Abstract
Salmonella enterica has the ability to form biofilms and large aggregates on produce surfaces, including on cilantro leaves. Aggregates of S. enterica serovar Thompson that remained attached to cilantro leaves after rigorous washing and that were present free or bound to dislodged leaf tissue in the wash suspension were observed by confocal microscopy. Measurement of S. Thompson population sizes in the leaf washes by plate counts failed to show an effect of 0.05% Tween 80 on the removal of the pathogen from cilantro leaves 2 and 6 days after inoculation. On the contrary, digital image analysis of micrographs of single cells and aggregates of green fluorescent protein (GFP)-S. Thompson present in cilantro leaf washes revealed that single cells represented 13.7% of the cell assemblages in leaf washes containing Tween 80, versus 9.3% in those without the surfactant. Moreover, Tween 80 decreased the percentage of the total S. Thompson cell population located in aggregates equal to or larger than 64 cells from 9.8% to 4.4% (P < 0.05). Regression analysis of the frequency distribution of aggregate size in leaf washes with and without Tween 80 showed that the surfactant promoted the dispersal of cells from large aggregates into smaller ones and into single cells (P < 0.05). Our study underlines the importance of investigating bacterial behavior at the scale of single cells in order to uncover trends undetectable at the population level by bacterial plate counts. Such an approach may provide valuable information to devise strategies aimed at enhancing the efficacy of produce sanitization treatments.
INTRODUCTION
Biofilms provide an important milieu that shields bacteria from a range of stresses and antimicrobial compounds and therefore limit the efficacy of physical and chemical approaches used for decontamination in the food production industry. Food-borne pathogens are known to have the ability to form biofilms on a variety of inert and biological surfaces, including plant surfaces (1–3), which naturally harbor large and mixed biofilm communities (4–6). While considerable attention has been given to the attachment of bacteria to surfaces and their production of biofilms, few investigations have focused on their detachment and dispersal (7). These processes allow for the dissemination of the bacterial cells into food production environments and their contamination of industrial systems. This implies that in freshly cut fruit and vegetable production, bacteria may disperse as single or aggregated cells to other plant surfaces and to washing and processing equipment, thereby potentially amplifying a contamination event if human pathogens are present on the produce. Wachtel and Charkowski demonstrated that one lettuce piece inoculated with Escherichia coli O157:H7 led to contamination of a large volume of cut lettuce pieces stored in water for 24 h (8). Strategies to sanitize produce wash water to prevent cross-contamination are likely mostly effective against single planktonic bacterial cells and may fail to eradicate cells residing in large aggregates, as was demonstrated with Mycobacterium in wastewater (9).
Plant surfaces are hydrophobic due to the waxy nature of their cuticle layer, and bacterial cell surface hydrophobicity was identified as a major factor mediating adherence of bacteria to leaves (10). This interaction may account partly for the failure of most aqueous sanitizers to decontaminate plant surfaces effectively. Hassan and Frank reported that highly hydrophobic compounds, such as the surfactant Tween 85, affect hydrophobic interactions between bacteria and plant surfaces, thereby disrupting their adhesion to the plant cuticle (11). Tween 80 (polysorbate 80) can reduce bacterial attachment to, and inhibit biofilm formation on, a variety of surfaces (12) and was shown to promote the dispersal of Salmonella enterica cells from biofilms in vitro on polyvinyl chloride (13). The aim of this study was to investigate the effect of Tween 80 on the detachment of S. enterica Thompson from leaf surfaces and its dispersal from cell aggregates. Microscopy and digital image analysis were used to determine the effect of Tween 80 in wash water on the frequency distribution of aggregate size of S. Thompson in cilantro leaf washes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Salmonella enterica serovar Thompson strain RM1987N is a spontaneous nalidixic acid-resistant mutant of a clinical isolate associated with an outbreak linked to cilantro in California (14, 15). It was labeled with the plasmid pGT-KAN, which harbors a constitutively expressed gfp gene, as described previously; this plasmid is stably maintained in S. Thompson RM1987N over multiple generations (1). This strain was grown to stationary phase at 28°C in Luria-Bertani (LB) broth amended with nalidixic acid at 50 μg/ml and gentamicin at 15 μg/ml. Cultured cells were washed twice in potassium phosphate buffer (KP buffer) (10 mM, pH 7) and resuspended in KP buffer. Suspensions for plant inoculation were prepared by adding washed cells to 1 mM KP buffer at a final concentration of 105 cells/ml.
Biofilm assay.
