Abstract
Chilo suppressalis and Sesamia inferens are two important lepidopteran rice pests that occur concurrently during outbreaks in paddy fields in the main rice-growing areas of China. Previous and current field tests demonstrate that the transgenic rice line Huahui 1 (HH1) producing a Cry1Ab-Cry1Ac hybrid toxin from the bacterium Bacillus thuringiensis reduces egg and larval densities of C. suppressalis but not of S. inferens. This differential susceptibility to HH1 rice correlates with the reduced susceptibility to Cry1Ab and Cry1Ac toxins in S. inferens larvae compared to C. suppressalis larvae. The goal of this study was to identify the mechanism responsible for this differential susceptibility. In saturation binding assays, both Cry1Ab and Cry1Ac toxins bound with high affinity and in a saturable manner to midgut brush border membrane vesicles (BBMV) from C. suppressalis and S. inferens larvae. While binding affinities were similar, a dramatically lower concentration of Cry1A toxin binding sites was detected for S. inferens BBMV than for C. suppressalis BBMV. In contrast, no significant differences between species were detected for Cry1Ca toxin binding to BBMV. Ligand blotting detected BBMV proteins binding Cry1Ac or Cry1Ca toxins, some of them unique to C. suppressalis or S. inferens. These data support that reduced Cry1A binding site concentration is associated with a lower susceptibility to Cry1A toxins and HH1 rice in S. inferens larvae than in C. suppressalis larvae. Moreover, our data support Cry1Ca as a candidate for pyramiding efforts with Cry1A-producing rice to extend the activity range and durability of this technology against rice stem borers.
INTRODUCTION
The striped stem borer (SSB) (Chilo suppressalis) and the pink stem borer (PSB) (Sesamia inferens) are two important lepidopterous pests distributed in the main rice-growing areas of China (1). Traditionally, intensive chemical control has been the most common method for control of these pests, which has led to the evolution of pest resistance (2). Transgenic rice expressing Bacillus thuringiensis insecticidal proteins (i.e., Bt rice) has been introduced as an environmentally sound alternative to control the damage caused by rice pests (3, 4). Especially in China, a number of transgenic rice lines transformed with genes encoding Bt Cry insecticidal proteins have been reported to be effective against lepidopteran rice pest complexes (5–10). In preliminary field tests, a transgenic rice line producing the Cry1Ac protein was effective in controlling C. suppressalis larvae (10) but had lower efficacy against S. inferens larvae (11). A later report demonstrated that only S. inferens survived in paddy fields planted with a transgenic Cry1Ac-producing rice line (12), suggesting that long-term utility of Cry1A-producing rice would be threatened by S. inferens outbreaks. The Huahui 1 transgenic rice line (HH1) producing a Cry1Ac-Cry1Ab hybrid protein was issued biosafety certificates in 2009 for its commercial production in Hubei Province (China), showing good prospects for commercial application. Field tests with the Huahui 1 rice line demonstrated good control of C. suppressalis but relatively low resistance to S. inferens (13), although the mechanism responsible for this differential susceptibility is not known. Knowledge of the mechanism responsible for differential susceptibility among species in this rice pest complex is needed to optimize transgenic insecticidal technologies and to extend their future utility through effective resistance management strategies.
We hypothesized that differential susceptibilities to Cry1A toxins between C. suppressalis and S. inferens larvae result from differences in the intoxication process. The most accepted model for Cry intoxication includes processing in the insect midgut fluids to yield a Cry1A toxin core that binds to receptors on the brush border membrane of the gut epithelium and inserts on the enterocyte membrane to create a pore leading to osmotic cell death (14). Studies of resistance mechanisms against Cry1 toxins expressed in Bt crops suggest that high levels of resistance are almost exclusively associated with alterations in toxin binding to midgut receptors (15). These high levels of resistance are expected in cases of field-evolved practical resistance to Bt crops (16). In the case of C. suppressalis, binding competition studies in larval brush border membrane vesicles (BBMV) revealed that Cry1A toxins share binding sites with Cry1Ba toxin (17) but they are not recognized by Cry2A, Cry9C, or Cry1C toxins (18, 19). Diverse studies have suggested aminopeptidase-N and cadherin-like proteins as Cry1A binding sites in C. suppressalis BBMV (20, 21). In contrast, no Cry toxin binding data are available for S. inferens.
In the present study, we aimed at comparing the efficacy of the transgenic rice line Huahui 1 with that of nontransgenic rice against C. suppressalis and S. inferens populations under field conditions and determining if toxin binding differences help explain differential susceptibilities to Cry1A toxins in these insects.
MATERIALS AND METHODS
Insects and rice lines.
Laboratory colonies of C. suppressalis and S. inferens used in this study were originally initiated from larvae collected from paddy fields in Shucheng (32°28′N, 116°55′E), Anhui Province, China, in 2010. Insects were reared on an artificial diet as previously described (22) without exposure to any Bt toxin for 5 generations before testing. All cultures were kept under constant conditions of a temperature of 27 ± 1°C, 70 to 80% relative humidity (RH), and a photoperiod of 16:8 h (light:dark) [16:8 h (L:D)].
The transgenic rice line Huahui 1 (HH1) and the corresponding nontransgenic isoline Minghui63 (MH63) were used in field tests. The HH1 line contains a cry1Ab-cry1Ac fusion gene consisting of 1,344 bp encoding the N terminus of cry1Ab and 486 bp encoding the C terminus of cry1Ac, with its expression under the control of the rice actinI promoter (6). All rice seeds used in this study were provided by Huazhong Agricultural University, Wuhan, China.
Insecticidal proteins.
Recombinant Escherichia coli strains were used to produce Cry1Ab, Cry1Ac, and Cry1Ca toxins, which were activated with trypsin, purified, and provided as lyophilized powder by Marianne Pusztai-Carey (Case Western Reserve University, Cleveland, OH, USA). Recombinant E. coli and B. thuringiensis strains kindly provided by the Biotechnology Laboratory of the Institute of Plant Protection (Chinese Academy of Agricultural Sciences, Beijing, China) were used to produce Cry2Aa and Cry1Ah toxins, respectively, which were activated and purified as previously described (23). Cultures of B. thuringiensis strain HD-73 were used for production of Cry1Ac protoxin, which was activated and purified as described previously (24) and used in binding and ligand blotting experiments.
