Abstract
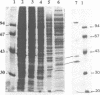
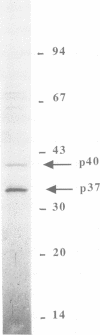
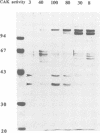
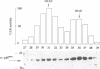
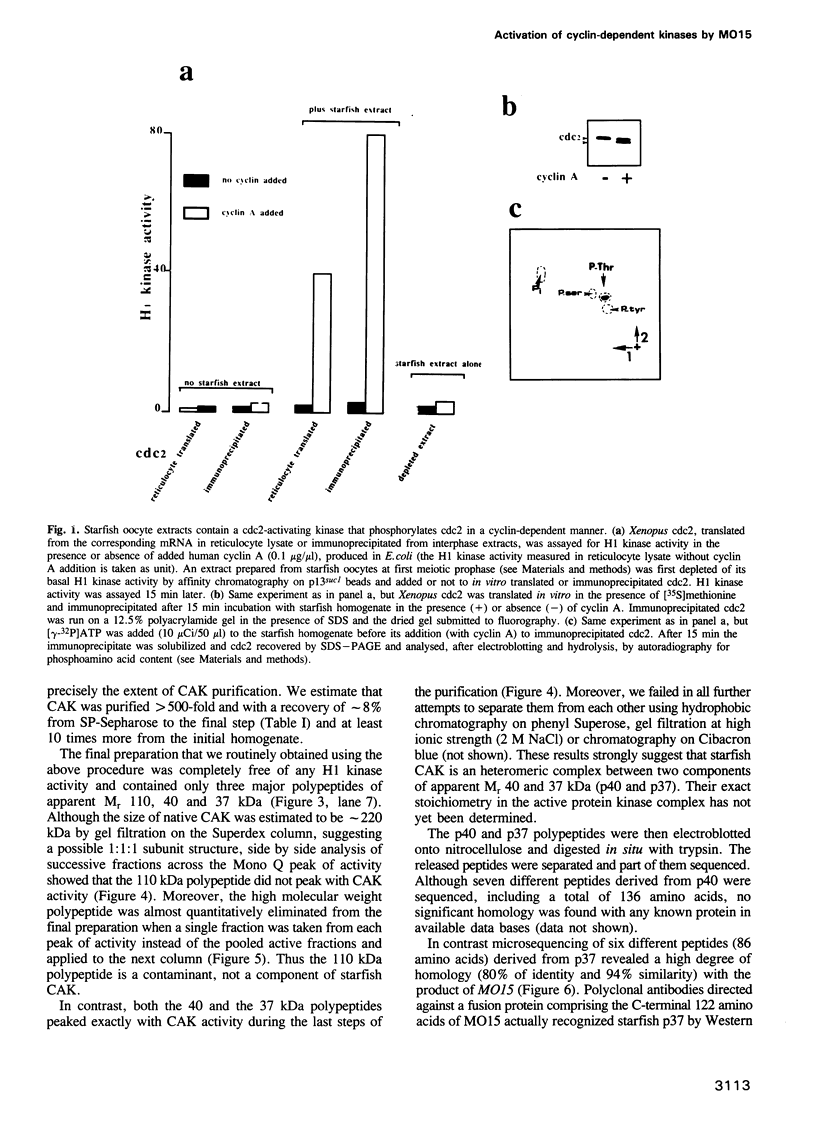
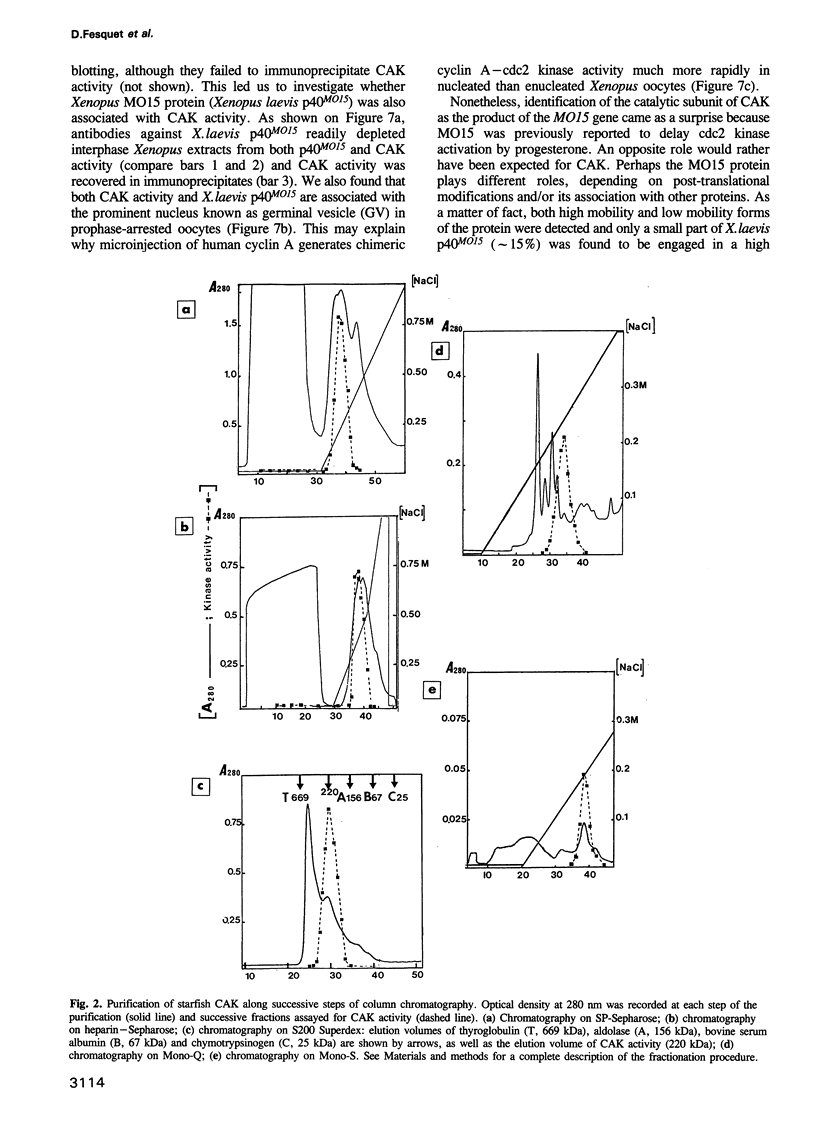
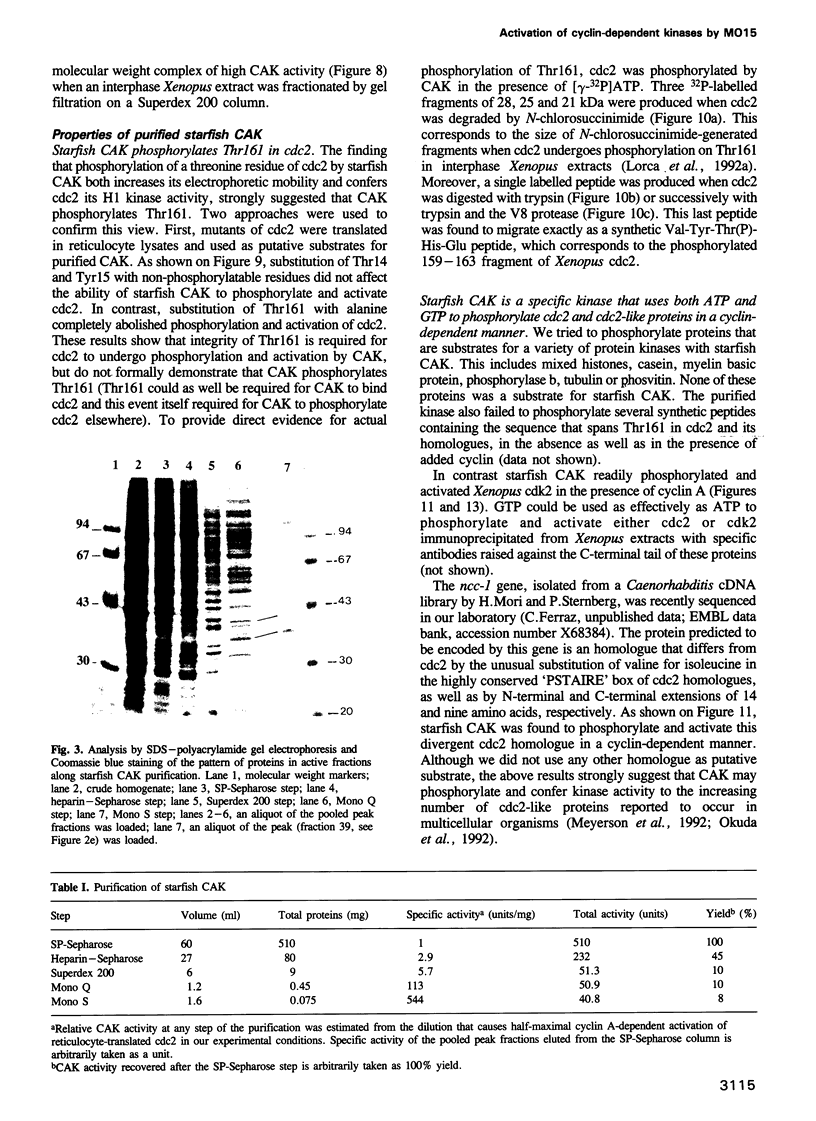
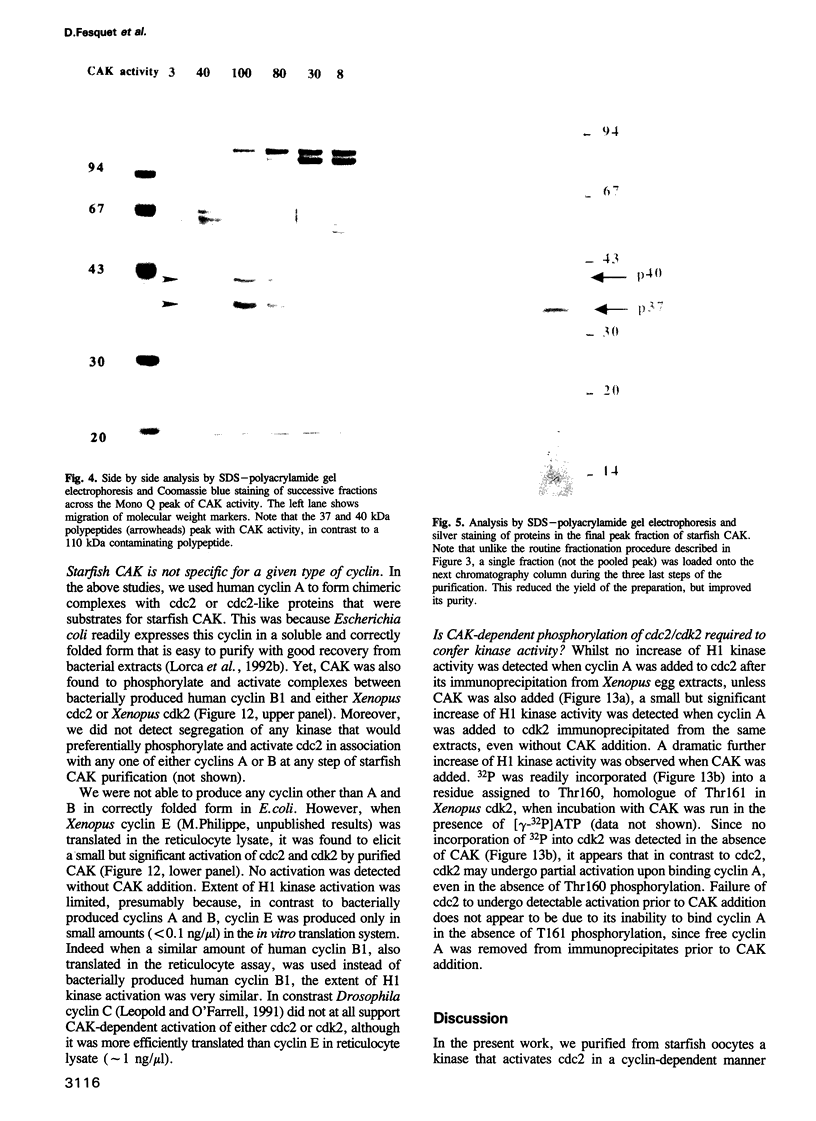
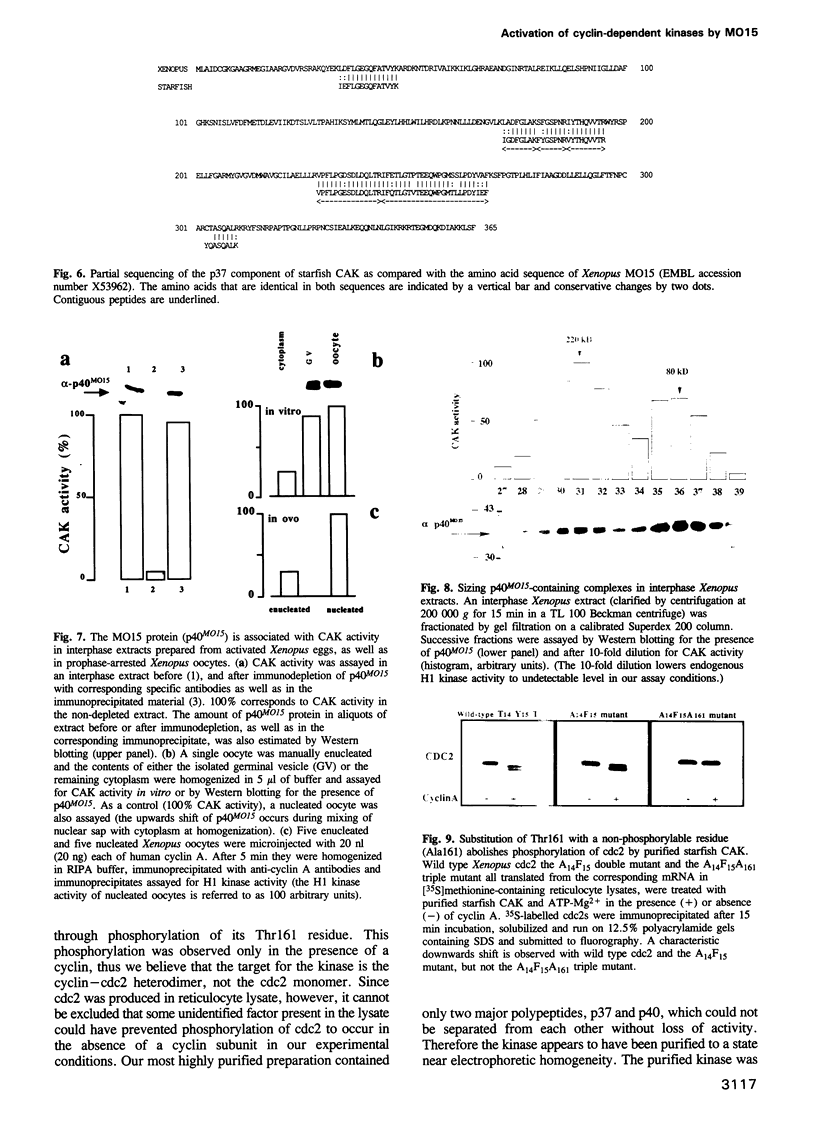
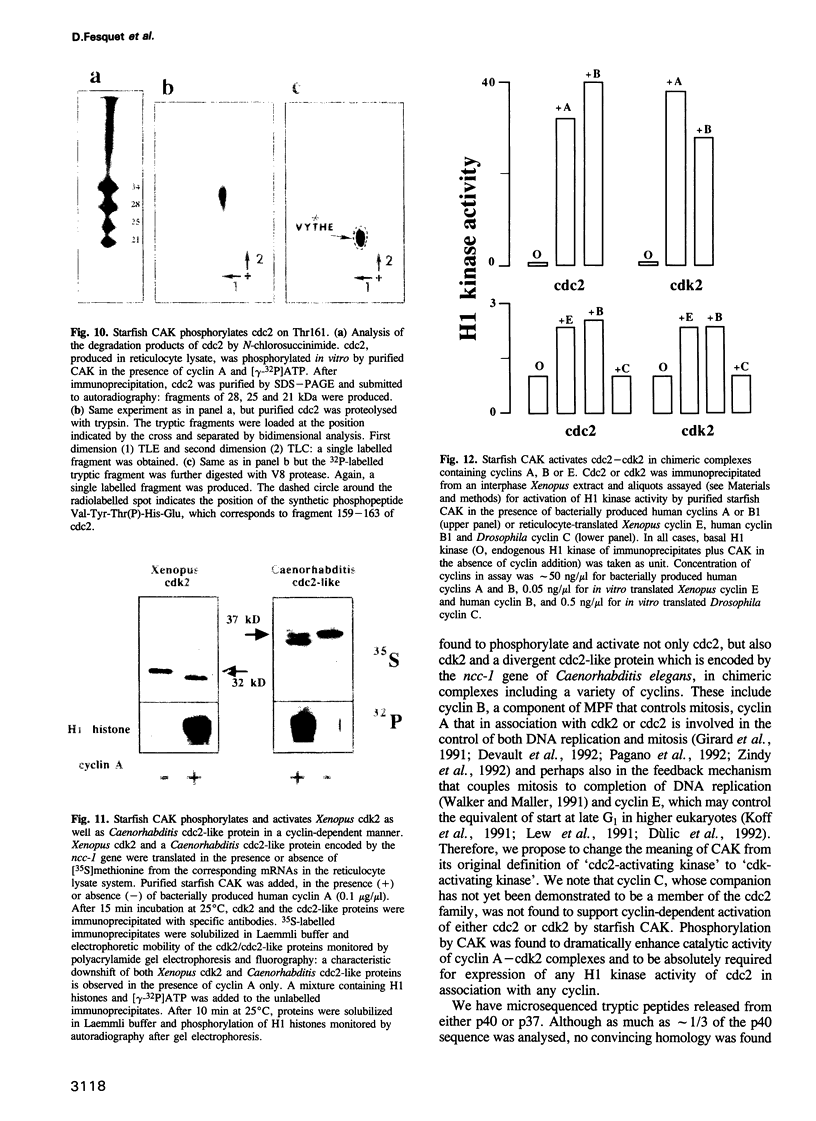
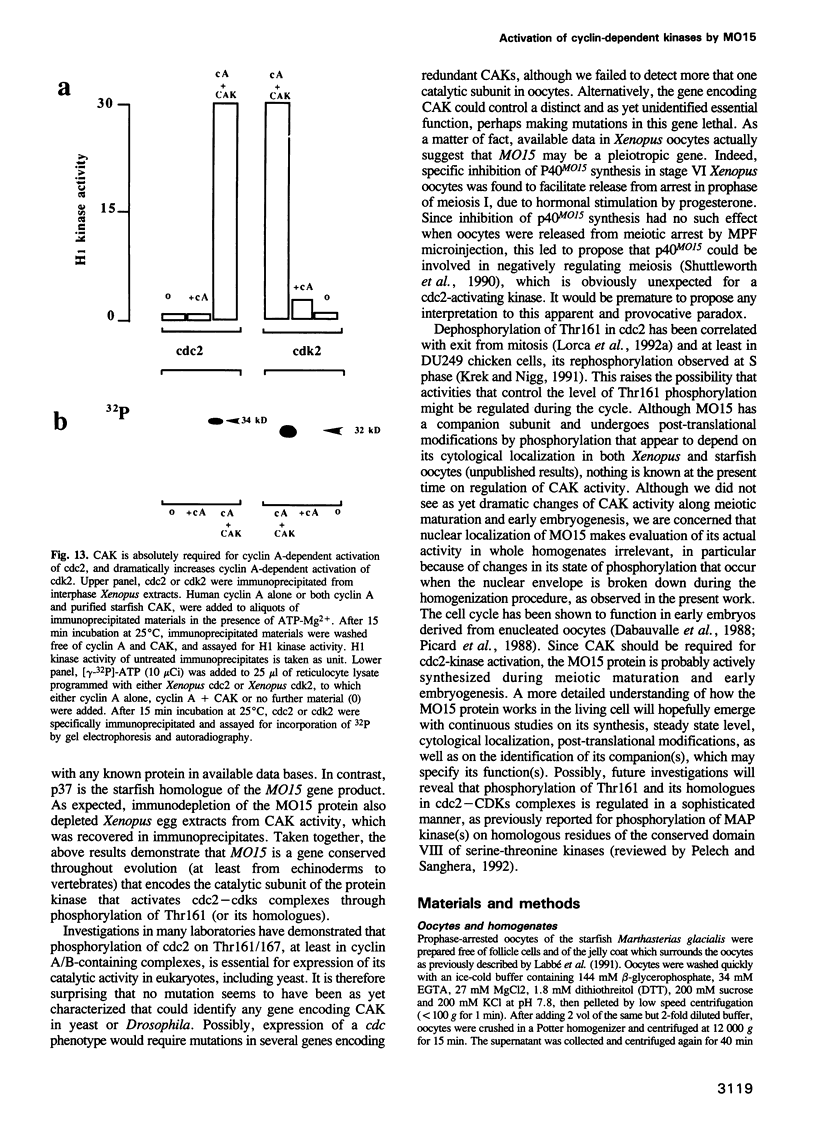
Phosphorylation of Thr161, a residue conserved in all members of the cdc2 family, has been reported to be absolutely required for the catalytic activity of cdc2, the major regulator of eukaryotic cell cycle. In the present work, we have purified from starfish oocytes a kinase that specifically activates cdc2 in a cyclin-dependent manner through phosphorylation of its Thr161 residue. Our most highly purified preparation contained only two major proteins of apparent M(r) 37 and 40 kDa (p37 and p40), which could not be separated from each other without loss of activity. The purified kinase was found to phosphorylate not only cdc2, but also cdk2 and a divergent cdc2-like protein from Caenorhabditis, in chimeric complexes including both mitotic and G1/S cyclins. Extensive microsequencing of p40 did not reveal any convincing homology with any known protein. In contrast, p37 is the starfish homologue of the M015 gene product, a kinase previously cloned by homology probing from a Xenopus cDNA library. As expected, immunodepletion of the MO15 protein depleted Xenopus egg extracts of CAK (cdk-activating kinase) activity, which was recovered in immunoprecipitates. Taken together, the above results demonstrate that MO15 is a gene conserved throughout evolution (at least from echinoderms to vertebrates) that encodes the catalytic subunit of a protein kinase that activates cdc2-cdks complexes through phosphorylation of Thr161 (or its homologues).