Abstract
Cystic echinococcosis is a zoonotic infection caused by the larval stage of the cestode Echinococcus granulosus. Chemotherapy currently employs benzimidazoles; however, 40% of cases do not respond favorably. With regard to these difficulties, novel therapeutic tools are needed to optimize treatment in humans. The aim of this work was to explore the in vitro and in vivo effects of tamoxifen (TAM) against E. granulosus. In addition, possible mechanisms for the susceptibility of TAM are discussed in relation to calcium homeostasis, P-glycoprotein inhibition, and antagonist effects on a putative steroid receptor. After 24 h of treatment, TAM, at a low micromolar concentration range (10 to 50 μM), inhibited the survival of E. granulosus protoscoleces and metacestodes. Moreover, we demonstrated the chemotherapeutic and chemopreventive pharmacological effects of the drug. At a dose rate of 20 mg/kg of body weight, TAM induced protection against the infection in mice. In the clinical efficacy studies, a reduction in cyst weight was observed after the administration of 20 mg/kg in mice with cysts developed during 3 or 6 months, compared to that of those collected from control mice. Since the collateral effects of high TAM doses have been largely documented in clinical trials, the use of low doses of this drug as a short-term therapy may be a novel alternative approach for human cystic echinococcosis treatment.
INTRODUCTION
Human cystic echinococcosis (CE), or hydatidosis, is a zoonotic infection caused by the larval stage of the cestode Echinococcus granulosus. The metacestode develops as a unilocular cyst that consists of an inner germinal layer with totipotent cells which produce brood capsules with multiple protoscoleces by asexual division, supported by a laminated acellular membrane, named the laminar layer. The treatment options for CE are surgery and/or chemotherapy, depending on the location, state, and number of the cysts. The only two drugs licensed to date are the benzimidazole carbamate derivatives albendazole and mebendazole (1). Therefore, a search for novel candidates for the treatment of hydatidosis has been proposed. The performances of potential therapeutic compounds have been investigated, applying antimicrobial and anticancer agents in in vitro and in vivo assays (2).
The effects of several drugs inhibiting proliferation of cancer cells were assayed on Echinococcus metacestodes and protoscoleces (3–5). Spicher et al. (4) demonstrated the in vitro and in vivo effects of an endogenous metabolite of estrogen, 2-methoxyestradiol, either alone or combined with albendazole, against Echinococcus multilocularis and E. granulosus. Furthermore, the isoflavone genistein and the genistein derivative Rm6423 exhibited profound in vitro activities against the mentioned parasites (3). The cytostatic drug imatinib had drastic effects on the in vitro development and survival of E. multilocularis (5).
Tamoxifen (TAM) is a competitive antagonist of the estrogen receptor alpha (ERα), classified as a nonsteroidal selective estrogen receptor modulator (SERM), widely used for treating primary breast cancer in premenopausal women and gynecomastia in men receiving hormonal therapy for prostatic carcinoma (6). TAM and its bioactive metabolites, 4-hydroxytamoxifen (4-OH-TAM) and N-desmethyl tamoxifen (ND-TAM), inhibit proliferation and induce apoptosis in several types of ER-positive and ER-negative breast cancer cells, rat mammary tumors, and other cancer types (7, 8). Interestingly, this antiestrogenic drug has also proved to be effective against several protozoan parasites, including Leishmania major, Leishmania braziliensis, Leishmania chagasi, Leishmania amazonensis, and Trypanosoma cruzi (9–11). Furthermore, the effect of TAM on cestode parasites has been studied in Taenia crassiceps and Taenia solium (12, 13). In fact, TAM inhibits T. crassiceps and T. solium proliferation and in vitro viability, whereas it induces protection against the infection in murine and hamster models, respectively (12, 13). Antiestrogenic drugs have never been studied in E. granulosus.
Estrogen receptors have an evolutionary conserved modular structure. It consists of a DNA-binding domain (DBD), a nuclear localization signal (NLS), and a ligand-binding domain (LBD) at the C terminus. The LBD of ERα has been crystallized with 17β-estradiol (E2), diethylstilbestrol (DES), and the SERMs TAM and raloxifene (14). The bulky side chain of these SERMs prevents sealing of the LBD, and this produces antiestrogenic action (15, 16). Typical nuclear receptors (NR) have been identified in Echinococcus spp. (ortholog of SmFTZ-F1) with the highest homologies (approximately 26% identical amino acids) of all identified cestode NR to the LBDs of vertebrate ERs (17).
TAM also acts via an estrogen receptor-independent mechanism. It has a wide range of effects on mammalian cell physiology, such as induction of intracellular calcium release, apoptosis, and autophagy. Moreover, it possesses antioxidant and antiangiogenesis properties (18). Consistent with this wide range of cellular effects, TAM has been shown to target mammalian proteins such as calmodulin (CaM), protein kinase C, phospholipase C, phosphoinositide kinase, P-glycoprotein (Pgp), and swelling-induced chloride channels (19).
Here, we report data showing the in vitro activity of TAM against protoscoleces and metacestodes and the in silico analysis of a possible interaction with a putative steroid receptor (SR). Moreover, we investigated the chemoprophylactic and clinical efficacy of TAM in mice infected with E. granulosus.
MATERIALS AND METHODS
Chemicals.
Culture-grade tamoxifen citrate was obtained from Sigma-Aldrich (USA). TAM was kept as a 100 mM stock solution in dimethyl sulfoxide at 20°C for in vitro assays. Dilutions were prepared in medium at concentrations of 5 to 200 μM. For in vivo experiments, oily solutions of TAM were prepared by taking aliquots from fresh alcoholic stock solutions, and adding the required volume of corn oil (Sigma-Aldrich) and maintained under refrigeration (3 to 5°C).
In vitro culture of E. granulosus protoscoleces and drug treatment.
Protoscoleces were removed under aseptic conditions from hydatid cysts of infected cattle slaughtered in two abattoirs located in the southeast of Buenos Aires province, Argentina. The area where the cattle came from is known to include only the G1 strain of E. granulosus. Viable, motile, and morphologically intact protoscoleces (n = 3,000) were cultured using medium 199 (Gibco) supplemented with antibiotics (20). Protoscoleces were incubated in 24-well culture dishes in the presence of increasing concentrations (5 to 100 μM) of TAM up to 7 days. Viability assays were performed daily until the death of all parasites (20). Five experiments were performed in triplicate, and results are expressed as the mean percentages of reduction of parasite numbers compared with those in untreated control wells. For scanning electron microscopy (SEM), samples were taken every 12 h and processed (20).
Detection of the cytosolic calcium levels in E. granulosus protoscoleces.
Changes in cytosolic free Ca2+ concentrations ([Ca2+]i) were fluorometrically monitored using a Fluo-3 acetoxymethyl ester(Fluo-3-AM) probe (20). The experiments were carried out with 5 × 103 protoscoleces, which were incubated with 10, 25, and 50 μM TAM for 2 h. Pretreatments with 1 mM EGTA or 100 μM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM) or both calcium chelators were carried out for 15 min. Then, fluorescence was registered with a spectrofluorimeter (model F-4500; Hitachi). Excitation was provided by the 488-nm line of a krypton-argon laser, and the emitted fluorescence was collected using band-pass filters (505 to 530 nm). At the end of registration, 1 mM EGTA plus 100 μM BAPTA-AM was added to chelate Ca2+. After 5 min, fluorescence was measured again every 1 min for 30 min (Fmin, total fluorescence of dye in the absence of free Ca2+). Each experiment was individually corrected for autofluorescence, and untreated controls were included in each replication.