Biofilms of S. Thompson were allowed to form at the liquid-air interface on glass and polystyrene tubes by incubating cultures inoculated at 107 cells/ml in 300 μl of LB without salt (to promote biofilm formation on inert surfaces) at 28°C without agitation. Biofilms formed more rapidly on glass than on polystyrene. Thus, cultures were incubated for 24 h and 48 h in glass and polystyrene tubes, respectively, at which time the suspensions were removed from the tubes and the tubes rinsed gently with H2O twice to fully remove unattached cells. Then the biofilms were incubated with 500 μl of Tween 80 (0.05% in H2O) or H2O without agitation for 30 min at 24°C and vortexed at the highest setting for 30 s to disperse the biofilms. The suspension was removed from the tubes, which were then rinsed with H2O twice. Measurement of the biofilms remaining on the tube surfaces was carried out with the crystal violet staining method as described by O'Toole et al. (16).
Inoculation of cilantro plants.
Cilantro plants (Coriandrum sativum cv. Leisure) grown to the fourth to sixth true-leaf stage were inoculated by immersion of the upper plant part in the bacterial suspension for 2 s. Six replicate pots of eight plants each were used per treatment. Immediately after inoculation, 18 leaves were removed at random from the six pots and assessed for initial S. Thompson population size on the leaves as described below. The plants were then placed at 28°C with a 12-h period per day of illumination under fluorescent lights in a humid chamber (90 to 100% relative humidity [RH]) that allowed for the presence of visible free water on part of the leaf surfaces.
Bacterial recovery from cilantro leaves for enumeration of CFU.
For estimation of S. Thompson population sizes on cilantro leaves, 18 leaves were removed at each sampling time (0, 2, and 6 days after inoculation) from the cilantro plants at random from the replicate pots and placed in 25 ml of 10 mM KP buffer with or without Tween 80 at a final concentration of 0.05% (vol/vol). The leaves were then sonicated in an Astramax Generator sonicator bath with adjustable power (Misonix Inc., Farmingdale, NY) at 250 W (frequency, 40 kHz) for 1 min at 4°C and vortexed vigorously for 30 s to dislodge the bacterial cells from the leaf surface and to break up bacterial aggregates. In order to test the effect of various sonication conditions on bacterial recovery from the leaves, sonication had been previously tested also at 100 W for 1 and 10 min; 250 W for 1 min was shown to be the most effective and thus was used for bacterial recovery from leaves (Table 1). The suspensions obtained from the leaf washes were dilution plated with an automated plater (Autoplate 4000; Spiral Biotech Inc., Norwood, MA) onto LB agar containing nalidixic acid at 50 μg/ml.
TABLE 1.
Effect of sonication on the recovery of S. enterica cells from cilantro leavesa
| Treatment | Log (cell concn ratio) |
|---|---|
| 100 W, 1 min | −0.024A (0.95) |
| 100 W, 10 min | +0.317B (2.07) |
| 250 W, 1 min | −0.097A (0.79) |
Leaves of cilantro plants inoculated with S. enterica were sampled and placed individually in KP buffer. Each leaf was then vortexed for 1 min, and an aliquot of the wash suspension was dilution plated immediately (presonication). Then the leaf was sonicated in a sonicator bath, followed by vortexing for 1 min, and the wash suspension dilution was plated (postsonication). Data represent the means of the log-transformed ratios of S. enterica concentration in the presonication wash over that in the postsonication wash for five replicate leaves. Different superscript letters indicate a significant difference between the means by Tukey's multiple-comparison test (P < 0.05). Values in parentheses represent the back-transformed arithmetic values of the means.
Bacterial recovery for quantification of aggregate sizes in the leaf washes and microscopy.
After dilution plating of a 50-μl aliquot of each leaf washing obtained as described above, the remaining leaf washes (about 25 ml) from six replicate leaves per treatment were filtered through Durapore filters (EMD Millipore, Billerica, MA) (0.2-μm pore size). Each filter, harboring the cells recovered from one leaf, was cut in half and each half was mounted separately with Aqua-Poly/mount (Polysciences, Warrington, PA) onto a glass slide with a coverslip. The green fluorescent protein (GFP)-labeled S. Thompson cells were visualized on the filter surface under a Leica DMRB microscope fitted with a 20×/0.5 HC PL FLUOTAR objective and GFP filter set 513847 (Leica Microsystems, Wetzlar, Germany). The fluorescence images were captured with a Hamamatsu (Bridgewater, NJ) Orca C4742-95 camera and acquired with the software Openlab, version 2.1 (Improvision, Lexington, MA). Ten different random fields of view were imaged per half disc for a total of 120 fields of view for six leaves for each type of wash solution. S. Thompson single cells and cell aggregates (defined as assemblages composed of two or more cells) that remained on the leaf surface after washing or were associated with plant debris in the washes collected on filters were visualized with a Leica spectral confocal microscope TCS SP5 (Leica Microsystems, Germany). Pseudo-three-dimensional (pseudo-3D) images were constructed by projected series of multiple optical scans in the z plane.