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amon A., Surana U., Muroff I., Nasmyth K. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature. 1992 Jan 23;355(6358):368–371. doi: 10.1038/355368a0. [DOI] [PubMed] [Google Scholar]
- Clarke P. R., Leiss D., Pagano M., Karsenti E. Cyclin A- and cyclin B-dependent protein kinases are regulated by different mechanisms in Xenopus egg extracts. EMBO J. 1992 May;11(5):1751–1761. doi: 10.1002/j.1460-2075.1992.tb05227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabauvalle M. C., Doree M., Bravo R., Karsenti E. Role of nuclear material in the early cell cycle of Xenopus embryos. Cell. 1988 Feb 26;52(4):525–533. doi: 10.1016/0092-8674(88)90465-5. [DOI] [PubMed] [Google Scholar]
- Devault A., Cavadore J. C., Fesquet D., Labbé J. C., Lorca T., Picard A., Strausfeld U., Dorée M. Concerted roles of cyclin A, cdc25+ mitotic inducer, and type 2A phosphatase in activating the cyclin B/cdc2 protein kinase at the G2/M phase transition. Cold Spring Harb Symp Quant Biol. 1991;56:503–513. doi: 10.1101/sqb.1991.056.01.057. [DOI] [PubMed] [Google Scholar]
- Devault A., Fesquet D., Cavadore J. C., Garrigues A. M., Labbé J. C., Lorca T., Picard A., Philippe M., Dorée M. Cyclin A potentiates maturation-promoting factor activation in the early Xenopus embryo via inhibition of the tyrosine kinase that phosphorylates cdc2. J Cell Biol. 1992 Sep;118(5):1109–1120. doi: 10.1083/jcb.118.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorée M. Control of M-phase by maturation-promoting factor. Curr Opin Cell Biol. 1990 Apr;2(2):269–273. doi: 10.1016/0955-0674(90)90018-a. [DOI] [PubMed] [Google Scholar]
