Abstract
Antimicrobial peptides (AMPs) are key elements of innate immunity, which can directly kill multiple bacterial, viral, and fungal pathogens. The medically important fungus Candida albicans colonizes different host niches as part of the normal human microbiota. Proliferation of C. albicans is regulated through a complex balance of host immune defense mechanisms and fungal responses. Expression of AMPs against pathogenic fungi is differentially regulated and initiated by interactions of a variety of fungal pathogen-associated molecular patterns (PAMPs) with pattern recognition receptors (PRRs) on human cells. Inflammatory signaling and other environmental stimuli are also essential to control fungal proliferation and to prevent parasitism. To persist in the host, C. albicans has developed a three-phase AMP evasion strategy, including secretion of peptide effectors, AMP efflux pumps, and regulation of signaling pathways. These mechanisms prevent C. albicans from the antifungal activity of the major AMP classes, including cathelicidins, histatins, and defensins leading to a basal resistance. This minireview summarizes human AMP attack and C. albicans resistance mechanisms and current developments in the use of AMPs as antifungal agents.
INTRODUCTION
Understanding host-fungus interactions is crucial for efficient treatment of fungal infections. The human host, upon contact with Candida albicans, utilizes an efficient complex immune response that leads to production of soluble effectors, including antimicrobial peptides (AMPs) and cytokines, or to activation of complement, which can directly damage the pathogen (1). Disturbance of human defense systems and activation of fungal virulence traits favor the transition from the commensal stage to the pathogenic stage of fungal infection (2). C. albicans defends itself against a multiplicity of innate immune components, including AMPs (3), to remain a successful commensal organism and eventually, to become a human pathogen that causes serious disease. This minireview summarizes human AMP production triggered by fungal recognition and C. albicans AMP resistance mechanisms and discusses novel applications in the use of AMPs as antifungal agents.
ACTIVITY OF HUMAN AMPs AGAINST C. ALBICANS
AMPs are small soluble defense molecules that kill or block growth of the pathogen by membrane permeabilization of microbial cells and by inactivation of cytoplasmic targets therein (4). AMPs are generated by proteolytic processing of one or more precursor peptides (3). Active peptides consist of 10 to 50 amino acids and are mostly positively charged because of several lysine and arginine residues, but they also contain a substantial proportion of hydrophobic residues (≥30%) (5). Peptide antimicrobial agents that can form an amphipathic, α-helical structure are classified on the basis of their structural characteristics; others, such as the defensins, are classified by the number of disulfide bonds they possess (6). At high AMP concentrations, pores are formed leading to cytoplasmic membrane dysfunction and depolarization by release of ATP and ions; these events effect osmotic dysregulation (Table 1) and finally lead to cell death (7). Beside antimicrobial activity, AMPs also act as immune modulators by promoting migration of neutrophils and monocytes to the site of infection, by upregulating tumor necrosis factor alpha (TNF-α) and by chemoattraction of immature dendritic and T cells to modify the adaptive immune response (reviewed in reference 8). The major antimicrobial peptides in humans include the cathelicidin LL-37, the histatins (Hst), and the defensins (4).
TABLE 1.
Mechanisms of human peptide antimicrobials against C. albicans
| AMP | Mechanism(s) | Reference(s) |
|---|---|---|
| LL-37 | Association with cell wall and cell membrane by binding to carbohydrates; massive disruption of cell membrane; ATP efflux | 7, 9, 11 |
| Histatin 5 | Intracellular accumulation; release of cellular ATP in the absence of cytolysis; induced the formation of reactive oxygen species (ROS) | 18, 89 |
| hNP-1 | Cellular ATP efflux | 21 |
| hBD1 | Membrane association; increasing the membrane permeability | 22 |
| Lactoferrin | Externalization of phosphatidylserine; DNA degradation; increases ROS production; ATP release | 61, 90 |
| hGAPDH | Initiating apoptosis; inhibiting C. albicans Sap1/2 (CaSap1/2) protease activity; inducing secretion of IL-8 and GM-CSF | 28 |
In C. albicans, LL-37 initially associates with the cell wall and/or the cytoplasmic membrane (9). Treatment of fungal cells with large amounts of the peptide resulted in a breakdown of the membrane into discrete vesicles and led to rapid efflux of small molecules, such as ATP, as well as larger molecules with molecular masses up to 40 kDa. The C. albicans cell wall β-1,3-exoglucanase Xog1 has been identified as a LL-37 receptor (10). Xog1–LL-37 interactions led to cell wall remodeling, and Xog1 enzyme activity was elevated, lowering C. albicans adhesion (10, 11). Subsequent to cleavage by a serine protease, processed forms of LL-37 were found at the human skin surface (KS-30 and RK-31) (12). Interestingly, the N-terminally truncated peptide RK-31 accumulated at the cell boundary but migrated into the cytoplasm over time, while the C-terminally truncated peptide was exclusively found in the cytoplasm, inducing leakage of nucleotides and proteins (13).
The fungicidal mechanism of histatin 5 (Hst 5) was reported as a multistep process (14). First, the peptide binds to the ATPase domain of cell envelope proteins Ssa1 and Ssa2 of C. albicans (15). Then, Hst 5 utilizes the C. albicans polyamine influx transporters Dur3 and Dur31 and accumulates intracellularly (16, 17), where it induces formation of reactive oxygen species (ROS) and efflux of ions and ATP, resulting in cell death (18, 19). Hst 5 downregulates specific mitochondrial proteins involved in C. albicans energy metabolism and upregulates biosynthetic proteins (14), which ultimately leads to a drastic decrease in mitochondrial ATP synthesis. The role of the candidacidal mechanism of Hst 5 is also discussed in detail in the accompanying minireview by Puri and Edgerton (20).
In neutrophils, the physiological concentrations of α-defensins 1 to 3 (human neutrophil peptide 1 [hNP-1] to -3) are very high (6 mg/ml). hNP-1 acts on energy metabolism by causing depletion of intracellular ATP and increasing extracellular ATP concentrations to kill C. albicans (21). The β-defensins hBD1 (human β-defensin 1) to hBD3 cause membrane permeabilization, leading to cell death (22). Interestingly, unlike hBD1 and hBD2, hBD3 kills C. albicans by energy-independent mechanisms. In addition to LL-37, hBD3 elevates Xog1 activity, resulting in reduced C. albicans adherence (10).
Cationic peptides derived from the N-terminal portion of human mucin MUC7 associate with the fungal plasma membrane but are also internalized to exert fungicidal activity (23). Like hBD3, MUC7-derived peptides induce killing of Candida without affecting cellular metabolic activity.