Ethics statement.
Animal procedures and management protocols were carried out in accordance with the 2011 revised form of The Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. Unnecessary animal suffering was avoided throughout the study.
Mouse infection and procedures for in vitro incubation of cysts.
Infection of mice and obtaining of cysts were carried out as previously described (21). Briefly, female CF1 mice (body weight, 25 ± 5 g) were infected by intraperitoneal infection of 1,500 protoscoleces in 0.5 ml of medium 199 to produce experimental secondary hydatid disease. Animals were housed in a temperature-controlled (22 ± 1°C), light-cycled (12-h light/dark cycle) room. Food and water were provided ad libitum. At 6 months postinfection (p.i.), mice were euthanized, and necropsy was carried out immediately thereafter. At necropsy, the peritoneal cavity was opened, and the hydatid cysts were carefully removed.
Groups of 10 to 15 E. granulosus murine cysts with diameters between 3 and 5 mm were incubated in Leighton tubes in the presence of increasing concentrations (5 to 100 μM) of TAM. Metacestode viability was assessed daily through an inverted light microscope (until the viability control was <90%, approximately 6 days). The criteria for cyst viability assessment included the loss of turgidity and the collapse of the germinal layers. Each experiment was assayed for three replicates and repeated three times.
Analysis of E. granulosus Pgp and tegumental gamma-glutamyl-transpeptidase activities of in vitro TAM-treated protoscoleces and metacestodes.
Pgp transport activity was measured in the absence and presence of 25 μM TAM from fresh samples of protoscoleces (n = 1,000) and intact metacestodes (n = 10) on 96-well plates using a calcein AM assay (Molecular Probes, USA) (22). Each experiment was assayed for three replicates and repeated three times. Protoscoleces were also imaged with an inverted confocal laser scanning microscope (confocal microscope C1; Nikon).
Gamma-glutamyl-transpeptidase (GGT), associated with damage of the internal tegument, was determined following the Szasz method from protoscoleces and metacestodes (23).
Chemoprophylactic efficacy study.
Experimental animals were infected and housed as described above. At the time point of infection, 20 CF-1 mice were allocated into 1 of 2 experimental groups (10 animals/group): (i) an unmedicated control group, receiving corn oil as a placebo, and (ii) a treated group, receiving TAM dissolved in corn oil. In the chemoprophylactic efficacy study, treatments were performed every 48 h (40 doses) by intragastric administration (0.3 ml/animal) at the dose rate of 20 mg/kg of body weight. Six months after infection, the mice were euthanized, and necropsies were carried out immediately thereafter.
Clinical efficacy study.
Two different experimental designs were used.
(i) Experiment 1.
At 6 months p.i., mice were allocated to one of two experimental groups (10 animals/group): (a) an unmedicated control group, receiving corn oil as a placebo, and (b) a TAM suspension-treated group (experiment 1 [E1]), receiving a dose rate of 20 mg/kg every 48 h (40 doses).
(ii) Experiment 2.
At 3 months p.i., mice were allocated to one of three experimental groups (10 animals/group): (a) an unmedicated control group, receiving corn oil as a placebo, (b) a TAM suspension-treated group (experiment 2a [E2a]), receiving 20 mg/kg every 48 h (40 doses), and (c) a TAM suspension-treated group (experiment 2b [E2b]), receiving 100 mg/kg/day every 48 h (16 doses). In all cases, the treatments were performed by intragastric administration (0.3 ml/animal). The final dose received by mice in group E2b was double the final dose administered to groups E1 and E2a. At the end of the treatment period, the animals were euthanized, and necropsies were carried out immediately thereafter.
Determination of efficacy of in vivo treatments.
At necropsy in both the prophylactic and efficacy studies, the peritoneal cavity was opened, and the hydatid cysts were carefully removed. The weight of the cysts collected from each individual animal was recorded using an analytical balance. The efficacy of treatments (based on the weights of the cysts from infected mice) was calculated using the formula (mean weight of the control group − mean cyst weight of the treated group)/mean cyst weight of the control group × 100. Samples of cysts recovered from each mouse were processed for SEM or transmission electron microscopy (TEM) as described by Elissondo et al. (21).
Analysis of TAM and its metabolites.
A spectrophotometric method, based on the formation of a chloroform-soluble ion-association complex between TAM and the dye alizarin red-S in acidic medium, was used for the determination of intracystic TAM. Cystic samples were obtained from the sacrificed animals. Cystic liquids were extracted, and the precipitated protein was removed by centrifugation at 2,000 × g for 15 min. The supernatants were transferred to sample vials, and the vials were capped and stored at −20°C until analysis by the colorimetric method (24). The colored complexes between the dye, TAM, and its metabolites were determined at 440 nm.
Expression and sequence analysis of Echinococcus SR.
A BLASTp search for ER homologs in the E. granulosus genome database (http://www.sanger.ac.uk/Projects/Echinococcus) was carried out using Homo sapiens ERα (GenBank accession no. NP_000116) and ERβ (GenBank accession no. AAC05985) as queries. These data allowed the identification of an ortholog of EmuJ_000814300 and SmFTZ-F1, whose predicted open reading frame encodes a putative E. granulosus SR (EgrG_000814300 included in the EgG_scaffold_0001 at position 7377598 to 7382189) named E. granulosus SR (17). Specific primers for the putative E. granulosus sr1 gene were designed (srFw, 5′-ATTTGCGGGGACAAGATATCGGGTTATCAT-3′, and sr-Rv, 5′-CAATTTTGAAGAAGCAATATCTGATCATCTGT-3′) for its amplification. Total RNA extractions from E. granulosus protoscoleces and metacestodes were performed (23). The partial-length amplified cDNAs were obtained through direct sequencing of a PCR fragment (897 bp).
Sequence alignments were generated with the ClustalX software program, and modeling of the E. granulosus SR tertiary structure was obtained from the deduced primary structure using GenTHREADER (http://bioinf.cs.ucl.ac.uk/psipred/). Predictions of NLSs and hydrophobic cores were analyzed with cNLS Mapper (http://nls-mapper.iab.keio.ac.jp/cgi-bin/NLS_Mapper_form.cgi) and ProtScale (http://web.expasy.org/protscale).
Statistics.
Data within experiments were compared, and the significance was determined using the Student t test and nonparametric Mann-Whitney test. All data are shown as arithmetic means ± standard errors of the mean (SEM), and P values for each assay are indicated.
RESULTS
TAM treatment increases intracellular calcium in E. granulosus protoscoleces.