- Ducommun B., Brambilla P., Félix M. A., Franza B. R., Jr, Karsenti E., Draetta G. cdc2 phosphorylation is required for its interaction with cyclin. EMBO J. 1991 Nov;10(11):3311–3319. doi: 10.1002/j.1460-2075.1991.tb04895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulić V., Lees E., Reed S. I. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992 Sep 25;257(5078):1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991 Oct 4;67(1):189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Wu M., Gerhart J. C., Martin G. S. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991 Apr;11(4):1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix M. A., Cohen P., Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 1990 Mar;9(3):675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Minshull J., Lohka M., Glotzer M., Hunt T., Maller J. L. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990 Feb 9;60(3):487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Gautier J., Solomon M. J., Booher R. N., Bazan J. F., Kirschner M. W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991 Oct 4;67(1):197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Girard F., Strausfeld U., Fernandez A., Lamb N. J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991 Dec 20;67(6):1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Moreno S., Owen D. J., Sazer S., Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991 Nov;10(11):3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Gu Y., Rosenblatt J., Morgan D. O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992 Nov;11(11):3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Honda R., Ohba Y., Yasuda H. The cell cycle regulator, human p50weel, is a tyrosine kinase and not a serine/tyrosine kinase. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1333–1338. doi: 10.1016/s0006-291x(05)81552-9. [DOI] [PubMed] [Google Scholar]