Image analysis for determination of frequency distribution of aggregate size.
The frequency distribution of the size of S. Thompson aggregates present in the cilantro leaf washes with or without Tween 80 was obtained by digital image analysis using the software IPLab Spectrum, version 3.5.5, for Macintosh (Scanalytics, Fairfax, VA). The size of each S. Thompson aggregate was estimated as described previously (1, 17). Briefly, each S. Thompson single cell or aggregate in the image was identified by thresholding on the bright pixels from the GFP-labeled cells, which had greater fluorescence intensity than the background pixels from the nonfluorescent filter. This yielded a collection of objects (single cells or aggregates), each with an area measured as the sum of the individual pixels forming the object. The size of each aggregate was then assessed as the total number of GFP-labeled cells by dividing the area of each aggregate by the average number of pixels forming one bacterial cell (six pixels on average). The measurements were pooled for both filter halves for each leaf for all six leaves, and the frequency distribution of the size of all the aggregates was determined for each type of wash solution (KP buffer or Tween 80).
Statistical methods.
Statistical calculations were performed with the software Prism, version 5.02 (GraphPad Software, San Diego, CA).
RESULTS AND DISCUSSION
Whereas attachment of enteric bacterial pathogens to plant surfaces has been the subject of numerous investigations in the context of produce safety, research on the detachment of such pathogens from plants and their dispersal has been sparse. Current washing technologies for the postharvest processing of produce are ineffective at reducing pathogen loads on produce (18). Therefore, any approach that may contribute to releasing the pathogen cells adhering to the plant surface and dispersing them in order to inactivate them in the wash water may improve the microbial safety of the product. In this study, we used sonication in the absence or presence of the surfactant Tween 80, followed by agitation (vortexing) in the same solution, in order to determine the effect of the surfactant on the release of single and aggregated S. Thompson cells from the leaves of cilantro, an herb associated with outbreaks of salmonellosis (15). Cilantro was used successfully as a model plant to investigate the behavior of this human pathogen on leaf surfaces in previous studies (1, 14, 19).
Sonication was reported to improve the efficacy of sanitizers in reducing S. enterica and E. coli O157:H7 population sizes on minimally processed fruits and vegetables (20–22). Phyllosphere bacteriologists traditionally have used sonication at sublethal levels to dislodge bacterial cells from leaf surfaces in order to assess their population size in the resulting wash suspension (23). Table 1 shows that sonication of inoculated cilantro leaves in KP buffer for 1 min at 100 or 250 W, followed by vortexing, released more S. Thompson cells from cilantro than solely vortexing the leaves in KP buffer. On the contrary, sonication at 100 W for 10 min appeared to kill S. Thompson cells rather than enhancing their removal from the leaves, since the untransformed mean ratio of the S. Thompson population size on the leaves before sonication to that after sonication was 2.07. Although sonication for 1 min at 250 W was slightly more effective at bacterial removal than sonication at 100 W, this difference was not statistically significant (Tukey's multiple-comparison test, P > 0.05).
The waxy cuticle layer that lines aerial plant surfaces limits their wettability. Thus, compounds such as surfactants that can break the surface tension of leaves may increase their wettability and allow for aqueous solutions to more effectively remove phyllosphere bacterial colonists, particularly cells located in hidden microsites. Additionally, surfactants may effect bacterial detachment from the leaf surface by disrupting the hydrophobic interactions between bacterial cells and the cuticle (10). The hydrophobic surfactant polyoxyethylene sorbitan trioleate (Tween 85) in combination with sodium chloride and sodium bicarbonate was previously shown to remove a significantly greater number of E. coli O157:H7 cells from the intact surface of lettuce leaves than water and similar solutions of Tween 20 and 60 (11). Tween 80 (polysorbate 80) is widely used to emulsify and disperse substances in the cosmetic and pharmaceutical industries as well as in the food industry at concentrations up to 1% (http://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm091048.htm#ftnP). Tween 80 concentrations as low as 0.001% inhibited biofilm formation by Pseudomonas aeruginosa (12), and its addition at 0.25% accelerated S. enterica biofilm dispersal from a polyvinyl chloride surface (13). In the present study, 0.05% Tween significantly enhanced the removal of S. Thompson biofilms from polystyrene and glass surfaces compared with water alone, by 16% and 23%, respectively, (Student t test, P < 0.03 and P < 0.0001) (data not shown). In contrast, log-transformed S. Thompson population sizes in cilantro leaf washes containing Tween 80 were not statistically different than those with KP buffer alone at 6 days postinoculation (P > 0.05) (Fig. 1). This failure of plate counts to reveal an effect of the surfactant on the removal of Salmonella cells from cilantro leaves was observed also with Tween 20 and Salmonella cell removal from parsley leaves (24).