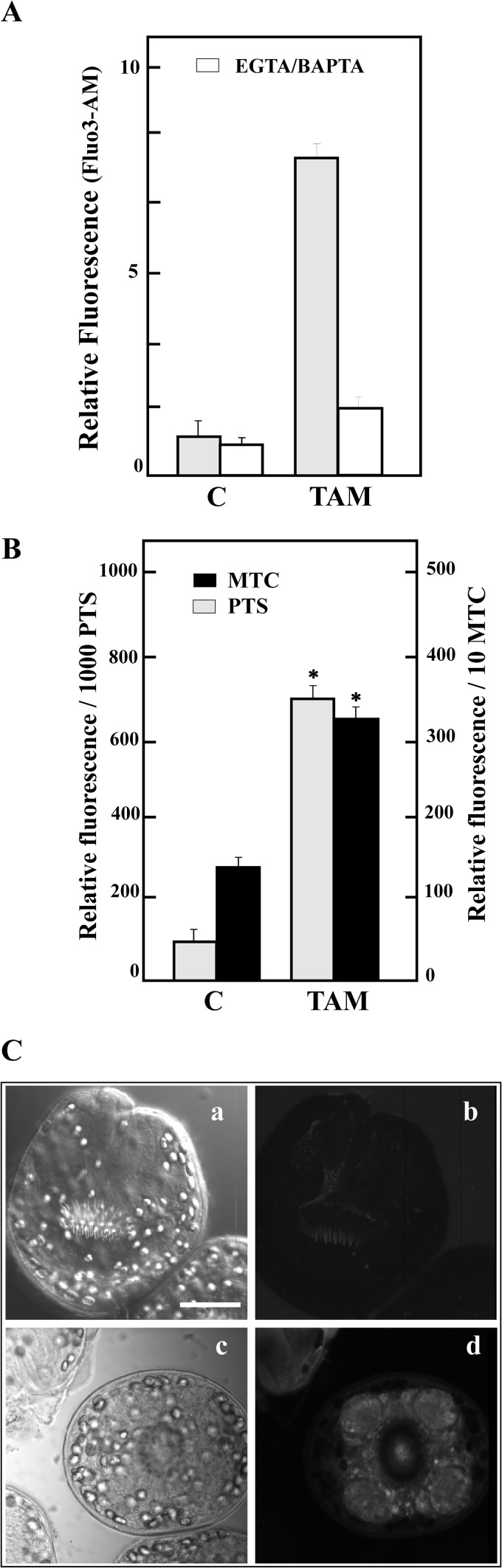
TAM exposure (20 μM) increased free [Ca2+]i 7-fold over a 2-h observation period compared with that in the controls (Fig. 1A). To determine whether the TAM-induced increase was from the intracellular stores or the extracellular space, we pretreated parasites with BAPTA-AM (a membrane-permeable calcium chelator) and EGTA (an extracellular chelator) immediately prior to addition of the drug. The fluorescence decreased to about control values only with both chelators in the medium, indicating that these drugs were able to release [Ca2+]i from extracellular and intracellular stores in E. granulosus. Normalization of cytosolic calcium was also consistently delayed in TAM-treated cultures compared with that in the controls (data not shown).
FIG 1.
In vitro effect of TAM treatment in Echinococcus granulosus protoscoleces and metacestodes. (A) Determination of changes in the cytosolic free Ca2+ concentration ([Ca2+]i) from control protoscoleces incubated with buffer (C, control) or with 20 μM TAM for 2 h and then loaded with Fluo-3-AM (▩). Pretreatments with 1 mM EGTA plus 100 μM BAPTA-AM (□) for 15 min were carried out before the Fluo-3-AM incubations. Data are the means ± SD from five independent experiments. (B) Relative fluorescence levels in protoscoleces (PTS) and dissected metacestodes (MTC) in the presence of 20 μM TAM compared with the control levels. Treated parasites showed a significant increase in fluorescence (*, P < 0.005) with respect to that of the control, indicating that TAM is a Pgp inhibitor. The values represent means ± standard deviations. (C) Confocal imaging revealed calcein accumulation in the tegument and suckers. Shown are light field (a and c) and fluorescence field (b and d) photomicroscopy; control protoscoleces (a and b) and protoscoleces treated with TAM (c and d). Bar, 50 μm.
TAM inhibits E. granulosus Pgp activity in protoscoleces and metacestodes.
Pgp activity is inversely proportional to the accumulation of intracellular fluorescent calcein. Control protoscoleces and metacestodes (sections and intact cysts) showed basal and stable E. granulosus Pgp activity, because the calcein-AM was extruded from the cells (typically 75 ± 5 fluorescent units [FU] and 125 ± 15 FU, respectively) (Fig. 1B). In the presence of 20 μM TAM, protoscoleces and metacestodes increased 10- and 3-fold in relative FU, respectively, over controls (Fig. 1B). Pgp activity was detected by confocal microscopy, particularly in suckers and tegument (Fig. 1C).
In vitro activity of TAM against E. granulosus protoscoleces and metacestodes.
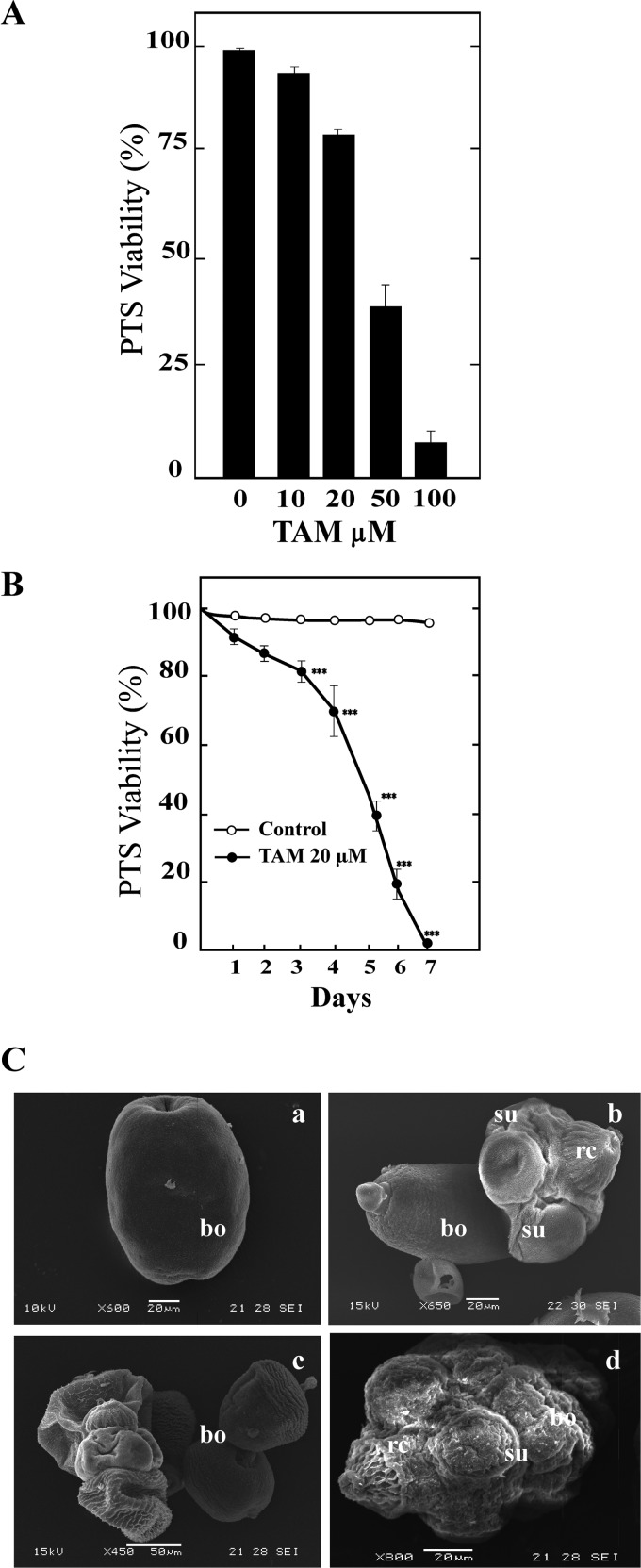
Viability decreased as a function of drug concentration in both protoscoleces and metacestodes (Fig. 2 and 3). TAM had an effect from a concentration of 10 μM and at 50 μM reduced the viability of treated protoscoleces to 45% ± 2.3% after 24 h (Fig. 2A). The protoscolex mortality rate was 100% after incubation for 7 days with 20 μM TAM (Fig. 2B). Untreated protoscoleces remained at least 99% ± 1.0% viable during the complete experiment. After 24 h of treatment, the ultrastructure of protoscoleces incubated with 20 μM TAM showed significant differences compared with that of control samples (Fig. 2C). Protoscoleces showed the a complete alteration of the tegument, shedding of microtriches, loss of hooks, and contraction of the soma region. After 24 h or 72 h, metacestodes incubated with 50 and 20 μM TAM presented detachment of the germinal layer in 100% of cysts (Fig. 3). The calculated 50% inhibitory concentration (IC50) against metacestodes was 18 ± 3 μM (Fig. 3A, inset).