- Hunt T. Maturation promoting factor, cyclin and the control of M-phase. Curr Opin Cell Biol. 1989 Apr;1(2):268–274. doi: 10.1016/0955-0674(89)90099-9. [DOI] [PubMed] [Google Scholar]
- Inaba T., Matsushime H., Valentine M., Roussel M. F., Sherr C. J., Look A. T. Genomic organization, chromosomal localization, and independent expression of human cyclin D genes. Genomics. 1992 Jul;13(3):565–574. doi: 10.1016/0888-7543(92)90126-d. [DOI] [PubMed] [Google Scholar]
- Koff A., Cross F., Fisher A., Schumacher J., Leguellec K., Philippe M., Roberts J. M. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991 Sep 20;66(6):1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- Krek W., Marks J., Schmitz N., Nigg E. A., Simanis V. Vertebrate p34cdc2 phosphorylation site mutants: effects upon cell cycle progression in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1992 May;102(Pt 1):43–53. doi: 10.1242/jcs.102.1.43. [DOI] [PubMed] [Google Scholar]
- Krek W., Nigg E. A. Differential phosphorylation of vertebrate p34cdc2 kinase at the G1/S and G2/M transitions of the cell cycle: identification of major phosphorylation sites. EMBO J. 1991 Feb;10(2):305–316. doi: 10.1002/j.1460-2075.1991.tb07951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusubata M., Tokui T., Matsuoka Y., Okumura E., Tachibana K., Hisanaga S., Kishimoto T., Yasuda H., Kamijo M., Ohba Y. p13suc1 suppresses the catalytic function of p34cdc2 kinase for intermediate filament proteins, in vitro. J Biol Chem. 1992 Oct 15;267(29):20937–20942. [PubMed] [Google Scholar]