Field tests.
Field tests in this study were conducted in Shucheng (32°28′ N, 116°55′ E), Anhui Province, China, during the summers of 2009 and 2010. Treatments included the transgenic HH1 rice line and its nontransgenic isoline MH63. The field experimental design was a randomized complete block, with three replications for each treatment. A 1-m buffer area was left between plots to reduce the possibility of larval movement among treatments. There were six plots in total, and each plot was ca. 120 m2. The rice seeds were sown in a seedling bed on 20 May 2009 and 10 May 2010. Transplantation of seedlings at the four-leaf stage in each plot began on 20 June 20 2009 and 10 June 10 2010 at the rate of two seedlings per hill, with a plant-to-plant distance of 20 cm. The design and all agronomic practices were the same for all plots. In each plot, 20 sites with a total of 100 hills of rice plants were sampled once at random in a parallel line pattern. Investigations of egg density and natural larval infestation were conducted from mid-July to mid-September, and samples were taken once every 10 days in both 2009 and 2010, weather permitting. A thorough whole-plant survey was conducted, and the numbers of eggs and larvae were counted for each sampled plant (n = 100). Egg and larval densities for C. suppressalis and S. inferens were computed for each treatment according to the rule for investigation and forecast of rice stem borer (National Standard of the People's Republic of China GB/T 15792-1995). Prior to statistical analyses, the number of eggs or larvae collected was transformed to a standardized unit of eggs or larvae per hundred plants. All the data were tested for normality (Kolmogorov-Smirnov test) and homogeneity of variance (Bartlett's test), with square root transformations used when data did not pass normality and homogeneity tests. Egg or larval densities were subjected to two-way analysis of variance (ANOVA) with a repeated measure on two factors (sampling data and pest species or rice types) within each year. The data analysis was generated using JMP software v.4 of SAS (SAS Institute Inc., Cary, NC, USA).
Cry toxin bioassays.
Diet incorporation bioassays were used for dose-response testing to estimate 50% lethal concentrations (LC50s) for Cry1Ab, Cry1Ac, Cry1Ah, Cry1Ca, and Cry2Aa toxins in C. suppressalis and S. inferens larvae. Preliminary tests were conducted to determine the proper range of toxin concentrations to use. Stock solutions were prepared by dissolving lyophilized toxin powder in 50 mM Na2CO3 (pH 9.5) buffer, and then different toxin concentration solutions were obtained by serial dilution of the stock using phosphate-buffered saline (PBS) buffer (135 mM NaCl, 2 mM KCl, 10 mM Na2PO4, 1.7 mM KH2PO4, pH 7.4). Toxin solutions were thoroughly incorporated into the artificial diet, with an equal volume of PBS buffer used as the control. All assays included ca. 5 to 8 concentrations for each toxin and insect tested. A diet disc (1.6-cm diameter, about 0.5 g) containing Cry toxin was put into each well of a 24-well plate, and a neonate was placed on each well, with ca. 40 to 50 neonates used in each replicate, and the bioassay was replicated four times for each treatment. Neonate mortalities were determined after 6 days at 27 ± 1°C, 16: 8 h (L:D), and 60 to 80% RH. Mortalities were corrected for natural death (mortality in controls) using Abbott's formula (25). The POLO-PC software package (26) was used for probit analysis to estimate LC50s and for testing goodness of fit. Responses were considered significantly different when 95% fiducial limits for the LC50s did not overlap. Relative susceptibility ratios were calculated as the LC50 ratios between S. inferens and C. suppressalis for a toxin.
BBMV preparation.
Fourth-instar C. suppressalis and S. inferens larvae were anesthetized on ice and their midguts dissected and kept at −80°C until used to prepare BBMV using a differential centrifugation method (27) with minor modifications (41). Isolated BBMV proteins were quantified in a fluorometer (Qubit, Invitrogen) and then kept at −80°C until used (less than 2 months). Specific activity of aminopeptidase-N (APN) using leucine-p-nitroanilide as the substrate was used as a marker for brush border enzyme enrichment in the BBMV preparations as described elsewhere (28). APN activities in the final BBMV preparations were enriched 5- to 7-fold compared to the APN activities in the initial midgut homogenates.
Cry toxin radiolabeling.
Activated Cry1Ab, Cry1Ac, and Cry1Ca toxins (10 μg) were radioiodinated with 0.5 mCi Na125I (PerkinElmer, Boston, MA) using chloramine T as previously described (29). Labeled toxins were purified from free iodine using a PD-10 desalting column (GE Healthcare Life Sciences) equilibrated in column buffer (20 mM Tris-HCl, 150 mM NaCl, 0.1% bovine serum albumin [BSA], pH 8.65). The presence and purity of the radiolabeled toxins in the eluted fractions were established by measuring radioactivity in a Wizard2 gamma counter (PerkinElmer) and by SDS–8% PAGE, followed by autoradiography of the dried gel at −80°C (data not shown). The specific activities of labeled toxins were 0.46 μCi/μg for Cry1Ab, 1.54 μCi/μg for Cry1Ac, and 0.36 μCi/μg for Cry1Ca.
Binding assays with 125I-labeled Cry toxins.