FIG 2.
In vitro TAM treatment of Echinococcus granulosus protoscoleces. (A) Protoscolicidal activity of TAM from cultures maintained for 24 h. Bars show means ± standard errors from five independent experiments. (B) Long-term effect of 20 μM TAM on E. granulosus protoscolex viability over 7 days. Each point represents the mean percentage of vital protoscoleces from three different experiments. ***, Statistically significant difference (P < 0.05) from the corresponding control. (C) Ultrastructural changes of protoscoleces detected by SEM after incubation with TAM (20 μM, 48 h). Control (a, invaginated protoscolex; b, evaginated protoscolex) and TAM-treated (c and d) protoscoleces are shown. Note the extensive drug-induced damage with contraction of the soma region, alterations in the tegument, and loss of rostellar hooks and microtriches in the scolex region. Bars, 20 μm (a, b, and d) and 50 μm (c). rc, rostellar cone; su, suckers; bo, body.
FIG 3.
In vitro TAM treatment of Echinococcus granulosus metacestodes. (A) Metacestode viability measured on the basis of vesicle integrity. The IC50 is shown in the inset. (B) Macroscopic photographs showing the detachment of the germinal layer from the laminar layer of the metacestodes (20 μM, 48 h). Control (C, without morphological changes) and treated (TAM) metacestodes show increased permeability (culture medium inside cysts) and collapsed germinal membranes (arrows).
The ultrastructural alterations were accompanied by the release of E. granulosus GGT into the medium supernatant in a dose-dependent manner (data not shown). An increase in the E. granulosus GGT activity in the culture supernatants of cysts and protoscoleces incubated in vitro with 20 μM TAM was detected. After 24 h of incubation, total E. granulosus GGT activity in the protoscolex culture supernatants was measured (42 ± 5 nmol min−1 mg−1). This value was two times higher than that in metacestodes (18 ± 5 nmol min−1 mg−1). E. granulosus GGT activity was barely detectable in control supernatants.
Chemoprophylactic efficacy study.
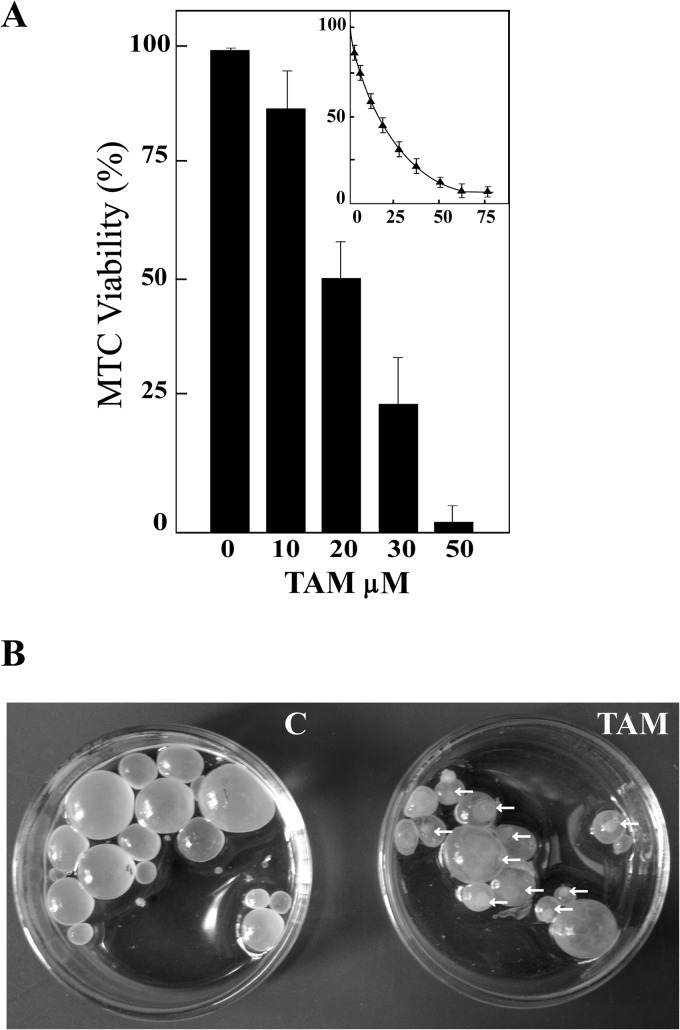
All of the infected mice from the untreated control group (10/10) developed metacestodes in the abdominal cavity, whereas in 4 out of the 10 treated mice, the infection did not progress. Statistically significant differences (P < 0.05) were observed in the weights of the cysts recovered from untreated mice (7.2 ± 3.4 g) compared with those from the TAM-treated group (0.64 ± 0.35 g) (Fig. 4A).
FIG 4.
Chemoprophylactic activity of TAM during Echinococcus granulosus cyst development. (A) Box plot showing the comparative distribution of the weight (g) of the cysts recovered from untreated and TAM-treated (20 mg/kg) mice. A significant cyst weight reduction (P < 0.005) was achieved in treated animals. (B) Representative SEM (a and b) and TEM (c and d) images of hydatid cysts recovered from untreated control mice (a and c) compared with those from TAM-treated mice (b and d). LL, laminar layer; MT, microtriches; DC, distal cytoplasm; GL, germinal layer.
All cysts removed from control mice appeared turgid, showing no observable collapse of the germinal layer, and no changes in ultrastructure were detected by SEM (Fig. 4Ba). TEM analysis of cysts recovered from the untreated control group revealed the typical features of E. granulosus metacestodes, with a distinct acellular outer laminated layer and a germinal layer without alterations (Fig. 4Bc). In contrast, the ultrastructural study of cysts developed in mice treated with TAM suspension revealed changes in the germinal layer. Regarding the ultrastructural study with SEM, only cell debris was observed (Fig. 4Bb). TEM analysis of cysts from treated mice revealed completely damaged germinal layers with internal tissue extensively distorted, vacuolated areas, and numerous lipid droplets (Fig. 4Bd).
Clinical efficacy study.
