were applied to cultured Caco-2 cells for 1 h. After several washes to remove unattached bacteria, the treated cells were then fixed, mounted, and visualized by confocal microscopy. Similarly to previous reports using purified mucus, we detected significantly higher numbers of wild-type strain GG adherent to cultured cells than of the isogenic spaC mutant or the E. coli K-12 control (Fig. 1A and B). Strain GG has the capacity to bind intestinal mucus of disparate species; therefore, to further assess the binding properties of strain GG in vivo, we assayed bacterial adhesion within the mouse small intestine (22). For this purpose, GFP-expressing strains of strain GG, its isogenic spaC mutant, or E. coli were fed to wild-type mice, and then the number of adherent bacteria was measured by both confocal microscopy and direct plating as detailed in Materials and Methods. Both methods detected significantly higher numbers of strain GG bacteria adhering to the murine small intestinal mucosa (by at least 2 orders of magnitude) than of the isogenic spaC mutant or E. coli control (Fig. 1C and D). Notably, differences in bacterial numbers were not observed when bacteria were recovered from unwashed tissue, indicating that differences in the numbers of strain GG and GGΩspaC bacteria recovered from washed tissue are due to differences in bacterial adherence and not differences in the mutant's ability to survive within the gastrointestinal (GI) tract. Finally, fluorescence in situ hybridization (FISH) was performed to visually determine the effects of the spaC mutation on the ability of strain GG to adhere and persist in the intestine. Wild-type mice were fed a daily dose of strain GG, its isogenic spaC mutant, or E. coli for 3 days and sacrificed 24 h after the last dose. Intestinal sections were subjected to FISH using a Lactobacillus-specific probe, Lcas467. Confocal analysis of the tissue revealed that strain GG, but not the spaC mutant, formed a layer directly luminal of the intestinal epithelial cells in both colon and small intestine samples (Fig. 1E).
FIG 1.
The L. rhamnosus GG SpaC pilin protein is required for adherence to cultured intestinal epithelial cells and murine intestinal mucosa. (A) Adhesion of GG, GGΩspaC, and E. coli strains to cultured, confluent Caco-2 intestinal epithelial cells. Bacteria (2 × 107 CFU) were stained with a fluorescent, cell-permeant dye; coincubated with epithelial cells in a chamber slide format for 1 h; gently washed with HBSS; and then prepared for confocal microscopy as described in Materials and Methods. Green, bacteria; red, cellular actin. Representative results are shown. (B) Quantification of cell-adherent bacteria from confocal images averaged from 20 random fields. Experiments were repeated at least 3 times with similar results. ****, P < 0.0001. Error bars show calculated standard errors of the means. (C) Adhesion of GFP-expressing GG, GGΩspaC, and E. coli strains to murine intestinal mucosa. Bacteria (2 × 1010 CFU) were fed to mice by oral gavage, 1 h after which a 2-cm section of the proximal jejunum was removed, washed, frozen in OCT medium, mounted, and stained for confocal microscopy. Blue, mucus; green, GFP-expressing bacteria; red, cellular actin. (D) Quantification of mucosa-attached bacteria. Two-centimeter sections of proximal jejunum tissue were obtained from mice treated as described for panel C. This tissue was then washed, homogenized, and plated on antibiotic-containing medium. Error bars show calculated standard errors of the means (n ≥ 4). ***, P < 0.001. (E) FISH of murine intestines using a Lactobacillus-specific probe, Lcas467 (white arrows). Samples were taken 24 h after mice were given the last of 3 daily doses of 2 × 109 CFU of bacteria and prepared as described in Materials and Methods.
