Abstract
The reference broth microdilution (BMD) antimicrobial susceptibility testing method for telavancin was revised to include dimethyl sulfoxide (DMSO) as a solvent and diluent for frozen-form panel preparation, following the CLSI recommendations for water-insoluble agents. Polysorbate 80 (P-80) was also added to the test medium to minimize proven drug losses associated with binding to plastic surfaces. Four hundred sixty-two Gram-positive isolates, including a challenge set of organisms with reduced susceptibilities to comparator agents, were selected and tested using the revised method for telavancin, and the MIC results were compared with those tested by the previously established method and several Sensititre dry-form BMD panel formulations. The revised method provided MIC results 2- to 8-fold lower than the previous method when tested against staphylococci and enterococci, resulting in MIC50 values of 0.03 to 0.06 μg/ml for staphylococci and 0.03 and 0.12 μg/ml for Enterococcus faecium and Enterococcus faecalis, respectively. Less-significant MIC decreases (1 to 2 log2 dilution steps) were observed when testing streptococci in broth supplemented with blood, which showed similar MIC50 values for both methods. However, Streptococcus pneumoniae had MIC50 results of 0.008 and 0.03 μg/ml when tested by the revised and previous methods, respectively. Highest essential agreement rates (≥94.0%) were noted for one candidate dry-form panel formulation compared to the revised test. The revised BMD method provides lower MIC results for telavancin, especially when tested against staphylococci and enterococci. This is secondary to the use of DMSO for panel production and the presence of P-80, which ensure the proper telavancin testing concentration and result in a more accurate MIC determination. Moreover, earlier studies where the previous method was applied underestimated the in vitro drug potency.
INTRODUCTION
Telavancin is a lipoglycopeptide antibiotic with potent in vitro bactericidal activity when tested against Gram-positive bacteria, including methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant S. aureus (MRSA), vancomycin-intermediate S. aureus (VISA), heterogeneous VISA (hVISA), and multidrug-resistant (MDR) streptococci and enterococci (1, 2). Telavancin is approved in the United States and Canada for the treatment of patients with complicated skin and skin structure infections due to susceptible Gram-positive pathogens and in the United States and Europe for the treatment of hospital-acquired bacterial pneumonia, including ventilator-associated bacterial pneumonia (HABP/VABP) due to susceptible isolates of S. aureus (MRSA strains only in Europe), when alternative medicines are unsuitable (3).
During the development of dalbavancin, also a lipoglycopeptide, the use of polysorbate 80 (P-80) (0.002%, final testing concentration) was shown to be essential for accurate MIC susceptibility testing determinations (4). Subsequent investigations for oritavancin (another lipoglycopeptide) demonstrated that the addition of P-80 to MIC testing broth was also necessary for test performance reliability via minimizing the drug binding to plastic 96-well panels (5), similar to dalbavancin. The antimicrobial susceptibility testing for these lipoglycopeptide agents was revised (6, 7), and updated quality control (QC) ranges for dalbavancin and oritavancin were established and published by the Clinical and Laboratory Standards Institute (CLSI), in M100-S24 and previous documents (8). Surfactants, such as P-80, act as wetting agents and are commonly used in commercially prepared antimicrobial agent susceptibility testing panels or as part of the inoculum for broth microdilution assays to aid in the homogenous dispersal of reagents or to ensure their quantitative recovery from solution (4, 5).
With these precedents, the effect of the addition of P-80 on telavancin broth microdilution (BMD) testing was deemed prudent, as well as addressing the need for changes in solvents and/or diluents to achieve optimal drug solubilization. Further investigations proposed the use of dimethyl sulfoxide (DMSO) as the solvent for stock solution preparation, as well as a stock solution diluent for panel preparation. In addition, P-80 was incorporated into the test medium. These changes were shown to improve drug solubility during panel preparation (DMSO) and drug availability in the 96-well plastic plates (P-80), resulting in a more accurate in vitro assessment of telavancin MIC determinations (data on file; Theravance, Inc.). Initial studies using this revised method observed that the MIC50 results for telavancin were 4- to 8-fold lower than those obtained by the previous applied method (use of DMSO and water as solvent and diluent for panel preparation, respectively, and no P-80 supplementation) when tested against staphylococci and enterococci, but minimal differences were observed when testing streptococci (data on file; JMI Laboratories).