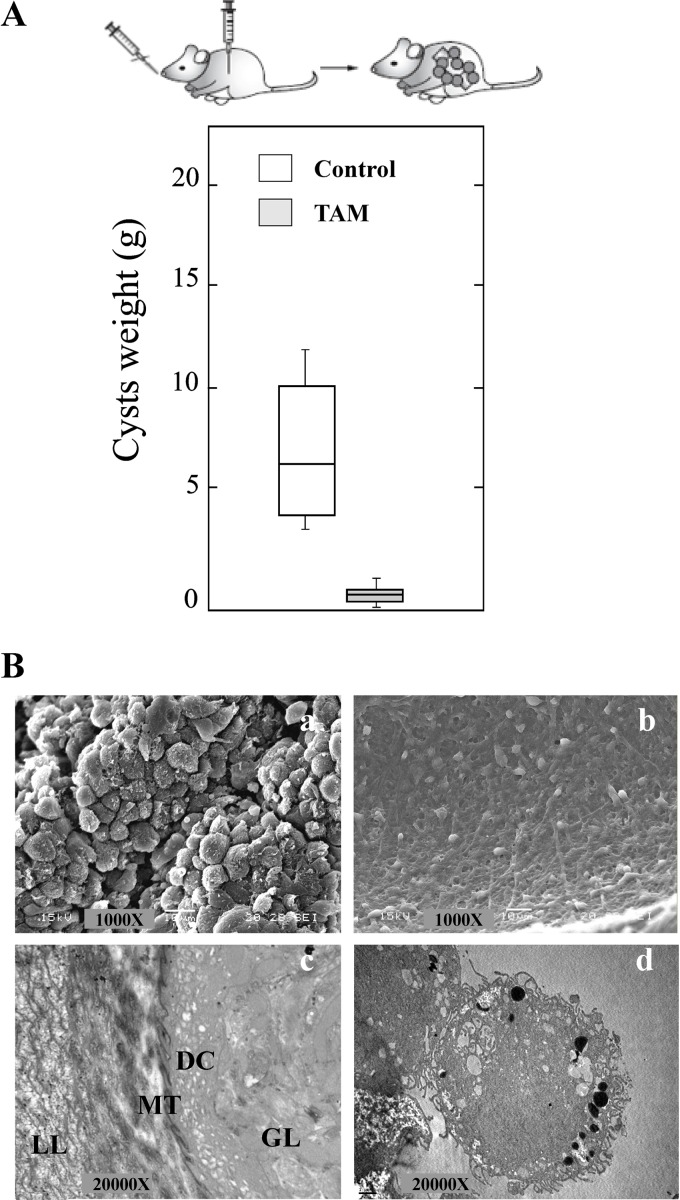
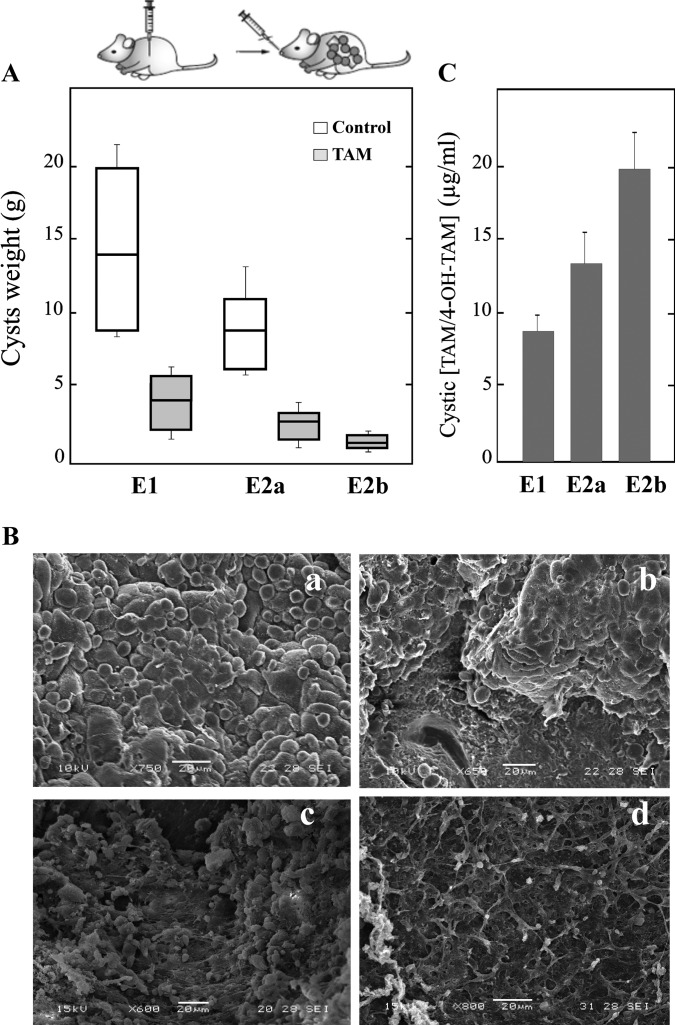
All infected animals involved in the efficacy study developed cysts in their abdominal cavity. All treatments resulted in statistically significant reductions in the cyst weights compared with those obtained from unmedicated mice (E1 and E2) (Fig. 5A).
FIG 5.
In vivo TAM treatment of Echinococcus granulosus-infected mice. (A) Box plots indicate the distribution of cyst weights (g) in the different treatment groups. Significant reductions of recovered parasite weights in relation to those in the control groups were achieved. (B) Representative images obtained by SEM of cysts from TAM-treated mice. (a) Control after 6 months p.i.; (b) TAM-treated mice from E1; (c) E2a; (d) E2b. (C) Intracystic concentrations of TAM and its metabolites from the experiments whose results are presented in panel B.
When the treatment was initiated after 6 months p.i. (E1), the efficacy of TAM was 61%. TAM treatment resulted in a reduction in the cyst weights (4.9 ± 1 g) compared with those obtained from unmedicated mice (12.5 ± 6.2 g). This reduction was statistically significant (P < 0.01). On the other hand, during E2, TAM treatment was initiated after cysts developed for 3 months. Two different therapeutic schemes were developed: 20 mg/kg every 48 h (E2a) and 100 mg/kg every 48 h (E2b). This 5-fold increase in the dose improved the efficacy of the treatment (83% versus 68%), but no statistically significant differences were found between the weights of cysts recovered from the two groups (P > 0.05) (Fig. 5A). There were statistical differences (P < 0.05) between the weight of cysts recovered from the unmedicated group (6.3 ± 2 g) and those from the E2a and E2b treated groups (2.0 ± 0.92 g and 1.1 ± 0.6 g, respectively).
All cysts removed from the unmedicated control mice at 3 or 6 months p.i. appeared turgid, showing no observable collapse of the germinal layer, in which no alteration in ultrastructure was observed by SEM (Fig. 5Ba). On the other hand, the ultrastructural study of cysts developed in mice treated with TAM revealed alterations in the germinal layer. However, the extent of damage appeared to be broader when the treatment was initiated at 3 months p.i. (E2) (Fig. 5Bc and Bd). Moreover, a complete loss of cells from the germinal layer of cysts was observed with the highest dose (100 mg/kg) (Fig. 5Bd).
TAM and its metabolites were detected in cysts obtained from treated mice (Fig. 5C). A dose-concentration relationship was detected for E2b (100 mg/kg, 20 ± 3 μg/ml) versus E2a (20 mg/kg, 12 ± 2 μg/ml).
In silico analysis of putative Echinococcus SR.
Reverse transcription (RT)-PCR analysis showed that E. granulosus sr1 is expressed in control conditions from protoscoleces and metacestodes (data not shown). E. granulosus SR is a typical NR (Fig. 6). It contains a DBD (residues 340 to 550) with two highly conserved zinc finger motifs (CI and CII, called the P and D boxes) responsible for the sequence-specific DNA recognition and the dimerization (Fig. 6A). The hinge region between the DBD and the LBD is a poorly conserved sequence in E. granulosus SR as in all NR, due to its function as a flexible region. In H. sapiens ER, this region is involved in CaM binding (residues 248 to 317), and it showed 38% similarity to E. granulosus SR (residues 430 to 500). The C-terminal LBD of E. granulosus SR (residues 580 to 770 compared with residues 340 to 530 of H. sapiens ER) is the most highly hydrophobic portion of the protein in agreement with orthologs (Fig. 6B). This region contains two partially conserved motifs (LXXLL, named the NR box) and key polar amino acids, which might be involved in hydrogen bonds with 17β-estradiol and estrogen derivates.