L. rhamnosus GGΩspaC is a less potent inducer of ROS generation in cultured epithelial cells and the murine intestine.
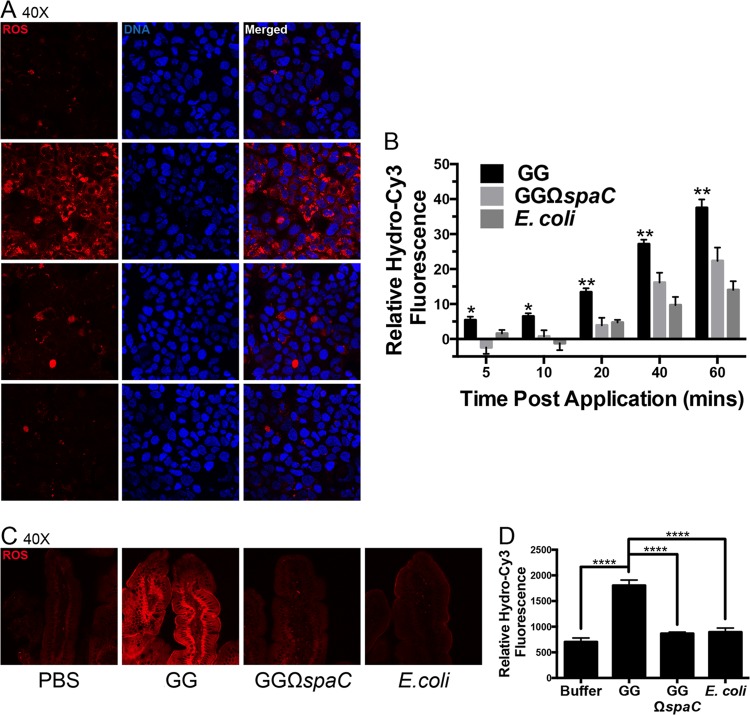
Our research group recently reported that contact of cultured cells with strain GG induces the generation of physiological levels of ROS (8). Here, we examine whether strain GG must be in physical contact with intestinal enterocytes to induce ROS generation. Cultured Caco-2 cells were treated with the cell-permeant ROS indicator hydrocyanine-3 dye (hydro-Cy3) before being overlaid with bacteria (23). Confocal analysis revealed markedly lower induction of cellular ROS in cells contacted by the spaC mutant than in cells contacted by wild-type strain GG (Fig. 2A). In addition, fluorometric quantification confirmed significantly reduced levels of ROS generation induced by the pilin mutant (Fig. 2B). An increase in ROS generation was not observed when cells were treated with strain GG-conditioned medium (data not shown). We also recapitulated these observations in vivo. Mice were intraperitoneally administered hydro-Cy3 and subsequently fed strain GG, its isogenic spaC mutant, or E. coli by gavage. Microscopic analysis of whole-mounted sections of the jejunum revealed a near-total loss of ROS generation following feeding of the spaC mutant compared to feeding of the wild-type strain GG (Fig. 2C and D).
FIG 2.
L. rhamnosus GGΩspaC is compromised for bacterium-induced cellular ROS generation. (A) Cellular ROS generation induced by GG, GGΩspaC, and E. coli strains within Caco-2 intestinal epithelial cells. Cell monolayers were preloaded with hydro-Cy3, washed, and incubated with 1 × 108 CFU bacteria in a chamber slide format for 1 h, after which the slide was mounted and visualized by confocal microscopy. Representative results are shown. Red, ROS; blue, DNA. (B) Quantification of cellular ROS induction in Caco-2 cells by the GG, GGΩspaC, or E. coli strain. Cell monolayers grown in 96-well microplates were preloaded with hydro-Cy3, washed, and incubated with 1 × 108 CFU bacteria. ROS was measured at various time points up to 1 h in a fluorescence microplate reader. Experiments were repeated at least 3 times. Error bars show calculated standard errors of the means. *, P < 0.05; **, P < 0.01. (C) Cellular ROS generation in the murine intestine induced by the GG, GGΩspaC, or E. coli strain. Hydro-Cy3 was administered i.p. 15 min before oral gavage of 2 × 109 CFU bacteria. One hour after gavage, the mice were sacrificed and their proximal jejunums were prepared for whole mount as described in Materials and Methods and examined by confocal microscopy. Representative images at ×40 magnification are shown. Red, ROS. (D) Quantification of ROS in the murine intestine induced by the GG, GGΩspaC, or E. coli strain as shown in panel C. Average ROS fluorescence intensity was measured at 5 random fields within the epithelia and averaged. Five mice were examined per treatment. Error bars show calculated standard errors of the means (n = 5). ****, P < 0.0001.
L. rhamnosus GG SpaC is required for efficient ERK phosphorylation in polarized cultured cells and the murine small intestine.
Previous studies from our laboratory have shown that contact of strain GG with the apical surface of polarized, cultured epithelial cells induces rapid ERK phosphorylation without provoking the proinflammatory NF-κB or the proapoptotic Jun N-terminal kinase (JNK) signaling pathways (12). Additionally, we demonstrated that ERK induction occurs by a redox-sensitive mechanism (9). Therefore, we examined the extent to which strain GG-induced phosphorylation of ERK is dependent on SpaC-mediated bacterial contact. Polarized T84 cells were overlaid with strain GG or the spaC mutant for up to 30 min before analysis of cellular lysates by immunoblotting. Contact with wild-type strain GG induced significantly higher levels of ERK phosphorylation than did contact with the isogenic spaC mutant (Fig. 3A). Notably, inclusion of the antioxidant N-acetyl-cysteine virtually abolished bacterium-dependent ERK phosphorylation, confirming that strain GG-stimulated activation of this mitogen-activated protein kinase (MAPK) requires intracellular ROS generation as described previously (9). These events were also studied in vivo; intrarectal administration of wild-type strain GG induced significantly higher levels of ERK phosphorylation in colonic epithelial cells than did administration of the spaC mutant or controls (Fig. 3B and C).