The revised method and subsequent differences in MIC results prompted the reestablishment of QC ranges for telavancin (9) and interpretive breakpoints (3). The revised method, along with QC ranges and updated breakpoints, was approved by the Food and Drug Administration (FDA) and incorporated into the labeling supplement for the product Vibativ (telavancin) (3). The revised method and respective QC ranges are also currently available in the CLSI M100-S24 document (8). The purpose of this study was to fully evaluate telavancin MIC results when using the revised BMD method compared with those obtained by the previous CLSI method when tested against a larger collection of clinically relevant strains. In addition, the telavancin MIC results obtained with the revised method were compared with several candidate dry-form formulation panels.
MATERIALS AND METHODS
Clinical and reference isolates.
A total of 462 clinical isolates were included in this study. Initially, Gram-positive clinical strains collected during previous worldwide surveillance programs (89.6% from the 2009 surveyed year) were selected. These strains originated predominantly in U.S. (51.7%) and European (47.8%) hospitals and included S. aureus (100 strains), coagulase-negative staphylococci (CoNS) (101 strains), Enterococcus faecalis (61; 15 VanA and 5 VanB resistance phenotypes and 41 vancomycin-susceptible strains), Enterococcus faecium (44; 17 VanA and 6 VanB resistance phenotypes and 21 vancomycin-susceptible strains), Streptococcus pneumoniae (50 strains), viridans group streptococci (VGS) (25 strains), and beta-hemolytic streptococci (BHS) (25 strains).
Second, a challenge set of organisms (56 strains) displaying decreased antimicrobial susceptibilities to several key comparator agents were selected and included in this study, as follows: hVISA (11 strains), VISA (5 strains), vancomycin-resistant S. aureus (VRSA) (6 strains), vancomycin-resistant enterococci (VRE) (4 E. faecalis [2 VanA and 2 VanB types] and 6 E. faecium [4 VanA and 2 VanB-types]), daptomycin-nonsusceptible staphylococci (6 S. aureus and 7 CoNS), and linezolid-resistant staphylococci (4 S. aureus and 7 Staphylococcus epidermidis). Some of the isolates included in this set (22 strains) were provided by the Network on Antimicrobial Resistance in S. aureus (NARSA) (www.narsa.net).
Antimicrobial susceptibility testing.
Telavancin stock solutions were dissolved and diluted in DMSO, following CLSI recommendations for water-insoluble agents for the preparation of frozen-form panels according to the revised method (see Table 8B in CLSI document M100-S24 [8]). Briefly, the dry powder was dissolved in 100% DMSO in a glass vial to obtain a concentration of 1,600 μg/ml. This stock solution was diluted again using DMSO (100%) in order to obtain intermediate concentrations. These were further diluted (100×) in Mueller-Hinton broth (MHB) containing P-80 (0.002%, final testing concentration). Aliquots (50 μl) of these final concentrations were dispensed into 96-well plates.
Frozen-form panels produced according to the previously established susceptibility testing method were manufactured, following the previous CLSI recommendations (M100-S23) (10). Several Sensititre dry-form broth microdilution panel candidate formulations (eight) were manufactured and tested simultaneously with the previous and revised frozen-form panels. All 96-well panels were manufactured by ThermoFisher Scientific (formerly Trek Diagnostics Systems/Sensititre, Cleveland, Ohio), following the recommendations described in the M07-A9 document (11). MHB was supplemented with 2.5 to 5% lysed horse blood (LHB) for testing fastidious streptococci.
Validation of the MIC values was performed by concurrent testing of American Type Culture Collection (ATCC) QC strains: S. aureus ATCC 29213, E. faecalis ATCC 29212, and S. pneumoniae ATCC 49619 (8). All telavancin MIC QC values obtained by frozen-form panels prepared according to the previous and revised methods were within the ranges published in the M100-S23 and M100-S24 documents, respectively (3, 8–10). Telavancin MIC values obtained by the revised method were considered reference results for these analyses. MIC values obtained by the previous frozen-form and dry-form formulation panels that were between ±1 log2 dilution step compared to the revised method were considered essential agreement (EA). The minimal acceptable criteria for EA was targeted at ≥90% (12).