FIG 6.
Sequence analysis of Echinococcus granulosus steroid receptor (Eg-SR). Multiple sequence alignment of DBD (A) and LBD (B) among invertebrate and vertebrate species: Aplysia californica (Ac-ER) (NP_001191648, Mollusca) and Homo sapiens (Hs-ER) (NP_000116, Vertebrata). Consensus is indicated in the last line: total (capital letters), partial (lowercase letters), conservative changes (asterisks), and nonconservative substitutions (dots). Nuclear localization signals (NLS) are indicated with gray-shaded boxes. In the N-terminal DBD, the alignments of highly conserved zinc finger motifs (CI [C-X2-C-X13-C-X2-C-] and CII [C-X5/10-C-X9-C-X2-C]; dashed-line boxes) and the cysteine residues (arrows) are indicated. The CaM binding region is underlined, and three lysine residues involved in direct association with the CaM ER are indicated with arrowheads. In the C-terminal LBD, the hydrophobic portion involved in the ligand interaction is boxed, the conserved NR box motifs (residues shown with pound signs) and the key polar amino acids (residues shown with ! in H. sapiens ER corresponding to E353, R394, and H524) are indicated.
DISCUSSION
E. granulosus and E. multilocularis metacestodes exhibit tumor-like properties, as reflected by their seemingly unlimited growth and proliferation potential and their abilities to modulate the immune response and to form metastases (4). In this study, we demonstrate that TAM exhibits promising in vitro and in vivo activity against E. granulosus.
TAM treatment of protoscoleces (50 μM) and metacestodes (20 μM) resulted in the killing of 50% of parasites after 24 h compared with those in the controls (Fig. 2 and 3). As assessed by E. granulosus GGT activity, the protoscolex tegument was completely contracted (Fig. 2C), and the cyst germinal layer was detached (Fig. 3B). Taken together, all of the above results showed that 20 μM TAM has severe effects on the in vitro survival of the E. granulosus larval stage in a time-dependent manner, demonstrating the potent effect of the parental drug. While TAM exerts its toxic effects within a short time (24 to 48 h), other previously characterized drugs such as benzimidazoles (21, 25) and praziquantel (25) presented pharmacological effects only after 5 to 12 days of treatment. In future studies, we will assay the effect of TAM at shorter times (minutes) in order to evaluate its possible application as a scolicidal agent during surgery or puncture-aspiration-injection-reaspiration (PAIR).
As was mentioned previously, TAM induces protection against the in vivo establishment of T. solium and T. crassiceps (12, 13). Although the conditions used in our experiments are different, since E. granulosus is a tissue parasite, our results coincide. Protection against the infection was observed during the chemoprophylactic efficacy study. Furthermore, this study describes for the first time the clinical efficacy of TAM for a cestode parasite.
After the oral administration of TAM to mice, the drug demonstrated a chemoprophylactic effect. First, 20% of TAM-treated mice did not develop any cysts, while the infection progressed in all unmedicated mice. A deleterious drug effect on E. granulosus protoscoleces at the time of infection may help to explain the lack of cyst development. Additionally, statistically significant differences in cyst weight were detected between the treated and control groups. Therefore, TAM may not only reduce the number of cysts that develop but may also inhibit the development of secondary hydatidosis in mice. It is important to highlight the fact that the obtained results coincide with those for other treatment schedules previously assayed (26, 27). SEM and TEM evaluations revealed that TAM induces a number of characteristic alterations in the cyst germinal layer. The ultrastructural changes induced by TAM were similar to those described for other compounds such as albendazole, praziquantel, and flubendazole (26, 27), including distorted internal tissue, vacuolated areas, and the presence of abundant lipid droplets.
Hydatid cysts developed in all of the infected animals involved in the clinical efficacy studies. A clear reduction in cyst weight was observed after the administration of TAM in mice with cysts that developed during 3 or 6 months compared with that in the unmedicated control mice (Fig. 5A). Not surprisingly, greater effectiveness of the treatment was observed when TAM was administered 3 months p.i. This coincides with the results obtained by Urrea-París et al. (28), and a possible explanation is that the most pronounced development of cystic layers might protect the cyst against the drug. The germinal layer of cysts recovered from TAM-treated mice was markedly altered. Nevertheless, the ultrastructural damage extension appeared to be greater when the drug was administered earlier (3 months p.i.). Furthermore, when the dose was increased (100 mg/kg), complete destruction of the germinal layer was observed. Once again, the ultrastructural changes induced by TAM were similar to those described for other drugs, such as albendazole and flubendazole (21, 29, 30).
Despite the promising results, TAM has a parasitostatic rather than parasiticidal effect, as is also the case for benzimidazoles (4). Further studies should be conducted to focus on adjusting the dose of TAM to obtain a parasiticidal effect.
TAM, due to its high lipid partition coefficient, has been shown to be strongly incorporated in biomembranes (31). This TAM incorporation is increased when the cholesterol concentration is low. To be accurate, E. granulosus organisms do not synthesize cholesterol, and the amount of this lipid is limited in their cell membranes; thus, this aspect may favor accumulation of TAM in the cells. Besides, TAM is a potent inhibitor of the Pgp efflux transporter (32, 33). Our results in E. granulosus correlate well with these findings (Fig. 1B and C). Thus, this TAM retention in Echinococcus cells and cysts might enhance the pharmacological effects (Fig. 1B and C and 4 and 5). Moreover, we demonstrated that TAM and its metabolites were accumulated in the cysts in adequate quantities after in vivo treatment of infected mice. Our results coincide with the concentrations reported by Robinson et al. (34) in mouse tissues.
Signaling pathways involving sterol-responsive nuclear receptors are conserved from invertebrates to mammals and regulate metabolism and development (35). Recent evidence revealed that estrogens can affect Taenia physiology (13, 36). Within this line of evidence, the efficiency of chemoprotective TAM treatment suggests a possible role for the estrogens in Echinococcus development. Interestingly, all NRs have been recently identified in E. multilocularis and E. granulosus genomes, and one of them showed the highest homology to the LBDs of vertebrate ER (17). In this work, we verified in the E. granulosus larval stage the constitutive expression of this putative SR, E. granulosus SR, an EmuJ_000814300 ortholog, which displayed similarities in structure to the H. sapiens ER and an invertebrate prototype ER (Fig. 6).
Furthermore, it has been proposed that during estrogen receptor evolution, specificity has been determined by the early requirement for the aromatized A ring, the final step in a conserved estrogen synthesis pathway beginning with cholesterol (37). Therein, a promiscuous ancestral SR is activated by nonsteroidal ER agonists such as DES and genistein and competitively inhibited by ER antagonists such as SERMs (37). Tapeworms, like flukes, lack the ability to synthesize cholesterol de novo, and until now there has been no conclusive research on the biosynthesis of estrogens, progesterone, and androgens in cestodes (17). Metabolic and transcriptomic studies have demonstrated that sterol synthesis in E. granulosus is interrupted at the level of farnesyl or nerolidol pyrophosphate, that the cholesterol derives from the host, and that for signaling actions, the host sterols may be modified by the parasite (38). But it is highly likely that if Echinococcus spp. have sensitivity to estrogens, the candidate receptor might be the E. granulosus SR.