Binding saturation assays were performed using BBMV proteins at a constant concentration (20 μg) as binding sites and increasing amounts of 125I-Cry1Ab, 125I-Cry1Ac or 125I-Cry1Ca as ligands. Final reaction volumes were 0.1 ml in binding buffer (PBS [pH 7.4], 0.1% BSA). Reaction mixtures were incubated at room temperature for 1 h, and reactions were stopped by centrifugation (14,500 × g for 10 min), after which pellets were washed with 0.5 ml of ice-cold binding buffer twice. The radioactivity in the final pellets was measured in a Wizard2 gamma counter (PerkinElmer). Specific binding was obtained by subtracting nonspecific binding, obtained by including an excess (300-fold the highest ligand concentration used) of unlabeled homologous toxin in binding mixtures, from total binding obtained in the absence of competitor. The relative percentages of nonspecific binding differed between labeled toxins, insect BBMV, and ligand input, but it did not surpass 12% of total Cry1Ab binding, 30% of total Cry1Ca binding, and 30% of total Cry1Ac binding at the highest ligand concentration tested for the insect with the highest detected total binding (C. suppressalis). Data from at least two replicated experiments performed in duplicate for each toxin were pooled and analyzed using SigmaPlot v.12.0 software (Systat Software, San Jose, CA) to obtain the apparent dissociation constant (Kd) and concentration of binding sites (Bmax).
Ligand blot assays.
BBMV proteins (20 μg) from C. suppressalis and S. inferens were separated by SDS–8% PAGE and then transferred to polyvinylidene difluoride (PVDF) filters as described elsewhere (30). After electrotransfer, the filters were blocked in blocking buffer (PBS buffer [pH 7.4] with 3% BSA and 0.1% Tween 20) and then probed with 1.0 nM 125I-Cry1Ac or 125I-Cry1Ca in blocking buffer for 1 h at room temperature with constant slow orbital shaking. Filters were then washed with washing buffer (PBS buffer [pH 7.4] with 0.1% BSA and 0.1% Tween 20) on a rotary shaker for three washes of 10 min each, air dried, and used in autoradiography at −80°C.
RESULTS
Field tests.
Egg and larva density dynamics for both C. suppressalis and S. inferens differed among rice lines and growing seasons, with populations in 2009 being distinctly higher than in 2010 (Table 1). Repeated-measures ANOVA tests revealed that mean egg densities did not differ significantly between C. suppressalis and S. inferens either on MH63 or HH1 rice lines in 2009 (F1,4 = 0.07, P = 0.801, for MH63; F1,4 = 3.63, P = 0.130, for HH1) or 2010 (F1,4 = 1.15, P = 0.345, for MH63; F1,4 = 0.62, P = 0.476, for HH1) (Table 1).
TABLE 1.
Mean numbers of eggs and larvae of C. suppressalis and S. inferens per 100 plants of transgenic (HH1) and nontransgenic (MH63) rice linesa
| Yr | Rice line | No. of eggs/100 plants (mean ± SE) |
No. of larvae/100 plants (mean ± SE) |
||
|---|---|---|---|---|---|
| C. suppressalis | S. inferens | C. suppressalis | S. inferens | ||
| 2009 | MH63 | 147.8 ± 48.6 A | 150.4 ± 44.1 A | 32.1 ± 12.2 A | 24.6 ± 8.3 A |
| HH1 | 139.7 ± 37.7 A | 158.3 ± 49.3 A | 9.7 ± 4.7 B | 31.3 ± 9.2 A | |
| 2010 | MH63 | 76.6 ± 23.4 A | 67.8 ± 18.7 A | 22.8 ± 8.8 A | 17.0 ± 5.7 A |
| HH1 | 59.4 ± 14.4 A | 65.2 ± 18.4 A | 6.7 ± 3.1 B | 16.3 ± 6.3 A | |
Different letters within a row indicate significant differences as determined using a repeated measure two-way ANOVA (P < 0.05). SE, standard error of the mean.
Comparison of larval densities for C. suppressalis between HH1 and MH63 rice (Table 1) detected significantly reduced larval densities on the HH1 rice line in both years (F1,4 = 425.65, P < 0.05, in 2009; F1,4 = 72.05, P < 0.05, in 2010), while no significant differences were detected for S. inferens larval densities between the rice lines (F1,4 = 5.86, P = 0.073, in 2009; F1,4 = 0.08, P = 0.787, in 2010). Moreover, significantly more S. inferens larvae than C. suppressalis larvae survived on HH1 rice for both years (F1,4 = 913.51, P < 0.05, in 2009; F1,4 = 37.25, P < 0.05, in 2010). In contrast, no significant differences in larval densities between C. suppressalis and S. inferens were detected on the control (MH63) rice line (F1,4 = 6.67, P = 0.061, in 2009; F1,4 = 5.17, P = 0.085, in 2010) (Table 1).
Cry toxin bioassays.
Laboratory bioassays with purified activated Cry toxins showed that Cry1Ab and Cry1Ac toxicity against S. inferens larvae was drastically lower than that against C. suppressalis larvae (Table 2). Large LC50 differences were detected between the two species for Cry1Ab (39-fold) and Cry1Ac (20-fold). In comparison, a modest difference was detected for Cry1Ah (3-fold), while Cry1Ca and Cry2Aa were similarly active, with Cry1Ca being the only Cry toxin highly active against both species (Table 2).
TABLE 2.
Susceptibility of C. suppressalis (SSB) and S. inferens (PSB) to five different Bt toxins in diet incorporation bioassaysa
| Bt toxin | Species | No. of larvae | Slope ± SEa | LC50 (mg/liter) | 95% FL | χ2 | df | Pb | Relative ratioc |
|---|---|---|---|---|---|---|---|---|---|
| Cry1Ac | SSB | 1,120 | 2.25 ± 0.15 | 38.69 | 29.17–48.22 | 5.15 | 5 | 0.40 | 20.21 |
| PSB | 900 | 2.23 ± 0.25 | 782.11 | 651.11–900.35 | 4.19 | 3 | 0.24 | ||
| Cry1Ab | SSB | 1,120 | 1.95 ± 0.13 | 1.84 | 1.47–2.21 | 8.86 | 5 | 0.11 | 39.13 |
| PSB | 1,056 | 2.08 ± 0.17 | 71.99 | 62.56–83.91 | 5.27 | 4 | 0.26 | ||
| Cry1Ah | SSB | 960 | 2.33 ± 0.14 | 20.70 | 18.12–23.53 | 5.32 | 3 | 0.15 | 3.53 |
| PSB | 1,056 | 1.74 ± 0.14 | 73.12 | 62.70–85.04 | 4.22 | 4 | 0.38 | ||
| Cry2Aa | SSB | 960 | 1.93 ± 0.16 | 152.88 | 131.79–179.45 | 3.51 | 3 | 0.32 | 1.85 |
| PSB | 1,056 | 2.06 ± 0.17 | 282.48 | 244.83–332.17 | 3.67 | 4 | 0.45 | ||
| SSB | 1120 | 1.49 ± 0.12 | 4.17 | 2.95–5.42 | 1.27 | 5 | 0.87 | 1.58 | |
| PSB | 900 | 1.85 ± 0.20 | 6.57 | 5.54–8.14 | 4.68 | 3 | 0.20 |
SE, standard error of the mean; FL, fiducial limit.