RESULTS AND DISCUSSION
Overall, the majority (345/462 [74.7%]) of telavancin MIC results obtained by the previous method were ≥2 log2 dilution steps higher than those obtained with the revised reference method, which translated into low EA between the two methods (Table 1). When telavancin was tested using the previously established BMD method, >96% of S. aureus and CoNS clinical isolates had telavancin MIC results 2 to 3 doubling dilutions higher than those obtained by the revised method (Table 1). These lower results obtained by the revised BMD method translated into telavancin modal MIC and MIC50 values of 0.03 and 0.06 μg/ml for S. aureus and CoNS, respectively, which were 8- and 4-fold lower than those obtained by the previously established BMD method (all 0.25 μg/ml) (Table 2).
TABLE 1.
MIC result variations and summary of essential agreement rates between previously established broth microdilution method and revised reference method for telavancin
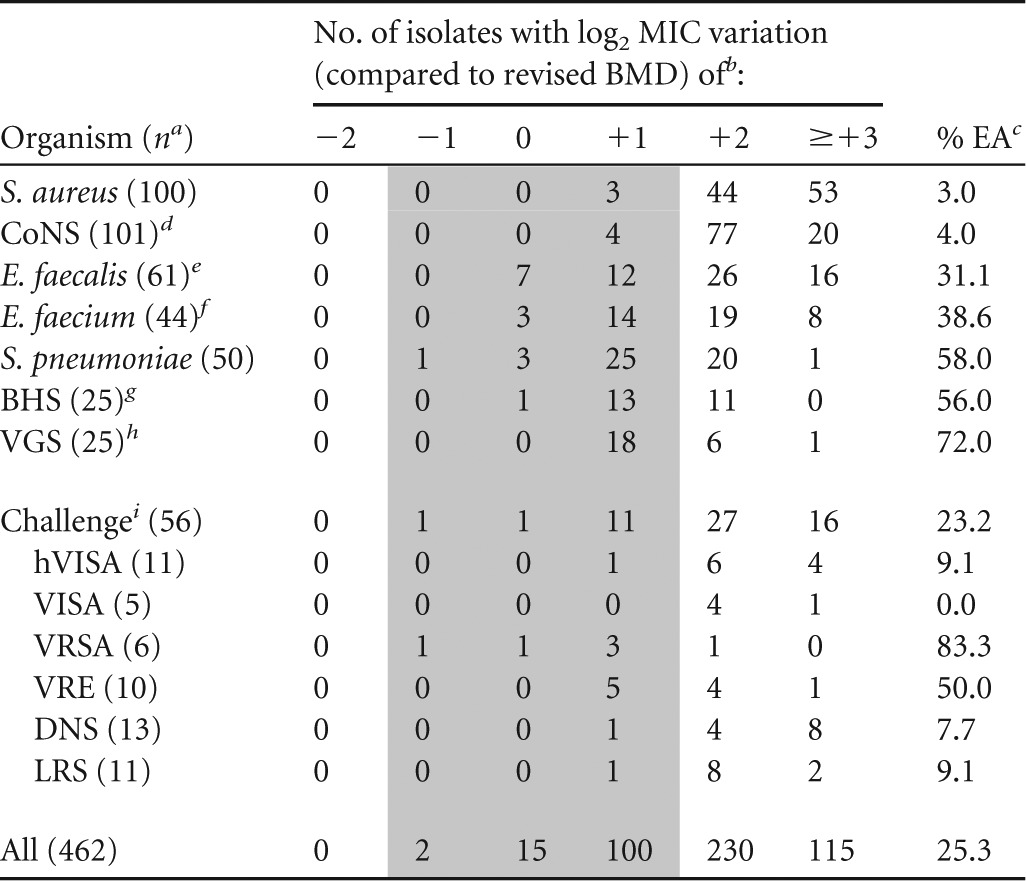
n, no. of isolates tested.
Previously established BMD panels prepared with DMSO and water as the solvent and diluent, respectively, and no P-80 supplementation versus a revised BMD panel (DMSO as solvent and diluent and P-80 supplementation [0.002%]).
Percentage of essential agreement (±1 log2 dilution step), represented by the shaded area.