- Labbé J. C., Capony J. P., Caput D., Cavadore J. C., Derancourt J., Kaghad M., Lelias J. M., Picard A., Dorée M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989 Oct;8(10):3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J. C., Cavadore J. C., Dorée M. M phase-specific cdc2 kinase: preparation from starfish oocytes and properties. Methods Enzymol. 1991;200:291–301. doi: 10.1016/0076-6879(91)00147-o. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Lorca T., Labbé J. C., Devault A., Fesquet D., Capony J. P., Cavadore J. C., Le Bouffant F., Dorée M. Dephosphorylation of cdc2 on threonine 161 is required for cdc2 kinase inactivation and normal anaphase. EMBO J. 1992 Jul;11(7):2381–2390. doi: 10.1002/j.1460-2075.1992.tb05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T., Labbé J. C., Devault A., Fesquet D., Strausfeld U., Nilsson J., Nygren P. A., Uhlen M., Cavadore J. C., Dorée M. Cyclin A-cdc2 kinase does not trigger but delays cyclin degradation in interphase extracts of amphibian eggs. J Cell Sci. 1992 May;102(Pt 1):55–62. doi: 10.1242/jcs.102.1.55. [DOI] [PubMed] [Google Scholar]
- Léopold P., O'Farrell P. H. An evolutionarily conserved cyclin homolog from Drosophila rescues yeast deficient in G1 cyclins. Cell. 1991 Sep 20;66(6):1207–1216. doi: 10.1016/0092-8674(91)90043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y. Towards understanding the control of the division cycle in animal cells. Biochem Cell Biol. 1992 Oct-Nov;70(10-11):920–945. doi: 10.1139/o92-138. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Ewen M. E., Strom D. K., Kato J. Y., Hanks S. K., Roussel M. F., Sherr C. J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992 Oct 16;71(2):323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Roussel M. F., Ashmun R. A., Sherr C. J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991 