Besides “professional” AMPs dedicated solely to antimicrobial defense, humans produce peptides with AMP activity, which are derived from proteins with different functions. “Moonlighting” proteins include RNase 7, expressed by human keratinocytes, which exhibits antimicrobial activity against C. albicans independent of its RNase activity (24). AMP activity of RNase 7 is inhibited by a RNase inhibitor protein expressed in epidermal keratinocytes and is in turn activated, when the inhibitor is cleaved by serine proteases (25). Lactoferrin is a 77-kDa iron-binding glycoprotein present in different body secretions that is active against C. albicans. This property is due to a highly basic N-terminal region containing a 25-amino-acid domain termed lactoferricin (LF) that elicits antifungal properties (26). Similar to LL-37, lactoferricin mostly affects membrane morphology, resulting in disintegration of the membrane bilayer, as well as efflux of ATP and proteins in C. albicans cells (27).
Recently, a peptide derived from human glyceraldehyde-3-phosphate dehydrogenase (hGAPDH) was also shown to exhibit antimicrobial activity (28). The hGAPDH peptide killed the fungus by initiating apoptosis and also inhibited Sap1/2 protease activity; in addition, the peptide induced secretion of interleukin 8 (IL-8) and granulocyte-macrophage colony-stimulating factor (GM-CSF) that attracted immune cells to the site of infection (28).
It should be noted that on the one hand, humans produce many more cationic peptides, with yet undefined AMP activity (reviewed in reference 29); on the other hand, a myriad of microbes cohabit the human host, some of which produce AMPs that inhibit C. albicans (reviewed in reference 30).
REGULATED AMP ACTIVITY AGAINST C. ALBICANS
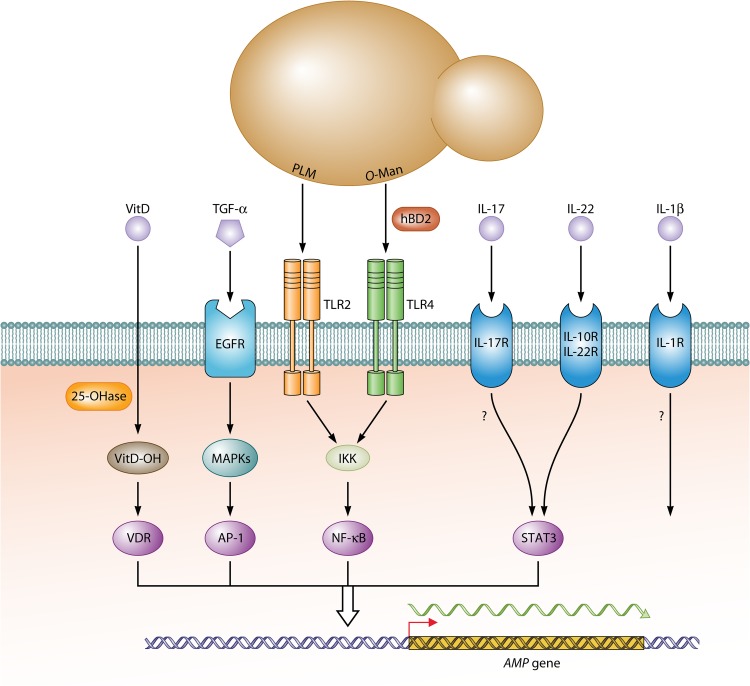
How does C. albicans influence the synthesis of AMPs? Many epithelia produce a basal level of AMPs constitutively, which provides a first line of protection against C. albicans and other pathogens (reviewed in references 3 and 31). In a commensal scenario, continuous but relatively infrequent PAMP-PRR interactions trigger low levels of NF-κB activation that drive basal transcription of AMP-encoding genes. Binding of C. albicans phospholipomannan by Toll-like receptor 2 (TLR2) may be especially important for this process in keratinocytes (32). Upon increased microbial colonization and in case of epithelial damage or inflammation, AMP transcripts increase dramatically (Fig. 1). This boosted “damage” response is caused predominantly by a strong upregulation of extracellular signal-regulated kinase (ERK)/Jun N-terminal protein kinase (JNK) mitogen-activated protein (MAP) kinase (MAPK) activities and activation of their dedicated transcription factors (e.g., activator protein 1 [AP-1]) (33). In this condition, epithelial cells produce proinflammatory lymphokines, including IL-1β, IL-6, and IL-8 that activate cohabiting immune cells, of which TH-17 cells producing IL-17, IL-22, and TNF-α are especially relevant for AMP production (34–36). Hyphal formation by C. albicans is a decisive factor for the initial release of proinflammatory lymphokines through activation of the inflammosome complex (reviewed in reference 37), but prolonged exposure to hyphae downregulates again the production of β-defensins (38). Inflammatory signaling of epithelial cells is activated further by cohabiting neutrophils, which induce upregulation of TLR4 (39), which in normal human skin may already be upregulated by contact with Gram-positive bacteria (40). TLR4 not only binds O-mannans in the C. albicans cell wall but also the defensin hBD2, which generates an autostimulatory feedback loop in epithelial cells; in addition, an autocrine IL-1β loop augments transcriptional responses (41). Zones of fungal infection become increasingly inflamed due to the chemoattractive activities of AMPs that recruit more lymphocytes (reviewed in reference 42). Transcriptional activation in epithelial cells is mediated by transcription factors NF-κB, AP-1, and charged vitamin D receptor (VDR) that target specific promoter sequences of genes encoding hBD2 and human CAP18 (18-kDa cationic antimicrobial protein) (hCAP18) (43). TLR signaling increases CYP27B1 (cytochrome p450 27B1) hydroxylase enzyme activity to promote the conversion of 25(OH)D to 1,25(OH)2D, which in turn binds to VDR to trigger synthesis of LL-37. The importance of sufficient vitamin D and its maturation for AMP production by human epithelial cells (but surprisingly not by mouse epithelial cells) provide an example for the impact of nutrition on antimicrobial defenses.
FIG 1.
Epithelial signaling pathways directing AMP gene expression. Binding of C. albicans PAMPs phospholipomannan (PLM) and O-mannan (O-Man) to epithelial TLR2/4 proteins leads initially to activation of NF-κB via IkB kinase (IKK). Epithelial proinflammatory lymphokines including IL-1β, IL-6, and IL-8 secreted by epithelial cells trigger cohabiting immune cells to produce IL-1β, IL-17, IL-22, and TNF-α, which boost immune responses of epithelial cells by binding to their dedicated surface receptors IL-1R (IL-1 receptor), IL-17R, IL10R/IL-22R, and EGFR, respectively. MAP kinases (MAPKs) ERK/JNK activate AP-1, which binds with NF-κB and STAT3 transcription factors to promoters of AMP genes to enhance their expression. Production and binding of hBD2, as well as of IL-1β, generates positive-feedback loops in epithelial cells that further increase immune responses. Nutritional input for AMP gene expression is provided by vitamin D (VitD), which is modified by 1α-hydroxylase (25-OHase) to its hydroxylated form (VitD-OH) that binds and activates the promoter of the LL-37-encoding gene.