CoNS, coagulase-negative staphylococci.
Includes 20 VRE (15 VanA and 5 VanB phenotypes).
Includes 23 VRE (17 VanA and 6 VanB phenotypes).
BHS, beta-hemolytic streptococci.
VGS, viridans group streptococci.
hVISA, heterogeneous vancomycin-intermediate S. aureus; VISA, vancomycin-intermediate S. aureus; VRSA, vancomycin-resistant S. aureus; VRE, vancomycin-resistant enterococci (4 E. faecalis isolates [2 VanA and 2 VanB types] and 6 E. faecium isolates [4 VanA and 2 VanB types]); DNS, daptomycin-nonsusceptible staphylococci; LRS, linezolid-resistant staphylococci).
TABLE 2.
In vitro MIC results for telavancin when tested against Gram-positive isolates using previously established broth microdilution method and revised reference method
| Organism (na) | Methodb | MIC (μg/ml) |
MIC50/MIC90 ratioc | |||
|---|---|---|---|---|---|---|
| Range | Mode | 50% | 90% | |||
| All (462) | Previous | ≤0.004–>8 | 0.25 | 0.25 | 2 | 4/8 |
| Revised | ≤0.004–8 | 0.03 | 0.06 | 0.25 | ||
| S. aureus (100) | Previous | 0.06–0.5 | 0.25 | 0.25 | 0.5 | 8/8 |
| Revised | 0.015–0.25 | 0.03 | 0.03 | 0.06 | ||
| CoNS (101)d | Previous | 0.06–0.5 | 0.25 | 0.25 | 0.5 | 4/8 |
| Revised | 0.015–0.12 | 0.06 | 0.06 | 0.06 | ||
| E. faecalis (61)e | Previous | 0.12–>8 | 0.5 | 0.5 | 8 | 4/2 |
| Revised | 0.03–8 | 0.12 | 0.12 | 4 | ||
| E. faecalis VanSf (41) | Previous | 0.12–1 | 0.5 | 0.5 | 1 | 4/8 |
| Revised | 0.03–0.25 | 0.12 | 0.12 | 0.12 | ||
| E. faecium (44)g | Previous | 0.06–4 | 0.12, 4h | 0.25 | 4 | 8/2 |
| Revised | 0.015–4 | 0.03 | 0.03 | 2 | ||
| E. faecium VanSf (21) | Previous | 0.06–0.5 | 0.12 | 0.12 | 0.25 | 4/8 |
| Revised | 0.015–0.06 | 0.03 | 0.03 | 0.03 | ||
| S. pneumoniae (50) | Previous | ≤0.004–0.12 | 0.03 | 0.03 | 0.06 | 4/2 |
| Revised | ≤0.004–0.06 | 0.008 | 0.008 | 0.03 | ||
| BHS (25)i | Previous | 0.03–0.12 | 0.06 | 0.06 | 0.12 | 2/4 |
| Revised | 0.015–0.06 | 0.03 | 0.03 | 0.03 | ||
| VGS (25)j | Previous | 0.03–0.12 | 0.06 | 0.06 | 0.12 | 2/4 |
| Revised | 0.015–0.06 | 0.03 | 0.03 | 0.03 | ||
| Challenge (56)k | Previous | 0.06–8 | 0.25, 0.5h | 0.5 | 4 | 8/1 |
| Revised | 0.015–8 | 0.06 | 0.06 | 4 | ||
n, no. of isolates tested.
Previously established BMD panels prepared with DMSO and water as the solvent and diluent, respectively, and no P-80 supplementation versus a revised BMD panel (DMSO as solvent and diluent and P-80 supplementation [0.002%]).
MIC50 obtained by the previous method/MIC50 obtained by the revised method; and MIC90 obtained by the previous method/MIC90 obtained by the revised method.
CoNS, coagulase-negative staphylococci.
Includes 20 VRE (15 VanA and 5 VanB phenotypes).
VanS, vancomycin susceptible.
Includes 23 VRE (17 VanA and 6 VanB phenotypes).
Bimodal MIC distribution (two modal values).
BHS, beta-hemolytic streptococci.
VGS, viridans group streptococci.