FIG 3.
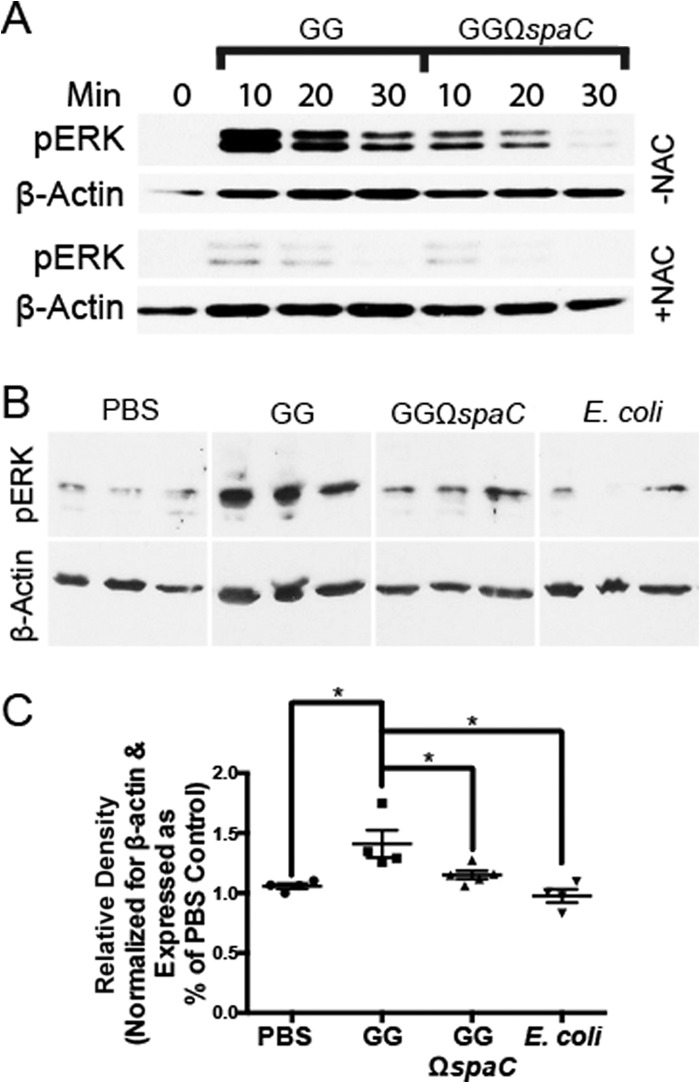
The SpaC pilin subunit is required for efficient L. rhamnosus GG-dependent ERK phosphorylation. (A) Phosphorylation of cellular ERK within cultured epithelial cells after contact by strain GG or GGΩspaC. Bacteria (1 × 108 CFU) were applied apically to polarized T84 cell monolayers grown on transwell inserts and incubated for various times before immunoblotting for phospho-ERK and β-actin (as described in Materials and Methods). NAC, N-acetyl-cysteine. (B) Phosphorylation of cellular ERK in murine colonic epithelial cells after treatment with the GG, GGΩspaC, or E. coli strain. Colon epithelial cells were harvested from mice 7 min after intrarectal injections with 1 × 106 CFU bacteria. Images were taken from the same blot but rearranged for clarity. (C) Quantification of ERK phosphorylation as shown in panel B. The amount of phosphorylated ERK was normalized for β-actin and compared to a sample treated with PBS. Error bars show calculated standard errors of the means (n ≥ 4). *, P < 0.05.
L. rhamnosus GG SpaC contributes to bacterium-dependent cellular proliferation.
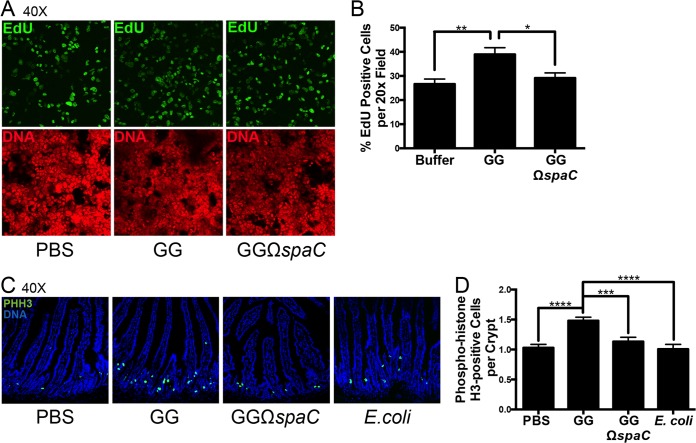
The ERK1/2 MAPK is involved in diverse, critical cellular functions, including differentiation, motility, and proliferation (24, 25). We have previously demonstrated that strain GG applied directly to cultured epithelial cells can induce cellular proliferation (9). Thus, we next considered whether direct, SpaC-mediated contact of strain GG with intestinal cells is necessary to induce a proproliferative response. After incubation of cultured Caco-2 cells with strain GG or the spaC mutant, the thymidine nucleoside analog EdU was added to measure active DNA synthesis. Consistent with our previous report, the application of strain GG significantly increased the ratio of proliferating cells within the population, whereas no significant difference from medium control was detected following treatment with the spaC mutant (Fig. 4A and B).