It should be noted that transcriptional activation does not proceed equally for all AMPs. For example, increased transcription of the β-defensin 3 (human β-defensin 3 [hBD3])-encoding gene requires TNF-α, which is released from its membrane precursor by the ADAM17 “sheddase”; TNF-α binds to the epidermal growth factor receptor (EGFR) and thereby, through MAP kinases, activates AP-1 (but not NF-κB) (41, 44). While transcriptional activation is the principal mechanism of C. albicans-induced AMP induction, AMP processing and/or release is also relevant for cells, including neutrophils, keratinocytes, and Paneth intestinal cells that store precursors or mature AMPs in intracellular vesicles. In neutrophils, azurophil granules accumulate α-defensins, which fuse with phagosomes to attack ingested microbes intracellularly (45). On the other hand, Paneth cells release α-defensins into intestinal crypts, and for unknown reasons, this secretion is enhanced not by fungi but by bacterial cohabitants (46). Another described antimicrobial mechanism of neutrophils is the formation of neutrophil extracellular traps (NETs). These structures are composed of DNA in association with histones as well as granular proteins, such as elastase and myeloperoxidase, and several cytoplasmic proteins (reviewed in reference 47). Interestingly, LL37 stimulates the release of NETs by neutrophils via CD32 (reviewed in reference 48). Immune cells that store the cathelicidin precursor in granules or lamellar bodies include neutrophils, natural killer cells, and mast cells (reviewed in reference 3); upon activation by C. albicans or other microbes, these cells degranulate and release the inactive hCAP18 precursor into the extracellular environment, where it is processed and activated. The human skin is known to be hypoxic in deeper layers with oxygen levels ranging between 1.5 and 5.0% (49). Cellular adaptation to low-oxygen environments is orchestrated by the human transcriptional regulator hypoxia-inducible factor 1α (HIF-1α), which also upregulates cathelicidin expression of keratinocytes (50). Interestingly, by inducing c-Fos and AP-1 activity, some antifungal drugs enhance defensin production in keratinocytes, conceivably contributing to the therapeutic success of such antifungals (51). In conclusion, upregulation of AMP production is differentially regulated and is initiated by a variety of PAMP-PRR interactions, as well as by inflammatory signaling and other environmental stimuli.
ESCAPE STRATEGIES FROM ANTIMICROBIAL PEPTIDES
It has been suggested that AMPs and AMP resistance mechanisms have coevolved, leading to a transient host-pathogen balance (5). Several bacterial AMP resistance mechanisms have been reported. Examples include the secretion of AMP-binding proteins that redirect peptide antimicrobials away from microbial structures (52). As presented below, similar mechanisms were also shown for C. albicans (53–58).
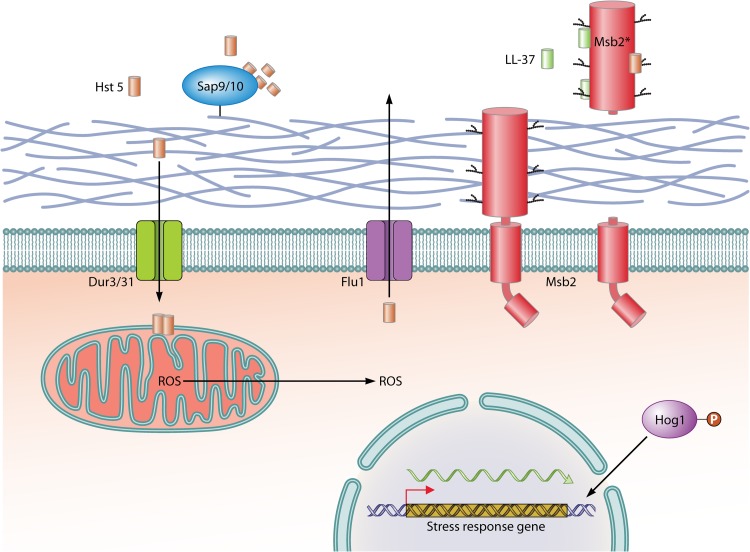
To overcome antifungal activity of AMPs, C. albicans has developed a three-phase AMP evasion strategy, including secretion of peptide effectors, AMP efflux pumps, and regulation of signaling pathways. The first line of fungal defense includes secreted proteins that inhibit AMP activity by degradation or binding. To combat antifungal activity of histatin 5, which constitutes the initial barrier against orally invading pathogens, C. albicans uses the glycosylphosphatidylinositol (GPI)-anchored proteases Sap9 and Sap10, which are highly and consistently expressed during oral infection; cleavage by Sap9 and Sap10 inactivates the salivary peptide (53) (Fig. 2). Degradation of Hst 5 by purified proteases resulted in complete loss of AMP killing capacity. In addition to this cell surface-associated AMP effector, C. albicans secretes the Msb2 glycoprotein, which is a broad-range protectant against peptide antimicrobials (54). The precursor of the plasma membrane protein Msb2 is cleaved, and its extracellular glycodomain is released in considerable amounts into the fungal environment during growth in liquid or on a surface. Extracellularly, it acts to protect the pathogen against AMPs. It is currently debated if secreted aspartic proteases (SAPs) are responsible for the cleavage and release of the secreted Msb2 glycodomain, referred to as Msb2* (55, 59). Msb2* inactivates a wide range of AMPs, including the human cathelicidin LL-37, Hst 5, hNP-1, and hBD1 by tight binding (Fig. 2). By microscale thermophoresis technology, a high affinity (73.1 nM) of LL-37 binding to the shed Msb2* glycodomain was determined (54). In agreement with a protective role of Msb2*, the C. albicans msb2 mutant was found to be supersensitive to LL-37. It was shown that effective binding and protection under normoxia and hypoxia required native folding and correct O-glycosylation of Msb2 by protein-O-mannosyltransferases 1 and 2 (Pmt1/Pmt2) (54, 55). This protective effect was not restricted to C. albicans, because the mannosylated glycofragment also rescued Escherichia coli from the action of LL-37 and Hst 5 (55).
FIG 2.
C. albicans mechanisms to evade AMP responses. Histatin 5 (Hst 5) is taken up by the C. albicans influx transporters Dur3 and Dur31 and induces the formation of reactive oxygen species (ROS); in addition, Hst 5 acts by promoting the efflux of ions and ATP. The Hog1 MAP kinase pathway is activated during AMP stress and upregulates antioxidative and other response mechanisms to overcome AMP activity. The toxicity of Hst 5 is decreased further by its extrusion from fungal cells via the polyamine efflux transporter Flu1. The cell wall-anchored protease Sap9 cleaves and inactivates Hst 5 on the outside of fungal cells. In addition, the shed exodomain fragment of the Msb2 membrane sensor (Msb2*) binds several AMPs extracellularly to provide broad-range protection against AMPs. P, phosphate.