Calcium-dependent activation of H. sapiens ER is mediated by CaM, and CaM antagonists prevent 17β-estradiol from stimulating the transcription (39, 40). CaM binds to H. sapiens ER in the hinge domain (residues 248 to 317) and contributes to the ER dimerization and to the ER transcriptional activity in combination with 17β-estradiol and calcium (39, 40). The E. granulosus SR conserves this hydrophobic region in the LBD involved in association with the CaM ER (Fig. 6) (40). On the other hand, in our attempt to understand the TAM mechanism in E. granulosus, several findings suggested that TAM interfered with calcium homeostasis in eukaryotic cells (41). Different research on mammalian cells and yeast clearly showed that TAM targets endoplasmic reticulum calcium pumps, protein kinase C, and/or CaM. Consequently, this targeting induces an increase in intracellular calcium as a non-estrogen receptor-dependent effect (18). Our in vitro results on the TAM effects suggest a calcium cell deregulation in treated protoscoleces (Fig. 1A) through independent or indirectly ER-dependent mechanisms, which must be confirmed by further studies on the Echinococcus larval stage.
In the search for an effective drug against E. granulosus, we showed that TAM, at low micromolar concentrations, inhibits the survival of protoscoleces and metacestodes. Moreover, we demonstrated the chemotherapeutic and chemopreventive pharmacological effects of TAM. Since the collateral effects of high TAM doses have been largely documented in clinical trials, the use of low doses of this drug as a short-term therapy may be a novel alternative approach for human CE treatment. Nevertheless, this is a preliminary study performed in a murine model, and for this reason, more exhaustive evaluation should be carried out in humans to fully demonstrate the therapeutic usefulness of TAM.
ACKNOWLEDGMENTS
This work was supported by CONICET (grant PIP 0029), ANPCyT (grants PICT 2012 2668 and 1164), and the Universidad Nacional de Mar del Plata (grants EXA 572/12 and 581/12), Argentina.
We gratefully acknowledge M. Oppedisano for technical assistance with the electronic microscopy. We also appreciate the use of the facilities at the IIB-CONICET-UNMdP. The E. multilocularis and E. granulosus genome sequence data mentioned were produced by the Pathogen Sequencing Group of the Wellcome Trust Sanger Institute (Program of Helminth Sequencing; project manager, Matt Berriman).
Footnotes
Published ahead of print 16 June 2014
REFERENCES
- 1.Vuitton DA. 2009. Benzimidazoles for the treatment of cystic and alveolar echinococcosis: what is the consensus? Expert Rev. Anti Infect. Ther. 7:145–149. 10.1586/14787210.7.2.145 [DOI] [PubMed] [Google Scholar]
- 2.Hemphill A, Stadelmann B, Scholl S, Müller J, Spiliotis M, Müller N, Gottstein B, Siles-Lucas M. 2010. Echinococcus metacestodes as laboratory models for the screening of drugs against cestodes and trematodes. Parasitology 137:569–587. 10.1017/S003118200999117X [DOI] [PubMed] [Google Scholar]
- 3.Naguleswaran A, Spicher M, Vonlaufen N, Ortega-Mora LM, Torgerson P, Gottstein B, Hemphill A. 2006. In vitro metacestodicidal activities of genistein and other isoflavones against Echinococcus multilocularis and Echinococcus granulosus. Antimicrob. Agents Chemother. 50:3770–3778. 10.1128/AAC.00578-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spicher M, Naguleswaran A, Ortega-Mora LM, Müller J, Gottstein B, Hemphill A. 2008. In vitro and in vivo effects of 2-methoxyestradiol, either alone or combined with albendazole, against Echinococcus metacestodes. Exp. Parasitol. 119:475–482. 10.1016/j.exppara.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 5.Hemer S, Brehm K. 2012. In vitro efficacy of the anticancer drug imatinib on Echinococcus multilocularis larvae. Int. J. Antimicrob. Agents. 40:458–462. 10.1016/j.ijantimicag.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 6.Radmacher M, Simon R. 2000. Estimation of tamoxifen's efficacy for preventing the formation and growth of breast tumors. J. Natl. Cancer Inst. 92:48–53. 10.1093/jnci/92.1.48 [DOI] [PubMed] [Google Scholar]
- 7.Mandlekar S, Hebbar V, Christov K, Kong A. 2000. Pharmacodynamics of tamoxifen and its 4-hydroxy and N-desmethyl metabolites: activation of caspases and induction of apoptosis in rat mammary tumors and in human breast cancer cell lines. Cancer Res. 60:6601–6606 [PubMed] [Google Scholar]
- 8.Salami S, Karami-Tehrani F. 2003. Biochemical studies of apoptosis induced by tamoxifen in estrogen receptor positive and negative breast cancer cell lines. Clin. Biochem. 36:247–253. 10.1016/S0009-9120(03)00007-9 [DOI] [PubMed] [Google Scholar]
- 9.Miguel DC, Yokoyama-Yasunaka JK, Andreoli WK, Mortara RA, Uliana SR. 2007. Tamoxifen is effective against Leishmania and induces a rapid alkalinization of parasitophorous vacuoles harbouring Leishmania (Leishmania) amazonensis amastigotes. J. Antimicrob. Chemother. 60:526–534. 10.1093/jac/dkm219 [DOI] [PubMed] [Google Scholar]
- 10.Miguel DC, Yokoyama-Yasunaka JK, Uliana SR. 2008. Tamoxifen is effective in the treatment of Leishmania amazonensis infections in mice. PLoS Negl. Trop. Dis. 2:e249. 10.1371/journal.pntd.0000249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miguel DC, Ferraz ML, Alves RDO, Yokoyama-Yasunaka JK, Torrecilhas AC, Romanha AJ, Uliana SR. 2010. The anticancer drug tamoxifen is active against Trypanosoma cruzi in vitro but ineffective in the treatment of the acute phase of Chagas disease in mice. Mem. Inst. Oswaldo Cruz 105:945–948. 10.1590/S0074-02762010000700021 [DOI] [PubMed] [Google Scholar]
- 12.Vargas-Villavicencio JA, Larralde C, De Leon-Nava MA, Escobedo G, Morales-Montor J. 2007. Tamoxifen treatment induces protection in murine cysticercosis. J. Parasitol. 93:1512–1517. 10.1645/GE-1191.1 [DOI] [PubMed] [Google Scholar]
- 13.Escobedo G, Palacios-Arreola MI, Olivos A, López-Griego L, Morales-Montor J. 2013. Tamoxifen treatment in hamsters induces protection during taeniosis by Taenia solium. Biomed. Res. Int. 2013:280496. 10.1155/2013/280496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. 1998. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95:927–937. 10.1016/S0092-8674(00)81717-1 [DOI] [PubMed] [Google Scholar]
- 15.Paulmurugan R, Gambhir SS. 2006. An intramolecular folding sensor for imaging estrogen receptor-ligand interactions. Proc. Natl. Acad. Sci. U. S. A. 103:15883–15888. 10.1073/pnas.0607385103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maximov PY, Myers CB, Curpan RF, Lewis-Wambi JS, Jordan VC. 2010. Structure-function relationships of estrogenic triphenylethylenes related to endoxifen and 4-hydroxytamoxifen. J. Med. Chem. 53:3273–3283. 10.1021/jm901907u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Förster S, Günthel D, Kiss F, Brehm K. 2011. Molecular characterisation of a serum-responsive, DAF-12-like nuclear hormone receptor of the fox-tapeworm Echinococcus multilocularis. J. Cell Biochem. 112:1630–1642. 10.1002/jcb.23073 [DOI] [PubMed] [Google Scholar]