FIG 4.
L. rhamnosus GG-induced cellular proliferation is SpaC dependent. (A) Detection of EdU-positive cells in cultured epithelial cells following contact by either the GG or GGΩspaC strain. Bacteria (1 × 108 CFU) were applied to semiconfluent Caco-2 cell monolayers and assayed for proliferation as described in Materials and Methods. Representative results are shown. Green, EdU; red, DNA. (B) Quantification of cellular proliferation after contact of Caco-2 cell monolayers by strain GG or GGΩspaC as shown in panel A. The average number of EdU-positive cells was quantified as a ratio of the total number of cells counted at 10 random fields. Experiments were repeated at least 3 times. Error bars show calculated standard errors of the means. *, P < 0.05; **, P < 0.01. (C) Visualization of phospho-histone H3 (PHH3)-positive proliferating epithelial cells in the murine proximal small intestine following administration of the GG, GGΩspaC, or E. coli strain. Bacteria (2 × 109 CFU) were given by oral gavage, and cell staining was performed as described in Materials and Methods. Green, PHH3; blue, DNA. (D) Quantification of small intestinal epithelial proliferation as shown in panel C. The average number of PHH3-positive cells is expressed as a ratio of the number of crypts counted in 5 random fields for each mouse. Error bars show calculated standard errors of the means (n ≥ 4). ***, P < 0.001; ****, P < 0.0001.
Our group has recently reported that strain GG, when administered orally, increases proliferative cell numbers in the murine small intestinal crypts in the intact gut (8), while transrectal administration of strain GG can stimulate colonic epithelial proliferation and migration postinjury (14). In order to investigate the importance of SpaC for strain GG-induced cellular proliferation, we quantified phospho-histone H3 (PHH3) in the proximal murine small intestine after oral administration of strain GG, its isogenic spaC mutant, or E. coli. As previously demonstrated, feeding of strain GG leads to a significant increase in epithelial cell proliferation; however, mice fed either the spaC mutant or E. coli did not exhibit significantly increased numbers of PHH3-positive cells compared to those in mice fed buffer alone (Fig. 4C and D).
The L. rhamnosus GG SpaC pilin subunit contributes to cellular protection from radiation-induced intestinal injury.
Previous research has established that strain GG can protect small intestines from radiation-induced injury and reduce the amount of cellular apoptosis observed in intestinal crypts after exposure to gamma irradiation (18). To investigate whether the SpaC subunit is required for this protection, mice were orally gavaged with PBS, strain GG, its isogenic spaC mutant, or E. coli prior to whole-body irradiation. Staining for broken DNA by the TUNEL method in histological sections revealed that mice treated with strain GG, but not the spaC mutant, had markedly reduced amounts of apoptosis in their jejunal crypts compared to controls (Fig. 5A and B).
FIG 5.
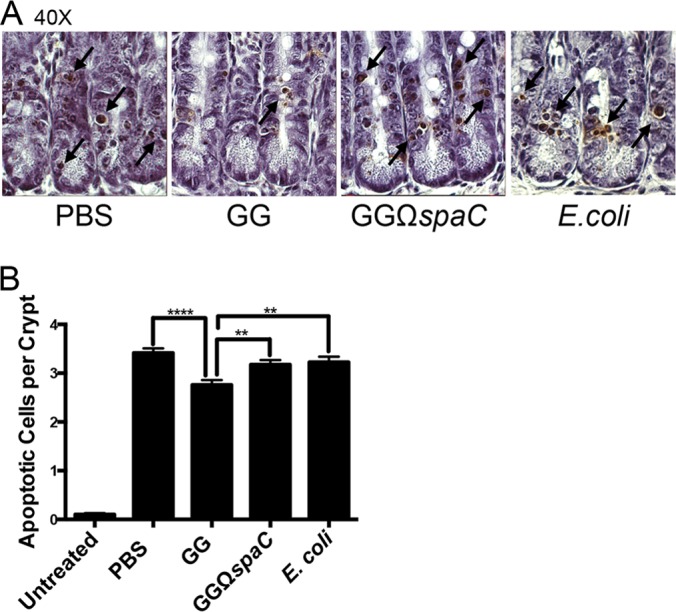
L. rhamnosus GG-induced in vivo cellular protection is SpaC dependent. (A) Visualization of TUNEL-positive apoptotic cells (black arrows) in murine jejunum following administration of the GG, GGΩspaC, or E. coli strain and irradiation treatment. Mice were orally gavaged with 2 × 109 CFU bacteria once daily for 4 days and then exposed to 12-Gy whole-body irradiation. Cell staining was performed as described in Materials and Methods. (B) Quantification of apoptosis in the jejunum as shown in panel A and expressed as average number of apoptotic cells per jejunal crypt. Error bars show calculated standard errors of the means (50 crypts per mouse, n = 4). **, P < 0.01; ****, P < 0.0001.