May 17;65(4):701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- McGowan C. H., Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J. 1993 Jan;12(1):75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson M., Enders G. H., Wu C. L., Su L. K., Gorka C., Nelson C., Harlow E., Tsai L. H. A family of human cdc2-related protein kinases. EMBO J. 1992 Aug;11(8):2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Blow J. J., Hunt T. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell. 1989 Mar 24;56(6):947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W. Creative blocks: cell-cycle checkpoints and feedback controls. Nature. 1992 Oct 15;359(6396):599–604. doi: 10.1038/359599a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Norbury C., Blow J., Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991 Nov;10(11):3321–3329. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent J. H., Alfa C. E., Young T., Hyams J. S. Conserved structural motifs in cyclins identified by sequence analysis. J Cell Sci. 1991 Jul;99(Pt 3):669–674. doi: 10.1242/jcs.99.3.669. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Okuda T., Cleveland J. L., Downing J. R. PCTAIRE-1 and PCTAIRE-3, two members of a novel cdc2/CDC28-related protein kinase gene family. Oncogene. 1992 Nov;7(11):2249–2258. [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992 Mar;11(3):961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J., Le Guellec R., Couturier A., Le Guellec K., Omilli F., Camonis J., MacNeill S., Philippe M. Cloning by differential screening of a Xenopus cDNA coding for a protein highly homologous to cdc2. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1039–1043. doi: 10.1073/pnas.88.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L. L., Atherton-Fessler S., Piwnica-Worms H. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2917–2921. doi: 10.1073/pnas.89.7.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. MAP kinases: charting the regulatory pathways. Science. 1992 Sep 4;257(5075):1355–1356. doi: 10.1126/science.1382311. [DOI] [PubMed] [Google Scholar]
- Picard A., Harricane M. C., Labbe J. C., Doree M. Germinal vesicle components are not required for the cell-cycle oscillator of the early starfish embryo. Dev Biol. 1988 Jul;128(1):121–128. doi: 10.1016/0012-1606(88)90273-4. [DOI] [PubMed] [Google Scholar]
- Poon R. Y., Yamashita K., Adamczewski J. P., Hunt T., Shuttleworth J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 1993 Aug;12(8):3123–3132. doi: 10.1002/j.1460-2075.1993.tb05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I. The role of p34 kinases in the G1 to S-phase transition. Annu Rev Cell Biol. 1992;8:529–561. doi: 10.1146/annurev.cb.08.110192.002525. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J., Gu Y., Morgan D. O. Human cyclin-dependent kinase 2 is activated during the S and G2 phases of the cell cycle and associates with cyclin A. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2824–2828. doi: 10.1073/pnas.89.7.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987 May 22;49(4):559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986 Apr 11;45(1):145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Shuttleworth J., Godfrey R., Colman A. p40MO15, a cdc2-related protein kinase involved in negative regulation of meiotic maturation of Xenopus oocytes. EMBO J. 1990 Oct;9(10):3233–3240. doi: 10.1002/j.1460-2075.1990.tb07522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M. J., Glotzer M., Lee T. H., Philippe M., Kirschner