Represent strains with key resistance phenotypes (11 hVISA, 5 VISA, 6 VRSA, 10 VRE, 13 daptomycin-nonsusceptible staphylococci, and 11 linezolid-resistant staphylococci).
Similarly, for E. faecalis and E. faecium, when they were tested by the previous method, most MIC results were 2 log2 dilutions higher than those obtained by the revised BMD method (Table 1). The previous method generated results against all E. faecalis (MIC50, 0.5 μg/ml) and E. faecium (MIC50, 0.25 μg/ml) 4- and 8-fold higher than those with the revised method (MIC50 values of 0.12 and 0.03 μg/ml, respectively) (Table 2). Differences in MIC results between frozen-form BMD methods were less significant for the streptococci, where the majority of MIC values obtained by the previous method were only 1 doubling dilution step higher than those obtained by the revised method (Table 1). This observation translated into greater equivalence between methods for both BHS and VGS, which exhibited telavancin MIC50 values of 0.06 μg/ml by the previous method, while 2-fold-lower MIC50 results were noted for the revised method (i.e., 0.03 μg/ml). The previous method produced most MIC results against S. pneumoniae that were 1 (50.0%; 25/50) or 2 (40.0%; 20/50) log2 dilutions higher than those obtained by the revised method, with final MIC50 results with the previous method (0.03 μg/ml) 4-fold higher than those with the latter (0.008 μg/ml) (Tables 1 and 2). Among candidate dry-form panels tested, all had EA rates above the minimal acceptable target (i.e., ≥90%), and one formulation had highest overall EA rates (98.7%) compared to those with the revised method (Table 3). EA rates of ≥99.0% were observed for all species or groups of organisms except for S. pneumoniae (94.0%) and the challenge set (96.4%).
TABLE 3.
MIC result variations and summary of essential agreement rates between dry-form broth microdilution formulation panel (Sensititre) and revised reference method for telavancin
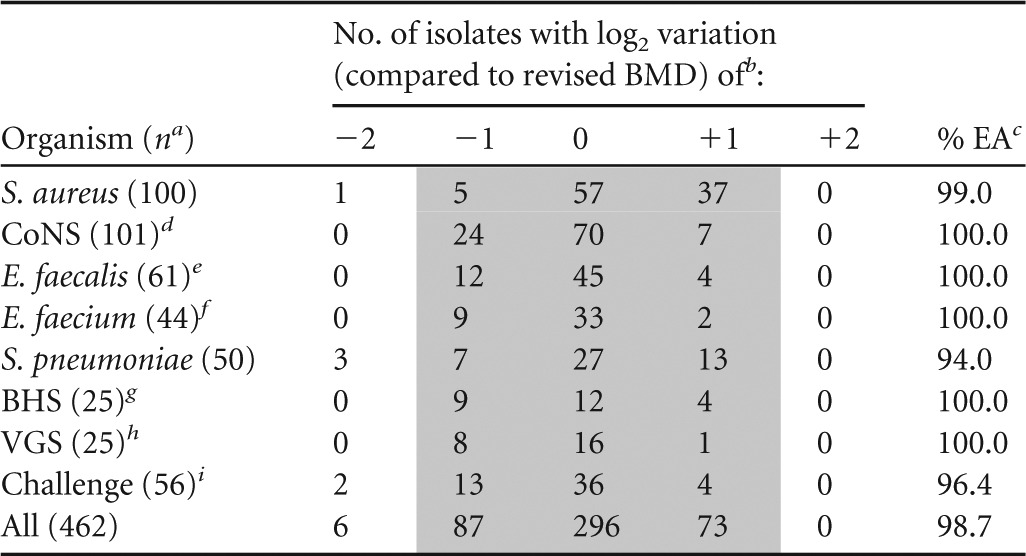
n, no. of isolates tested.
Previously established BMD panels prepared with DMSO and water as the solvent and diluent, respectively, and no P-80 supplementation versus a revised BMD panel (DMSO as solvent and diluent and P-80 supplementation [0.002%]).
Percentage of essential agreement (±1 log2 dilution step), represented by the shaded area.
CoNS, coagulase-negative staphylococci.
Includes 20 VRE (15 VanA and 5 VanB phenotypes).
Includes 23 VRE (17 VanA and 6 VanB phenotypes).