DISCUSSION
Our investigations show that the L. rhamnosus GG SpaC pilin subunit is required for strain GG adhesion to cultured epithelial cells or to the intestinal mucosa. In addition, a functional SpaC is required for strain GG-mediated gut epithelial responses previously described by our research group (8, 9). These include the rapid induction of strain GG-mediated ROS generation in epithelial cells, the induction of ERK phosphorylation in epithelial cells, increased cell proliferation rates in intestinal crypts, and strain GG-mediated cytoprotection against radiological insult (18).
The taxonomic, anatomic, and functional characterization of the mammalian intestinal microbiota has become a subject of increasing interest. Nucleic acid-based techniques have allowed detailed descriptions of this microbial community structure in both spatial and temporal distribution while identifying intriguing correlations with normal intestinal functions as well as under disease conditions. One specific realization from this body of work is that the intestinal microbiota can exist in a luminal, planktonic state or can occupy a mucosa-associated niche. While the former are often bacteria with fermentative capacity, recent work suggests that mucosa-associated microbes are responsible for many of the physiological functions normally attributed to the entire population. These taxa, which include Lactobacillus, Bifidobacterium, and Bacteroides, have a wide variety of functions ascribed to them: for example, Lactobacillus reuteri can inhibit Western-diet-associated obesity (26), Bifidobacterium infantis-conditioned medium enhances intestinal epithelial cell barrier function (27), Bacteroides thetaiotaomicron is known to induce gene regulatory events in the upper GI tract (28), and Bacteroides fragilis has been shown to influence immune development and enhance barrier function (29). Studies from our research group and others have shown that lactobacilli stimulate epithelial ROS generation and activate motility and proliferation events. Thus, an emerging paradigm of host-commensal interactions suggests key roles for specific mucosa-associated taxa.
As the mucosal epithelial membrane is insulated from direct contact with the microbiota by a glycocalyx and layers of secreted mucins, the study of commensal interactions with these complex carbohydrates has become a topic of considerable interest. Pathogens, such as Helicobacter pylori, typically reside within this mucous layer, and a functional class of bacteria, often with mucolytic capacity, has also been identified in this environment (30). The mucous layer not only provides a constant and renewable energy source but also forms a substrate for adhesion and may play a role in bacterial persistence.
The recent discovery of a sortase-dependent pilus spaCBA gene cluster in the well-characterized probiotic L. rhamnosus GG, which is required for mucus attachment, may be indicative of a wider phylogenetic distribution of such genes in commensal bacteria (15). Here, we advance the functional characterization of the SpaC protein by showing that (i) SpaC is necessary for strain GG to maintain contact with the host mucous layer overlaying the intestinal epithelia in vivo and (ii) SpaC-mediated bacterium-host contact is essential for strain GG to elicit its cellular modulatory responses. Specifically, we show that strain GG, but not its isogenic spaC mutant, efficiently elicits ROS generation at physiological levels, that is to say, at concentrations of ROS relevant to cellular signaling pathways but below the levels that function as antimicrobial molecules or lead to macromolecular damage. Nonradical forms of ROS, such as H2O2, function as signaling molecules at these concentrations (31). Here, redox cell signaling is mediated by sensory proteins, which possess regulatory factors whose activity can be modulated by ROS. These redox-sensitive proteins are regulated by reversible nonradical ROS-mediated oxidation of active site cysteine residues, thus allowing for graded perception of intracellular ROS levels and an exquisitely sensitive and rapid cellular responses (32). ROS generation in response to long-term lactobacillus colonization is likely to be spatially and temporally variable, where ROS generation, cytoplasmic ROS levels, and cell proliferation rates are dynamically modulated. These events are the focus of current investigations within our research group.