Recently, it was shown that AMPs can be actively extruded from the cytoplasm of fungal cells. The polyamine efflux transporter Flu1, a member of the MDR (multidrug resistance) family, was found to mediate efflux of Hst 5 in C. albicans, resulting in reduction of AMP toxicity (60). Concordantly, deletion of FLU1 reduced the efflux of Hst 5 and therefore increased sensitivity to the AMP. This finding suggests that various AMPs may represent substrates for fungal efflux transporters, which provide resistance.
Fungal stress response pathways have been shown to be essential for basal resistance to histatins. Physiological levels of Hst 5 activated the mitogen-activated protein kinase (MAPK) Hog1 (61) in wild-type cells, while hog1 mutants were hypersensitive to the AMP. Nonhuman AMPs (as discussed below) or human β-defensins were shown to trigger Hog1 activity by interaction with the upstream MAPK kinase Pbs2 to orchestrate a cell damage compensatory response (56, 62). Accordingly, both hog1 and pbs2 mutants were supersensitive to treatment with the human β-defensins 2 and 3 (56). It has been reported that the high-osmolarity glycerol (HOG) pathway is involved in regulating ROS production and ATP demand by mitochondria (63). On the other hand, ROS production and ATP efflux are often induced by AMPs (Table 1). Thus, the HOG pathway may function as a key element in survival of C. albicans to various AMPs by downregulation of ROS production and of ATP efflux. Some response pathways, which mediate basal resistance to host defense peptides, are distinct from general stress adaptation. The C. albicans RNA-binding protein Ssd1, a component of the RAM (regulation of Ace2 and morphogenesis) pathway, and its downstream transcription factor Bcr1 desensitize susceptibility to AMPs by maintaining mitochondrial integrity and by reducing membrane permeabilization (57, 64). Thus, ssd1 mutants were significantly more susceptible to AMPs. The RAM pathway governs multiple processes such as cell wall integrity (65); therefore, Ssd1 may regulate cell wall and membrane adaptive modification to adapt to specific AMPs, including helical cationic polypeptide protamine and hBD-2 (57). The human salivary mucin MUC7 12-mer activated both the calcineurin pathway and the activity of the 20S and 26S proteasome. Consequently, inactivation of the calcineurin pathway led to hypersensitivity to MUC7 (58). Interestingly, calcineurin signaling is essential for protection against membrane perturbations and conveys tolerance to antifungals (66, 67); therefore, activation of the calcineurin pathway partially protects C. albicans from mucin-derived antimicrobial peptides. In C. albicans, calcineurin, Hog1, and PKC (protein kinase C) cell wall integrity pathways coordinately regulate chitin synthesis in response to cell wall stress (68). Peptide antimicrobials, including Hst 5, hNP-1, and short lactoferrin peptides, suppress the synthesis of chitin (69), while stimulation of chitin synthesis was shown to rescue C. albicans from the action of echinocandin, a glucan synthesis inhibitor (70). Therefore, upregulation of chitin levels by calcineurin, Hog1, and Mkc1 may increase protection against various peptide antimicrobials. Thus, activation and regulation of different signaling pathways mediate a direct fungal response to increase basal AMP resistance.
AMPs are produced by organisms of all types, including insects, vertebrates, plants, and microorganisms (71). Some of these peptides, e.g., daptomycin and vancomycin, are of clinical importance, because they are used as reserve antibiotics for the treatment of multiresistant Gram-positive infections (72). Bacterially produced AMPs, including nisin, contribute to the huge AMP diversity in the human body (73), and continuous AMP interactions may lead to the evolution of resistance mechanisms in pathogens. C. albicans exists in many niches in the human body, where fungi may network with many other microbial species, including bacterial pathogens. Fungus-bacterium interactions affect survival, colonization, and pathogenesis of both organisms (74, 75). Such a beneficial situation is represented by the complex polymicrobial biofilm formed by C. albicans and Staphylococcus aureus, in which the bacterial partner is protected from the action of peptide antibiotics in planktonic and biofilm cohabitation (76). The shed Msb2* from C. albicans inactivated the lipopeptide antibiotic daptomycin and provided daptomycin salvage for different bacterial pathogens, including S. aureus (54). This action also provided bacterial survival during cohabitation in polymicrobial C. albicans-bacterium biofilms. Thus, fungal AMP protectants can improve survival of bacterial pathogens and mediate cross-kingdom resistance against novel human and therapeutic AMPs. This mode of resistance was referred to as “quorum resistance” because it depends on AMP concentrations that depend on C. albicans cell numbers. A practical consequence of this finding extends to treatment of multiresistant Gram-positive infections with daptomycin. Thus, multiresistant S. aureus infections should be tested for polymicrobial C. albicans infestation and consequently treated concurrently with antifungals to guarantee the success of antibacterial antibiotic therapy.
AMPs: NEW ANTIFUNGAL AGENTS FOR THERAPY?
At present, treatment of C. albicans infection is primarily based on antifungal agents of the four distinct major classes azoles, polyenes, pyrimidine analogues, and caspofungin/candins (77). Resistance to almost all major antifungal agents, including caspofungin, has been reported in clinical isolates of C. albicans (78, 79). Unlike currently used antimicrobial agents, peptide antimicrobials show little or no toxicity toward human cells (80). Besides their direct microbial killing properties, AMPs can neutralize bacterial lipopolysaccharides and regulate host defense mechanisms, as described above (8). Although many AMPs are sensitive to pH and temperature changes, several AMPs are active over a broad range of pH values, and some AMPs are temperature stable up to 100°C (81). The combination of the human broad-spectrum AMP lactoferrin with amphotericin B or fluconazole has been reported to synergistically increase the activity of the antifungals against Candida spp. Furthermore, lactoferrin inhibited hypha formation in fluconazole-resistant strains of C. albicans (82). Recently, an Hst 5-spermidine conjugate has been developed, which enhanced fungicidal activity compared to nonconjugated Hst 5 (83). It is known that Hst 5 utilizes the polyamine transporters Dur3 and Dur31 for its uptake in C. albicans (16); therefore, the Hst 5 peptide (Hst 5 with amino acids 4 to 15 [Hst 54-15]) conjugated with a GGG linker and spermidine was rapidly taken up, leading to higher in vitro and in vivo candicidal activity than with nonconjugated Hst 5 (83). Collectively, these results suggest that the development and investigation of AMP conjugates could lead to promising new antifungals.