FIG 1.
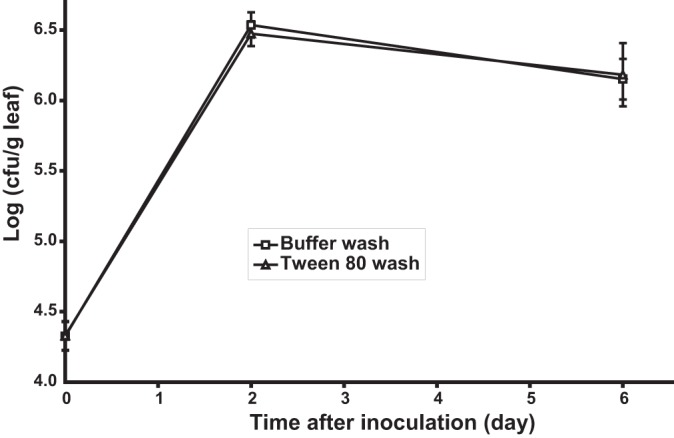
S. enterica serovar Thompson population sizes in the washes from cilantro leaves of plants incubated at 28°C under moist conditions after inoculation, as revealed by bacterial plate counts on growth agar. The leaves were washed with KP buffer with (△) or without (□) 0.05% Tween 80. Datum points represent the means of the log values of the S. Thompson population size in the wash suspension from each of 18 leaves. Bars indicate SEMs.
Bacterial population sizes in the phyllosphere tend to show large leaf-to-leaf variations (25), and this variance may obscure significant differences unless a very large number of replicate sampling units is used to generate mean population sizes per leaf. Monier and Lindow demonstrated that epiphytic bacterial populations are aggregated at the microscopic scale (26). In our previous study, we used fluorescence microscopy and digital image analysis to quantify bacterial population sizes on leaves at the scale of single bacterial cells and estimated that 54% of the total S. Thompson cell population on leaves of cilantro of plants incubated under moist conditions resided in aggregates or biofilms composed of more than 128 cells (1). In the present study, confocal microscopy clearly revealed that despite thorough washing of contaminated cilantro leaves, large S. Thompson aggregates remained attached to the leaf tissue (Fig. 2A) and were present in the wash water bound to leaf material that was released during washing (Fig. 2B). Given that such bacterial aggregates are difficult to break up into single cells, plate counts would considerably underestimate the number of cells recovered from leaves, since CFU arising from aggregates would still be considered equivalent to one cell. This may partly account for the apparent decrease in population size of S. Thompson in the cilantro phyllosphere in the period between 2 and 6 days after inoculation (Fig. 1).
FIG 2.
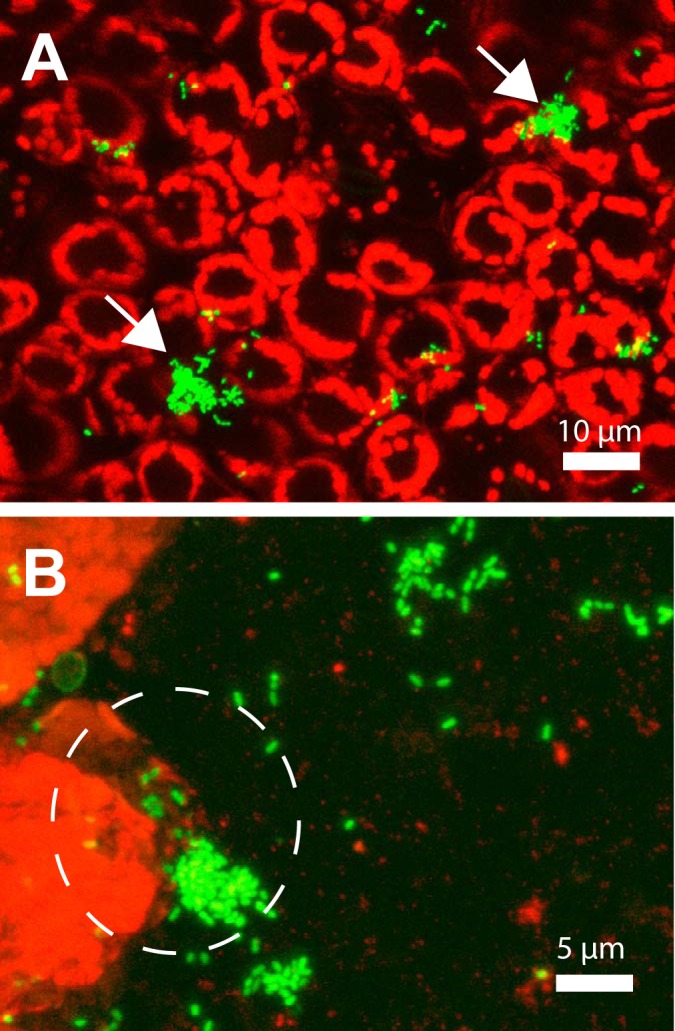
Pseudo-3D confocal micrographs of GFP-labeled S. enterica serovar Thompson present as single cells or aggregates (arrows) on cilantro leaves after washing the leaves by sonication and agitation in KP buffer (A) and present as single cells or aggregates in the wash suspension per se (B). Note the large aggregate of S. Thompson cells still bound to detached leaf tissue and detected in the leaf wash (dashed circle). The pathogen was incubated on cilantro plants for 6 days before the leaves were washed and the washings collected on filters, as described in Materials and Methods. The red fluorescent signal is that of the autofluorescence of the chloroplasts in the leaf epidermal cells.