Unsurprisingly, other lactobacilli display mucosal adhesive properties, and additional species with the capacity to bind mucins are actively being identified, as are the proteins involved in these interactions (33). In 2002, Roos and Jonsson identified a protein in L. reuteri 1063 that could bind pig and hen mucus. This protein, named Mub due to its mucus binding capacity, contained an LPQTG domain and displayed robust mucus adhesion at pHs below neutral, potentially indicating that its capacity to bind mucus was most important under the acidic conditions that would be encountered during passage through a digestive tract (34). Lactobacillus plantarum contains a gene that has been designated msa (mannose-specific adhesion) due to its ability to bind the common epithelial sugar mannose. The Msa protein is homologous to Mub and also contains an LPxTG-like domain. L. plantarum strains expressing Msa can agglutinate the yeast Saccharomyces cerevisiae, a finding that might explain how these strains competitively inhibit intestinal pathogens (35). L. plantarum Lp6 has the capacity to bind rat mucus and can also agglutinate S. cerevisiae; this strain also likely carries an msa gene since the addition of d-mannose inhibits the abovementioned properties (36). GroEL, identified in Lactobacillus johnsonii La1, binds mucus and HT29 cells in a pH-dependent manner and, similarly to Msa, has the ability to agglutinate H. pylori (37).
Until recently, it was widely held that commensal bacteria elicited their positive influences on the intestine solely by mechanisms such as competitive exclusion of pathogens or the fermentation of complex dietary carbohydrates. However, recent reports by our research group and others show that commensal microbes are actively involved in modulating host cellular signaling processes. Genes that initially evolved to facilitate gastrointestinal colonization through physical attachment may have subsequently adapted to ensure persistence through more complex interactions. Experiments comparing the binding capacities of wild-type L. plantarum 299v and an msa-deficient mutant showed that Msa is not required for binding jejunal mucosa in pigs. While the wild-type lactobacilli had a slight competitive advantage over the msa mutant in direct competition, the study indicated that Msa was more important for host gene regulation than physical adhesion (38). Two secreted strain GG proteins, p40 and p70, can contribute to intestinal epithelial homoeostasis by preventing apoptosis as well as promoting proliferation (39, 40). Interestingly, the Lactobacillus casei BL23 homologues of these factors, known as CmuA or CmuB, can hydrolyze muropeptides and bind mucin in addition to their homeostatic capacity (41).
In our past studies, we showed that the bacterial product N-formyl-Met-Leu-Phe (fMLF) is a ligand for the formyl peptide receptor (FPR) located on the apical side of epithelial cells, interfacing with the microbiota (12). Moreover, fMLF binding to FPR potentiated the specific and ROS-dependent activation of the ERK MAPK signaling pathway. The elevated capacity of commensal bacteria to bind the mucous layer may result in more frequent and sustained contact of fMLF peptides with cell surface-localized FPRs. However, fMLF-FPR binding is certainly only one example of a commensal bacterium-host ligand-receptor interaction. Additional interactions between bacterial proteins/products and cellular pattern recognition receptors, such as Toll-like or Nod-like receptors, clearly influence host signaling (42, 43). Importantly, whether strain GG displays specific surface decorations that can directly bind host cellular receptors remains an open question.
Abnormal composition of the microbiota, known as a “dysbiotic flora,” has been implicated in the pathogenesis of inflammatory bowel diseases and some systemic immune disorders (5). We show here that the beneficial effects of strain GG, a well-established probiotic, and, by extension, the effects of a normal microbiota, are potentiated by intimate and persistent bacterial contact with the intestinal mucous layer. The appearance of mucus binding proteins in gut commensal inhabitants facilitates a mutualistic relationship where the bacteria benefit by securing a stable niche and the host benefits from a wide range of activities provided by the microbiota. Importantly, the abundance of mucus binding proteins, such as SpaC, in the intestinal microbiota may be an indicator of the condition of this microbial population and its potential to elicit beneficial influences on host health. Conversely, the absence of mucus binding genes may be a hallmark of a dysbiotic flora. The development of metagenomic and deep-sequencing techniques will facilitate the identification of these genes. Moreover, application of these techniques will advance the spatial and temporal characterization of these indicator genes along the entire gastrointestinal tract and will provide insights into the establishment of a healthy microbiota in the mammalian gut.
ACKNOWLEDGMENTS
We thank Soile Tykkynen for providing the L. rhamnosus GGΩspaC strain and Niren Murthy for providing the hydrocyanine-3 dye.
This work was supported by NIH grants RO1AL64462 (to A.S.N.), K12GM000680 (to J.W.M.), and R01DK098391 (to R.M.J.).