Some antimicrobial peptides are currently in clinical trials or under development (reviewed in reference 84). Part of commercial AMPs are derived from human peptides such as lactoferrin or human defensins modified in their structure, e.g., cyclization to prevent degradation. Alternative AMP-based approaches include plant defensins, which are active both against phytopathogenic and also against human-pathogenic fungi such as C. albicans. Besides their antifungal activity, plant AMPs are advantageous because they are nontoxic to human cells (85). Plant defensins have a wide variety of functions and differ in their mechanisms of antifungal activity. It was shown that antifungal protein 2 of radish (Raphanus sativus AFP2 [RsAFP2]) interacts with glucosylceramides in the C. albicans cell wall and induces an intracellular signaling pathway, which leads to apoptosis of the fungal pathogen (86). The defensin NaD1 from Nicotiana alata interacts with the fungal cell surface and causes membrane permeabilization, which leads to entry of AMPs into the cytoplasm and to increased production of ROS (62). The modes of action of plant AMPs compared to human AMP peptides could also reveal new fungal “Achilles' heels” for antifungal compounds to be used in therapy.
Recently, synthetic peptide mimics of transmembrane regions (TMPMs) of the ABC (ATP-binding cassette) efflux pump Cdr1 (Candida drug resistance 1) were found to bind to Cdr1 and to block the efflux of antifungal agents (87). By optimizing TMPMs to avoid aggregation of the highly hydrophobic peptides, azole-resistant C. albicans cells were chemosensitized to azoles. This approach may possibly be applied to other targets, e.g., secreted aspartyl proteases or to the MDR family member Flu1, to inhibit essential fungal resistance functions. In this scenario, AMPs are used not to kill the fungal pathogen directly but to inhibit essential functions in growth, morphogenesis, and resistance of fungi.
CONCLUSIONS
During the last years, the numbers of fungal morbidity and mortality cases caused by C. albicans have increased, not only because of diagnostics of invasive infections but also because of limited efficacy of current antifungals. Humans express a large number of various peptides with antimicrobial and, specifically, antifungal action that are key effectors of innate immunity. In addition to their role in combating microbial pathogens by direct killing or recruitment of immune cells to the site of infection, AMPs are involved in the processes of wound healing and angiogenesis. AMPs have many potential uses in treatment of complex infections, and their mode of action can be exploited for the generation of novel antifungal molecules. Various applications of human and nonhuman AMPs show the effectiveness of small peptides against the human-pathogenic fungus C. albicans (62, 83, 86, 87). Recently, different AMP resistance mechanisms that allow C. albicans to become either a successful commensal or a human fungal pathogen were discovered (15, 17, 54–56, 61, 88). These fungal AMP resistance mechanisms include secreted AMP-blocking proteins and proteins regulating signaling pathways, which collectively may prevent AMP-mediated killing. The molecular understanding of AMP activity may reveal novel antifungal targets and aid in the design of new strategies and agents in the fight against drug-resistant fungi. In future research, it will important to clarify the molecular details of C. albicans AMP resistance to fully understand interactions between the host and pathogen in fungal disease. Importantly, novel therapeutic peptide antifungals should be evaluated in the light of fungal AMP-resistance mechanisms.
ACKNOWLEDGMENTS
We thank E. Román, J. Pla, and M. Juchimiuk for useful comments on the manuscript.
Our work is funded by the Deutsche Forschungsgemeinschaft (ER47-13) and by BMBF-ERA-NET PathoGeNoMics projects “Glycoshield” and “OXYstress.”
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 20 June 2014
REFERENCES
- 1.Cheng SC, Joosten LA, Kullberg BJ, Netea MG. 2012. Interplay between Candida albicans and the mammalian innate host defense. Infect. Immun. 80:1304–1313. 10.1128/IAI.06146-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hube B. 2004. From commensal to pathogen: stage- and tissue-specific gene expression of Candida albicans. Curr. Opin. Microbiol. 7:336–341. 10.1016/j.mib.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 3.Vandamme D, Landuyt B, Luyten W, Schoofs L. 2012. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell. Immunol. 280:22–35. 10.1016/j.cellimm.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 4.Lehrer RI, Ganz T. 1999. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 11:23–27. 10.1016/S0952-7915(99)80005-3 [DOI] [PubMed] [Google Scholar]
- 5.Peschel A, Sahl HG. 2006. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4:529–536. 10.1038/nrmicro1441 [DOI] [PubMed] [Google Scholar]
- 6.van't Hof W, Veerman EC, Helmerhorst EJ, Amerongen AV. 2001. Antimicrobial peptides: properties and applicability. Biol. Chem. 382:597–619 [DOI] [PubMed] [Google Scholar]
- 7.Vylkova S, Sun JN, Edgerton M. 2007. The role of released ATP in killing Candida albicans and other extracellular microbial pathogens by cationic peptides. Purinergic Signal 3:91–97. 10.1007/s11302-006-9040-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinstraesser L, Kraneburg U, Jacobsen F, Al-Benna S. 2011. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology 216:322–333. 10.1016/j.imbio.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 9.den Hertog AL, van Marle J, van Veen HA, Van 't Hof W, Bolscher JG, Veerman EC, Nieuw Amerongen AV. 2005. Candidacidal effects of two antimicrobial peptides: histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem. J. 388:689–695. 10.1042/BJ20042099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang HT, Tsai PW, Huang HH, Liu YS, Chien TS, Lan CY. 2012. LL37 and hBD-3 elevate the beta-1,3-exoglucanase activity of Candida albicans Xog1p, resulting in reduced fungal adhesion to plastic. Biochem. J. 441:963–970. 10.1042/BJ20111454 [DOI] [PubMed] [Google Scholar]
- 11.Tsai PW, Yang CY, Chang HT, Lan CY. 2011. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS One 6:e17755. 10.1371/journal.pone.0017755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami M, Lopez-Garcia B, Braff M, Dorschner RA, Gallo RL. 2004. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J. Immunol. 172:3070–3077. 10.4049/jimmunol.172.5.3070 [DOI] [PubMed] [Google Scholar]