- 18.Dolan K, Montgomery S, Buchheit B, Didone L, Wellington M, Krysan DJ. 2009. Antifungal activity of tamoxifen: in vitro and in vivo activities and mechanistic characterization. Antimicrob. Agents Chemother. 53:3337–3346. 10.1128/AAC.01564-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman ZY. 1998. Recent advance in the molecular mechanisms of tamoxifen action. Cancer Invest. 16:391–396. 10.3109/07357909809115779 [DOI] [PubMed] [Google Scholar]
- 20.Cumino AC, Lamenza P, Denegri GM. 2010. Identification of functional FKB protein in Echinococcus granulosus: its involvement in the protoscolicidal action of rapamycin derivates and in calcium homeostasis. Int. J. Parasitol. 40:651–661. 10.1016/j.ijpara.2009.11.011 [DOI] [PubMed] [Google Scholar]
- 21.Elissondo MC, Ceballos L, Alvarez L, Sánchez Bruni S, Lanusse C, Denegri G. 2009. Flubendazole and ivermectin in vitro combination therapy produces a marked effect on Echinococcus granulosus protoscoleces and metacestodes. Parasitol. Res. 105:835–842. 10.1007/s00436-009-1469-y [DOI] [PubMed] [Google Scholar]
- 22.Nicolao MC, Denegri GM, Cárcamo JG, Cumino AC. 2014. P-glycoprotein expression and pharmacological modulation in larval stages of Echinococcus granulosus. Parasitol. Int. 63:1–8. 10.1016/j.parint.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 23.Cumino AC, Nicolao MC, Loos JA, Denegri G, Elissondo MC. 2012. Echinococcus granulosus tegumental enzymes as in vitro markers of pharmacological damage: a biochemical and molecular approach. Parasitol. Int. 61:579–585. 10.1016/j.parint.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 24.Jordan VC, Bain RR, Brown RR, Gosden B, Santos MA. 1983. Determination and pharmacology of a new hydroxylated metabolite of tamoxifen observed in patient sera during therapy for advanced breast cancer. Cancer Res. 43:1446–1450 [PubMed] [Google Scholar]
- 25.Urrea-París MA, Moreno MJ, Casado N, Rodriguez-Caabeiro F. 2000. In vitro effect of praziquantel and albendazole combination therapy on the larval stage of Echinococcus granulosus. Parasitol. Res. 86:957–964. 10.1007/PL00008526 [DOI] [PubMed] [Google Scholar]
- 26.Casado N, Urrea-París M, Moreno M, Rodriguez-Caabeiro F. 2001. Combined praziquantel and albendazole chemoprophylaxis in experimental hydatidosis. Parasitol. Res. 87:787–789. 10.1007/s004360100443 [DOI] [PubMed] [Google Scholar]
- 27.Ceballos L, Elissondo C, Confalonieri A, Denegri G, Alvarez L, Lanusse C. 2010. Chemoprophylactic activity of flubendazole in cystic echinococcosis. Chemotherapy 56:386–392. 10.1159/000316827 [DOI] [PubMed] [Google Scholar]
- 28.Urrea-París MA, Moreno MJ, Casado N, Rodríguez-Caabeiro F. 2002. Relationship between the efficacy of praziquantel treatment and the cystic differentiation in vivo of Echinococcus granulosus metacestode. Parasitol. Res. 88:26–31. 10.1007/s004360100468 [DOI] [PubMed] [Google Scholar]
- 29.Ceballos L, Elissondo C, Moreno L, Dopchiz M, Bruni SS, Denegri G, Alvarez L, Lanusse C. 2008. Albendazole treatment in cystic echinococcosis: pharmacokinetics and clinical efficacy of two different aqueous formulations. Parasitol. Res. 103:355–362. 10.1007/s00436-008-0980-x [DOI] [PubMed] [Google Scholar]
- 30.Ceballos L, Elissondo M, Bruni SS, Denegri G, Alvarez L, Lanusse C. 2009. Flubendazole in cystic echinococcosis therapy: pharmaco-parasitological evaluation in mice. Parasitol. Int. 58:354–358. 10.1016/j.parint.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 31.Kisanga ER, Gjerde J, Guerrieri-Gonzaga A, Pigatto F, Pesci-Feltri A, Robertson C, Serrano D, Pelosi G, Decensi A, Lien EA. 2004. Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clin. Cancer Res. 10:2336–2343. 10.1158/1078-0432.CCR-03-0538 [DOI] [PubMed] [Google Scholar]
- 32.Callaghan R, Higgins CF. 1995. Interaction of tamoxifen with the multidrug resistance P-glycoprotein. Br. J. Cancer 71:294–299. 10.1038/bjc.1995.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hennessy M, Spiers JP. 2007. A primer on the mechanics of P-glycoprotein the multidrug transporter. Pharmacol. Res. 55:1–15. 10.1016/j.phrs.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 34.Robinson SP, Langan-Fahey SM, Johnson DA, Jordan CV. 1991. Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab. Dispos. 19:36–43 [PubMed] [Google Scholar]
- 35.Wollam J, Antebi A. 2011. Sterol regulation of metabolism, homeostasis, and development. Annu. Rev. Biochem. 80:885–916. 10.1146/annurev-biochem-081308-165917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernández-Bello R, Escobedo G, Guzman C, Ibarra-Coronado EG, López-Griego L, Morales-Montor J. 2010. Immunoendocrine host-parasite interactions during helminth infections: from the basic knowledge to its possible therapeutic applications. Parasite Immunol. 32:633–643. 10.1111/j.1365-3024.2010.01232.x [DOI] [PubMed] [Google Scholar]
- 37.Eick GN, Colucci JK, Harms MJ, Ortlund EA, Thornton JW. 2012. Evolution of minimal specificity and promiscuity in steroid hormone receptors. PLoS Genet. 8:e1003072. 10.1371/journal.pgen.1003072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obal G, Ramos AL, Silva V, Lima A, Batthyany C, Bessio MI, Ferreira F, Salinas G, Ferreira AM. 2012. Characterisation of the native lipid moiety of Echinococcus granulosus antigen B. PLoS Negl. Trop. Dis. 6:e1642. 10.1371/journal.pntd.0001642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Li Z, Sacks DB. 2005. The transcriptional activity of estrogen receptor is dependent on Ca2/calmodulin. J. Biol. Chem. 280:13097–13104. 10.1074/jbc.M410642200 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Li Z, Sacks DB, Ames JB. 2012. Structural basis for Ca2+-induced activation and dimerization of estrogen receptor α by calmodulin. J. Biol. Chem. 287:9336–9344. 10.1074/jbc.M111.334797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsons AB, Lopez A, Givini TE, Williams DE, Gray CA, Porter J, Chua G, Sopko R, Brost RL, Ho CH, Wang J, Ketela T, Brenner C, Brill JA, Fernandez GE, Lorenz TC, Payne GS, Ishihara S, Ohya Y, Andrews B, Hughes TR, Frey BJ, Graham TR, Andersen RJ, Boone C. 2006. Exploring the mode-of action of bioactive compounds by chemical-genetic profiling in yeast. Cell 126:611–625. 10.1016/j.cell.2006.06.040 [DOI] [PubMed] [Google Scholar]