Footnotes
Published ahead of print 13 June 2014
REFERENCES
- 1.Hooper LV, Gordon JI. 2001. Commensal host-bacterial relationships in the gut. Science 292:1115–1118. 10.1126/science.1058709 [DOI] [PubMed] [Google Scholar]
- 2.Neish AS. 2009. Microbes in gastrointestinal health and disease. Gastroenterology 136:65–80. 10.1053/j.gastro.2008.10.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas CM, Versalovic J. 2010. Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes 1:148–163. 10.4161/gmic.1.3.11712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. 2005. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. U. S. A. 102:99–104. 10.1073/pnas.0405979102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sartor RB. 2008. Microbial influences in inflammatory bowel diseases. Gastroenterology 134:577–594. 10.1053/j.gastro.2007.11.059 [DOI] [PubMed] [Google Scholar]
- 6.Neish AS. 2013. Redox signaling mediated by the gut microbiota. Free Radic. Res. 47:950–957. 10.3109/10715762.2013.833331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambeth JD. 2004. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4:181–189. 10.1038/nri1312 [DOI] [PubMed] [Google Scholar]
- 8.Jones RM, Luo L, Ardita CS, Richardson AN, Kwon YM, Mercante JW, Alam A, Gates CL, Wu H, Swanson PA, Lambeth JD, Denning PW, Neish AS. 2013. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 32:3017–3028. 10.1038/emboj.2013.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wentworth CC, Alam A, Jones RM, Nusrat A, Neish AS. 2011. Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3. J. Biol. Chem. 286:38448–38455. 10.1074/jbc.M111.268938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, Pan ZQ, Jones DP, Neish AS. 2007. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 26:4457–4466. 10.1038/sj.emboj.7601867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar A, Wu H, Collier-Hyams LS, Kwon YM, Hanson JM, Neish AS. 2009. The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation. J. Immunol. 182:538–546. 10.4049/jimmunol.182.1.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wentworth CC, Jones RM, Kwon YM, Nusrat A, Neish AS. 2010. Commensal-epithelial signaling mediated via formyl peptide receptors. Am. J. Pathol. 177:2782–2790. 10.2353/ajpath.2010.100529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanson PA, II, Kumar A, Samarin S, Vijay-Kumar M, Kundu K, Murthy N, Hansen J, Nusrat A, Neish AS. 2011. Enteric commensal bacteria potentiate epithelial restitution via reactive oxygen species-mediated inactivation of focal adhesion kinase phosphatases. Proc. Natl. Acad. Sci. U. S. A. 108:8803–8808. 10.1073/pnas.1010042108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam A, Leoni G, Wentworth CC, Kwal JM, Wu H, Ardita CS, Swanson PA, Lambeth JD, Jones RM, Nusrat A, Neish AS. 2014. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 7:645–655. 10.1038/mi.2013.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, Satokari R, Vesterlund S, Hendrickx AP, Lebeer S, De Keersmaecker SC, Vanderleyden J, Hamalainen T, Laukkanen S, Salovuori N, Ritari J, Alatalo E, Korpela R, Mattila-Sandholm T, Lassig A, Hatakka K, Kinnunen KT, Karjalainen H, Saxelin M, Laakso K, Surakka A, Palva A, Salusjarvi T, Auvinen P, de Vos WM. 2009. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl. Acad. Sci. U. S. A. 106:17193–17198. 10.1073/pnas.0908876106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Ossowski I, Reunanen J, Satokari R, Vesterlund S, Kankainen M, Huhtinen H, Tynkkynen S, Salminen S, de Vos WM, Palva A. 2010. Mucosal adhesion properties of the probiotic Lactobacillus rhamnosus GG SpaCBA and SpaFED pilin subunits. Appl. Environ. Microbiol. 76:2049–2057. 10.1128/AEM.01958-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebeer S, Claes I, Tytgat HL, Verhoeven TL, Marien E, von Ossowski I, Reunanen J, Palva A, Vos WM, Keersmaecker SC, Vanderleyden J. 2012. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 78:185–193. 10.1128/AEM.06192-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciorba MA, Riehl TE, Rao MS, Moon C, Ee X, Nava GM, Walker MR, Marinshaw JM, Stappenbeck TS, Stenson WF. 2012. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 61:829–838. 10.1136/gutjnl-2011-300367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cormack BP, Valdivia RH, Falkow S. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33–38. 10.1016/0378-1119(95)00685-0 [DOI] [PubMed] [Google Scholar]
- 20.Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quevedo B, Giertsen E, Zijnge V, Luthi-Schaller H, Guggenheim B, Thurnheer T, Gmur R. 2011. Phylogenetic group- and species-specific oligonucleotide probes for single-cell detection of lactic acid bacteria in oral biofilms. BMC Microbiol. 11:14. 10.1186/1471-2180-11-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinkinen M, Westermarck E, Salminen S, Ouwehand AC. 2003. Absence of host specificity for in vitro adhesion of probiotic lactic acid bacteria to intestinal mucus. Vet. Microbiol. 97:55–61. 10.1016/S0378-1135(03)00183-4 [DOI] [PubMed] [Google Scholar]