A P value of ≥0.05 indicates a significant fit between the observed and expected regression lines in a probit analysis.
Relative ratio = PSB LC50/SSB LC50.
Specific binding of 125I-labeled Cry toxins to C. suppressalis and S. inferens BBMVs.
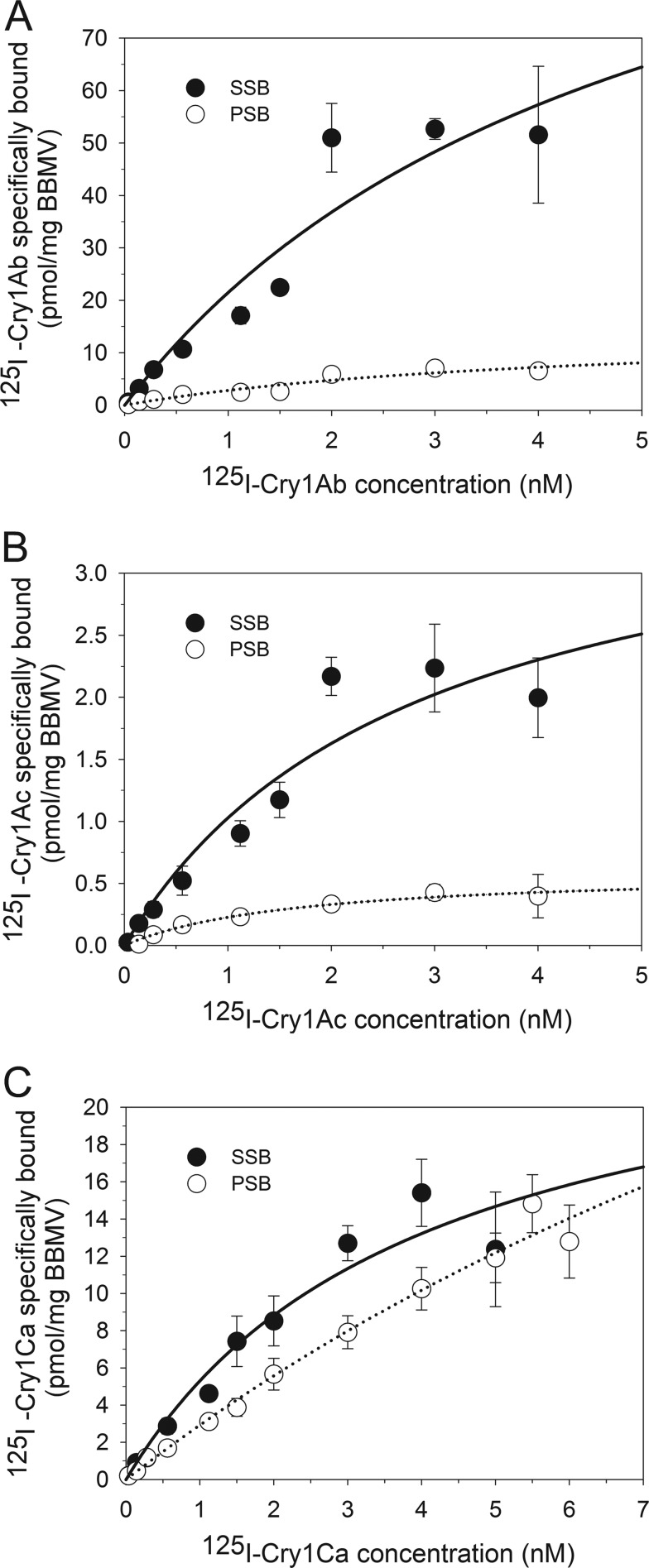
In saturation binding experiments, binding of 125I-labeled Cry1Ab, Cry1Ac, or Cry1Ca to BBMV proteins from both C. suppressalis and S. inferens larvae was specific and saturable (Fig. 1). Maximum specific Cry1Ab and Cry1Ac binding to BBMV proteins from C. suppressalis and S. inferens was observed at a 3.0 nM ligand concentration (Fig. 1A and B), while Cry1Ca binding to BBMV proteins from both species seems to saturate at 4.0 nM and 5.5 nM ligand concentrations, respectively (Fig. 1C). Analysis of binding data using nonlinear regression demonstrated that the binding data were best described by a model considering a single population of binding sites (correlation coefficient [R], >0.97) for all BBMV proteins and each of the three toxins tested. This model would support the existence of a single type of binding protein or a population of diverse binding proteins with similar binding affinities for the toxin in the BBMV. Calculated apparent dissociation constant (Kd) values (Table 3) indicated high-affinity binding of all three toxins, with no significant differences detected (t test, P < 0.05) in Kd estimates between C. suppressalis and S. inferens BBMV proteins for the same toxin or between toxins. In contrast, drastic differences were observed when comparing estimates of binding site concentration (Bmax) for Cry1Ab and Cry1Ac toxins in BBMV proteins from S. inferens and C. suppressalis larvae (Table 3). While the biggest difference (about 8-fold) was detected for Cry1Ab, statistical significance (t test, P < 0.05) was detected only for the difference in Bmax values for Cry1Ac between S. inferens and C. suppressalis BBMV proteins (>6-fold). In contrast, a small (∼2-fold) not significant difference (t test, P < 0.05) was detected for Cry1C Bmax values in BBMV proteins from C. suppressalis and S. inferens (Table 3).
FIG 1.