Because Tween 80 increased S. Thompson biofilm dispersal on inert surfaces, as described above, a digital image analysis approach was used to investigate its potential to disperse aggregates of the human pathogen that had formed on the cilantro leaves over 6 days of incubation. In this scheme, bacteria recovered from leaves washed with or without Tween 80 were collected on filters and the frequency distribution of GFP-S. Thompson aggregate size was obtained by analysis of epifluorescence images of the bacteria on the filters. Based on quantitative analysis of 120 digital micrographs per wash treatment, there were, on average, two times more cells recovered from the leaves washed with Tween 80 than from those washed with KP buffer (mean of log 10 CFU/leaf equal to 4.01 and 3.75, respectively). However, these mean population sizes were not significantly different (Student t test, P = 0.19).
As reported previously also for Pseudomonas syringae populations on bean leaves (26), the aggregate sizes of S. Thompson in the cilantro leaf washes followed a positively skewed frequency distribution, with most of the aggregates being small and large cell assemblages representing rare events (Fig. 3A and B). Single cells represented 13.7% of the cell assemblages washed off with Tween 80 but only 9.3% of the cell assemblages washed off with KP buffer. Additionally, the difference between the cumulative percentages of cells located in small aggregates (two to 16 cells) washed off with Tween 80 and with water decreased as the size of the small aggregates increased. The latter observations indicate that the presence of Tween 80 in the wash resulted in the recovery of a greater number of single cells and small aggregates (Fig. 3A and B). Due to the great discrepancy between the frequency of single cells or small aggregates and that of large aggregates, which generated a broad scale on the y axis of the frequency distribution histograms and rendered differences in the number of large aggregates difficult to observe, the aggregate size data were plotted as the percentage of cells in the total cell population residing in aggregates equal to or larger than bin values (Fig. 3C). This revealed that a considerable portion of the total cell population was present in large aggregates, since 9.8% of the cells in the washes without Tween 80 were located in aggregates equal to or greater than 64 cells. This was 2.25-fold greater than the percentage of cells located in aggregates of that size in the leaf washes containing Tween 80 (4.4%). Overall, Fig. 3C illustrates that this difference (represented as a ratio value above the bars) increased as the bin value increased, indicating that the washes without Tween 80 contained a larger portion of the S. Thompson population as aggregated cells. Along with the more efficient recovery of singles cells and small aggregates obtained with Tween 80, these results suggest that addition of the surfactant to the wash solution likely contributed to detaching single cells or small cell clumps from the leaf surface and/or to breaking up large aggregates into smaller ones and into single cells in the wash solution during agitation.
FIG 3.