- 23.Kundu K, Knight SF, Willett N, Lee S, Taylor WR, Murthy N. 2009. Hydrocyanines: a class of fluorescent sensors that can image reactive oxygen species in cell culture, tissue, and in vivo. Angew. Chem. Int. Ed. Engl. 48:299–303. 10.1002/anie.200804851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning G, Plowman GD, Hunter T, Sudarsanam S. 2002. Evolution of protein kinase signaling from yeast to man. Trends Biochem. Sci. 27:514–520. 10.1016/S0968-0004(02)02179-5 [DOI] [PubMed] [Google Scholar]
- 25.Ramos JW. 2008. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int. J. Biochem. Cell Biol. 40:2707–2719. 10.1016/j.biocel.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 26.Poutahidis T, Kleinewietfeld M, Smillie C, Levkovich T, Perrotta A, Bhela S, Varian BJ, Ibrahim YM, Lakritz JR, Kearney SM, Chatzigiagkos A, Hafler DA, Alm EJ, Erdman SE. 2013. Microbial reprogramming inhibits Western diet-associated obesity. PLoS One 8:e68596. 10.1371/journal.pone.0068596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. 2008. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 295:G1025–G1034. 10.1152/ajpgi.90227.2008 [DOI] [PubMed] [Google Scholar]
- 28.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. 2001. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291:881–884. 10.1126/science.291.5505.881 [DOI] [PubMed] [Google Scholar]
- 29.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122:107–118. 10.1016/j.cell.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 30.Ouwerkerk JP, de Vos WM, Belzer C. 2013. Glycobiome: bacteria and mucus at the epithelial interface. Best Pract. Res. Clin. Gastroenterol. 27:25–38. 10.1016/j.bpg.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 31.Jones RM, Mercante JW, Neish AS. 2012. Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications. Curr. Med. Chem. 19:1519–1529. 10.2174/092986712799828283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ray PD, Huang BW, Tsuji Y. 2012. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24:981–990. 10.1016/j.cellsig.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Callaghan J, O'Toole PW. 2013. Lactobacillus: host-microbe relationships. Curr. Top. Microbiol. Immunol. 358:119–154. 10.1007/82_2011_187 [DOI] [PubMed] [Google Scholar]
- 34.Roos S, Jonsson H. 2002. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148:433–442 [DOI] [PubMed] [Google Scholar]
- 35.Pretzer G, Snel J, Molenaar D, Wiersma A, Bron PA, Lambert J, de Vos WM, van der Meer R, Smits MA, Kleerebezem M. 2005. Biodiversity-based identification and functional characterization of the mannose-specific adhesin of Lactobacillus plantarum. J. Bacteriol. 187:6128–6136. 10.1128/JB.187.17.6128-6136.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J, Le GW, Shi YH, Su GW. 2007. Factors involved in binding of Lactobacillus plantarum Lp6 to rat small intestinal mucus. Lett. Appl. Microbiol. 44:79–85. 10.1111/j.1472-765X.2006.02031.x [DOI] [PubMed] [Google Scholar]
- 37.Bergonzelli GE, Granato D, Pridmore RD, Marvin-Guy LF, Donnicola D, Corthesy-Theulaz IE. 2006. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect. Immun. 74:425–434. 10.1128/IAI.74.1.425-434.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross G, van der Meulen J, Snel J, van der Meer R, Kleerebezem M, Niewold TA, Hulst MM, Smits MA. 2008. Mannose-specific interaction of Lactobacillus plantarum with porcine jejunal epithelium. FEMS Immunol. Med. Microbiol. 54:215–223. 10.1111/j.1574-695X.2008.00466.x [DOI] [PubMed] [Google Scholar]
- 39.Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, Chaturvedi R, Peek RM, Jr, Wilson KT, Polk DB. 2011. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Invest. 121:2242–2253. 10.1172/JCI44031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. 2007. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132:562–575. 10.1053/j.gastro.2006.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauerl C, Perez-Martinez G, Yan F, Polk DB, Monedero V. 2010. Functional analysis of the p40 and p75 proteins from Lactobacillus casei BL23. J. Mol. Microbiol. Biotechnol. 19:231–241. 10.1159/000322233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abreu MT, Fukata M, Arditi M. 2005. TLR signaling in the gut in health and disease. J. Immunol. 174:4453–4460. 10.4049/jimmunol.174.8.4453 [DOI] [PubMed] [Google Scholar]
- 43.Strober W, Murray PJ, Kitani A, Watanabe T. 2006. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 6:9–20. 10.1038/nri1747 [DOI] [PubMed] [Google Scholar]