Saturation binding experiments with BBMV proteins from C. suppressalis (striped stem borer [SSB]) and S. inferens (pink stem borer [PSB]) and increasing amounts of 125I-Cry proteins. Specific binding of 125I-Cry1Ab (A), 125I-Cry1Ac (B), or 125I-Cry1Ca (C) was calculated by subtracting nonspecific from total binding in the absence of unlabeled competitor. Data shown are the means and standard errors from at least two independent experiments performed in duplicate for each concentration of radiolabeled Cry toxin. The curves shown are derived from fitting the binding data to a model that considers a single population of binding sites as the best-fitting model.
TABLE 3.
Apparent dissociation constant (Kd) and concentration of binding sites (Bmax) calculated from Cry1 toxin saturation binding assay using C. suppressalis and S. inferens BBMV proteinsa
| Toxin |
C. suppressalis |
S. inferens |
||
|---|---|---|---|---|
| Kd ± SE (nM) | Bmax (pmol/mg BBMV) | Kd ± SE (nM) | Bmax (pmol/mg BBMV) | |
| Cry1Ab | 5.02 ± 3.99 | 129.28 ± 67.25 | 4.40 ± 3.00 | 15.19 ± 6.54 |
| Cry1Ac | 2.82 ± 1.65 | 3.93 ± 1.25 | 1.67 ± 0.44 | 0.61 ± 0.07 |
| Cry1Ca | 3.95 ± 1.72 | 26.29 ± 6.45 | 18.90 ± 9.95 | 58.28 ± 24.62 |
SE, standard error of the mean.
Ligand blotting.
Considering the detected differences in binding site concentrations, we performed ligand blot assays with radiolabeled Cry1Ac and Cry1C toxins to detect BBMV proteins binding these toxins. A higher intensity of Cry1Ac binding signal was detected for C. suppressalis BBMV proteins than for S. inferens BBMV proteins. In C. suppressalis BBMV, Cry1Ac mostly recognized proteins of approximately 15, 75, 115, and 200 kDa in size, while in S. inferens BBMV, this toxin bound to proteins of approximately 15, 42, 100, and 140 kDa (Fig. 2A). In contrast, Cry1Ca mostly recognized at least four proteins of less than 27 kDa in size in C. suppressalis BBMV and proteins of approximately 30 and 100 kDa in S. inferens BBMV (Fig. 2B). Weaker Cry1Ca binding to BBMV proteins of 33 and 40 kDa was also detected in both C. suppressalis and S. inferens.
FIG 2.
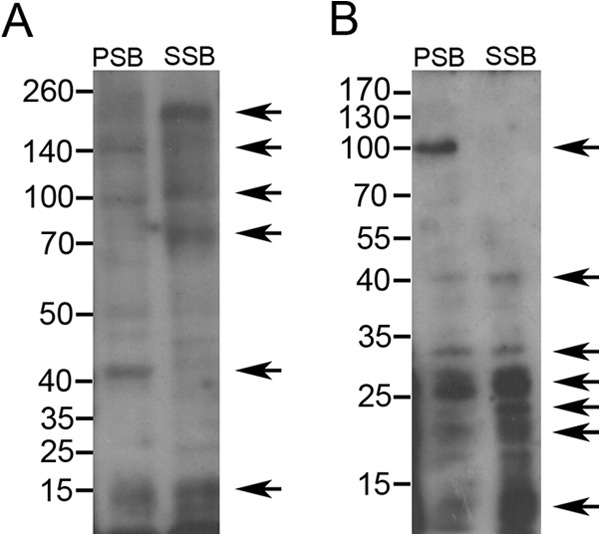
Ligand blot analysis of 125I-Cry1Ac (A) and 125I-Cry1Ca (B) toxin binding to BBMV proteins from C. suppressalis (SSB) and S. inferens (PSB). Proteins (20 μg) were separated by SDS–8% PAGE, transferred to a PVDF filter, and then probed with 125I-Cry1Ac or 125I-Cry1Ca toxins. Bound toxin was detected by autoradiography at −80°C. Arrows indicate main BBMV proteins recognized by each toxin in each BBMV sample.
DISCUSSION
Transgenic HH1 rice producing a Cry1Ab-Cry1Ac hybrid toxin is expected to dramatically impact rice farming in China by providing efficient control of the main lepidopteran rice pests (31). As with alternative Bt crops, productivity and maintained efficacy of Bt rice is threatened by the development of resistance in targeted pests and by an increase in nontarget pest populations resulting from reduced pesticide application (32, 33). In the case of pest complexes with diverse susceptibility to Bt crops, increased tolerance has been observed for species with low susceptibility, as exemplified in the budworm/bollworm complex in Bt cotton (34). In this example, the susceptible pest species (Heliothis virescens) is efficiently controlled by Bt cotton, while the pest with low susceptibility (Helicoverpa zea) is able to evolve increased tolerance and resistance (34). In the case of the Bt rice line HH1 and the C. suppressalis-S. inferens stem borer complex, data from this study and previous reports (13) support that HH1 rice is effective against C. suppressalis infestations but lacks effective control of S. inferens. Accordingly, these data predict that S. inferens may change its ecological status in transgenic rice fields to become the dominant pest species. Understanding the mechanism responsible for differential susceptibility to HH1 rice in the C. suppressalis-S. inferens complex is crucial to develop improved rice lines and insect resistance management strategies to secure sustainability of Bt rice.