- 13.den Hertog AL, Van Marle J, Veerman ECI, Valentijn-Benz M, Nazmi K, Kalay H, Grün CH, Van't Hof W, Bolscher JGM, Amerongen AVN. 2006. The human cathelicidin peptide LL-37 and truncated variants induce segregation of lipids and proteins in the plasma membrane of Candida albicans. Biol. Chem. 387:1495–1502 [DOI] [PubMed] [Google Scholar]
- 14.Komatsu T, Salih E, Helmerhorst EJ, Offner GD, Oppenheim FG. 2011. Influence of histatin 5 on Candida albicans mitochondrial protein expression assessed by quantitative mass spectrometry. J. Proteome Res. 10:646–655. 10.1021/pr100861k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun JN, Li W, Jang WS, Nayyar N, Sutton MD, Edgerton M. 2008. Uptake of the antifungal cationic peptide histatin 5 by Candida albicans Ssa2p requires binding to non-conventional sites within the ATPase domain. Mol. Microbiol. 70:1246–1260. 10.1111/j.1365-2958.2008.06480.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar R, Chadha S, Saraswat D, Bajwa JS, Li RA, Conti HR, Edgerton M. 2011. Histatin 5 uptake by Candida albicans utilizes polyamine transporters Dur3 and Dur31 proteins. J. Biol. Chem. 286:43748–43758. 10.1074/jbc.M111.311175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer FL, Wilson D, Jacobsen ID, Miramon P, Grosse K, Hube B. 2012. The novel Candida albicans transporter Dur31 is a multi-stage pathogenicity factor. PLoS Pathog. 8:e1002592. 10.1371/journal.ppat.1002592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helmerhorst EJ, Troxler RF, Oppenheim FG. 2001. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A. 98:14637–14642. 10.1073/pnas.141366998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koshlukova SE, Araujo MW, Baev D, Edgerton M. 2000. Released ATP is an extracellular cytotoxic mediator in salivary histatin 5-induced killing of Candida albicans. Infect. Immun. 68:6848–6856. 10.1128/IAI.68.12.6848-6856.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puri S, Edgerton M. 2014. How does it kill?: understanding the candidacidal mechanism of salivary histatin 5. Eukaryot. Cell 13:958–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgerton M, Koshlukova SE, Araujo MW, Patel RC, Dong J, Bruenn JA. 2000. Salivary histatin 5 and human neutrophil defensin 1 kill Candida albicans via shared pathways. Antimicrob. Agents Chemother. 44:3310–3316. 10.1128/AAC.44.12.3310-3316.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnakumari V, Rangaraj N, Nagaraj R. 2009. Antifungal activities of human beta-defensins HBD-1 to HBD-3 and their C-terminal analogs Phd1 to Phd3. Antimicrob. Agents Chemother. 53:256–260. 10.1128/AAC.00470-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Situ H, Wei G, Smith CJ, Mashhoon S, Bobek LA. 2003. Human salivary MUC7 mucin peptides: effect of size, charge and cysteine residues on antifungal activity. Biochem. J. 375:175–182. 10.1042/BJ20030779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harder J, Schroder JM. 2002. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 277:46779–46784. 10.1074/jbc.M207587200 [DOI] [PubMed] [Google Scholar]
- 25.Abtin A, Eckhart L, Mildner M, Ghannadan M, Harder J, Schroder JM, Tschachler E. 2009. Degradation by stratum corneum proteases prevents endogenous RNase inhibitor from blocking antimicrobial activities of RNase 5 and RNase 7. J. Investig. Dermatol. 129:2193–2201. 10.1038/jid.2009.35 [DOI] [PubMed] [Google Scholar]
- 26.Lupetti A, Paulusma-Annema A, Welling MM, Senesi S, van Dissel JT, Nibbering PH. 2000. Candidacidal activities of human lactoferrin peptides derived from the N terminus. Antimicrob. Agents Chemother. 44:3257–3263. 10.1128/AAC.44.12.3257-3263.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolscher J, Nazmi K, van Marle J, van 't Hof W, Veerman E. 2012. Chimerization of lactoferricin and lactoferrampin peptides strongly potentiates the killing activity against Candida albicans. Biochem. Cell Biol. 90:378–388. 10.1139/o11-085 [DOI] [PubMed] [Google Scholar]
- 28.Wagener J, Schneider JJ, Baxmann S, Kalbacher H, Borelli C, Nuding S, Kuchler R, Wehkamp J, Kaeser MD, Mailander-Sanchez D, Braunsdorf C, Hube B, Schild L, Forssmann WG, Korting HC, Liepke C, Schaller M. 2013. A peptide derived from the highly conserved protein GAPDH is involved in tissue protection by different antifungal strategies and epithelial immunomodulation. J. Investig. Dermatol. 133:144–153. 10.1038/jid.2012.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehra T, Koberle M, Braunsdorf C, Mailander-Sanchez D, Borelli C, Schaller M. 2012. Alternative approaches to antifungal therapies. Exp. Dermatol. 21:778–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F. 2012. Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J. Appl. Microbiol. 113:723–736. 10.1111/j.1365-2672.2012.05338.x [DOI] [PubMed] [Google Scholar]
- 31.Selsted ME, Ouellette AJ. 2005. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6:551–557. 10.1038/ni1206 [DOI] [PubMed] [Google Scholar]
- 32.Li M, Chen Q, Shen Y, Liu W. 2009. Candida albicans phospholipomannan triggers inflammatory responses of human keratinocytes through Toll-like receptor 2. Exp. Dermatol. 18:603–610. 10.1111/j.1600-0625.2008.00832.x [DOI] [PubMed] [Google Scholar]
- 33.Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, Naglik JR. 2010. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 8:225–235. 10.1016/j.chom.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eyerich S, Wagener J, Wenzel V, Scarponi C, Pennino D, Albanesi C, Schaller M, Behrendt H, Ring J, Schmidt-Weber CB, Cavani A, Mempel M, Traidl-Hoffmann C, Eyerich K. 2011. IL-22 and TNF-alpha represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur. J. Immunol. 41:1894–1901. 10.1002/eji.201041197 [DOI] [PubMed] [Google Scholar]
- 35.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. 2004. IL-22 increases the innate immunity of tissues. Immunity 21:241–254. 10.1016/j.immuni.2004.07.007 [DOI] [PubMed] [Google Scholar]
- 36.Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. 2004. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J. Immunol. 173:3482–3491. 10.4049/jimmunol.173.5.3482 [DOI] [PubMed] [Google Scholar]
- 37.Rehaume LM, Jouault T, Chamaillard M. 2010. Lessons from the inflammasome: a molecular sentry linking Candida and Crohn's disease. Trends Immunol. 31:171–175. 10.1016/j.it.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 38.Lu Q, Jayatilake JA, Samaranayake LP, Jin L. 2006. Hyphal invasion of Candida albicans inhibits the expression of human beta-defensins in experimental oral candidiasis. J. Investig. Dermatol. 126:2049–2056. 10.1038/sj.jid.5700346 [DOI] [PubMed] [Google Scholar]