M. W. Cyclin activation of p34cdc2. Cell. 1990 Nov 30;63(5):1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Lee T., Kirschner M. W. Role of phosphorylation in p34cdc2 activation: identification of an activating kinase. Mol Biol Cell. 1992 Jan;3(1):13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Murray A. W. S-phase feedback control in budding yeast independent of tyrosine phosphorylation of p34cdc28. Nature. 1992 Jan 23;355(6358):365–368. doi: 10.1038/355365a0. [DOI] [PubMed] [Google Scholar]
- Strausfeld U., Labbé J. C., Fesquet D., Cavadore J. C., Picard A., Sadhu K., Russell P., Dorée M. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature. 1991 May 16;351(6323):242–245. doi: 10.1038/351242a0. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Harlow E., Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Maller J. L. Role for cyclin A in the dependence of mitosis on completion of DNA replication. Nature. 1991 Nov 28;354(6351):314–317. doi: 10.1038/354314a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Connolly T., Futcher B., Beach D. Human D-type cyclin. Cell. 1991 May 17;65(4):691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Fukada S., Yoshikuni M., Bulet P., Hirai T., Yamaguchi A., Yasuda H., Ohba Y., Nagahama Y. M-phase-specific histone H1 kinase in fish oocytes. Purification, components and biochemical properties. Eur J Biochem. 1992 Apr 15;205(2):537–543. doi: 10.1111/j.1432-1033.1992.tb16810.x. [DOI] [PubMed] [Google Scholar]
- Zindy F., Lamas E., Chenivesse X., Sobczak J., Wang J., Fesquet D., Henglein B., Bréchot C. Cyclin A is required in S phase in normal epithelial cells. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1144–1154. doi: 10.1016/0006-291x(92)91851-g. [DOI] [PubMed] [Google Scholar]