BHS, beta-hemolytic streptococci.
VGS, viridans group streptococci.
Represent strains with key resistance phenotypes (11 hVISA, 5 VISA, 6 VRSA, 10 VRE, 13 daptomycin-nonsusceptible staphylococci, and 11 linezolid-resistant staphylococci).
As previously observed with dalbavancin (4) and oritavancin (5), the data presented here, using a large collection of clinically relevant strains, shows that the revised BMD method containing the addition of P-80 (common to all three lipoglycopeptides) provides lower MIC results than those obtained by the previous method, especially when tested against staphylococci and enterococci. In contrast, when tested against streptococci, the impact of the revised method on the telavancin MIC results was less pronounced, which was similar to those observed for the other lipoglycopeptides (4, 5). These results suggest that (i) P-80 is necessary for a more accurate MIC determination for telavancin and previous studies underestimated the drug's in vitro potency due to drug loss because of binding to plastic surfaces (1, 2, 13–15) and (ii) similar to dalbavancin and oritavancin, presence of LHB provides an effect similar to that of P-80. Arhin et al. (5) demonstrated that the recovery of radiolabeled [14C]oritavancin from 96-well plates decreased rapidly in the absence of P-80 or LHB, while the presence of either of these reagents or of both promoted nearly 100% recovery. Similar experiments were performed for telavancin, and similar results were obtained (data on file; Theravance, Inc.).
Also noteworthy were the 4- to 8-fold-lower telavancin MIC results obtained against S. aureus by the revised method, where P-80 was present throughout the manufacturing process of the 96-well panels and susceptibility testing. During the development of this revised method, previous telavancin MIC determinations obtained when P-80 was added only at the latest step (bacterial inoculation) resulted in MIC values against S. aureus clinical isolates that were only 2-fold lower (data on file; JMI Laboratories). These results also were observed for oritavancin, indicating that the presence of P-80 at 0.002% throughout the panel manufacturing process and susceptibility testing maximizes the reagent's (P-80) ability in promoting drug availability. This additional evidence supports that P-80 minimizes drug binding to plastic surfaces, rather than acting synergistically with telavancin. Otherwise, if synergistic activity were expected, results should have been similar, since the final testing concentration of P-80 was the same for both determinations but was just introduced at a different phase of susceptibility testing (5).
It is also important to mention that although this revised method provides lower MIC determinations for telavancin, the antimicrobial susceptibility profile remains similar to that established by using the previous BMD method (1, 2, 13–15). The results presented here show that telavancin remains less active against VRSA and VanA-phenotype enterococci, regardless of the susceptibility testing method applied, while higher antimicrobial activity was observed against other tested Gram-positive pathogens, including MRSA, VISA, hVISA and vancomycin-resistant VanB enterococci. These antimicrobial profile characteristics have been very well documented in studies performed during drug development or after regulatory approval when applying the previous BMD method (1, 2, 13–15).
In summary, these study results demonstrate that the previous BMD method adopted by CLSI (use of DMSO as a solvent and diluent for panel preparation and addition of P-80 to the broth) ensures a proper assessment of the telavancin MIC determination, especially when tested against staphylococci and enterococci. The results presented here also validate a commercial dry-form formulation panel, which can be used as an alternative method for telavancin susceptibility testing in the clinical microbiology setting, along with adequate QC ranges and interpretive breakpoints (3, 8, 9). Lastly, the telavancin in vitro MIC results tested against Gram-positive organisms by the revised BMD method are now comparable to those reported for other lipoglycopeptide agents (i.e., oritavancin and dalbavancin), for which results were also generated by BMD susceptibility testing methodologies similar to that presented here for telavancin (16, 17).
ACKNOWLEDGMENTS
This study was sponsored by an educational/research grant from Theravance, Inc. (South San Francisco, CA). D. J. Farrell, R. E. Mendes, P. R. Rhomberg, and R. N. Jones are employees of JMI Laboratories who receive grant funds to study telavancin and were paid consultants to Theravance in connection with the development of the manuscript. Coordination of scientific review of the draft manuscript by Theravance and partners was conducted by Suzanne Douthwaite, an employee of Envision Scientific Solutions, funded by Theravance.