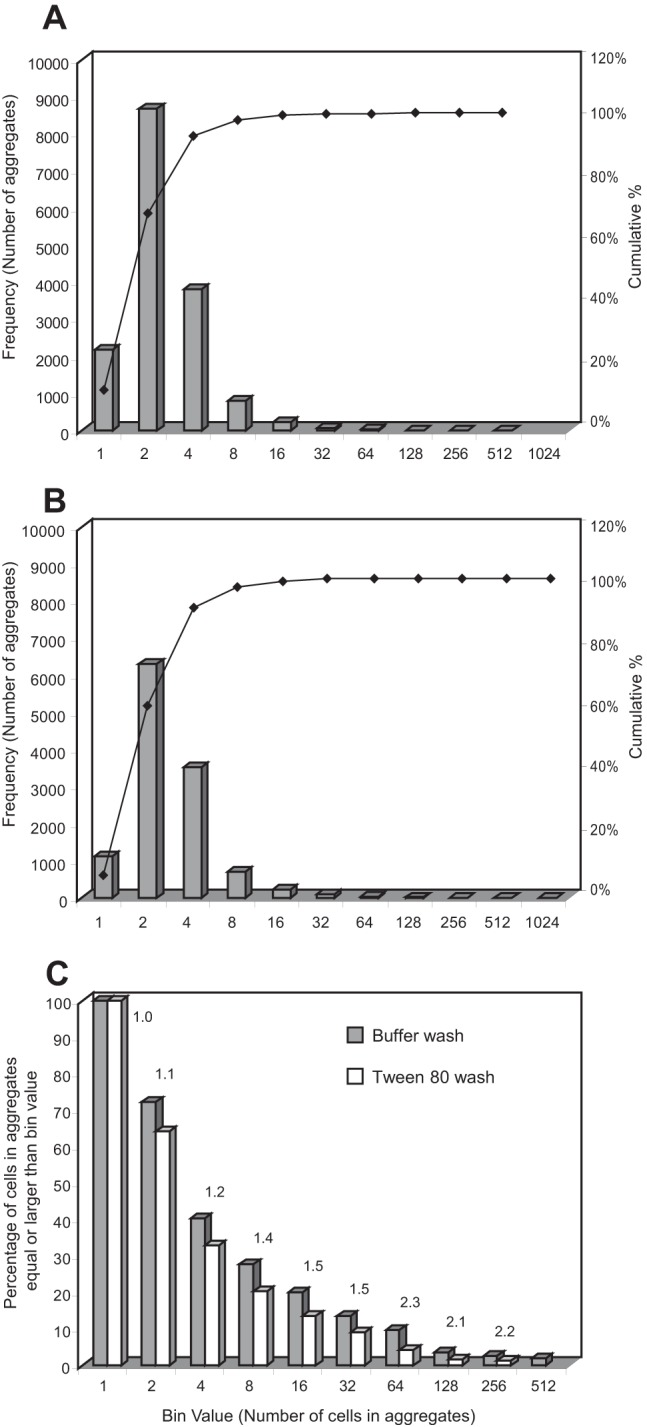
Frequency distribution of GFP-labeled S. enterica serovar Thompson aggregate size in cilantro leaf washes collected on filters and imaged under an epifluorescence microscope. Each frequency distribution is based on the analysis of a total of 120 images from six replicate leaves. Leaves were washed with KP buffer with (A) or without (B) 0.05% Tween 80. The bin values on the x axis in panels A and B represent single cells (bin value of 1) or the number of cells per aggregate (number of cells greater than bin value to the left and less than or equal to given bin value). The left y axis represents the number of single cells (bin value of 1) and the number of aggregates of a given size (bin values greater than 1). In panel C, bin values on the x axis represent single cells (bin value of 1) or number of cells per aggregate (number of cells equal to or greater than bin value), the y axis represents the percentage of the total cell population located in aggregate sizes equal to and greater than the bin value, and the numbers above datum bars represent the ratios of percentage for buffer washes (control) over percentage for Tween 80 washes.
The above conclusion is supported by our results shown in Fig. 4, which shows the regression analysis of the aggregate size data transformed into a Pareto distribution by binning together the smallest assemblages (one and two cells). In order to perform regression analysis on the Pareto distribution, the median of each aggregate size range was log transformed and the frequency of aggregate size was transformed into the log value (1 − cumulative relative frequency), as previously described (1, 27). The regression analysis revealed a linear relationship with high correlation coefficients for both the Tween and KP buffer washes (R2 = 0.992 and 0.991, respectively) and a significant difference in the slopes (F = 5.44, P < 0.05) (Fig. 4). The greater absolute value of the slope for the Tween wash than for the KP buffer wash indicates that the presence of Tween 80 allowed for the recovery of a larger number of smaller S. Thompson aggregates. It remains to be determined, however, whether Tween would have had a similar effect if simple agitation rather than sonication had been used for bacterial cell removal from the leaves, and if its enhanced dispersal of S. Thompson cells occurred during or following the process of removal from the leaf surface, or both.
FIG 4.