- 39.Weindl G, Naglik JR, Kaesler S, Biedermann T, Hube B, Korting HC, Schaller M. 2007. Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling. J. Clin. Invest. 117:3664–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawai K, Shimura H, Minagawa M, Ito A, Tomiyama K, Ito M. 2002. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J. Dermatol. Sci. 30:185–194. 10.1016/S0923-1811(02)00105-6 [DOI] [PubMed] [Google Scholar]
- 41.Pahl R, Brunke G, Steubesand N, Schubert S, Bottner M, Wedel T, Jurgensen C, Hampe J, Schafer H, Zeissig S, Schreiber S, Rosenstiel P, Reiss K, Arlt A. 2011. IL-1beta and ADAM17 are central regulators of beta-defensin expression in Candida esophagitis. Am. J. Physiol. Gastrointest. Liver Physiol. 300:G547–G553. 10.1152/ajpgi.00251.2010 [DOI] [PubMed] [Google Scholar]
- 42.Wuerth K, Hancock RE. 2011. New insights into cathelicidin modulation of adaptive immunity. Eur. J. Immunol. 41:2817–2819. 10.1002/eji.201142055 [DOI] [PubMed] [Google Scholar]
- 43.Campbell Y, Fantacone ML, Gombart AF. 2012. Regulation of antimicrobial peptide gene expression by nutrients and by-products of microbial metabolism. Eur. J. Nutr. 51:899–907. 10.1007/s00394-012-0415-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steubesand N, Kiehne K, Brunke G, Pahl R, Reiss K, Herzig KH, Schubert S, Schreiber S, Folsch UR, Rosenstiel P, Arlt A. 2009. The expression of the beta-defensins hBD-2 and hBD-3 is differentially regulated by NF-kappaB and MAPK/AP-1 pathways in an in vitro model of Candida esophagitis. BMC Immunol. 10:36. 10.1186/1471-2172-10-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. 1997. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90:2796–2803 [PubMed] [Google Scholar]
- 46.Rumio C, Besusso D, Palazzo M, Selleri S, Sfondrini L, Dubini F, Menard S, Balsari A. 2004. Degranulation of Paneth cells via Toll-like receptor 9. Am. J. Pathol. 165:373–381. 10.1016/S0002-9440(10)63304-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinkmann V, Zychlinsky A. 2007. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 5:577–582. 10.1038/nrmicro1710 [DOI] [PubMed] [Google Scholar]
- 48.Mantovani A, Cassatella MA, Costantini C, Jaillon S. 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11:519–531. 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- 49.Stewart FA, Denekamp J, Randhawa VS. 1982. Skin sensitization by misonidazole: a demonstration of uniform mild hypoxia. Br. J. Cancer 45:869–877. 10.1038/bjc.1982.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peyssonnaux C, Boutin AT, Zinkernagel AS, Datta V, Nizet V, Johnson RS. 2008. Critical role of HIF-1alpha in keratinocyte defense against bacterial infection. J. Investig. Dermatol. 128:1964–1968. 10.1038/jid.2008.27 [DOI] [PubMed] [Google Scholar]
- 51.Kanda N, Kano R, Ishikawa T, Watanabe S. 2011. The antimycotic drugs itraconazole and terbinafine hydrochloride induce the production of human beta-defensin-3 in human keratinocytes. Immunobiology 216:497–504. 10.1016/j.imbio.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 52.Frick IM, Åkesson P, Rasmussen M, Schmidtchen A, Björck L. 2003. SIC, a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. J. Biol. Chem. 278:16561–16566. 10.1074/jbc.M301995200 [DOI] [PubMed] [Google Scholar]
- 53.Meiller TF, Hube B, Schild L, Shirtliff ME, Scheper MA, Winkler R, Ton A, Jabra-Rizk MA. 2009. A novel immune evasion strategy of Candida albicans: proteolytic cleavage of a salivary antimicrobial peptide. PLoS One 4:e5039. 10.1371/journal.pone.0005039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swidergall M, Ernst AM, Ernst JF. 2013. Candida albicans mucin Msb2 is a broad-range protectant against antimicrobial peptides. Antimicrob. Agents Chemother. 57:3917–3922. 10.1128/AAC.00862-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szafranski-Schneider E, Swidergall M, Cottier F, Tielker D, Roman E, Pla J, Ernst JF. 2012. Msb2 shedding protects Candida albicans against antimicrobial peptides. PLoS Pathog. 8:e1002501. 10.1371/journal.ppat.1002501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Argimon S, Fanning S, Blankenship JR, Mitchell AP. 2011. Interaction between the Candida albicans high-osmolarity glycerol (HOG) pathway and the response to human beta-defensins 2 and 3. Eukaryot. Cell 10:272–275. 10.1128/EC.00133-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung SI, Finkel JS, Solis NV, Chaili S, Mitchell AP, Yeaman MR, Filler SG. 2013. Bcr1 functions downstream of Ssd1 to mediate antimicrobial peptide resistance in Candida albicans. Eukaryot. Cell 12:411–419. 10.1128/EC.00285-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lis M, Liu TT, Barker KS, Rogers PD, Bobek LA. 2010. Antimicrobial peptide MUC7 12-mer activates the calcium/calcineurin pathway in Candida albicans. FEMS Yeast Res. 10:579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puri S, Kumar R, Chadha S, Tati S, Conti HR, Hube B, Cullen PJ, Edgerton M. 2012. Secreted aspartic protease cleavage of Candida albicans Msb2 activates Cek1 MAPK signaling affecting biofilm formation and oropharyngeal candidiasis. PLoS One 7:e46020. 10.1371/journal.pone.0046020 (Author Correction, 8:10.1371/annotation/13ef5e14-c192-4bb2-af91-886024500b4b, 2013. ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li R, Kumar R, Tati S, Puri S, Edgerton M. 2013. Candida albicans Flu1-mediated efflux of salivary histatin 5 reduces its cytosolic concentration and fungicidal activity. Antimicrob. Agents Chemother. 57:1832–1839. 10.1128/AAC.02295-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vylkova S, Jang WS, Li W, Nayyar N, Edgerton M. 2007. Histatin 5 initiates osmotic stress response in Candida albicans via activation of the Hog1 mitogen-activated protein kinase pathway. Eukaryot. Cell 6:1876–1888. 10.1128/EC.00039-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayes BM, Bleackley MR, Wiltshire JL, Anderson MA, Traven A, van der Weerden NL. 2013. Identification and mechanism of action of the plant defensin NaD1 as a new member of the antifungal drug arsenal against Candida albicans. Antimicrob. Agents Chemother. 57:3667–3675. 10.1128/AAC.00365-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alonso-Monge R, Carvaihlo S, Nombela C, Rial E, Pla J. 2009. The Hog1 MAP kinase controls respiratory metabolism in the fungal pathogen Candida albicans. Microbiology 155:413–423. 10.1099/mic.0.023309-0 [DOI] [PubMed] [Google Scholar]