Footnotes
Published ahead of print 14 July 2014
REFERENCES
- 1.Mendes RE, Sader HS, Farrell DJ, Jones RN. 2012. Telavancin activity tested against a contemporary collection of Gram-positive pathogens from USA hospitals (2007-2009). Diagn. Microbiol. Infect. Dis. 72:113–117. 10.1016/j.diagmicrobio.2011.09.023 [DOI] [PubMed] [Google Scholar]
- 2.Farrell DJ, Krause KM, Benton BM. 2011. In vitro activity of telavancin and comparator antimicrobial agents against a panel of genetically defined staphylococci. Diagn. Microbiol. Infect. Dis. 69:275–279. 10.1016/j.diagmicrobio.2010.09.017 [DOI] [PubMed] [Google Scholar]
- 3.Theravance, Inc. 2014. Vibativ, package insert. Theravance, Inc., South San Francisco, CA: http://www.vibativ.com Accessed 25 April 2014 [Google Scholar]
- 4.Rennie RP, Koeth L, Jones RN, Fritsche TR, Knapp CC, Killian SB, Goldstein BP. 2007. Factors influencing broth microdilution antimicrobial susceptibility test results for dalbavancin, a new glycopeptide agent. J. Clin. Microbiol. 45:3151–3154. 10.1128/JCM.02411-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arhin FF, Sarmiento I, Belley A, McKay GA, Draghi DC, Grover P, Sahm DF, Parr TR, Jr, Moeck G. 2008. Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob. Agents Chemother. 52:1597–1603. 10.1128/AAC.01513-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderegg TR, Biedenbach DJ, Jones RN. 2003. Initial quality control evaluations for susceptibility testing of dalbavancin (BI397), an investigational glycopeptide with potent Gram-positive activity. J. Clin. Microbiol. 41:2795–2796. 10.1128/JCM.41.6.2795-2796.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arhin FF, Tomfohrde K, Draghi DC, Aranza M, Parr TR, Jr, Sahm DF, Moeck G. 2008. Newly defined in vitro quality control ranges for oritavancin broth microdilution testing and impact of variation in testing parameters. Diagn. Microbiol. Infect. Dis. 62:92–95. 10.1016/j.diagmicrobio.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing: 24th informational supplement M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9.Ross JE, Mendes RE, Jones RN. 11 June 2014. Quality control MIC ranges used for telavancin with application of a revised CLSI reference broth microdilution method. J. Clin. Microbiol. 10.1128/JCM.01210-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing: 23rd informational supplement M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M07-A9, 8th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 12.U.S. Food and Drug Administration. 2009. Guidance for industry and FDA. Class II special controls guidance document: antimicrobial susceptibility test (AST) systems. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm080564.htm Accessed 12 February 2014
- 13.Mendes RE, Sader HS, Farrell DJ, Jones RN. 2011. Update on the telavancin activity tested against European staphylococcal clinical isolates (2009-2010). Diagn. Microbiol. Infect. Dis. 71:93–97. 10.1016/j.diagmicrobio.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 14.Draghi DC, Benton BM, Krause KM, Thornsberry C, Pillar C, Sahm DF. 2008. Comparative surveillance study of telavancin activity against recently collected Gram-positive clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:2383–2388. 10.1128/AAC.01641-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draghi DC, Benton BM, Krause KM, Thornsberry C, Pillar C, Sahm DF. 2008. In vitro activity of telavancin against recent Gram-positive clinical isolates: results of the 2004-05 Prospective European Surveillance Initiative. J. Antimicrob. Chemother. 62:116–121. 10.1093/jac/dkn124 [DOI] [PubMed] [Google Scholar]
- 16.Jones RN, Flamm RK, Sader HS. 2013. Surveillance of dalbavancin potency and spectrum in the United States (2012). Diagn. Microbiol. Infect. Dis. 76:122–123. 10.1016/j.diagmicrobio.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 17.Mendes RE, Sader HS, Flamm RK, Farrell DJ, Jones RN. 18 February 2014. Oritavancin activity against Staphylococcus aureus causing invasive infections in USA and European hospitals. A five-year international surveillance program. Antimicrob. Agents Chemother. 10.1128/AAC.02482-13 [DOI] [PMC free article] [PubMed] [Google Scholar]