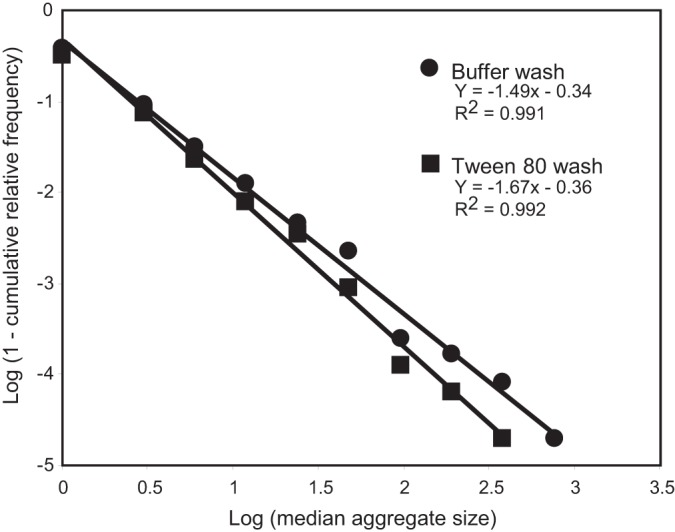
Regression analysis of the log-transformed value (1 − cumulative relative frequency of aggregate size) over the log-transformed median aggregate size, based on the frequency distribution of S. enterica Thompson aggregate sizes washed off cilantro leaves with KP buffer or 0.05% Tween 80, as presented in Fig. 3. For a given median aggregate size, each datum point represents the mean of the log value of 1 minus the cumulative relative frequency of aggregate size from six replicate leaves. The regression analysis shows that the frequency distribution of aggregate size differed significantly between the KP buffer (●) and the Tween 80 (◼) washes (P < 0.05), with the surfactant treatment yielding overall smaller aggregates.
Studies of the behavior of bacterial cells at the population level reveal only gross differences in overall population sizes and fail to inform about fine aspects of their biology that may be relevant to their persistence on plants or in other habitats. Although population sizes of S. Thompson in leaf washes as estimated by plate counts did not show a significant effect of Tween 80 on its removal from cilantro leaves, analysis at the single-cell scale uncovered the ability of the surfactant to wash off a greater number of single cells and/or to disperse aggregated cells. This study is the first to examine the effect of a produce wash treatment at the scale of single bacterial cells and demonstrates the potential of this approach to provide important information for the development of sanitization strategies that can be integrated into hurdle technologies in order to minimize contamination of produce.
ACKNOWLEDGMENTS
This research was supported by the U.S. Department of Agriculture, Agricultural Research Service CRIS project 5325-42000-046.
Thanks are given to Yaguang Zhou for technical assistance.
Footnotes
Published ahead of print 6 June 2014
REFERENCES
- 1.Brandl MT, Miller WG, Bates AH, Mandrell RE. 2005. Production of autoinducer 2 in Salmonella enterica serovar Thompson contributes to its fitness in chickens but not on cilantro leaf surfaces. Appl. Environ. Microbiol. 71:2653–2662. 10.1128/AEM.71.5.2653-2662.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olmez H, Temur SD. 2010. Effects of different sanitizing treatments on biofilms and attachment of Escherichia coli and Listeria monocytogenes on green leaf lettuce. LWT Food Sci. Technol. 43:964–970. 10.1016/j.lwt.2010.02.005 [DOI] [Google Scholar]
- 3.Poza-Carrion C, Suslow T, Lindow S. 2013. Resident bacteria on leaves enhance survival of immigrant cells of Salmonella enterica. Phytopathology 103:341–351. 10.1094/PHYTO-09-12-0221-FI [DOI] [PubMed] [Google Scholar]
- 4.Morris CE, Monier JM. 2003. The ecological significance of biofilm formation by plant-associated bacteria. Annu. Rev. Phytopathol. 41:429–453. 10.1146/annurev.phyto.41.022103.134521 [DOI] [PubMed] [Google Scholar]
- 5.Rayner J, Veeh R, Flood J. 2004. Prevalence of microbial biofilms on selected fresh produce and household surfaces. Int. J. Food Microbiol. 95:29–39. 10.1016/j.ijfoodmicro.2004.01.019 [DOI] [PubMed] [Google Scholar]
- 6.Fett WF. 2000. Naturally occurring biofilms on alfalfa and other types of sprouts. J. Food Prot. 63:625–632 [DOI] [PubMed] [Google Scholar]
- 7.Kaplan JB. 2010. Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 89:205–218. 10.1177/0022034509359403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wachtel MR, Charkowski AO. 2002. Cross-contamination of lettuce with Escherichia coli O157:H7. J. Food Prot. 65:465–470 [DOI] [PubMed] [Google Scholar]
- 9.Bohrerova Z, Linden KG. 2006. Ultraviolet and chlorine disinfection of Mycobacterium in wastewater: effect of aggregation. Water Environ. Res. 78:565–571. 10.2175/106143006X99795 [DOI] [PubMed] [Google Scholar]
- 10.Fett WF. 1985. Relationship of bacterial cell surface hydrophobicity and charge to pathogenicity, physiologic race, and immobilization in attached soybean leaves. Phytopathology 75:1414–1418. 10.1094/Phyto-75-1414 [DOI] [Google Scholar]