The lower susceptibility to HH1 rice of S. inferens than of C. suppressalis is explained by the lower susceptibility to purified Cry1A toxins in S. inferens, which was previously observed (35). Lower susceptibility to Cry1 toxins in older larval instars of Lepidoptera is sometimes associated with reduced toxin binding to midgut receptors as a crucial step in the Cry intoxication process (36). Furthermore, high levels of resistance to Cry1A toxins and Bt pesticides are associated with reduced toxin binding due to alterations in midgut binding sites (15). Consequently, we hypothesized that the lower susceptibility to HH1 rice and Cry1A toxins in S. inferens than in C. suppressalis was related to differences in binding of the toxins to midgut sites. While we were unable to use the Cry1Ab-Cry1Ac hybrid toxin produced by HH1 rice in our assays, a direct correlation between susceptibility to purified Cry1A toxins and HH1 rice validated the use of purified Cry1A toxins to compare binding in S. inferens and C. suppressalis. Based on the toxin regions included in the Cry1Ab-Cry1Ac hybrid produced by HH1 rice (37), the binding specificity of this hybrid toxin is dictated by domain II of Cry1Ab and domain III of Cry1Ac. In agreement with previous reports (18, 19), we detected high-affinity binding of both Cry1Ab and Cry1Ac toxins to BBMV proteins from C. suppressalis. While similar high-affinity Cry1Ab and Cry1Ac binding was detected for S. inferens BBMV proteins, we detected a drastic reduction in the concentration of binding sites for the two toxins in S. inferens BBMV compared to that in C. suppressalis BBMV. We propose that these differences in Cry1A binding site concentration and the resulting reduced level of bound Cry1A toxins explain the lower relative susceptibility to these toxins and HH1 rice in S. inferens than in C. suppressalis larvae. According to current models of Cry intoxication (38), smaller amounts of Cry1A toxin bound to the midgut may result in reduced toxin oligomerization and subsequent pore formation, explaining the reduced susceptibility. In support of the relevance of binding site concentration for susceptibility, positive associations have been reported for Cry1Ac and Cry1Ba during development of Manduca sexta larvae (36), for Cry1A and Cry2A toxins in Marasmia patnalis (39), and for Cry2Ae in H. zea and H. virescens (29).
In contrast to the variability detected in Cry1A binding, we did not detect significant differences in susceptibility or binding parameters for Cry1Ca in S. inferens or C. suppressalis. This observation supports Cry1Ca as a candidate for pyramiding efforts with Cry1A toxins in next-generation Bt rice to expand the range of controlled rice stem borers (35). While discrepancies in toxin binding affinity were detected when comparing our data with previous reports of Cry1Ca and Cry1Ac binding to C. suppressalis, one notable coincidence is the relatively higher levels of Cry1Ca binding sites than of Cry1Ac binding sites (18, 19). The higher concentrations of Cry1Ca binding sites than of Cry1Ac binding sites are associated with the higher toxicity of Cry1Ca than of Cry1Ac in C. suppressalis, suggesting that binding site abundance may be important for susceptibility in this insect. In agreement with this observation, the highest binding site concentration in C. suppressalis was detected for Cry1Ab, the most active toxin against this insect. However, the large difference in Bmax values between Cry1Ab and Cry1Ca did not result in largely different susceptibilities in C. suppressalis larvae, suggesting that additional variables may affect the susceptibility to each toxin. In contrast to our data, previous reports found similar binding site concentrations for Cry1Ab and Cry1Ac toxins in BBMV from C. suppressalis (19). These discrepancies in toxin binding parameters may result from the quality of the BBMV preparations, toxin labeling method (iodobeads versus chloramine T in our study), and/or purity of the labeled toxins.
Given the significant differences in Cry1Ac binding site concentration between S. inferens and C. suppressalis BBMV, we used ligand blotting to provide a preliminary comparison of BBMV proteins binding Cry1Ac to identify binding sites relevant to high susceptibility. Despite the denaturing conditions used in this technique, differences in the pattern of BBMV proteins recognized by Cry1Ac could help identify effective Cry1Ac binding sites in C. suppressalis that are absent in S. inferens. The pattern of Cry1Ac binding proteins on ligand blots with BBMV proteins from C. suppressalis in this study are similar to that in previous reports (40) and support the existence of multiple Cry1Ac binding sites in these BBMV. Proteomic studies identified APN isoforms (approximately 130 and 150 kDa) and an EH domain-containing protein 1 (approximately 80 kDa) as Cry1Ac binding proteins in C. suppressalis BBMV (21). In comparison, a 119-kDa APN and a 197-kDa cadherin were reported to bind Cry1Ab on ligand blots of C. suppressalis BBMV (20). Considering that Cry1Ab and Cry1Ac toxins share all binding sites in BBMV of C. suppressalis (19), we speculate that the 115- and 200-kDa proteins detected in our Cry1Ac ligand blots may represent the 119-kDa APN and 197-kDa cadherin, respectively, reported to bind Cry1Ab. Interestingly, both of these protein bands were absent from Cry1Ac ligand blots with S. inferens BBMV, a finding that may identify binding to these proteins as critical for high susceptibility to Cry1A toxins. In ligand blots with Cry1Ca toxin, we detected BBMV proteins that were smaller in size than Cry1A binding proteins. A protein of about 100 kDa was unique to S. inferens BBMV, but the lack of differences in susceptibility or Cry1Ca binding between C. suppressalis and S. inferens suggests that common proteins may be more relevant to the susceptibility to this toxin. Further work is necessary to identify functional Cry1A and Cry1C toxin receptors and their comparative expression levels in C. suppressalis and S. inferens.
Differences in susceptibility to HH1 rice will favor increased tolerance and potential outbreaks of S. inferens compared to C. suppressalis. Pyramiding of toxin genes with diverse modes of action (i.e., recognizing diverse binding site populations) in next-generation transgenic rice can broaden the insecticidal spectrum and delay the development of resistance. According to our data, the Cry1Ab-Cry1Ac hybrid toxin produced by HH1 rice has a limited activity range. This and previous studies (35) support pyramiding of cry1A and cry1Ca genes in Bt rice as an optimal strategy to increase the control of S. inferens and reduce the risk of resistance evolution.
ACKNOWLEDGMENTS
This research was funded by the National Genetically Modified Organisms Key Breeding Projects of China (2014ZX08011-001A, 2014ZX08012-004 and 2012ZX08011003) and the National Natural Science Foundation of China (30800723). Partial support was provided by a Dupont Young Professors award to J. L. Jurat-Fuentes.