- 64.Gank KD, Yeaman MR, Kojima S, Yount NY, Park H, Edwards JE, Jr, Filler SG, Fu Y. 2008. SSD1 is integral to host defense peptide resistance in Candida albicans. Eukaryot. Cell 7:1318–1327. 10.1128/EC.00402-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bharucha N, Chabrier-Rosello Y, Xu T, Johnson C, Sobczynski S, Song Q, Dobry CJ, Eckwahl MJ, Anderson CP, Benjamin AJ, Kumar A, Krysan DJ. 2011. A large-scale complex haploinsufficiency-based genetic interaction screen in Candida albicans: analysis of the RAM network during morphogenesis. PLoS Genet. 7:e1002058. 10.1371/journal.pgen.1002058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cruz MC, Goldstein AL, Blankenship JR, Del Poeta M, Davis D, Cardenas ME, Perfect JR, McCusker JH, Heitman J. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546–559. 10.1093/emboj/21.4.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48:959–976. 10.1046/j.1365-2958.2003.03495.x [DOI] [PubMed] [Google Scholar]
- 68.Lenardon MD, Lesiak I, Munro CA, Gow NA. 2009. Dissection of the Candida albicans class I chitin synthase promoters. Mol. Genet. Genomics 281:459–471. 10.1007/s00438-009-0423-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanida T, Okamoto T, Ueta E, Yamamoto T, Osaki T. 2006. Antimicrobial peptides enhance the candidacidal activity of antifungal drugs by promoting the efflux of ATP from Candida cells. J. Antimicrob. Chemother. 57:94–103 [DOI] [PubMed] [Google Scholar]
- 70.Walker LA, Munro CA, De Bruijn I, Lenardon MD, McKinnon A, Gow NAR. 2008. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 4:e1000040. 10.1371/journal.ppat.1000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395. 10.1038/415389a [DOI] [PubMed] [Google Scholar]
- 72.Moise PA, North D, Steenbergen JN, Sakoulas G. 2009. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: facts and assumptions. Lancet Infect. Dis. 9:617–624. 10.1016/S1473-3099(09)70200-2 [DOI] [PubMed] [Google Scholar]
- 73.Akerey B, Le-Lay C, Fliss I, Subirade M, Rouabhia M. 2009. In vitro efficacy of nisin Z against Candida albicans adhesion and transition following contact with normal human gingival cells. J. Appl. Microbiol. 107:1298–1307. 10.1111/j.1365-2672.2009.04312.x [DOI] [PubMed] [Google Scholar]
- 74.Harriott MM, Noverr MC. 2009. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob. Agents Chemother. 53:3914–3922. 10.1128/AAC.00657-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mallick EM, Bennett RJ. 2013. Sensing of the microbial neighborhood by Candida albicans. PLoS Pathog. 9:e1003661. 10.1371/journal.ppat.1003661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harriott MM, Noverr MC. 2010. Ability of Candida albicans mutants to induce Staphylococcus aureus vancomycin resistance during polymicrobial biofilm formation. Antimicrob. Agents Chemother. 54:3746–3755. 10.1128/AAC.00573-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moudgal V, Sobel J. 2010. Antifungals to treat Candida albicans. Expert Opin. Pharmacother. 11:2037–2048. 10.1517/14656566.2010.493875 [DOI] [PubMed] [Google Scholar]
- 78.Katiyar S, Pfaller M, Edlind T. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892–2894. 10.1128/AAC.00349-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanglard D, Odds FC. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73–85. 10.1016/S1473-3099(02)00181-0 [DOI] [PubMed] [Google Scholar]
- 80.Velden WJ, van Iersel TM, Blijlevens NM, Donnelly JP. 2009. Safety and tolerability of the antimicrobial peptide human lactoferrin 1–11 (hLF1–11). BMC Med. 7:44. 10.1186/1741-7015-7-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tokle T, McClements DJ. 2011. Physicochemical properties of lactoferrin stabilized oil-in-water emulsions: effects of pH, salt and heating. Food Hydrocolloids 25:976–982. 10.1016/j.foodhyd.2010.09.012 [DOI] [Google Scholar]
- 82.Venkatesh MP, Rong L. 2008. Human recombinant lactoferrin acts synergistically with antimicrobials commonly used in neonatal practice against coagulase-negative staphylococci and Candida albicans causing neonatal sepsis. J. Med. Microbiol. 57:1113–1121. 10.1099/jmm.0.2008/001263-0 [DOI] [PubMed] [Google Scholar]
- 83.Tati S, Li R, Puri S, Kumar R, Davidow P, Edgerton M. 2014. Histatin 5-spermidine conjugates have enhanced fungicidal activity and efficacy as a topical therapeutic for oral candidiasis. Antimicrob. Agents Chemother. 58:756–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L, Falla TJ. 2010. Potential therapeutic application of host defense peptides. Methods Mol. Biol. 618:303–327. 10.1007/978-1-60761-594-1_19 [DOI] [PubMed] [Google Scholar]
- 85.Thevissen K, Kristensen HH, Thomma BP, Cammue BP, Francois IE. 2007. Therapeutic potential of antifungal plant and insect defensins. Drug Discov. Today 12:966–971. 10.1016/j.drudis.2007.07.016 [DOI] [PubMed] [Google Scholar]
- 86.Thevissen K, de Mello Tavares P, Xu D, Blankenship J, Vandenbosch D, Idkowiak-Baldys J, Govaert G, Bink A, Rozental S, de Groot PW, Davis TR, Kumamoto CA, Vargas G, Nimrichter L, Coenye T, Mitchell A, Roemer T, Hannun YA, Cammue BP. 2012. The plant defensin RsAFP2 induces cell wall stress, septin mislocalization and accumulation of ceramides in Candida albicans. Mol. Microbiol. 84:166–180. 10.1111/j.1365-2958.2012.08017.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maurya IK, Thota CK, Verma SD, Sharma J, Rawal MK, Ravikumar B, Sen S, Chauhan N, Lynn AM, Chauhan VS, Prasad R. 2013. Rationally designed transmembrane peptide mimics of the multidrug transporter protein Cdr1 act as antagonists to selectively block drug efflux and chemosensitize azole-resistant clinical isolates of Candida albicans. J. Biol. Chem. 288:16775–16787. 10.1074/jbc.M113.467159 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Jang WS, Bajwa JS, Sun JN, Edgerton M. 2010. Salivary histatin 5 internalization by translocation, but not endocytosis, is required for fungicidal activity in Candida albicans. Mol. Microbiol. 77:354–370. 10.1111/j.1365-2958.2010.07210.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Edgerton M, Koshlukova SE. 2000. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv. Dent. Res. 14:16–21. 10.1177/08959374000140010201 [DOI] [PubMed] [Google Scholar]
- 90.Andres MT, Viejo-Diaz M, Fierro JF. 2008. Human lactoferrin induces apoptosis-like cell death in Candida albicans: critical role of K+-channel-mediated K+ efflux. Antimicrob. Agents Chemother. 52:4081–4088. 10.1128/AAC.01597-07 [DOI] [PMC free article] [PubMed] [Google Scholar]