- 11.Hassan AN, Frank JF. 2003. Influence of surfactant hydrophobicity on the detachment of Escherichia coli O157:H7 from lettuce. Int. J. Food Microbiol. 87:145–152. 10.1016/S0168-1605(03)00062-X [DOI] [PubMed] [Google Scholar]
- 12.Toutain-Kidd CM, Kadivar SC, Bramante CT, Bobin SA, Zegans ME. 2009. Polysorbate 80 inhibition of Pseudomonas aeruginosa biofilm formation and its cleavage by the secreted lipase LipA. Antimicrob. Agents Chemother. 53:136–145. 10.1128/AAC.00500-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mireles JR, Toguchi A, Harshey RM. 2001. Salmonella enterica serovar Typhimurium swarming mutants with altered biofilm-forming abilities: surfactin inhibits biofilm formation. J. Bacteriol. 183:5848–5854. 10.1128/JB.183.20.5848-5854.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandl MT, Mandrell RE. 2002. Fitness of Salmonella enterica serovar Thompson in the cilantro phyllosphere. Appl. Environ. Microbiol. 68:3614–3621. 10.1128/AEM.68.7.3614-3621.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell VJ, Mohle-Boetani J, Reporter R, Abbott S, Farrar J, Brandl MT, Mandrell RE, Werner SB. 2001. An outbreak of Salmonella serotype Thompson associated with fresh cilantro. J. Infect. Dis. 183:984–987. 10.1086/319254 [DOI] [PubMed] [Google Scholar]
- 16.O'Toole GA, Pratt LA, Watnick PI, Newman DK, Weaver VB, Kolter R. 1999. Genetic approaches to the study of biofilms. Methods Enzymol. 310:91–109. 10.1016/S0076-6879(99)10008-9 [DOI] [PubMed] [Google Scholar]
- 17.Brandl MT, Monier J-M. 2006. Methods in microscopy for the visualization of bacteria and their behavior on plants, p 595–619 In Sapers GM, Gorny JR, Yousef A. (ed), Microbiology of fruits and vegetables. CRC Press LLC, Boca Raton, FL [Google Scholar]
- 18.Ramos B, Miller FA, Brandão TRS, Teixeira P, Silva CLM. 2013. Fresh fruits and vegetables—an overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 20:1–15. 10.1016/j.ifset.2013.07.002 [DOI] [Google Scholar]
- 19.Goudeau DM, Parker CT, Zhou Y, Sela S, Kroupitski Y, Brandl MT. 2013. The Salmonella transcriptome in lettuce and cilantro soft rot reveals a niche overlap with the animal host intestine. Appl. Environ. Microbiol. 79:250–262. 10.1128/AEM.02290-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seymour IJ, Burfoot D, Smith RL, Cox LA, Lockwood A. 2002. Ultrasound decontamination of minimally processed fruits and vegetables. Int. J. Food Sci. Technol. 37:547–557. 10.1046/j.1365-2621.2002.00613.x [DOI] [Google Scholar]
- 21.Zhou B, Feng H, Pearlstein AJ. 2012. Continuous-flow ultrasonic washing system for fresh produce surface decontamination. Innov. Food Sci. Emerg. Technol. 16:427–435. 10.1016/j.ifset.2012.09.007 [DOI] [Google Scholar]
- 22.Bilek SE, Turantas F. 2013. Decontamination efficiency of high power ultrasound in the fruit and vegetable industry, a review. Int. J. Food Microbiol. 166:155–162. 10.1016/j.ijfoodmicro.2013.06.028 [DOI] [PubMed] [Google Scholar]
- 23.Jacques MA, Morris CE. 1995. A review of issues related to the quantification of bacteria from the phyllosphere. FEMS Microbiol. Ecol. 18:1–14. 10.1111/j.1574-6941.1995.tb00158.x [DOI] [Google Scholar]
- 24.Kisluk G, Hoover DG, Kneil KE, Yaron S. 2012. Quantification of low and high levels of Salmonella enterica serovar Typhimurium on leaves. LWT Food Sci. Technol. 45:36–42. 10.1016/j.lwt.2011.07.029 [DOI] [Google Scholar]
- 25.Kinkel LL, Wilson M, Lindow SE. 1995. Effect of sampling scale on the assessment of epiphytic bacterial populations. Microb. Ecol. 29:283–297. 10.1007/BF00164891 [DOI] [PubMed] [Google Scholar]
- 26.Monier JM, Lindow SE. 2004. Frequency, size, and localization of bacterial aggregates on bean leaf surfaces. Appl. Environ. Microbiol. 70:346–355. 10.1128/AEM.70.1.346-355.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson NL, Kotz S. 1970. Distributions in statistics, vol 1 Continuous univariate distributions. John Wiley & Sons, New York, NY [Google Scholar]