Footnotes
Published ahead of print 13 June 2014
REFERENCES
- 1.Cheng JA. 1996. Rice pests. China Agricultural Press, Beijing, China [Google Scholar]
- 2.He Y, Zhang J, Gao C, Su J, Chen J, Shen J. 2013. Regression analysis of dynamics of insecticide resistance in field populations of Chilo suppressalis (Lepidoptera: Crambidae) during 2002-2011 in China. J. Econ. Entomol. 106:1832–1837. 10.1603/EC12469 [DOI] [PubMed] [Google Scholar]
- 3.Bajaj S, Mohanty A. 2005. Recent advances in rice biotechnology—towards genetically superior transgenic rice. Plant Biotechnol. J. 3:275–307. 10.1111/j.1467-7652.2005.00130.x [DOI] [PubMed] [Google Scholar]
- 4.Deka S, Barthakur S. 2010. Overview on current status of biotechnological interventions on yellow stem borer Scirpophaga incertulas (Lepidoptera: Crambidae) resistance in rice. Biotechnol. Adv. 28:70–81. 10.1016/j.biotechadv.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 5.Shu Q, Ye G, Cui H, Cheng X, Xiang Y, Wu D, Gao M, Xia Y, Hu C, Sardana R, Altosaar I. 2000. Transgenic rice plants with a synthetic cry1Ab gene from Bacillus thuringiensis were highly resistant to eight lepidopteran rice pest species. Mol. Breeding. 6:433–439. 10.1023/A:1009658024114 [DOI] [Google Scholar]
- 6.Tu J, Zhang G, Datta K, Xu C, He Y, Zhang Q, Khush GS, Datta SK. 2000. Field performance of transgenic elite commercial hybrid rice expressing Bacillus thuringiensis delta-endotoxin. Nat. Biotechnol. 18:1101–1104. 10.1038/80310 [DOI] [PubMed] [Google Scholar]
- 7.Ye GY, Shu QY, Yao HW, Cui HR, Cheng XY, Hu C, Xia YW, Gao MW, Altosaar I. 2001. Field evaluation of resistance of transgenic rice containing a synthetic cry1Ab gene from Bacillus thuringiensis Berliner to two stem borers. J. Econ. Entomol. 94:271–276. 10.1603/0022-0493-94.1.271 [DOI] [PubMed] [Google Scholar]
- 8.Ye GY, Tu J, Hu C, Datta K, Datta SK. 2001. Transgenic IR72 with fused Bt gene cry1Ab/cry1Ac from Bacillus thuringiensis is resistant against four lepidopteran species under field conditions. Plant Biotechnol. 18:125–133. 10.5511/plantbiotechnology.18.125 [DOI] [Google Scholar]
- 9.Han L, Wu K, Peng Y, Wang F, Guo Y. 2007. Efficacy of transgenic rice expressing Cry1Ac and CpTI against the rice leaffolder, Cnaphalocrocis medinalis (Guenée). J. Invertebr. Pathol. 96:71–79. 10.1016/j.jip.2007.02.015 [DOI] [PubMed] [Google Scholar]
- 10.Han LZ, Wu KM, Peng YF, Wang F, Guo YY. 2006. Evaluation of transgenic rice expressing Cry1Ac and CpTI against Chilo suppressalis and intrapopulation variation in susceptibility to Cry1Ac. Environ. Entomol. 35:1453–1459. 10.1603/0046-225X(2006)35[1453:EOTREC]2.0.CO;2 [DOI] [Google Scholar]
- 11.Han L, Liu P, Wu K, Peng Y, Wang F. 2008. Population dynamics of Sesamia inferens on transgenic rice expressing Cry1Ac and CpTI in southern China. Environ. Entomol. 37:1361–1370. 10.1603/0046-225X(2008)37[1361:PDOSIO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 12.Gao YL, Fu Q, Wang F, Lai FX, Lu J, Peng YF, Zhang ZT. 2006. Effects of transgenic rice harboring Cry1Ac and CpTI genes on survival of Chilo suppressalis and Sesamia inferens and field composition of rice stemborers. Chin. J. Rice Sci. 20:543–548 [Google Scholar]
- 13.Li Z, Sui H, Xu Y, Han L, Chen F. 2012. Effects of insect-resistant transgenic Bt rice with a fused Cry1Ab+Cry1Ac gene on population dynamics of the stem borers, Chilo suppressalis and Sesamia inferens, occurring in paddyfield. Acta Ecol. Sin. 32:1783–1789. 10.5846/stxb201102260222 [DOI] [Google Scholar]
- 14.Vachon V, Laprade R, Schwartz JL. 2012. Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: a critical review. J. Invertebr. Pathol. 111:1–12. 10.1016/j.jip.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 15.Ferré J, Van Rie J. 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47:501–533. 10.1146/annurev.ento.47.091201.145234 [DOI] [PubMed] [Google Scholar]
- 16.Tabashnik BE, Mota-Sanchez D, Whalon ME, Hollingworth RM, Carrière Y. 2014. Defining terms for proactive management of resistance to Bt crops and pesticides. J. Econ. Entomol. 107:496–507. 10.1603/EC13458 [DOI] [PubMed] [Google Scholar]
- 17.Fiuza L, Nielsen-Leroux C, Goze E, Frutos R, Charles J. 1996. Binding of Bacillus thuringiensis Cry1 toxins to the midgut brush border membrane vesicles of Chilo suppressalis (Lepidoptera: Pyralidae): evidence of shared binding sites. Appl. Environ. Microbiol. 62:1544–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MK, Aguda RM, Cohen MB, Gould FL, Dean DH. 1997. Determination of binding of Bacillus thuringiensis δ-endotoxin receptors to rice stem borer midguts. Appl. Environ. Microbiol. 63:1453–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alcantara EP, Aguda RM, Curtiss A, Dean DH, Cohen MB. 2004. Bacillus thuringiensis delta-endotoxin binding to brush border membrane vesicles of rice stem borers. Arch. Insect Biochem. Physiol. 55:169–177. 10.1002/arch.10128 [DOI] [PubMed] [Google Scholar]
- 20.Yu HK, Chen H, Zhang YJ, Wu KM, Liang GM, Liu ZW, Guo YY. 2010. Gene cloning and expression of aminopeptidase N and cadherin from midgut of the rice stem borer, Chilo suppressalis. Insect Sci. 17:393–399. 10.1111/j.1744-7917.2010.01333.x [DOI] [Google Scholar]
- 21.Ma W, Zhang Z, Peng C, Wang X, Li F, Lin Y. 2012. Exploring the midgut transcriptome and brush border membrane vesicle proteome of the rice stem borer, Chilo suppressalis (Walker). PLoS One 7:e38151. 10.1371/journal.pone.0038151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han LZ, Li SB, Liu PL, Peng YF, Hou ML. 2012. New artificial diet for continuous rearing of Chilo suppressalis (Lepidoptera: Crambidae). Ann. Entomol. Soc. Am. 105:253–258. 10.1603/AN10170 [DOI] [Google Scholar]
- 23.Xue J, Liang G, Crickmore N, Li H, He K, Song F, Feng X, Huang D, Zhang J. 2008. Cloning and characterization of a novel Cry1A toxin from Bacillus thuringiensis with high toxicity to the Asian corn borer and other lepidopteran insects. FEMS Microbiol. Lett. 280:95–101. 10.1111/j.1574-6968.2007.01053.x [DOI] [PubMed] [Google Scholar]
- 24.Perera OP, Willis JD, Adang MJ, Jurat-Fuentes JL. 2009. Cloning and characterization of the Cry1Ac-binding alkaline phosphatase (HvALP) from Heliothis virescens. Insect Biochem. Mol. Biol. 39:294–302. 10.1016/j.ibmb.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 25.Abbott WS. 1925. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 18:265–267 [Google Scholar]
- 26.LeOra Software. 1987. Polo-Plus, POLO for Windows. LeOra Software, Petaluma, CA [Google Scholar]
- 27.Wolfersberger M, Luthy P, Maurer A, Parenti P, Sacchi VF, Giordana B, Hanozet GM. 1987. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. A Comp. Physiol. 86:301–308. 10.1016/0300-9629(87)90334-3 [DOI] [Google Scholar]
- 28.Jurat-Fuentes JL, Karumbaiah L, Jakka SRK, Ning C, Liu C, Wu K, Jackson J, Gould F, Blanco CA, Portilla M, Perera OP, Adang M. 2011. Reduced levels of membrane-bound alkaline phosphatase are common to lepidopteran strains resistant to Cry toxins from Bacillus thuringiensis. PLoS One 6:e17606. 10.1371/journal.pone.0017606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gouffon C, Van Vliet A, Van Rie J, Jansens S, Jurat-Fuentes JL. 2011. Binding sites for Bacillus thuringiensis Cry2Ae toxin on heliothine brush border membrane vesicles are not shared with Cry1A, Cry1F, or Vip3A toxin. Appl. Environ. Microbiol. 77:3182–3188. 10.1128/AEM.02791-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurat-Fuentes JL, Adang MJ. 2001. Importance of Cry1 δ-endotoxin domain II loops for binding specificity in Heliothis virescens (L.). Appl. Environ. Microbiol. 67:323–329. 10.1128/AEM.67.1.323-329.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Zhang G, Du J, Liu B, Wang M. 2010. Influence of transgenic hybrid rice expressing a fused gene derived from cry1Ab and cry1Ac on primary insect pests and rice yield. Crop. Prot. 29:128–133. 10.1016/j.cropro.2009.10.004 [DOI] [Google Scholar]
- 32.Lu Y, Wu K, Jiang Y, Xia B, Li P, Feng H, Wyckhuys KA, Guo Y. 2010. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 328:1151–1154. 10.1126/science.1187881 [DOI] [PubMed] [Google Scholar]
- 33.Cannon RJC. 2000. Bt transgenic crops: risks and benefits. Integr. Pest Manag. Rev. 5:151–173. 10.1023/A:1011347122894 [DOI] [Google Scholar]
- 34.Tabashnik BE, Brevault T, Carriere Y. 2013. Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotechnol. 31:510–521. 10.1038/nbt.2597 [DOI] [PubMed] [Google Scholar]
- 35.Gao Y, Hu Y, Fu Q, Zhang J, Oppert B, Lai F, Peng Y, Zhang Z. 2010. Screen of Bacillus thuringiensis toxins for transgenic rice to control Sesamia inferens and Chilo suppressalis. J. Invertebr. Pathol. 105:11–15. 10.1016/j.jip.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 36.Gilliland A, Chambers CE, Bone EJ, Ellar DJ. 2002. Role of Bacillus thuringiensis Cry1 delta endotoxin binding in determining potency during lepidopteran larval development. Appl. Environ. Microbiol. 68:1509–1115. 10.1128/AEM.68.4.1509-1515.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu J, Datta K, Alam MF, Fan Y, Khush GS, Datta SK. 1998. Expression and function of a hybrid Bt toxin gene in transgenic rice conferring resistance to insect pest. Plant Biotechnol. 15:195–203. 10.5511/plantbiotechnology.15.195 [DOI] [Google Scholar]
- 38.Pardo-López L, Soberón M, Bravo A. 2013. Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 37:3–22. 10.1111/j.1574-6976.2012.00341.x [DOI] [PubMed] [Google Scholar]
- 39.Karim S, Dean DH. 2000. Toxicity and receptor binding properties of Bacillus thuringiensis delta-endotoxins to the midgut brush border membrane vesicles of the rice leaf folders, Cnaphalocrocis medinalis and Marasmia patnalis. Curr. Microbiol. 41:276–283. 10.1007/s002840010134 [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, Yang Y, Wu Y. 2009. Ligand blot analysis of Bt Cry1A toxin binding with the midgut brush border membrane vesicle receptors of Chilo suppressalis (Lepidoptera: Pyralidae). Acta Entomol. Sin. 52:153–158 [Google Scholar]
- 41.Jurat-Fuentes JL, Gould FL, Adang MJ. 2002. Altered glycosylation of 63- and 68-kilodalton microvillar proteins in Heliothis virescens correlates with reduced Cry1 toxin binding, decreased pore formation, and increased resistance to Bacillus thuringiensis Cry1 toxins. Appl. Environ. Microbiol. 68:5711–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]